Abstract
Objective
Heart failure (HF) is a known risk factor for cognitive impairment. Cardiac rehabilitation (CR) may attenuate poor neurocognitive outcomes in HF via improved physical fitness—a significant promoter of cognitive function. However, no study has examined the possible acute and lasting benefits of CR on cognitive function in persons with HF.
Methods and Results
52 patients with HF completed a 12-week Phase II CR program. All participants were administered neuropsychological testing and completed a brief physical fitness assessment at baseline, completion of CR (i.e. 12-weeks), and 12-month follow-up. Repeated measures analyses showed a significant time effect for both attention/executive function and memory (p < 0.05). Attention/executive function performance increased from baseline to 12-weeks and these gains remained up to 12-months; memory was unchanged from baseline to 12-weeks, but then improved between the 12-week and 12-month time points. Physical fitness improved from baseline to 12-weeks and these benefits were maintained 12-months later. Changes in physical fitness and cognitive function over time did not reach a statistically significant association, though poorer physical fitness was associated with decreased cognitive performance at the baseline and 12-month time points.
Conclusions
CR is associated with both acute and lasting cognitive benefits in patients with HF. Prospective studies with extended follow-ups are needed to clarify the mechanisms that underpin cognitive improvements following CR (e.g., improved cerebral perfusion) and whether CR can ultimately reduce risk for cognitive decline and conditions like Alzheimer’s disease in HF.
Keywords: Cardiac rehabilitation, heart failure, cognitive function, physical fitness
Introduction
Heart failure (HF) affects >5 million American adults and prevalence rates are expected to grow by 25% over the next 15 years.1 HF is a significant source of economic hardships with total costs projected to reach approximately $70 billion by the year 2030.1 These costs may largely be attributed to the association of HF with poor outcomes. For instance, older adults with HF are at elevated risk for premature death, recurrent hospital readmissions, and poor quality of life.1,2
Patients with HF are also at risk for severe neurological conditions such as Alzheimer’s disease and vascular dementia.3 More mild impairments on formal cognitive testing can also be found across multiple domains, including executive function, psychomotor speed, and memory.4 This pattern is concerning, as cognitive dysfunction is a predictor of higher mortality risk and reduced functional independence in persons with HF.5,6 In response to these findings, recent attention has been paid to potential correlates of cognitive dysfunction in HF and they include an array of demographic (e.g., age) and medical factors (e.g., cerebral hypoperfusion, HF severity).7–10
Poor physical fitness is also an important contributor to reduced neurocognitive function in HF. Decreased cardiovascular fitness accompanies HF due to exercise intolerance stemming from severe cardiac dysfunction and high rates of physical inactivity in this population.11,12 Fitness worsens with increasing HF severity and is a strong predictor of poor outcomes such as elevated mortality risk.13,14 There is an extant literature that also documents reduced cardiovascular fitness as a significant correlate of smaller brain volume and cognitive dysfunction across multiple domains in patients with HF.15,16 The impact of fitness on neurocognitive outcomes is also well-established in the general older adult population.17,18
Physical fitness is modifiable through exercise training,19 raising the possibility that cognitive deficits in HF may be partially reversible through participation in cardiac rehabilitation (CR). CR is critical in the management of HF and has been shown to reduce hospital readmission rates in this population.20 Consistent with this notion, a short (e.g., 18-weeks) exercise training program has been shown to improve attention/executive function among a preliminary sample (e.g., N = 20) of patients with severe HF.21 Past work also demonstrates CR leads to cognitive gains among more heterogeneous samples of patients with CVD,22,23 suggesting it as a possible intervention for cognitive dysfunction in HF. Unfortunately, past studies demonstrating cognitive improvements following CR in cardiovascular disease patients, or exercise training in the case of HF, is limited by short follow-up periods, minimal assessment time points, and inconclusive results for underlying mechanisms.21–23
The effects of CR on cognitive function are poorly understood and no study to date has examined the acute and long-term impact of CR on cognitive function among older adults with HF. The purpose of the current study was to determine whether a HF-specific CR program yielded acute (i.e., baseline to 12-weeks) and lasting (i.e., up to 12-months) improvements in cognitive function. We also sought to examine the effects of CR on physical fitness levels in HF and whether increased fitness may serve as one potential mechanism for improved cognitive function following CR.
