“…As the United States proceeds with its peaceful program in space science and exploration…many aspects of space and space technology can be helpful to all people.”
With these words, President Dwight D. Eisenhower announced the creation of the National Aeronautics and Space Administration on March 26, 1958, thereby launching a project that, in a little over a decade, would bring humankind to walk on the moon. While every American watched the unfolding of this adventure into outer space with enthusiasm and trepidation, scientists at the National Institutes of Health (NIH) were beginning a different journey, perhaps less glamorous and far from the clamor of the media, but certainly no less important for humankind.
That same momentous year, the foundation for another milestone was being poured when Nathan Shock, William W. Peter, and Arthur Norris at the National Institute on Aging advanced the idea that the aging process would be better understood by studying the same individuals throughout their life span, collecting serial assessments at multiple time points rather than by comparing individuals of different ages who may have been exposed to different historical events and environmental conditions. Although the idea of longitudinal study was not completely new, it was the first time that it was applied to the study of aging. The NIH group called for volunteers to join the Baltimore Longitudinal Study of Aging (BLSA), remain in the study throughout the rest of their lives, and donate their bodies to science after their death. While today, this study design appears straightforward, in 1958, the notion of following individuals for life to study aging was an extraordinary leap forward in the field, a jump that would transform aging research forever. Although questions about the nature of the aging process are probably as old as humankind, never before had anyone postulated that chronological and biological age could be dissected, and that aging and pathology may evolve along separate, although somewhat parallel, pathways. Today, 50 years later, the BLSA is one of the largest and longest-running longitudinal studies of aging in the United States and is still addressing the same question. What a vision! Over many years, the BLSA has made major contributions to our understanding of normal aging in humans.
I cannot even begin to summarize here the fruits of 50 years of labor, hundreds of scientific publications, and generous contributions of more than 3000 participants. This is common knowledge. Data from the BLSA have helped scientists explore age-associated changes in anatomical integrity and function across all physiological systems, including musculoskeletal, cardiovascular, endocrine, and renal, just to name a few examples (1–4). By analyzing long-term personality data, BLSA scientists were able to refute the popular belief that, as people age, they become irritable and sad, and found that, in fact, personality doesn’t change much after puberty (1,5). By exploring how the ability to think, learn, and remember changes over time in specific BLSA participants, scientists were able to show that those who experienced an accelerated decline in memory, verbal intelligence, and executive function were more likely to develop dementia (6). Above all, the BLSA indicated that, although aging and longevity are affected by genetic factors, the quality of our own aging is strongly affected by following a few simple rules, such as watching our weight and waist, eating a healthy diet, exercising regularly, and maintaining an active role in society (1,7–16).
Curiously, the BLSA has both rewarded and, in some sense, disappointed the scientists who designed it. There is absolutely no doubt that most of the deterioration in physiological, physical, and cognitive function that occurs in many aging individuals is attributable to disease, suggesting that perhaps aging and diseases evolve along separate biological paths. However, as medical technology has advanced, and measurement tools have become more precise and accurate, the line of demarcation between aging and disease has started to blur (2). Subtle structural and functional changes that previously escaped the “pathology” filter are now considered (subclinical) risk factors for diseases (17–19). We are discovering that aging is a progressive dysregulation of our homeostatic network, and that life is maintained owing to the incredible adaptability and resiliency that the network allows at all levels of integration, from mitochondrial function to societal and community adaptations. This circuitry of signals, receptors, and effectors is extremely complex because of the need to detect and characterize thousands of different stressors and build the most effective, rapid, and parsimonious homeostatic response (20). There is evidence that, with aging, this homeostatic network undergoes a progressive simplification and tends to generate responses that are more stereotypic, more costly, and less effective (21,22). We also are starting to realize that the dysfunction of the homeostatic network can be detrimental to health. For example, the chronic mild proinflammatory state, progressive insulin resistance, and reduced walking speed, which commonly affect older persons, have been interpreted as compensatory responses but are also important determinants of pathology. If the dysfunction of the homeostatic network is the primary cause of increased susceptibility to disease, then the distinction between aging and pathology, while still conceptually valid, is almost impossible to detect empirically and certainly cannot be summarized by a few single measures. Perhaps this is why, in spite of considerable resources, the search for unique “biomarkers of aging” has so far been extremely disappointing.
