Abstract
Background:
The formation of new connective periodontal attachment is contingent upon the elimination or marked reduction of pathogens at the treated periodontal site. An anti-microbial agent, i.e. moxifloxacin has been incorporated into the bone graft to control infection and facilitate healing during and after periodontal therapy.
Materials and Methods:
By purposive sampling, 15 patients with at least two contralateral vertical defect sites were selected. The selected sites in each individual were divided randomly into test and control sites according to split-mouth design. Test site received moxifloxacin-hydroxyapatite composite graft and control site received hydroxyapatite-placebo gel composite graft. Probing depth (PD) and Clinical attachment level (CAL) were assessed at baseline, 3, 6, 9, and 12 months. Bone probing depth (BPD) and hard tissue parameters such as amount of defect fill, percentage of defect fill, and changes in alveolar crest were assessed at baseline, 6, and 12 months. Changes in subgingival microflora were also assessed by culturing the subgingival plaque samples at baseline and at 3-month follow-up. The clinical, radiographic, and microbiological data obtained were subjected to statistical analysis using descriptive statistics, paired sample t-test, independent t-test, and contingency test.
Results:
On intragroup comparison at test and control sites, there was a significant improvement in all clinical and radiographic parameters. However, on intergroup comparison of the same, there was no statistically significant difference between test and control sites at any interval. Although test sites showed slightly higher amount of bone fill, it was not statistically significant. There was a significant reduction in the counts of Aggregatibacter actinomycetemcomitans and Porphyromonas gingivalis at both sites from baseline to 3 months. In addition, there was a significant reduction at test sites as compared to control sites at 3-month follow-up (P = 0.003 and P = 0.013).
Conclusion:
The reduction in microbial counts found in test sites at 3-month follow-up could not bring similar significant improvements in the clinical and radiographic parameters though the test sites showed slightly higher bone fill.
Key words: Biodegradable gel, clinical parameters, composite graft, hydroxyapatite, intrabony defects, moxifloxacin, radiographic parameters, subgingival microflora
Introduction
Regenerative approaches to treat periodontitis lesions offer exciting possibilities; however, they frequently fail to produce the desired clinical outcome due to infectious complications.[1] Since regenerative periodontal devices are placed in a potentially highly infected environment, their successful application depends on the prior removal or marked suppression of microbial pathogens at the treated site(s).[2]
Hence, various anti-microbial agents have been incorporated into the graft materials to control infection and facilitate healing during and after periodontal therapy. This is based on the concept of local drug delivery which was pioneered by Goodson et al. with the aim of achieving a high concentration of an antibiotic directly at the site of periodontal infection to inhibit the target pathogens, with minimal side effects and lesser reliance on patient's compliance for taking medication.[3]
Antibiotics that have been studied extensively in periodontal treatment include tetracyclines (minocyclines and doxycycline), pencillins (amoxicillin and augmentin), macrolides (azithromycin), lincomycin derivative (clindamycin), and nitroimidazole (metronidazole). However, due to the problems of bacterial resistance, alternatives for the currently used antibiotics are needed. Moxifloxacin is one such alternative antibiotic.[4]
Moxifloxacin is a fourth-generation fluoroquinolone antibiotic with a broad antimicrobial activity against aerobic and anaerobic bacteria. It exerts an excellent antibacterial activity against a wide range of putative periodontal pathogens. It also penetrates well into soft tissues and is effective against intracellular periodontal pathogens.[5]
In the present study, moxifloxacin-containing biodegradable gel has been delivered to the intrabony defect site by mixing it with synthetic hydroxyapatite bone graft, forming a moxifloxacin-hydroxyapatite composite graft, and it is evaluated for efficacy in the regeneration of intrabony defects. To the best of authors’ knowledge, there has been no study that has used moxifloxacin along with bone grafts. In addition, this is the first study that used biodegradable gel as the vehicle of drug delivery directly into the defect site by mixing with bone graft.
Materials and Methods
A total of thirty sites from 15 systemically healthy patients with chronic periodontitis were selected based on the following inclusion criteria. Sample size of 15 was derived keeping the power of study as 80% based on the prevalence of chronic periodontitis.
Patients within the age group of 30–55 years having chronic generalized periodontitis with contralateral intrabony periodontal defects, having periodontal pocket with probing depth (PD) of ≥6 mm, with radiographic evidence of bilateral vertical/angular defects, without any systemic problem which contraindicate surgical therapy, who have not taken antibiotics within last 6 months, and who have not undergone any type of regenerative periodontal therapy in the area to be treated 1 year prior to initial examination were included in the study.
Patients were excluded from the study if they failed to maintain adequate oral hygiene, were allergic to drug, had any deleterious habits such as smoking and tobacco chewing, and who were pregnant or lactating women.
All participants following the initial examination and treatment planning were subjected to full mouth scaling and root planing, and oral hygiene instructions were given. Occlusal equilibration, if required, was done, and routine laboratory investigations were performed. At re-evaluation, the oral hygiene maintenance was evaluated by the use of clinical indices, and only patients showing optimal oral hygiene were scheduled for the surgical procedure. The nature of the study was explained to the patients, and a written informed consent was obtained.
A split-mouth design was adopted in the present study. The selected sites in each individual were divided randomly into test site and control site.
Test site received moxifloxacin-hydroxyapatite composite graft.
Control site received hydroxyapatite-placebo gel composite graft.