Methods
Participants
The sample consisted of 52 persons with HF from a NIH-funded study examining the effects of CR on neurocognitive function in older adults with HF. All HF participants were stable ambulatory patients that were enrolled in a 12-week outpatient CR program as part of their routine clinical care at Summa Health System in Akron, Ohio. All participants in this program were approached for possible involvement in research prior to the start of CR and interested individuals were screened for study eligibility. Strict inclusion/exclusion criteria were implemented to minimize potential confounds. For inclusion, participants must have been between the ages of 50–85 years, English speaking, and had a diagnosis of New York Heart Association (NYHA) HF class II, III, or IV at the time of enrollment. Potential participants were excluded for a history or current diagnosis of a significant neurological disorder (e.g. dementia, stroke), head injury >10 minutes loss of consciousness, severe psychiatric disorder (e.g. schizophrenia, bipolar disorder), substance abuse/dependence, and/or Stage 5 Chronic Kidney Disease.
CR Program
The phase II CR program at Summa Health System’s Akron City Hospital is a comprehensive EKG-monitored exercise and education program. Program duration is up to 12-weeks with three exercise/education sessions per week. Each session consists of one hour of exercise and one half and hour of education. An individual, customized exercise plan consisting of warm-up, cool-down, stretching, and a 40-minute, five station circuit training regimen using a variety of aerobic exercise modalities including rowers, treadmills, stationary cycles, elliptical trainers, stationary steppers, and arm exercises is developed for each patient. The education classes are aimed at helping patients understand their heart conditions, make positive lifestyle changes, and reduce their risk of future cardiac events.
Measures
Physical Fitness
The 2-minute step test (2MST) assessed physical fitness in the current sample.24 The 2MST requires participants to step in place lifting his/her knees to a marked target set on the wall set at the midpoint between the kneecap and crest of the iliac for a 2-minute period. Greater step count reflects better physical fitness. Average step count for healthy males between the ages of 60–85 ranges from 71–115 and between 60–107 steps for females.24 Below average 2MST according to the average age of males in this sample (mean = 66.77, SD = 8.24) is < 87 while for females with the average age of the current sample (mean = 66.46, SD = 6.62) is < 73.24 The 2MST has been correlated with metabolic equivalents derived from stress testing and is also a sensitive predictor of neurocognitive outcomes in HF.15,25
Cognitive Function
A series of neuropsychological measures were administered to assess global cognitive function, attention/executive function, memory, and language. All measures demonstrate excellent psychometric properties. The domains and their respective measures include:
Global Cognitive Status. The Mini Mental State Examination (MMSE).26
Attention/Executive Function. Trail Making Test A and B,27 and Digit Symbol Coding28 operationalized attention and executive function.
Memory. The California Verbal Learning Test-Second Edition (CVLT-II) was administered to test memory abilities.29 An alternate form of this measure was given at the 12-week follow-up to decrease possible practice effects.
Language. The Boston Naming Test30 and the Animal Fluency Test31 were used to assess language.
Demographic and Medical History
Demographic and medical characteristics were ascertained through participant self-report and corroborated by medical record review.
Procedures
The local Institutional Review Board (IRB) approved the study procedures and all participants provided written informed consent prior to study enrollment. Participants completed a total of three study assessments: a baseline assessment prior to CR, 12-weeks upon completion of CR, and at a 12-month follow-up. During the baseline assessment, participants completed demographic and medical self-report measures. Participants also completed the 2MST and were administered a comprehensive neuropsychological test battery. These identical procedures were repeated at completion of CR (12 weeks later) and 12-month follow-up. All procedures were performed by a trained research assistant under the supervision of a licensed clinical neuropsychologist.
Statistical Analyses
All neuropsychological measures assessing attention/executive function, memory, and language were converted to T-scores using existing normative data that accounts for age, as well as gender in the case of memory. Consistent with clinical convention, an MMSE score < 27 and a T-score < 35 operationalized clinically-meaningful levels of cognitive impairment. Composite scores were computed for attention/executive function, memory, and language that consisted of the mean of the T-scores of the neuropsychological measures that comprise these domains. Although minimal, for cases with missing data on individual cognitive measures, the composite scores consisted of the mean of the remaining measures within the respective domain.
