Abstract
Background:
Conventional nonsurgical periodontal therapy has been proven to be an effective treatment for patients with chronic periodontitis. Coenzyme Q10 and tea tree oil (TTO) are known to have potential therapeutic benefits in chronic periodontitis.
Aims:
The aim of the study is to compare the efficacy of Coenzyme Q10 (Perio Q®) and tea tree oil (Melaleuca alternifolia) gel as an adjunct to scaling and root planing in the treatment of chronic periodontitis.
Materials and Methods:
Patients were divided equally into three groups: Group I (Control group): those receiving placebo gel + SRP, Group II (Test group I): those receiving Perio QTM gel + SRP, and Group III (Test group II): those receiving tea tree oil gel + SRP. A total of 15 patients with 45 sites were enrolled in the study. Clinical parameters evaluated were plaque index (PI), gingival bleeding index (GI), probing pocket depth (PPD), and clinical attachment level (CAL).
Statistical Analysis Used:
Paired t-test was applied using SPSS software.
Results:
Mean PPD reduction for Group I, Group II, and Group III was 0.50 ± 0.2, 2.95 ± 0.20, and 2.09 ± 0.15, respectively. Mean CAL reduction for Group I, Group II, and Group III was 0.45 ± 0.22, 2.33 ± 0.04, and 2.28 ± 0.09, respectively. Changes in mean PI scores for Group I, Group II, and Group III were 0.67 ± 017, 1.00 ± 0.11, and 1.08 ± 0.05 and GBI scores were 0.92 ± 0.29, 1.08 ± 0.13, and 0.88 ± 0.28, respectively.
Conclusions:
Coenzyme Q10 and tea tree oil gel proved to be effective in the treatment of chronic periodontitis.
Key words: Coenzyme Q10, free radicals, periodontitis, reactive oxygen species, tea tree oil
Introduction
Periodontitis is an immuno-inflammatory disease process resulting from the interaction of a bacterial attack and host inflammatory response. This leads to the inflammation of the supporting tissues of the teeth further leading to tissue destruction and tooth loss. Varieties of molecules are considered to mediate the inflammatory response at one time or another. Among these are free radicals (FRs) and reactive oxygen species (ROS) known to cause destruction such as superoxide anion radicals, hydrogen peroxide, hydroxyl radicals, and hypochlorous acid. All these molecules are capable of damaging either cell membranes or associated biomolecules. Periodontal pathogens can induce ROS overproduction and thus may cause collagen and periodontal cell breakdown. When ROS are scavenged by antioxidants, there is a reduction of collagen degradation.[1] Hence, antioxidants are emerging as prophylactic and therapeutic agents. Antioxidants delivered by the diet,[2] systemically,[3] locally,[4] and through a dentifrice[5] have been shown to cause significant improvements in the measures of gingivitis, periodontitis, and oxidative injury.
Coenzyme Q10 (CoQ10) was discovered in beef heart mitochondria at the University of Wisconsin.[6] CoQ10 is also known as ubiquinone because of its ubiquitous presence in nature and its Quinone structure (similar to that of Vitamin K).[7] It is also called as “coenzyme” because of its unique ability to participate in chemical reactions but remain at steady-state levels in the cell and plays a central role in energy metabolism. It has a positive inotropic effect.[8] The effects and mechanisms of action of CoQ10 include stabilization of calcium-dependent channels, inhibition of intracellular phospholipases, prostaglandin metabolism, FR scavenging, and direct membrane stabilization.[9] Co-Q10 is also known to play a crucial role in the generation of adenosine triphosphate (ATP) and cellular respiration. It exists in two molecular forms, ubiquinone, the oxidized form, and ubiquinol, the reduced form, which are the basis for its antioxidant properties.[10] Co-Q10 functions as an intercellular antioxidant by acting as a primary scavenger of FRs and ROS. It serves as an endogenous antioxidant, and its increased concentration in the diseased gingiva effectively suppresses advanced periodontal inflammation.