Parameters
Probing depth and clinical attachment level (CAL) were assessed at baseline, 3, 6, 9, and 12 months [Figure 1]. Bone probing depth and hard tissue parameters (amount and percentage alveolar defect fill and alveolar crest resorption) were assessed at baseline, 6, and 12 months. Along with the above parameters, subgingival microflora was also studied by culturing the subgingival plaque samples at baseline and at 3-month follow-up. Plaque index, gingival index, and gingival bleeding index were recorded at all recall intervals to assess the patients’ oral hygiene maintenance.
Figure 1.
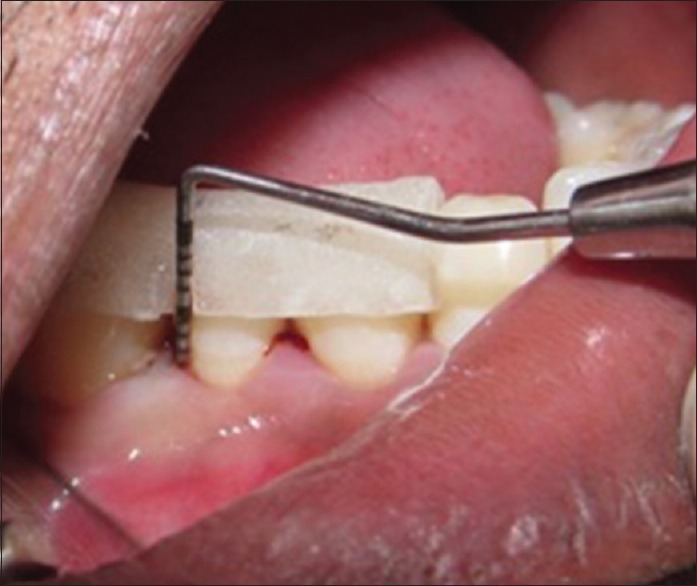
Preoperative probing
Measurements of clinical parameters which were recorded with UNC 15 probe were standardized using customized acrylic stents that were grooved in the area of defect to provide reproducible insertion axis [Figure 1].
Radiographic evaluation of the study was carried out by the conventional intraoral periapical radiographs (IOPARs) taken at baseline [Figures 2 and 3], 6 and 12 months. The long cone paralleling technique was used with conventional Kodak E Speed IOPAR films of size 2. An X-ray mesh gauge with grid markings of 1 mm2 was used along with IOPAR films. The XCP-Rinn holder was used to provide projection standardization. Exposures were made at 70 kvp, 8 mA, and 2 mm of aluminum filtration. Exposure time was standardized as per the patients’ built. The focus-to-film distance was maintained at 16 inches. The exposed films were processed with an automatic processor which was set at standardized processing time and temperature. The films were processed in sets in fresh developer and fixer solutions prepared as per the manufacturer's guidelines.
Figure 2.
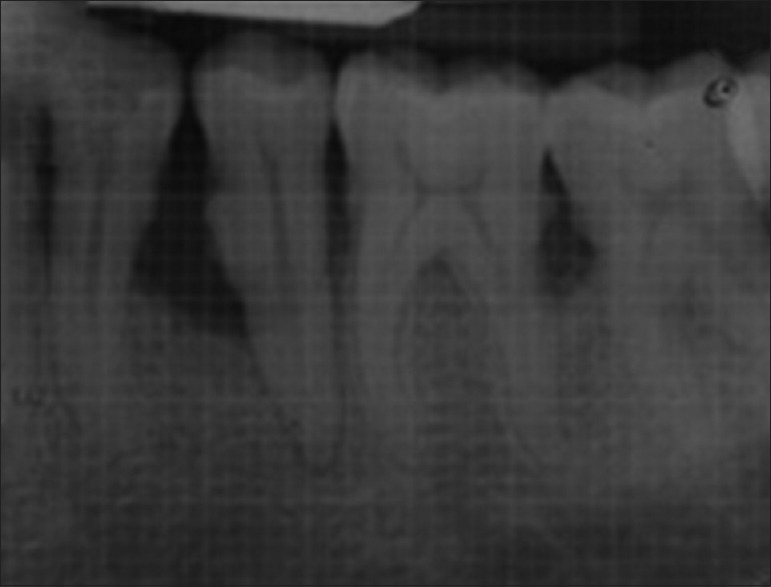
Intraoral periapical radiograph of test site at baseline
Figure 3.
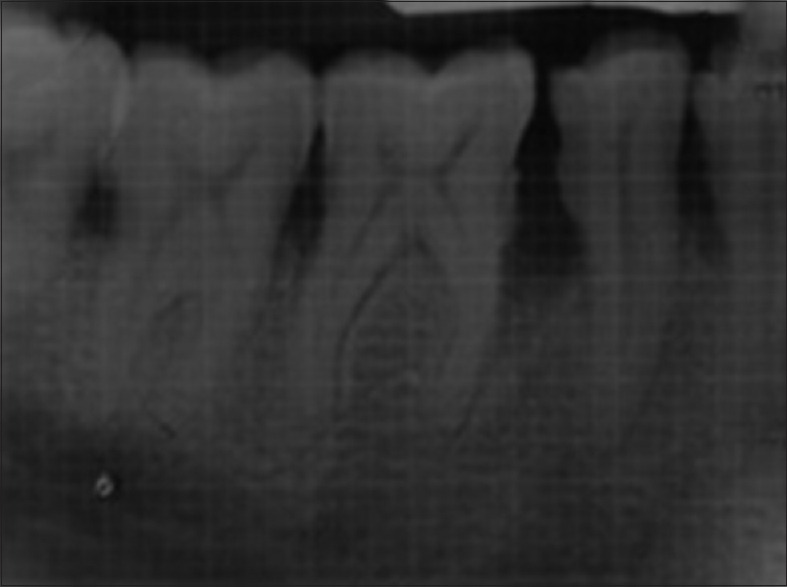
Intraoral periapical radiograph of control site at baseline
An IOPAR was taken for each selected site to measure the radiographic depth of the defect and to calculate the amount and percentage of bone defect fill. The radiographic measurements were carried out with the help of markings of grid on IOPAR. The cementoenamel junction (CEJ) was taken as the fixed reference point (FRP). The measurements of the radiographic images were done from CEJ to base of the defect (BOD) and CEJ to crest of the bone alveolar bone crest (AC).