Repeated measures analysis of variance examined changes in global cognitive status, the cognitive composite scores (e.g., attention/executive function, memory, and language) and the 2MST over the time points (i.e., baseline, 12-weeks, and 12-months). For those variables that exhibited significant changes, follow-up repeated measure analyses were then performed to clarify the nature of these changes between each time point. Hierarchical regression analyses were then conducted to determine whether changes in physical fitness predicted those cognitive domains that demonstrated significant improvements over time. Specifically, the dependent variable of these analyses included the 12-month cognitive domain. Block 1 of the model consisted of the baseline value of the dependent variable and baseline 2MST performance. Medical and demographic covariates were also entered in block 1 to control for their effects on neurocognitive function and they included age, sex (1 = male; 2 = female), and diagnostic history of hypertension and type 2 diabetes mellitus (T2DM; 1 = positive diagnostic history; 0 = negative diagnostic history). Block 2 included 12-month 2MST performance.
Results
Demographic, Medical and Clinical Characteristics
See Table 1 for a full summary of baseline demographic, medical, and clinical characteristics in the sample. Participants averaged 66.69 (SD = 7.80) years of age, were 25.0% female, and 90.4% Caucasian. The sample exhibited an average left ventricular ejection of 38.8 (SD = 11.5). Comorbid medical conditions were prevalent, including hypertension (55.8%), T2DM (28.8%), and elevated total cholesterol (59.6%). The prevalence of these conditions remained largely unchanged at the 12-week (hypertension: 53.8%; T2DM: 28.8%; elevated total cholesterol: 55.8%) and 12-month follow-up (hypertension: 63.5%; T2DM: 28.8%; elevated total cholesterol: 50.0%).
Table 1.
Demographic, Medical, and Clinical Characteristics of 52 Older Adults with Heart Failure Enrolled in Cardiac Rehabilitation
| Demographic Characteristics | Baseline |
|---|---|
| Age, mean (SD) | 66.69 (7.80) |
| Sex (% Women) | 25.0 |
| Race (% Caucasian) | 90.4 |
| Education, mean years (SD) | 14.0 (3.0) |
| Medical and Clinical Characteristics | |
| Left Ventricular Ejection Fraction, mean (SD) | 38.8 (11.5) |
| Diabetes (%) | 28.8 |
| Hypertension (%) | 55.8 |
| Sleep Apnea (%) | 21.2 |
Baseline Cognitive Function and Physical Fitness
Table 2 presents cognitive test performance in the sample across the three time points. At baseline, the sample exhibited an average MMSE of 28.0 (SD = 1.8) with 23.1% of sample scoring below a 27 on this measure. T-scores on all neuropsychological measures fell within the normative range. However, when using a T-score cutoff of 35, many participants demonstrated impairments across all domains. Most common impairments were noted on measures of attention/executive function and memory (up to 13.5% for both). Impairments were also common across the remaining time points (see Table 2).
Table 2.