A possible alternative to looking for specific biomarkers of aging is to search for an “aging signature,” a tag for individuals with age-associated, accelerated decline in function across multiple physiological systems. Although at first glance, this approach may seem unfamiliar, it is in fact consistent with the conceptualization of “frailty,” a syndrome that involves multiple physiological systems that is widely acknowledged in the geriatric literature. Scientists have even suggested that the number of systems involved is a measure of frailty severity and clinical progression (23,24). According to this view, expressions such as “aging brain” or “aging heart” are meaningful only in the context of their contribution to the aging of the whole individual. When deterioration occurs in isolated systems, it should be considered in the realm of pathology.
The conceptualization of frailty has contributed substantially to the description of the aging phenotype and its relevance to clinical practice. However, I have never been keen on the idea that frailty could be identified by simply “counting” affected systems. If we hope to someday decelerate aging and reduce the burden of disease and disability in older individuals, the only reasonable choice is to hunt for biological mechanisms and target them for treatment. What we need is a global theory that links together factors that both contribute to and participate in “accelerated aging.” When I became the Director of the BLSA in September 2002, I realized that, in spite of the available wealth of data, specimens, and long-term followup, I could not address the “homeostatic theory of aging” in the current BLSA design. In the mind of Nathan Shock and of most scientists who worked with him and after him, the BLSA constituted a recruitment core of volunteers for a number of unconnected longitudinal studies that targeted different aspects of the aging process. Although Dr. Shock acknowledged that the rate of aging may be parallel across different physiological systems and organs, this idea was never implemented in the study design. A substantial change in the design was required.
During my first 3 months at BLSA, I could not sleep. I felt like an inexperienced restorer asked to retouch a painting from Giotto or Masaccio. I wanted to continue the original BLSA mission of studying the aging process at the interface between aging and disease, but I also wanted to incorporate the emerging idea that aging is progressive dysfunction in some basic homeostatic mechanism whose effect is minimized by a full range of compensatory strategies.
Fortunately (or unfortunately…only time will tell), I was working with a group of extraordinarily talented scientists and, with their help, I was able to overcome my fear. The new scientific paradigm implemented in the BLSA was developed from a clinical protocol originally aimed at assessing geriatric patients with mobility problems in the geriatric hospital where I had worked in Italy. Studies have demonstrated that performance in mobility is a marker of health status superior to any other biomedical measure and one of the main determinants of quality of life in old age. Studies have also shown that poor lower extremity performance is a robust predictor of multiple negative health outcomes, including disability, health care resources utilization, nursing home admission, and mortality. In spite of this evidence, I believe that poor lower extremity performance is not the direct cause of such adverse outcomes, but rather a strong marker of homeostatic derangement and deteriorated health, which are the true causes of many adverse outcomes.
Because of the critical role that bipedal mobility played in human evolution, it is reasonable to assume that a number of compensatory strategies have been evolutionarily selected to maintain mobility despite considerable impairment. Decline in mobility only occurs after compensations fail. Thus, mobility may be interpreted as an overall measure of homeostatic stability, and understanding the causes of mobility disability may help explain the homeostatic disruption that occurs with aging.