At baseline on the day of surgery, radiographic defect depth and alveolar crest level (Alv) were measured. At 6 and 12 months, changes in Alv and amount and percentage of defect fill (PDF) were measured.
The radiographic defect depth was calculated as the linear distance (in mm) measured from the alveolar crest to the base of the bone defect, i.e. (FRP to BOD) − (FRP to AC)
Changes in Alveolar crest level (Alv) = (FRP to AC at baseline) − (FRP to AC at recall)
Amount of defect fill (ADF) = Initial defect depth − defect depth at recalled time interval
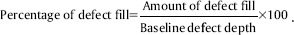
Materials
Materials used in the study were synthetic porous resorbable hydroxyapatite bone graft (Biograft HA), moxifloxacin gel 0.4%, and placebo gel.
Moxifloxacin gel 0.4% was formulated as follows:
Two percentage of hydroxypropyl methyl cellulose (HPMC) E15 LV and 1.5% of hydroxyethyl cellulose (HEC) (high viscosity grade 200) were weighed and completely dissolved in distilled water
Required quantity of moxifloxacin was dissolved in a small volume of methanol and was added to the preformed gel and it was properly mixed
Few drops of diluted solution of triethanolamine was added to the above mixture and stirred to get a clear gel. Benzal konium chloride (0.001% w/v) was added as a preservative
The final volume was made up with distilled water and gel was allowed to stand for 12 h
The final gel (HPMC E15 LV - 2%, HEC - 1.5%) was packed in sterile glass vials and stored till further use
The final gels were sterilized by gamma irradiation for a total dose of 2 KGray (60 Co source) for 20 min. Gamma irradiation of the samples was performed at room temperature (25°C and 60% RH) in air
The sterile moxifloxacin gels were used for the clinical study.
The gel formulated was tested for:
Compatibility between moxifloxacin and additives of gel
Compatibility between moxifloxacin and synthetic hydroxyapatite bone graft
Uniformity of drug content in gel
In vitro drug release studies of the gel.
Placebo gel was prepared in the same method as that of antibiotic gel except that the drug was not incorporated in to it.
Surgical procedure
After following the aseptic protocol, the defect sites were divided randomly as test site 1 and test site 2. The selected defect area was anesthetized using 2% lignocaine with 1:80,000 adrenaline using block and infiltration techniques. Sulcular incisions were given on the facial and lingual sides using bard parker knife with blade no. 12. A full-thickness mucoperiosteal flap was raised to provide access to the defect. The defect was cleared of granulation tissue and thoroughly root planed. The surgical area was then irrigated with normal saline and carefully inspected for any remaining granulation tissue or deposits. Any adherent granulation tissue was trimmed from the flaps.
The defects in the test site were filled with moxifloxacin-hydroxyapatite composite graft prepared as follows; the required quantity of hydroxyapatite was transferred from the vial to the dappen dish and mixed with 0.4% moxifloxacin gel. When it became a cohesive mass, it was delivered into the vertical defects. The defects in the control site were filled with hydroxyapatite-placebo gel composite graft prepared in the same way with placebo gel.
At all the sites, the same proportion of graft and gel were mixed, i.e. for every 0.1 g of bone graft, 0.1 ml of gel was mixed. Small increments of graft-gel mixture were added into the defect and condensed properly until the defect was completely filled. Flaps were repositioned and secured in place using (3-0) black-braided silk. Sling and interrupted sutures were placed to obtain the primary closure. The surgical sites were protected with a noneugenol periodontal dressing (Coe-Pack), and analgesics were prescribed. Postoperative instructions were given to all the patients. They were instructed to report back after 7 days. At 7 days following the surgeries, the dressings and sutures were removed, and the patients were enquired regarding discomfort, pain, and sensitivity.
Postsurgical evaluation
Patients were recalled at 3, 6, 9, and 12 months to evaluate oral hygiene maintenance and record PD and CAL. Bone probing depth and radiographic parameters were evaluated at 6 month [Figures 4 and 5] and 12 month intervals [Figures 6 and 7].
Figure 4.