Baseline, 12-week, and 12-Month Neuropsychological Test Performance
| Baseline Mean (SD) |
% T-score < 35 |
12-week Mean (SD) |
% T-score < 35 |
12-month Mean (SD) |
% T-score < 35 |
*p-value | |
|---|---|---|---|---|---|---|---|
| Global Cognition | |||||||
| MMSE | 28.0 (1.8) | -- | 28.1 (1.9) | -- | 28.3 (1.9) | -- | 0.44 |
|
Attention/Executive Function (T-scores) |
|||||||
| TMTA | 51.13 (8.36) | 5.9 | 53.30 (7.27) | 3.8 | 52.55 (8.15) | 5.8 | 0.07 |
| TMTB | 45.95 (18.21) | 13.5 | 48.39 (11.35) | 11.5 | 48.53 (13.89) | 15.4 | 0.29 |
| Digit Symbol Coding | 48.92 (8.13) | 9.6 | 50.67 (8.24) | 3.8 | 50.63 (7.66) | 3.8 | 0.001 |
| Memory | |||||||
| CVLT SDFR | 49.42 (9.73) | 3.8 | 51.35 (11.55) | 3.8 | 52.40 (9.05) | 0.0 | 0.04 |
| CVLT LDFR | 48.65 (10.01) | 7.7 | 49.71 (10.96) | 5.8 | 52.21 (10.26) | 7.7 | 0.02 |
| CVLT Recognition | 46.35 (11.42) | 13.5 | 44.04 (10.24) | 17.3 | 48.94 (10.31) | 7.7 | 0.01 |
| Language | |||||||
| Boston Naming Test | 54.08 (7.85) | 3.8 | 54.40 (8.90) | 2.0 | 55.47 (7.15) | 1.9 | 0.26 |
| Animals | 57.15 (10.79) | 0.0 | 58.12 (12.02) | 0.0 | 59.24 (11.20) | 0.0 | 0.46 |
Note. N for Baseline TMTA = 51; N for 12-week Boston Naming Test and Animals = 51; N for 12-month Animals = 51
p- values are based on repeated measures analysis of variance that examined mean changes across the three time points for each cognitive test; sample sizes for these analyses are based on complete data across the time points: N for TMT A = 51; N for Boston Naming Test = 51; and N for Animals = 50; the N for all other tests is the full sample size of 52.
Abbreviations—MMSE = Mini Mental State Examination; TMTA = Trail Making Test A; TMTB = Trail Making Test B; CVLT = California Verbal Learning Test; LDFR = Long Delay Free Recall; SDFR = Short Delayed Free Recall
In terms of physical fitness, the average 2MST performance in the sample at baseline was 72.08 (SD = 20.12). According to the average age of males and females in this sample, average 2MST performance for males (mean (SD) = 74.23 (20.10)) and females (mean (SD) = 65.62 (19.50)) fell in the below average range.
Cognitive Function Over Time
See Table 3 for a summary of repeated measures ANOVAs. At the 12-month follow-up, the sample had an average MMSE score of 28.3 (SD = 1.9); although this represented improvements in MMSE scores, it did not reach statistical significance (p > 0.05). At the 12-month follow-up, the sample prevalence of impaired global cognitive status (i.e., MMSE <27) decreased to 15.4%.
Table 3.
Changes in Cognitive Function and Physical Fitness Over Time
| Baseline | 12-weeks | 12-Months | F | p-value | Partial-eta | |
|---|---|---|---|---|---|---|
| Attention/EF | 48.67 (9.56) | 50.79 (7.52) | 50.57 (8.23) | 5.04 | 0.01 | 0.17 |
| Memory | 48.14 (9.13) | 48.37 (9.08) | 51.19 (8.23) | 4.66 | 0.01 | 0.16 |
| Language | 55.62 (7.78) | 56.52 (9.06) | 57.36 (7.32) | 2.18 | 0.12 | 0.08 |
| MMSE | 27.96 (1.77) | 28.10 (1.85) | 28.25 (1.89) | 0.83 | 0.44 | 0.03 |
| 2MST | 72.08 (20.12) | 76.27 (22.91) | 77.08 (27.74) | 2.60 | 0.08 | 0.09 |
Note. EF = executive function; 2MST = 2-minute step test
Analyses showed a significant time effect for attention/executive function (F(2, 50) = 5.04, p = 0.01) and memory (F(2, 50) = 4.66, p = 0.01). Follow-up repeated measures analyses showed that attention/executive function significantly improved from baseline to 12-week (F(1, 51) = 7.09, p = 0.01) and such benefits persisted to 12-months (F(1, 51) = 0.09, p = 0.76). There were significant improvements in attention/executive function between the baseline and 12-month time point (F(1, 51) = 8.44, p = 0.01).