Tea tree oil (TTO) is derived from the paper bark tea tree.[11] TTO has a broad-spectrum antimicrobial, antifungal, antiviral, antioxidant, and anti-inflammatory effect. A few studies have shown the therapeutic effect of the local application of TTO on periodontal tissues.[11]
Thus, the aim of the study was to compare and evaluate the efficacy of Coenzyme Q10 and TTO gel as an adjunct to SRP in the treatment of chronic periodontitis.
Materials and Methods
Fifteen patients (6 males, 9 females) were selected from the Outpatient Department of Periodontology. Ethical clearance was obtained from the Institutional Ethical Committee. Systemically healthy patients in the age group of 20-60 (37.4 ± 9.76) years of both the genders who were diagnosed with chronic periodontitis clinically and radiographically were included in the study.[12,13]
Subjects included in the study were patients with untreated moderate to severe chronic periodontitis, sites with probing pocket depth (PPD) of >5 mm and clinical attachment loss >4 mm.
Subjects excluded from the study were patients with systemic disease that affects the periodontium, pregnant or lactating females, patients who received any anti-inflammatory drugs, antibiotics or vitamins within the previous 3 months, patients who were smokers (>10 cigarettes/day), had received scaling and root planing (SRP) or subgingival instrumentation <2 months before the baseline examination.[14,15]
Materials
Perio Q® gel
Perio Q® gel (PerioQ Inc., Manchester, USA) is a mixture of CoQ10 in a vegetable oil base in a ratio of 1:9. It is supplied as a pack of gel and was stored at a temperature between 4°C and 8°C for maintaining its shelf-life.
Tea-tree oil gel preparation
Gel formulation was prepared from commercially available TTO (Allin Exporters, India). It was used as a locally delivered sustained release subgingival gel and was prepared as follows: To prepare the methyl cellulose gel, 7 g of methyl cellulose powder was dissolved in 100 ml boiled water. To make the TTO gel 5%, 5 ml of TTO was then mixed into the methylcellulose gel. The gel was sterilized in the autoclave at 110°C for 20 min.[16]
Placebo gel
The placebo gel preparation was same as that of tea-tree oil gel preparation except for the addition of tea-tree oil. Placebo gel was used to reduce any bias related to patient acceptance.
Sites treated
A total number of 15 patients with 45 sites was included in the study. In each patient, three different sites were randomly divided by lottery method. Thus, a single patient was treated with all three treatment modalities to avoid any intersubject bias.
Treatment sites in patients were randomly divided into three groups.
Group I (Control Group): SRP plus placebo gel
Group II (Test Group 1): SRP plus Coenzyme Q10 gel delivery
Group III (Test Group 2): SRP plus TTO gel delivery.
Clinical parameters
In total, 15 patients with 45 sites were enrolled in the study. Following clinical parameters were evaluated at baseline and 1 month follow-up.
Four clinical parameters evaluated were plaque index (PI),[17] sulcus bleeding index, PPD[18] and clinical attachment level.[18]
Treatment procedure
At the baseline, SRP was performed in all the groups. In Group I, placebo gel was placed into periodontal pocket. In Group II, the Local application of Coenzyme Q10 gel was performed using special needles designed to deliver gel in the pocket. In Group III, local delivery of TTO gel was performed after SRP [Figures 1 and 2].
Figure 1.
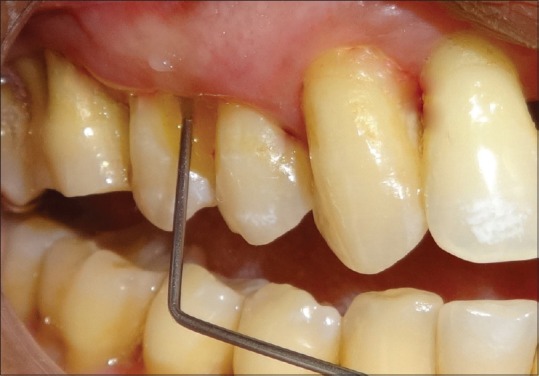
Coenzyme Q10 application
Figure 2.