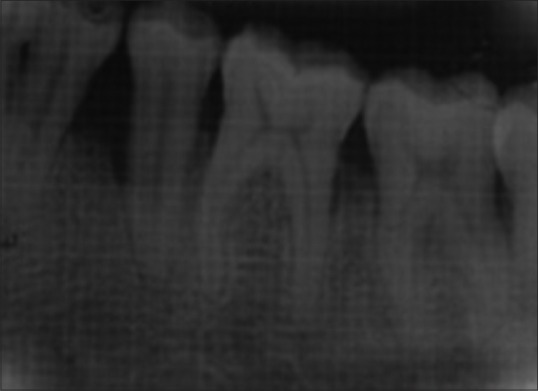
Intraoral periapical radiograph of test site at 6 months
Figure 5.
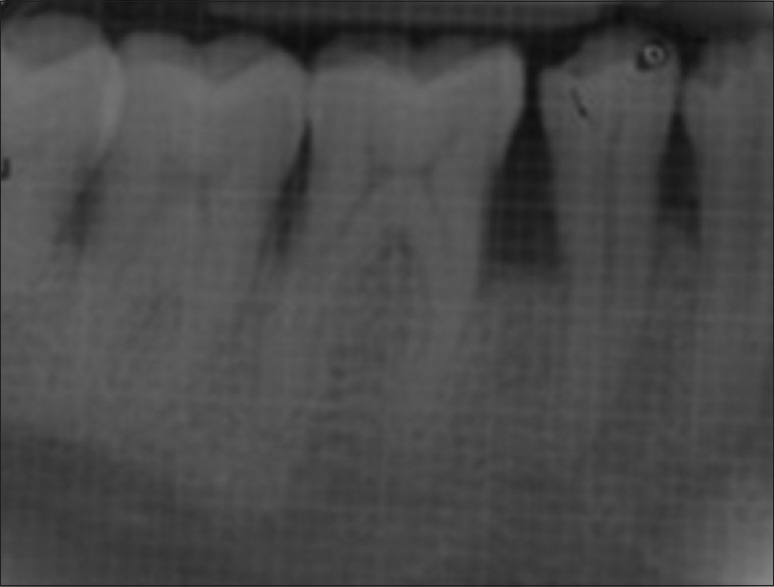
Intraoral periapical radiograph of control site at 6 months
Figure 6.
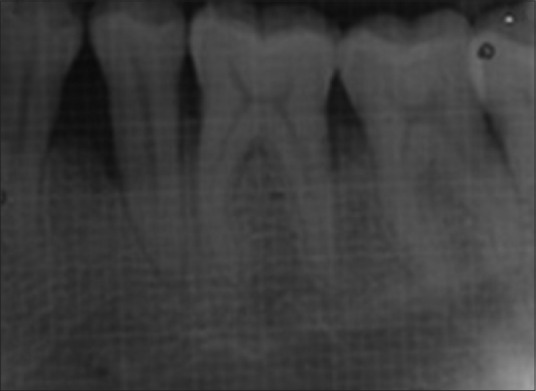
Intraoral periapical radiograph of test site at 12 months
Figure 7.
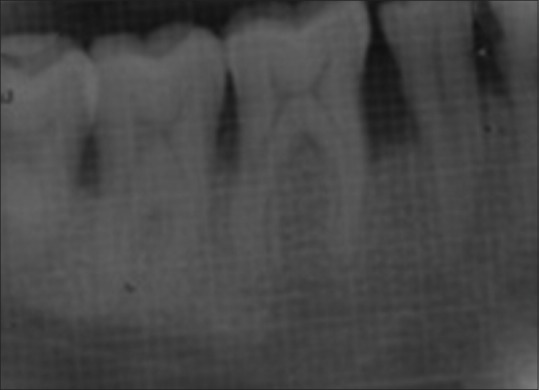
Intraoral periapical radiograph of control site at 12 months
Microbiologic analysis
To assess changes in the subgingival microflora, subgingival plaque samples were collected at the baseline and 3 month recall as follows. Supragingival plaque, if any, was removed from the surface of the selected teeth to prevent contamination of the flora. Then, the area was isolated using sterile cotton rolls, dried, and the subgingival plaque was collected using a sterile Gracey curette by inserting it subgingivally in the deepest portion of the periodontal pocket parallel to the long axis of the tooth and moved coronally by scraping along the root surface. The samples were directly inoculated. The procedure was again repeated after 3 months.
One sample was collected from each site. Each sample was then separately mixed in the thioglycolate broth. From this thioglycolate broth, each sample was again divided into two, one for the identification of Aggregatibacter actinomycetemcomitans and the other for Porphyromonas gingivalis. The subgingival plaque was further processed in the laboratory as follows.
Identification of Aggregatibacter actinomycetemcomitans
From the thioglycolate broth, subcultures were made on tryptone soya serum bacitracin vancomycin agar and incubated. Simultaneously, a Gram-stain smear study was done from the thioglycolate broth to know the microbiota. The inoculated plates were incubated as follows and examined for the growth of aerobic and anaerobic bacteria. The plates were arranged in the rack. The lid and the rim of the anaerobic jar were applied with grease. GasPak™ EZ gas-generating anaerobic sachet (containing inorganic carbonate, activated carbon, ascorbic acid, and water) was added to the anaerobic jar to maintain the anaerobic environment. The rack was placed in the anaerobic jar immediately. Clips were secured in place. The anaerobic jar was kept in the incubator at 37°C for 3 days. The semi-quantitative colony count was expressed in colony-forming units/milliliter (CFU/ml).