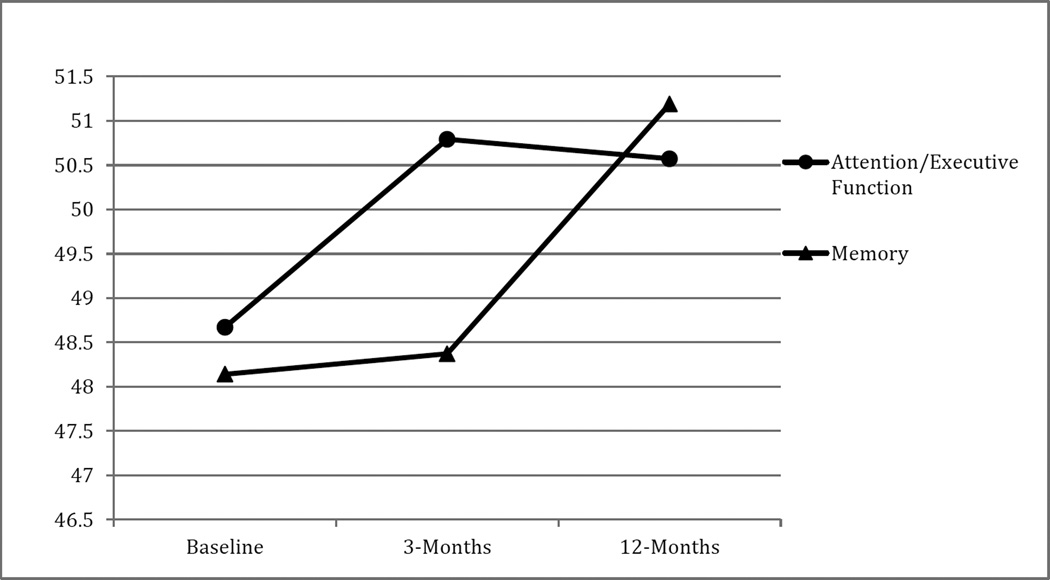
Memory remained stable from baseline to 12 weeks (F(1, 51) = 0.04, p = 0.85), though significant improvements emerged between the 12-week and 12-month time points (F(1, 51) = 8.02, p = 0.01). Baseline to 12-month memory improvements also reached significance (F(1, 51) = 5.88, p = 0.02). Although both attention/executive and memory improved significantly over time, performances remained in the average range relative to normative data across the time points. See Figure 1 for the trajectories of attention/executive function and memory over time. There was no significant time effect for language (F(2, 50) = 2.18, p = 0.12).
Figure 1. The Acute and Long-Term Effects of Cardiac Rehabilitation on Cognitive Function in Older Adults with Heart Failure.
Values on the Y-axis are T-scores. Higher scores reflect better cognitive function
p-value across the three time points for attention/executive function and memory is 0.01
p-value for attention/executive function from baseline to 12-weeks = 0.01, baseline to 12-months = 0.01, and 12-weeks to 12-months = 0.76
p-value for memory from baseline to 12-weeks = 0.85, baseline to 12-months = 0.02, and 12-weeks to 12-months = 0.01
Changes in Physical Fitness
Repeated measures ANOVA showed a trend for improved 2MST performance across the three time points (F(2, 50) = 2.60, p = 0.08). Further repeated measures analyses showed significant improvements in 2MST performance from baseline to the 12-week time point (F(1, 51) = 4.42), p = 0.04), and these gains remained up to the 12-month time point (F(1, 51) = 0.08, p = 0.78).
Predictive Validity of Physical Fitness on Cognitive Function
Regression analyses controlling for baseline values, and baseline demographic and medical variables showed that improved 2MST performance over time did not predict better attention/executive function or memory abilities (p > 0.05). However, follow-up partial correlations controlling for age, sex, and diagnostic history of hypertension, and T2DM showed that baseline 2MST performance was associated with baseline performances on the Trail Making Test B (r(46) = 0.37, p = 0.01), Animals (r(46) = 0.38, p = 0.01), Boston Naming Test (r(46) = 0.31, p = 0.03), and Digit Symbol Coding (r(46) = 0.41, p < 0.01). There was also a trend with the MMSE (r(46) = 0.27, p = 0.07). Better physical fitness was associated with increased cognitive function at the baseline time point. Likewise, higher 2MST performance at the 12-month follow-up also corresponded with better 12-month performances on the Trail Making Test A (r(46) = 0.37, p = 0.01), Digit Symbol Coding (r(46) = 0.28, p = 0.05), Animals (r(45) = 0.43, p < 0.01), the Boston Naming Test (r(46) = 0.36, p = 0.01), and strong trends emerged for the MMSE (r(46) = 0.26, p = 0.08), and Trail Making Test B (r(46) = 0.27 = 0.06).