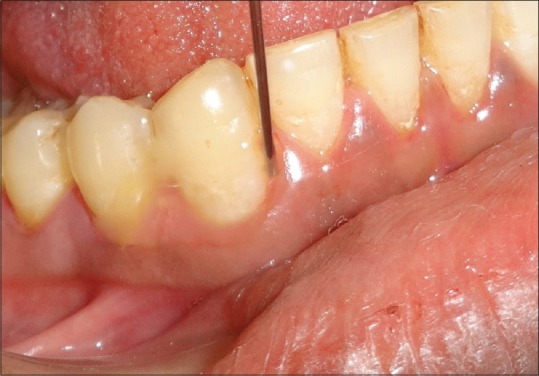
Tea tree oil gel Q10 application
After application of gel periodontal pack placement was done to avoid flowing away of the gel and for its sustained release into the periodontal pocket. Patients were recalled after 7 days for removal of pack and 1 month for follow-up.
Statistical analysis
All the results were tabulated and statistically analyzed using Statistical Package for Social Science (SPSS Version 12, IBM Inc, Chicago, IL, USA). P < 0.05 was defined as statistically significant.
Results
Probing pocket depth
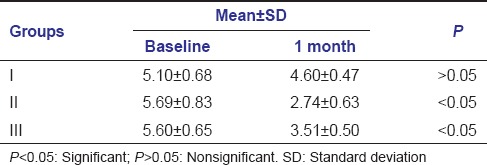
Mean pocket depth at baseline and 1 month was found to be 5.10 ± 0.68-4.60 ± 0.47, 5.69 ± 0.83-2.74 ± 0.63, 5.60 ± 0.65-3.51 ± 0.50 in Group I, Group II, Group III, respectively.
In Group I, mean pocket depth changes at baseline and 1 month were nonsignificant. In Group II and Group III, the difference in mean pocket depth at baseline and 1 month was statistically significant [Table 1].
Table 1.
Mean values of probing pocket depth at baseline and 1 month follow-up
Clinical attachment level
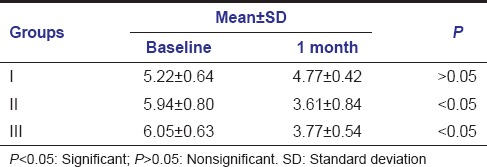
Mean clinical attachment level changes at baseline and 1 month were found to be 5.22 ± 0.64-4.77 ± 0.42, 5.94 ± 0.80-3.61 ± 0.84, 6.05 ± 0.63-3.77 ± 0.54 in Group I, Group II, Group III, respectively.
In Group I, mean clinical attachment level changes at baseline and 1 month were nonsignificant. In Group II and Group III, mean clinical attachment level changes from baseline to 1 month were significant [Table 2].
Table 2.
Mean values of clinical attachment level at baseline and 1 month follow-up
Plaque index
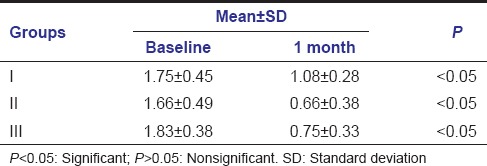
Mean PI scores at baseline and 1 month were 1.75 ± 0.45-1.08 ± 0.28, 1.66 ± 0.49-0.66 ± 0.38, and 1.83 ± 0.38-0.75 ± 0.33 in Group I, Group II, and Group III, respectively. The changes in mean PI scores from baseline to 1 month follow-up were significant in all three groups [Table 3].
Table 3.
Mean values of plaque index at baseline and 1 month follow-up
Gingival bleeding index
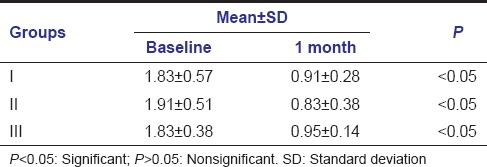
Mean gingival bleeding index scores changes from baseline to 1 month were 1.83 ± 0.57-0.91 ± 0.28, 1.91 ± 0.51-0.83 ± 0.38, 1.83 ± 0.38-0.95 ± 0.14 in Group I, Group II, Group III, respectively. Changes from baseline to 1 month follow-up were significant in all three groups (Group I, Group II, Group III) [Table 4].