Identification of Porphyromonas gingivalis
From the thioglycolate broth, subcultures were made on blood agar and incubated aerobically as well as anaerobically. Sheep blood agar plates were used for anaerobic culture. Simultaneously, a Gram-stain smear study was done from the thioglycolate broth to know the microbiota. The inoculated plates were incubated for 72 h, and examined for the growth of aerobic and anaerobic bacteria. The black pigmented colonies in the anaerobic plate were confirmed as P. gingivalis by smear examination. Colony counting was done. The colonies were further subjected to the following tests for the identification of the specific organism: ultraviolet-light microscopy, agglutination of sheep erythrocytes, iodole test, and fermentation test - sucrose and lactose.
Colony counting was done using colony counter as follows:
0= <10,000 CFU/μl or not detected
1+ =10,000-25,000 CFU/ml
2+ =25,000-50,000 CFU/ml
3+ =50,000-75,000 CFU/ml
4+ = >75,000 CFU/ml.
Results
All patients tolerated the surgical procedure well, experienced no postoperative complications, complied with the study protocol, and completed the 12-month follow-up. All the patients involved in the study maintained a good oral hygiene. All the parameters were recorded by a single trained examiner who was blinded throughout the study. The observations recorded were subjected to statistical analysis.
Clinical evaluation of PDs revealed that the reduction in PD noted at test sites was 1.8000 ± 0.41404 at 3 months, 2.7333 ± 0.45774 at 6 months, 3.4667 ± 0.51640 at 9 months, and 4.0667 ± 0.59362 at 12 months [Table 1]. In the control sites, reduction was 1.8667 ± 0.51540 at 3 months, 2.800 ± 0.56061 at 6 months, 3.400 ± 0.50709 at 9 months, and 4.0000 ± 0.53452 at 12 months [Table 1].
Table 1.
Intragroup comparison of probing depth and clinical attachment level test
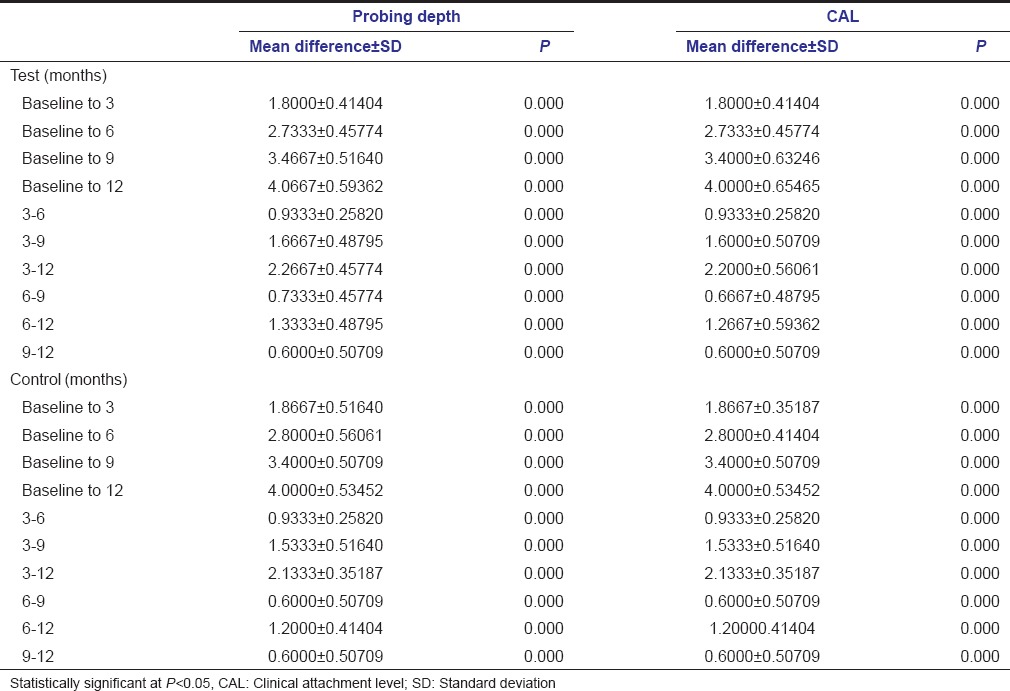
The gain in clinical attachment noted at the test site was 1.8000 ± 0.41404 at 3 months, 2.7333 ± 0.45774 at 6 months, 3.4000 ± 0.63246 at 9 months, and 4.0000 ± 0.65465 at 12 months [Table 1]. At control sites, it was 1.8667 ± 0.35157 at 3 months, 2.8000 ± 0.41404 at 6 months, 3.4000 ± 0.50709 at 9 months, and 4.0000 ± 0.53452 at 12 months [Table 1].
With reference to PD and CAL, there was no statistical difference observed between the test and control sites at any of the time intervals in the present study [Table 2]. However, within the test and control sites, the reduction in PD and gain in CAL were significant between the various time intervals [Table 2].
Table 2.