Discussion
This study is the first to examine the acute and long-term effects of CR on cognitive function among older adults with HF. We found that CR was associated with long-term improvements in multiple cognitive domains, though the pattern of gains varied across domains. CR also resulted in increased physical fitness levels, but changes in fitness and cognitive function over time did not reach a statistically significant association. Several aspects of these findings warrant further discussion.
The current study found that CR was associated with improvements in both attention/executive function and memory at the 12-month follow-up. CR has been previously linked with cognitive gains in attention/executive function and memory in cardiovascular disease patients22 and a brief exercise programs has also been associated with cognitive benefits in persons with HF.21 CR has been shown to confer many advantages to persons with HF, largely due to the beneficial effects of exercise on cardiac function, endothelial functioning, and cerebral blood flow.22,32,33 Such vascular benefits likely serve as the possible mechanisms by which CR improves cognition in HF. For instance, cerebral hypoperfusion is common in HF34 and believed to underlie cognitive impairment in this population.10 Past work in aging adults shows that exercise is associated with improved cognitive function possibly due to the observed increases in cerebral blood flow.35 Interestingly, aerobic interval training has been linked with better cerebral hemodynamics in patients with HF.36 Because HF patients are at elevated risk for dementia,3 CR may attenuate poor neurocognitive outcomes in this population via improved brain perfusion. Cognitive gains from CR may also help to preserve functional independence in HF in light of the documented effects of cognitive impairment on impaired self-care ability in this population (e.g., medication management, driving).5 Future studies with extended follow-ups are needed to determine whether cognitive benefits following CR last >12-months and possibly translate to better outcomes in this population such as reduced dementia and mortality risk.
In contrast to attention/executive function, there were no acute improvements for memory in this sample (i.e., baseline to 12-weeks) despite the benefits emerging at 12-months. Indeed, acute exercise (e.g., 18-weeks) in HF has previously been shown to benefit attention/executive function while other cognitive domains remained unchanged.21 Memory impairments are common in HF and this population is at risk for neurological conditions characterized by rapid declines in memory abilities (i.e., Alzheimer’s disease).3 Memory structures have been suggested to be particularly sensitive to ischemic/hypoxic episodes stemming from cerebral hypoperfusion.37 As an example, atrophy of brain structures such as mammillary bodies, fornix fiber, and other medial temporal lobe regions are prevalent in HF and theorized to contribute to impaired memory in this population.37,38 Taken together, it is possible that the vulnerability of memory to the pathophysiological effects of HF may make this domain less responsive to the short-term effects of CR. Instead, memory may improve in small increments and thus a longer duration of time may be needed to capture clinically meaningful changes in memory that stem from CR or exercise training regimens. Regardless, the exact reasoning for improvements in memory from 12-weeks to 12-months remains unclear and requires further investigation. For instance, it is also possible that a subgroup of individuals that continued living a healthy lifestyle after the CR program may help to explain continued improvements in cognitive function. Clearly, future work with longer follow-up periods is needed to fully elucidate the trajectories of cognitive improvements across domains following CR in patients with HF.
We also found that CR resulted in acute improvements in physical fitness and such benefits were maintained 12-months later. CR and exercise training are associated with acute improvements in exercise tolerance and fitness levels in HF.20–22,33 However, the current study extends these findings by showing that CR is associated with lasting improvements in physical fitness. Such findings are perhaps unexpected, as there is extant evidence that suggests physical fitness levels decline following the end CR.39 In-turn, the exact reason for our findings is not entirely clear, but may involve study limitations such as indirect assessment of physical fitness. Alternatively, there may be an unidentified subgroup of patients with HF in this sample that continued at home physical activity training and it is this cohort of individuals that largely account for the preservation of physical fitness following CR. There is some support for this notion in the literature, as HF patients that continue physical training after CR have been shown to maintain or even improve peak oxygen consumption over time.39 Future work that employs more sensitive measures of physical fitness and that assesses continued participation in physical activity during the months after CR is much needed to elucidate the long-term effects of CR on physical fitness.