Table 4.
Mean values of gingival bleeding index at baseline and 1 month follow-up
Discussion
Antioxidant therapy is believed to be effective in periodontitis.[19] However, studies exploring the role of antioxidants have shown mixed results.[20,21,22] In this study, two antioxidant gels that are Coenzyme Q10 gel and TTO gel were used for the treatment of periodontitis. Coenzyme Q10 gel was available commercially (Perio Q®) and TTO gel was prepared using the protocols described previously. However, there are limited data regarding the comparison of Coenzyme Q10 and TTO gel as an antioxidant in the treatment of chronic periodontitis.
Healing and repair of periodontal tissue require efficient energy production, and the metabolic functions of the periodontal tissues depend on an adequate supply of CoQ10.[6] Gingival biopsies revealed subnormal tissue level of CoQ10 in 60–96% patients with periodontal disease, indicating that periodontal disease is frequently associated with CoQ10 deficiency.[6] Physiologically, CoQ10 plays four major roles. It has an essential role in mitochondrial energy (ATP) production through redox activity in the respiratory chain, transporting electrons between enzymes. Second, it plays a role in extramitochondrial redox activity in the cell membrane and endomembranes. CoQ10 also functions as an antioxidant, inhibiting lipid peroxidation, and scavenging FRs. Finally, it plays an important role in membrane stabilization and fluidity.[7] The antioxidant nature of CoQ10 is derived from its energy carrier function. As an energy carrier, the CoQ10 molecule is continuously going through an oxidation-reduction cycle. CoQ10 inhibits lipid peroxidation by preventing the production of lipid peroxyl radicals. In addition, the reduced form of CoQ10 effectively regenerates Vitamin E from the α-tocopheroxyl radical. Furthermore, during oxidative stress, interaction of H2 O2 with metal ions bound to DNA generates hydroxyl radicals and CoQ10 efficiently prevents the oxidation of bases, particularly in mitochondrial DNA.[23]
Saxer et al. demonstrated that TTO (Melaleuca alternifolia) results in a reduction of inflammation due to the presence of 1,8-cineole and terpinen-4-ol.[24] TTO also results in less plaque formation as reported by Takarada et al. who demonstrated that TTO could inhibit the adhesion of Streptococcus mutans and Porphyromonas gingivalis.[25] These properties of TTO could account for the antiplaque and antigingivitis activities of the formulated gel. TTO is efficacious against oral bacteria, with antiseptic, fungicidal, and bactericidal effects due to its active ingredients.[24]
Results showed that there was a statistically significant reduction in the mean PI and sulcular bleeding index scores during the study in all three groups as compared with baseline values, and there was no significant difference between the three groups during the study. The reduction in plaque scores could be due to the proper oral hygiene maintenance after SRP. This was in agreement with the clinical results reported by Arweiler et al. who described the efficacy of a TTO used as mouthwash on plaque formation. They found that the PI in the group treated by TTO mouthwash did not differ from placebo mouthwash on any day from the study periods.[26] Furthermore, our results were in agreement with the other clinical results reported by Soukoulis and Hirsch, who performed a double-blind, longitudinal study to evaluate the effect of TTO gel (2.5%) applied to toothbrush twice daily. They found that TTO did not reduce plaque scores.[11]
In this study, there was significant reduction in PPD and clinical attachment level in Group II (Coenzyme Q10) and Group III (TTO gel) scores as compared to Group I (SRP + placebo). The results are consistent with the previous study done by Hans et al., Chatterjee et al. regarding the use of Coenzyme Q10.[6,27] The use of TTO also resulted improvement in all clinical parameters evaluated. The results using TTO gel are consistent with the previous study by Elgendy et al.[15]
The results of the present study show that there was no significant difference between the two test groups. However, patients in Group III reported with slight change in taste after the application of TTO gel. No such change in taste was reported after the use of Coenzyme Q10 gel.