Intergroup comparison of clinical and radiographic parameters
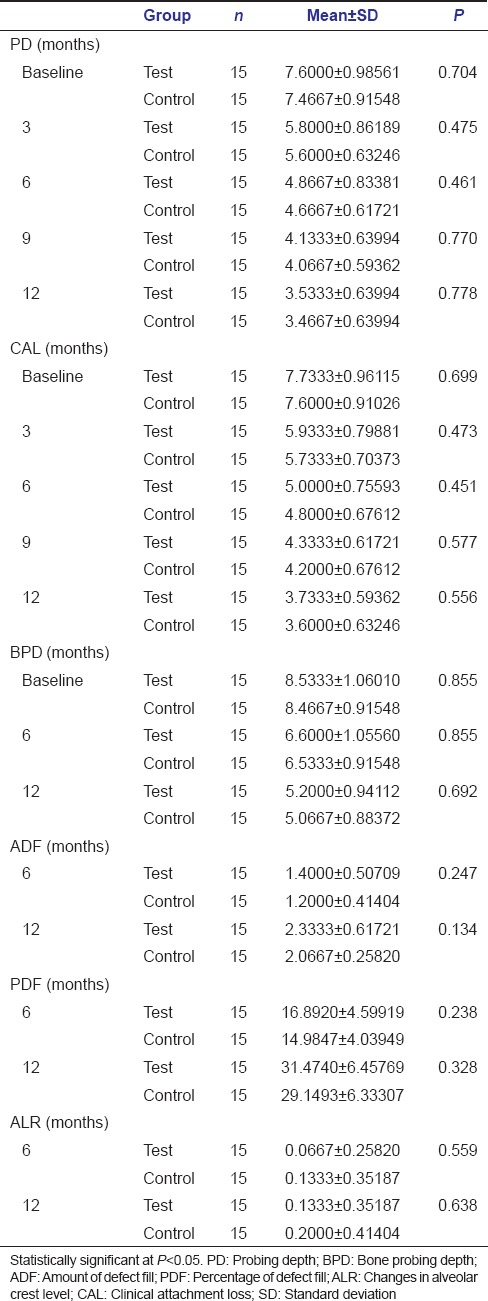
The present study showed a significant mean reduction in BPD at both test and control sites at any time interval with no significant differences between the two sites [Tables 2 and 3]. The mean reduction in bone PD for test site was 1.9333 ± 0.25820 mm at 6 months and 3.3333 ± 0.48795 mm at 12 months. The mean reduction in bone PD for control was 1.9333 ± 0.25820 mm at 6 months and 3.4000 ± 0.50709 mm at 12 months [Table 3].
Table 3.
Intragroup comparison of bone probing depth and radiographic parameters
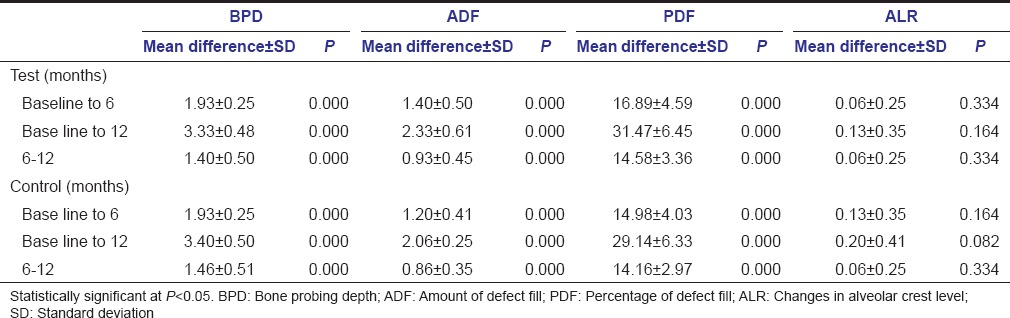
The ADF at test site was 1.4000 ± 0.50709 (16.9%) at 6 months and 2.3333 ± 0.6172 (31.45%) at 12 months. At control sites, the values were 1.2000 ± 0.41404 (14.98%) at 6 months and 2.0667 ± 0.25820 (29.14%) at 12 months [Table 3].
There was a significant improvement in the defect fill (ADF and PDF) at 6 and 12 months at both the sites. Although the improvement was slightly more in test sites at 6 and 12 months as compared to control sites, it was not statistically significant.
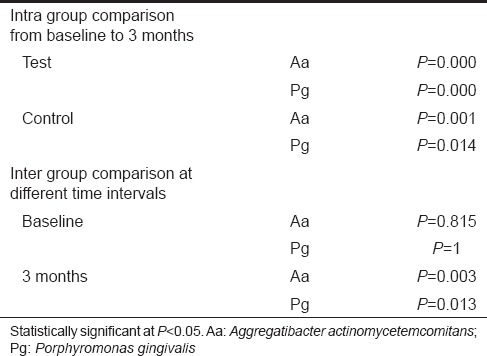
In the present study, there was a significant reduction in the counts of Aa and Pg in both the sites from baseline to 3-month follow-up. On intergroup comparison, there was a significant reduction in the counts of Aa and Pg at test sites compared to control sites at 3-month postoperative follow-up period [Tables 4 and 5].
Table 4.
Bacterial counts of Aggregatibacter actinomycetemcomitans and Porphyromonas gingivalis in test and control sites at baseline and 3 months
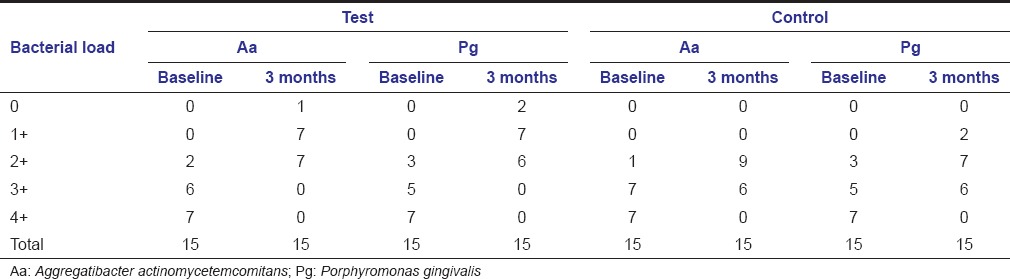
Table 5.