The beneficial effects of CR on physical fitness levels are noteworthy, as reduced fitness in HF is associated with heightened mortality risk13 and also negatively impacts cognitive function in this population.15 While the current findings support reduced physical fitness as a risk factor for cognitive dysfunction, improvements in physical fitness did not predict cognitive gains over time. This is surprising because exercise and better physical fitness are direct promoters of vascular factors (e.g., cerebral blood flow, endothelial function) believed to underlie cognitive dysfunction in HF.10,17 However, past work shows that up to 21% of HF patients do not exhibit improved physical fitness following CR due to factors such as greater medical comorbidities.40 In turn, it is possible that a portion of the unhealthiest HF patients that did not exhibit improved fitness is driving the current analyses. Similarly, as a whole, changes in fitness were relatively small and may not have reached threshold to benefit vascular or cognitive function. Given that fitness and cognitive function both improved, it is also possible that these factors are indeed a function of one another but there may be some indirect mechanism linking them or their effects were not captured by the statistical methods employed in this study. Lastly, CR also targets other lifestyle changes linked with cognitive function such as improved adherence to medication adherence and dietary regimens.5,41 Although this awaits empirical test, these behavior modifications may also benefit cognitive function. Future work is much needed to clarify the effects of improved fitness on cognitive function over time in HF and identify mechanisms (e.g., cerebral perfusion) by which CR improves cognition.
Several limitations of the current study warrant brief discussion. Although practice effects may have contributed to cognitive improvements, the length of the study time points and the use of alternate test versions argue against this possibility. Regardless, future work that employs healthy controls and non-CR HF patients is needed to confirm our findings and clarify whether CR may attenuate cognitive decline in this population. Despite the lack of a control in this study, we found cognitive improvements over time which is in contrast to the extant literature demonstrating cognitive decline on formal testing in this population. In addition, the 2MST is a validated measure of fitness, though assessment of metabolic equivalents (METs) may be more sensitive to fitness derived cognitive changes following CR22 and future studies should employ such methods to better understand the short- and long-term effects of CR on cognitive function and fitness. Future work that employs advanced neuroimaging will also help elucidate mechanisms underlying improved cognitive function following CR, including the role of cerebral blood flow and/or structural brain changes. Indeed, brain injury is common in HF and physical fitness has been identified as a correlate of gray matter and cortical thickness in this population.16 Lastly, future work should examine the effects of CR on lifestyle modifications (e.g., medication and diet adherence) and their corresponding changes with cognitive function, as this has not yet been examined. Likewise, the beneficial effects of CR on cognition may also be mediated by psychological gains. For example, depression and anxiety are common in HF and known to influence cognitive outcomes in this population. Fortunately, CR has been shown to improve psychological status in HF patients42 and such findings may translate to better cognitive abilities and future work should test this possibility.
In brief summary, CR in older adults with HF is associated with acute and long-term benefits in cognitive function and physical fitness. Prospective studies with extended follow-ups (e.g., 2–3 years) are needed to determine whether CR can reduce risk for accelerated cognitive decline and dementia in older adults with HF. Future work is also needed to clarify the underlying mechanisms by which CR improves cognitive function, including the role of systemic and cerebral perfusion.
Acknowledgments
Support for this work included National Institutes of Health (NIH) grants DK075119 and HL089311.
Footnotes
The authors have no competing interests to report.