Thus, results of the present study brighten the futuristic aspect of using antioxidant gels as a local drug delivery system in subgingival sites. The limitation of the present clinical trial was the small sample size and the short duration for determining the efficacy of the experimental drug. Thus, further studies are recommended with larger sample size for the evaluation of the efficacy of this antioxidants in the form of gel in the treatment of chronic periodontitis. The concomitant biochemical and microbial analysis could also help in better interpretation of findings.
Conclusion
Within the limitations of the present study, coenzyme Q10, and TTO gel as an adjunct to SRP proved to be effective in the treatment of periodontitis. Furthermore, it emphasizes the potential therapeutic value of two agents as an antioxidant which can reduce inflammation and promote healing of periodontal tissues.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Prakash S, Sunitha J, Hans M. Role of coenzyme Q(10) as an antioxidant and bioenergizer in periodontal diseases. Indian J Pharmacol. 2010;42:334–7. doi: 10.4103/0253-7613.71884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wood N, Johnson RB. The relationship between tomato intake and congestive heart failure risk in periodontitis subjects. J Clin Periodontol. 2004;31:574–80. doi: 10.1111/j.1600-051X.2004.00531.x. [DOI] [PubMed] [Google Scholar]
- 3.Chandra RV, Prabhuji ML, Roopa DA, Ravirajan S, Kishore HC. Efficacy of lycopene in the treatment of gingivitis: A randomised, placebo-controlled clinical trial. Oral Health Prev Dent. 2007;5:327–36. [PubMed] [Google Scholar]
- 4.Chandra RV, Sandhya YP, Nagarajan S, Reddy BH, Naveen A, Murthy KR. Efficacy of lycopene as a locally delivered gel in the treatment of chronic periodontitis: Smokers vs nonsmokers. Quintessence Int. 2012;43:401–11. [PubMed] [Google Scholar]
- 5.Maruyama T, Tomofuji T, Endo Y, Irie K, Azuma T, Ekuni D, et al. Supplementation of green tea catechins in dentifrices suppresses gingival oxidative stress and periodontal inflammation. Arch Oral Biol. 2011;56:48–53. doi: 10.1016/j.archoralbio.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 6.Hans M, Prakash S, Gupta S. Clinical evaluation of topical application of perio-Q gel (Coenzyme Q(10)) in chronic periodontitis patients. J Indian Soc Periodontol. 2012;16:193–9. doi: 10.4103/0972-124X.99261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soni S, Agrawal PK, Sharma N, Chander S. Coenzyme Q10 and periodontal health: A review. Int J Oral Maxillofac Pathol. 2012;3:21–6. [Google Scholar]
- 8.Littarru GP, Tiano L. Bioenergetic and antioxidant properties of coenzyme Q10: Recent developments. Mol Biotechnol. 2007;37:31–7. doi: 10.1007/s12033-007-0052-y. [DOI] [PubMed] [Google Scholar]
- 9.Pepping J. Coenzyme Q10. Am J Health Syst Pharm. 1999;56:519–21. doi: 10.1093/ajhp/56.6.519. [DOI] [PubMed] [Google Scholar]
- 10.Al-Hasso S. Coenzyme Q10: A review. Hosp Pharm. 2000;35:51–5. [Google Scholar]
- 11.Soukoulis S, Hirsch R. The effects of a tea tree oil-containing gel on plaque and chronic gingivitis. Aust Dent J. 2004;49:78–83. doi: 10.1111/j.1834-7819.2004.tb00054.x. [DOI] [PubMed] [Google Scholar]
- 12.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 13.Schätzle M, Löe H, Lang NP, Heitz-Mayfield LJ, Bürgin W, Anerud A, et al. Clinical course of chronic periodontitis. III. Patterns, variations and risks of attachment loss. J Clin Periodontol. 2003;30:909–18. doi: 10.1034/j.1600-051x.2003.00401.x. [DOI] [PubMed] [Google Scholar]