Intra- and inter-group comparison of microbial counts of Aggregatibacter actinomycetemcomitans and Porphyromonas gingivalis
The compatibility testing of moxifloxacin with excipients (components used in the gel) and bone graft was studied using Fourier-transform infrared spectrophotometer, and the results confirm that there are no interactions with the drug and other components of the gel and also with the bone graft.
The results of content uniformity studies indicated that moxifloxacin was uniformly distributed in the prepared gel. The drug content was 98.80 ± 0.35% (n = 3), proved the proper dispersion of drug in the gel.
The in vitro drug release (diffusion) studies using open (diffusion) tube apparatus showed that 98.91 ± 0.69% (n = 3) of the drug was released in 84 h, proving that the drug release was in a controlled manner for the stated period, which improves the efficacy of treatment.
Discussion
In the present study, a moxifloxacin-containing biodegradable gel has been formulated and mixed with synthetic hydroxyapatite bone graft forming a moxifloxacin-hydroxyapatite composite graft, and it is evaluated for efficacy in the regeneration of intrabony defects. This method was employed in an attempt to (1) suppress the periodontal pathogens in the intrabony defect, (2) prevent their recolonization, and (3) preclude any possible infection of the grafted area.
Moxifloxacin was selected for this present study because of its excellent antibacterial efficacy. It exerts a bactericidal effect by specifically inhibiting adenosine triphosphate-dependent topoisomerase IV and topoisomerase II (DNA gyrase). It exhibits antibacterial activity against a wide range of putative periodontal pathogens, including P. gingivalis, Tannerella forsythia, Prevotella spp., Fusobacterium nucleatum, Actinomyces spp., Peptostreptococcus spp., Campylobacter rectus, and A. actinomycetemcomitans. It penetrates well into soft tissues and is effective against intracellular periodontal pathogens. It shows an excellent bioavailability and tolerability, a long half-life, and a good tissue penetration.[5]
Moxifloxacin was used at 0.4% concentration in this study because Flemmig et al. in their study proved that 0.4% moxifloxacin gel was most effective as a local drug delivery agent. Furthermore, in the same study, it was reported that this particular concentration (4000 mg/L) exceeded the MICs for putative periodontal pathogens by a factor of 102−106.[5]
Biodegradable gel was used in the present study as a vehicle to carry and retain the moxifloxacin and hydroxyapatite at the treated site, to release drug in slow and sustained manner, to function as a system preventing particle scatter, and to facilitate graft retention. Gels were formulated in a clean environment and dispensed in transparent glass containers with air-tight caps and were sterilized under gamma irradiation (10 kilo gray). In the present study, in all the sites, same proportion of graft and gel were mixed, i.e., for every 0.1 g of bone graft, 0.1 ml of gel was mixed.
With reference to PD and CAL, there was no statistical difference observed between test and control sites at any of the time intervals in the present study. However, within the test and control sites, the reduction in PD and gain in CAL were significant between the various time intervals.
The results of the present study are in accordance with the following studies. Agarwal et al. in their study evaluated the regenerative outcomes of bone graft with and without local doxycycline and reported that both the groups showed a significant reduction in PD and gain in CAL from baseline to 6 months, but there was no statistically significant difference between both groups.[6] Kaur and Sikri in their study reported that mean reduction in PD and gain in CAL were greater in test group (doxycycline-loaded allograft) than in control group (allograft alone) at the end of 24 weeks, but the difference was statistically nonsignificant.[7] Stavropoulos et al. in their study reported that at the end of 1 year, there was a reduction in mean PD and gain in clinical attachment when gentamicin was used along with Bio-Osss, but it was not significant.[8]
Contrary to the present study results, Pepelassi et al. reported in their study that sites treated with the tricalcium phosphate, Plaster of Paris, and doxycycline composite graft had a significant reduction in PDs and gain in clinical attachment when compared to the surgically debrided control sites at the end of 6 months.[9]
In the present study, surgical re-entry was not considered to avoid the second surgical procedure which may depend on patient acceptability and cause ethical concerns. Thus, clinical and radiographic methods were employed in this study.
There was a significant improvement in the amount and PDF at 6 and 12 months at both the sites. Although the improvement was slightly more in test sites at 6 and 12 months as compared to control sites, it was not statistically significant.
The results of the present study are in accordance with the following studies. Agarwal et al. evaluated the regenerative outcomes of bone graft with or without local doxycycline in noncontained infrabony periodontal defects. Radiographic defect fill was 18% in control and 20.7% in test at the end of 6 months. Both the groups showed significant defect fill at 6 months, but the difference was not significant.[6] Masters et al. in a clinical trial to evaluate the use of demineralized freeze-dried bone allograft reconstituted with 50 mg/ml tetracycline hydrochloride in the treatment of intrabony periodontal defects reported that at the end of 12 months, the amount of bone fill in the sites filled with decalcified freeze-dried bone allograft (DFDBA) and tetracycline, DFDBA only, and debridement alone was 2.27 mm, 2.20 mm, and 1.27 mm, respectively. Although the grafted groups showed a greater bone fill, there was no statistically significant difference between the treatment groups.[10] In a study by Stavropoulos et al., it was revealed that the radiographic bone level gain in sites treated with guided tissue regeneration (GTR) and Bio-Osss impregnated with gentamicin (4.7 mm) was superior to those treated with GTR alone (3.1 mm) or GTR along with Bio-Osss (2.8 mm), but the difference was not significant.[8]
In contrast to the present study, Kaur and Sikri in their study evaluated clinically and radiographically the regenerative potential of doxycycline-loaded allograft with that of allograft alone in the treatment of human periodontal infrabony defects and reported that both the groups exhibited a highly significant linear bone fill at the end of 12 and 24 weeks. Intergroup comparison showed a statistically significant gain in bone fill in test group (doxycycline + allograft) as compared to control group (allograft alone).