The new BLSA study paradigm is illustrated in Figure 1. On the right side of the figure is mobility, the key outcome measure. In the middle are physiological domains that are relevant for mobility. In brief, the central nervous system generates the motivation for mobility and also creates, refines, and provides feedback to motor programs wired through the peripheral nervous system. Muscles are effectors that move bones and joints. Mobility requires that energy be generated, transported, and delivered locally, and that somatosensory systems provide continuous feedback from the environment. The hypothesis underlying this paradigm is that mobility problems in older persons result from impairment in multiple physiological domains, even when the clinical presentation suggests one precipitating cause. On the left side of the figure is a first attempt to identify the essential elements of the homeostatic network. We have hypothesized that the aging process affects multiple physiological systems in parallel (harmonically) because the signaling network that maintains a stable homeostasis and adequate distribution/utilization of energy becomes progressively less efficient and less able to adapt to stress with aging. The primary elements required for energy generation are provided by nutrition (in this context, oxygen is considered a nutrient). Most energy expenditure is accounted for by resting metabolic rate and physical activity. The production of energy during aerobic metabolism generates reactive oxygen species (ROS; oxidative stress) that are scavenged by antioxidant mechanisms. A dynamic stability of the internal environment is maintained by the combined effects of hormones and the autonomic nervous system. Finally, the integrity of the “self” is maintained by the immune system through inflammatory processes. Although it is useful to discuss these homeostatic systems separately, they appear to belong to the same signaling network and function in a very integrated way (25).
Figure 1.
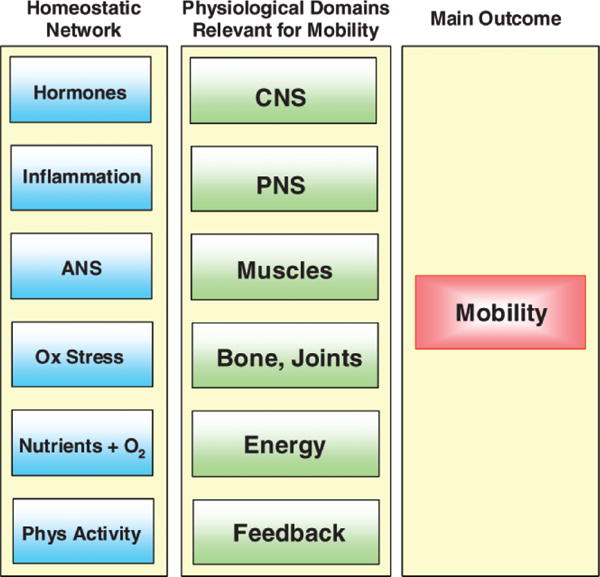
The new Baltimore Longitudinal Study of Aging paradigm of functional aging. CNS = central nervous system; PNS = peripheral nervous system; ANS = autonomic nervous system.
Hopefully, the systems biology approach implemented in the BLSA will tear the seals from the well-concealed mysteries of aging. In the past, geriatrics broke new ground when the field identified integrated whole-body functions as an important complement to disease nosology as a way to understanding aging. However, while functional assessment offered new and successful management approaches, we lacked an understanding of the pathophysiology of disability and, therefore, we could not fully exploit the profound biological implications of our discovery. The result was that geriatrics became somewhat marginalized within the greater culture of medicine. The outstanding research of the last decade has definitively overthrown this limitation. Rather than considering aging as an accumulation of somewhat unrelated individual disease processes, observational studies are uncovering common underlying processes that manifest across multiple body systems. These shared mechanisms modulate disease susceptibility and, therefore, are potential novel targets for prevention and treatment of age-related problems. This unique emerging body of knowledge has implications for future research, the organization and delivery of health care, for medical education, and for communication with the greater public. The renovated BLSA design fully acknowledges these new opportunities.