- 14.Killen JD, Fortmann SP, Telch MJ, Newman B. Are heavy smokers different from light smokers? A comparison after 48 hours without cigarettes. JAMA. 1988;260:1581–5. [PubMed] [Google Scholar]
- 15.Elgendy EA, Ali SA, Zineldeen DH. Effect of local application of tea tree (Melaleuca alternifolia) oil gel on long pentraxin level used as an adjunctive treatment of chronic periodontitis: A randomized controlled clinical study. J Indian Soc Periodontol. 2013;17:444–8. doi: 10.4103/0972-124X.118314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Enshaieh S, Jooya A, Siadat AH, Iraji F. The efficacy of 5% topical tea tree oil gel in mild to moderate acne vulgaris: A randomized, double-blind placebo-controlled study. Indian J Dermatol Venereol Leprol. 2007;73:22–5. doi: 10.4103/0378-6323.30646. [DOI] [PubMed] [Google Scholar]
- 17.Silness J, Loe H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condtion. Acta Odontol Scand. 1964;22:121–35. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 18.Ramfjord SP. The periodontal disease index (PDI) J Periodontol. 1967;38:602–10. doi: 10.1902/jop.1967.38.6.602. [DOI] [PubMed] [Google Scholar]
- 19.Battino M, Bullon P, Wilson M, Newman H. Oxidative injury and inflammatory periodontal diseases: The challenge of anti-oxidants to free radicals and reactive oxygen species. Crit Rev Oral Biol Med. 1999;10:458–76. doi: 10.1177/10454411990100040301. [DOI] [PubMed] [Google Scholar]
- 20.Tüter G, Kurtis B, Serdar M. Interleukin-1beta and thiobarbituric acid reactive substance (TBARS) levels after phase I periodontal therapy in patients with chronic periodontitis. J Periodontol. 2001;72:883–8. doi: 10.1902/jop.2001.72.7.883. [DOI] [PubMed] [Google Scholar]
- 21.Battino M, Ferreiro MS, Fattorini D, Bullon P. In vitro antioxidant activities of mouthrinses and their components. J Clin Periodontol. 2002;29:462–7. doi: 10.1034/j.1600-051x.2002.290512.x. [DOI] [PubMed] [Google Scholar]
- 22.Neiva RF, Steigenga J, Al-Shammari KF, Wang HL. Effects of specific nutrients on periodontal disease onset, progression and treatment. J Clin Periodontol. 2003;30:579–89. doi: 10.1034/j.1600-051x.2003.00354.x. [DOI] [PubMed] [Google Scholar]
- 23.Bentinger M, Tekle M, Dallner G. Coenzyme Q - Biosynthesis and functions. Biochem Biophys Res Commun. 2010;396:74–9. doi: 10.1016/j.bbrc.2010.02.147. [DOI] [PubMed] [Google Scholar]
- 24.Saxer UP, Stäuble A, Szabo SH, Menghini G. Effect of mouthwashing with tea tree oil on plaque and inflammation. Schweiz Monatsschr Zahnmed. 2003;113:985–96. [PubMed] [Google Scholar]
- 25.Takarada K, Kimizuka R, Takahashi N, Honma K, Okuda K, Kato T. A comparison of the antibacterial efficacies of essential oils against oral pathogens. Oral Microbiol Immunol. 2004;19:61–4. doi: 10.1046/j.0902-0055.2003.00111.x. [DOI] [PubMed] [Google Scholar]
- 26.Arweiler NB, Donos N, Netuschil L, Reich E, Sculean A. Clinical and antibacterial effect of tea tree oil - A pilot study. Clin Oral Investig. 2000;4:70–3. doi: 10.1007/s007840050118. [DOI] [PubMed] [Google Scholar]
- 27.Chatterjee A, Kandwal A, Singh N, Singh A. Evaluation of Co-Q10 anti-gingivitis effect on plaque induced gingivitis: A randomized controlled clinical trial. J Indian Soc Periodontol. 2012;16:539–42. doi: 10.4103/0972-124X.106902. [DOI] [PMC free article] [PubMed] [Google Scholar]