In the present study, there was a significant reduction in the counts of Aa and Pg in both the sites from baseline to 3-month follow up. On intergroup comparison, there was a significant reduction in the counts of Aa and Pg at test sites compared to control sites at 3-month postoperative follow-up period. This is because moxifloxacin is effective against many putative periodontal pathogens including Aa and Pg, which has been proved in previous studies.[5] Flemmig et al. reported in their study that there was a reduction in the counts of Pg from baseline to 6 weeks when 0.4% moxifloxacin gel was used as a local drug delivery agent in adjunct to SRP.
Thus, within the limitations of the present study, test sites showed a significant reduction in the counts of Aa and Pg compared to the control sites 3 months postoperatively. Regarding clinical and radiographic parameters, both the test and control sites showed a significant improvement from baseline to consecutive follow-ups, but there was no significant difference between both groups at any time interval. Even though test sites showed slightly higher bone fill, it was not statistically significant.
Conclusion
The moxifloxacin incorporated in the bone graft brought a significant reduction in microbial counts at test sites, but it could not bring similar significant improvements in clinical and radiographic parameters.
Further studies with larger sample size are required to confirm the beneficial effects of mixing different antibiotics with bone grafts in various methods and proportions to enhance treatment outcomes.
Financial support and sponsorship
We express our sincere thanks to the Indian Council of Medical Research, Department of Health Resource, Ministry of Health and Family Welfare, Ansari Nagar, New Delhi, for providing research grant to our study (Ref no. 3/2/2012/PG-thesis-HRD-25, dated 29-8-2013).
Conflicts of interest
There are no conflicts of interest.
References
- 1.Nowzari H, London R, Slots J. The importance of periodontal pathogens in guided periodontal tissue regeneration and guided bone regeneration. Compend Contin Educ Dent. 1995;16:1042–58. [PubMed] [Google Scholar]
- 2.Zimmerli W, Lew PD, Waldvogel FA. Pathogenesis of foreign body infection. Evidence for a local granulocyte defect. J Clin Invest. 1984;73:1191–200. doi: 10.1172/JCI111305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goodson JM, Offenbacher S, Farr DH, Hogan PE. Periodontal disease treatment by local drug delivery. J Periodontol. 1985;56:265–72. doi: 10.1902/jop.1985.56.5.265. [DOI] [PubMed] [Google Scholar]
- 4.Guentsch A, Jentsch H, Pfister W, Hoffmann T, Eick S. Moxifloxacin as an adjunctive antibiotic in the treatment of severe chronic periodontitis. J Periodontol. 2008;79:1894–903. doi: 10.1902/jop.2008.070493. [DOI] [PubMed] [Google Scholar]
- 5.Flemmig TF, Petersilka G, Völp A, Gravemeier M, Zilly M, Mross D, et al. Efficacy and safety of adjunctive local moxifloxacin delivery in the treatment of periodontitis. J Periodontol. 2011;82:96–105. doi: 10.1902/jop.2010.100124. [DOI] [PubMed] [Google Scholar]
- 6.Agarwal A, Bhattacharya HS, Srikanth G, Singh A. Comparative evaluation of decalcified freeze dried bone allograft with and without local doxycycline in non-contained human periodontal infrabony defects. J Indian Soc Periodontol. 2013;17:490–4. doi: 10.4103/0972-124X.118322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaur K, Sikri P. Evaluation of the effect of allograft with doxycycline versus the allograft alone in the treatment of infrabony defects: A controlled clinical and radiographical study. Dent Res J (Isfahan) 2013;10:238–46. doi: 10.4103/1735-3327.113359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stavropoulos A, Karring ES, Kostopoulos L, Karring T. Deproteinized bovine bone and gentamicin as an adjunct to GTR in the treatment of intrabony defects: A randomized controlled clinical study. J Clin Periodontol. 2003;30:486–95. doi: 10.1034/j.1600-051x.2003.00258.x. [DOI] [PubMed] [Google Scholar]
- 9.Pepelassi EM, Bissada NF, Greenwell H, Farah CF. Doxycycline-tricalcium phosphate composite graft facilitates osseous healing in advanced periodontal furcation defects. J Periodontol. 1991;62:106–15. doi: 10.1902/jop.1991.62.2.106. [DOI] [PubMed] [Google Scholar]
- 10.Masters LB, Mellonig JT, Brunsvold MA, Nummikoski PV. A clinical evaluation of demineralized freeze-dried bone allograft in combination with tetracycline in the treatment of periodontal osseous defects. J Periodontol. 1996;67:770–81. doi: 10.1902/jop.1996.67.8.770. [DOI] [PubMed] [Google Scholar]