Longevity combined with good health and functioning until near the end of life is the goal of most individuals. Finding ways to promote this condition is the raison d’être of aging research. Although research has been done on the correlates of long life and functional decline, we still know relatively little about why certain individuals live in excellent health into their 80s while others fail much sooner. To address this important question, the BLSA has launched the project IDEAL (Insight into the Determinants of Exceptional Aging and Longevity). Over the next 5 years, the BLSA will be enrolling several hundred exceptionally long-lived, healthy, and high-functioning individuals. These extremely rare subjects are living proof that longevity combined with good health is possible. Studying them in comparison to individuals born in the same period but who experience the diseases and functional decrements that occur in most aging individuals may help identify the critical elements required for aging successfully. The rapidly increasing number of long-lived healthy individuals deserves to know what they can do to avoid or delay sickness and functional decline as long as possible. Unfortunately, there is no certainty that the preventive strategies that were developed for the general population will work in these very special individuals. We are confident that the IDEAL project will shed some light on these issues.
Looking ahead, it is fun to fantasize about what we will learn in the next round of discoveries from the BLSA. The possibilities are so large that my head spins. I hope that any wish list will be focused on issues that are relevant for the health, functional status, and quality of life of our older population. In the end, this is why we became geriatricians and gerontologists.
Happy 50th birthday BLSA—I wish you a long and productive life!
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute on Aging, National Institute of Health, Baltimore, MD.
References
- 1.Shock NW, Greulich RC, Aremberg D, Costa PT, Lakatta EG, Tobin JD. Normal Human Aging: The Baltimore Longitudinal Study of Aging. Washington, D.C: National Institutes of Health; 1984. [Google Scholar]
- 2.Najjar SS, Scuteri A, Lakatta EG. Arterial aging: is it an immutable cardiovascular risk factor? Hypertension. 2005;46:454–462. doi: 10.1161/01.HYP.0000177474.06749.98. [DOI] [PubMed] [Google Scholar]
- 3.National Institute on Aging. Aging Hearts & Arteries. Bethesda, MD: National Institutes of Health; 2005. [Google Scholar]
- 4.Najjar SS, Scuteri A, Shetty V, et al. Pulse wave velocity is an independent predictor of the longitudinal increase in systolic blood pressure and of incident hypertension in the Baltimore Longitudinal Study of Aging. J Am Coll Cardiol. 2008;51:1377–1383. doi: 10.1016/j.jacc.2007.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costa PT, Jr, McCrae RR. Psychological research in the Baltimore Longitudinal Study of Aging. Zeitschrift fur Gerontologie. 1993;26:138–141. [PubMed] [Google Scholar]
- 6.Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal magnetic resonance imaging studies of older adults: a shrinking brain. J Neurosci. 2003;23:3295–3301. doi: 10.1523/JNEUROSCI.23-08-03295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimokata H, Tobin JD, Muller DC, Elahi D, Coon PJ, Andres R. Studies in the distribution of body fat: I. effects of age, sex, and obesity. J Gerontol. 1989;44:M66–M73. doi: 10.1093/geronj/44.2.m66. [DOI] [PubMed] [Google Scholar]
- 8.Shimokata H, Andres R, Coon PJ, Elahi D, Muller DC, Tobin JD. Studies in the distribution of body fat. II. Longitudinal effects of change in weight. Intl J Obes. 1989;13:455–464. [PubMed] [Google Scholar]
- 9.National Institute on Aging. With the Passage of Time: The Baltimore Longitudinal Study of Aging. Bethesda, MD: National Institutes of Health; 1993. [Google Scholar]
- 10.Reynolds MW, Fredman L, Langenberg P, Magaziner J. Weight, weight change, mortality in a random sample of older community-dwelling women. J Am Geriatr Soc. 1999;47:1409–1414. doi: 10.1111/j.1532-5415.1999.tb01558.x. [DOI] [PubMed] [Google Scholar]
- 11.Iwao N, Iwao S, Muller DC, Elahi D, Shimokata H, Andres R. A test of recently proposed BMI standards with respect to old age. Aging (Milan, Italy) 2000;12:461–469. doi: 10.1007/BF03339878. [DOI] [PubMed] [Google Scholar]
- 12.Iwao S, Iwao N, Muller DC, Elahi D, Shimokata H, Andres R. Does waist circumference add to the predictive power of the body mass index for coronary risk? Obesity Res. 2001;9:685–695. doi: 10.1038/oby.2001.93. [DOI] [PubMed] [Google Scholar]
- 13.Morrow-Howell N, Hinterlong J, Rozario PA, Tang F. Effects of volunteering on the well-being of older adults. J Gerontol. 2003;58:S137–S145. doi: 10.1093/geronb/58.3.s137. [DOI] [PubMed] [Google Scholar]
- 14.Newby PK, Muller D, Hallfrisch J, Qiao N, Andres R, Tucker KL. Dietary patterns and changes in body mass index and waist circumference in adults. Am J Clin Nutr. 2003;77:1417–1425. doi: 10.1093/ajcn/77.6.1417. [DOI] [PubMed] [Google Scholar]
- 15.Tucker KL, Hallfrisch J, Qiao N, Muller D, Andres R, Fleg JL. The combination of high fruit and vegetable and low saturated fat intakes is more protective against mortality in aging men than is either alone: the Baltimore Longitudinal Study of Aging. J Nutr. 2005;135:556–561. doi: 10.1093/jn/135.3.556. [DOI] [PubMed] [Google Scholar]
- 16.National Institute on Aging. Exercise and Physical Activity: Your Everyday Guide from the National Institute on Aging. Bethesda, MD: National Institute on Aging; 2008. [Google Scholar]
- 17.Manolio TA, Burke GL, Psaty BM, Newman AB, Haan M, Powe N, Tracy RP, O’Leary DH. Black-white differences in subclinical cardiovascular disease among older adults: the Cardiovascular Health Study. CHS Collaborative Research Group. J Clin Epidemiol. 1995;48:1141–1152. doi: 10.1016/0895-4356(94)00240-q. [DOI] [PubMed] [Google Scholar]
- 18.Newman AB, Arnold AM, Naydeck BL, et al. “Successful aging”: effect of subclinical cardiovascular disease. Arch Intern Med. 2003;163:2315–2322. doi: 10.1001/archinte.163.19.2315. [DOI] [PubMed] [Google Scholar]
- 19.Newman AB, Siscovick D. The Cardiovascular Health Study: risk factors, subclinical disease, and clinical cardiovascular disease in older adults. Am J Geriatr Cardiol. 2004;13:59–60. doi: 10.1111/j.1076-7460.2004.02126.x. [DOI] [PubMed] [Google Scholar]
- 20.Lipsitz LA. Dynamics of stability: the physiologic basis of functional health and frailty. J Gerontol A Biol Sci Med Sci. 2002;57:B115–B125. doi: 10.1093/gerona/57.3.b115. [DOI] [PubMed] [Google Scholar]
- 21.Lipsitz LA. Age-related changes in the “complexity” of cardiovascular dynamics: a potential marker of vulnerability to disease. Chaos (Woodbury, NY) 1995;5:102–109. doi: 10.1063/1.166091. [DOI] [PubMed] [Google Scholar]
- 22.Lipsitz LA. Physiological complexity, aging, and the path to frailty. Sci Aging Knowl Environ. 2004:pe16. doi: 10.1126/sageke.2004.16.pe16. [DOI] [PubMed] [Google Scholar]
- 23.Rockwood K, Hogan DB, MacKnight C. Conceptualisation and measurement of frailty in elderly people. Drugs Aging. 2000;17:295–302. doi: 10.2165/00002512-200017040-00005. [DOI] [PubMed] [Google Scholar]
- 24.Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62:722–727. doi: 10.1093/gerona/62.7.722. [DOI] [PubMed] [Google Scholar]
- 25.Walston J, Hadley EC, Ferrucci L, et al. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc. 2006;54:991–1001. doi: 10.1111/j.1532-5415.2006.00745.x. [DOI] [PubMed] [Google Scholar]