Conflict of Interest
The authors declare no conflict of interests.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bracata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2013 Update: A report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bennett SJ, Oldridge NB, Eckert GJ, Embree JL, Browning S, Hou N, Chui M, Deer M, Murray MD. Comparison of quality of life measures in heart failure. Nurs Res. 2003;52:207–216. doi: 10.1097/00006199-200307000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Qiu C, Winblad B, Marengoni A, Klarin I, Fastbom J, Fratiglioni L. Heart failure and risk of dementia and Alzheimer disease: a population-based cohort study. Arch Intern Med. 2006;166:1003–1008. doi: 10.1001/archinte.166.9.1003. [DOI] [PubMed] [Google Scholar]
- 4.Pressler SJ, Subramanian U, Kareken D, Perkins SM, Gradus-Pizlo I, Sauve MJ, Ding Y, Kim J, Sloan R, Jaynes H, Shaw RM. Cognitive deficits in chronic heart failure. Nurs Res. 2010;59:127–139. doi: 10.1097/NNR.0b013e3181d1a747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alosco ML, Spitznagel MB, Raz N, Cohen R, Sweet LH, Colbert LH, Josephson R, van Dulmen M, Hughes J, Rosneck J, Gunstad J. Executive dysfunction is independently associated with reduced functional independence in heart failure. J Clin Nurs. 2013 doi: 10.1111/jocn.12214. [epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zuccala G, Pedone C, Cesari M, Onder G, Pahor M, Marzetti E, Lo Monaco MR, Cocchi A, Carbonin P, Bernabel R. The effects of cognitive impairment on mortality among hospitalized patients with heart failure. Am J Med. 2003;115:97–103. doi: 10.1016/s0002-9343(03)00264-x. [DOI] [PubMed] [Google Scholar]
- 7.Van den Hurk K, Reijmer YD, van den Berg E, Alssema M, Nijpels G, Kostense PJ, Stehouwer CD, Paullus WJ, Kamp O, Dekker JM, Biessels GJ. Heart failure and cognitive function in the general population: the Hoorn Study. Eur J Heart Fail. 2011;13:1362–1369. doi: 10.1093/eurjhf/hfr138. [DOI] [PubMed] [Google Scholar]
- 8.Vogels RL, Oosterman JM, van Harten B, Gouw AA, Schroeder-Tanka JM, Scheltens P, van der Flier WM, Weinstein HC. Neuroimaging and correlates of cognitive function among patients with heart failure. Dement Geriatr Cogn Disord. 2007;24:418–423. doi: 10.1159/000109811. [DOI] [PubMed] [Google Scholar]
- 9.Baldasseroni S, Mossello E, Romboli B, Orso F, Colombi C, Fumagalli S, Ungar A, Tarantini F, Masotti G, Marchionni N. Relationship between cognitive function and 6-minute walking test in older outpatients with chronic heart failure. Aging Clin Exp Res. 2010;22:308–313. doi: 10.1007/BF03324936. [DOI] [PubMed] [Google Scholar]
- 10.Alosco ML, Brickman AM, Spitznagel MB, Garcia SL, Narkhede A, Griffith EY, Raz N, Cohen R, Sweet LH, Colbert LH, Josephson R, Hughes J, Rosneck J, Gunstad J. Cerebral perfusion is associated with white matter hyperintensities in older adults with heart failure. Congest Heart Fail. 2013;19:E29–E34. doi: 10.1111/chf.12025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alosco ML, Spitznagel MB, Miller L, Raz N, Cohen R, Sweet LH, Colbert LH, Josephson R, Waechter D, Hughes J, Rosneck J, Gunstad J. Depression is associated with reduced physical activity in persons with heart failure. Health Psychol. 2012;31:754–762. doi: 10.1037/a0028711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pina IL. Exercise and heart failure. Circulation. 2003;107:1210–1225. doi: 10.1161/01.cir.0000055013.92097.40. [DOI] [PubMed] [Google Scholar]
- 13.Boxer R, Klepppinger A, Ahmad A, Annis K, Hager D, Kenny A. The 6-minute walk is associated with frailty and predicts mortality in older adults with heart failure. Congest Heart Fail. 2010;16:208–213. doi: 10.1111/j.1751-7133.2010.00151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wegrzynowska-Teodorczyk K, Rudzinska E, Jankowska E, Grzeslo A, Nowakowska K, Lazorczyk M, Banasiak W, Ponikowski P, Wozniewski M. Determinant of physical fitness in males with systolic heart failure. Kardiol Pol. 2010;68:146–154. [PubMed] [Google Scholar]
- 15.Garcia S, Alosco ML, Spitznagel MB, Cohen R, Raz N, Sweet L, Josephson R, Hughes J, Rosneck J, Oberle ML, Gunstad J. Cardiovascular fitness associated with cognitive performance in heart failure patients enrolled in cardiac rehabilitation. BMC Cardiovasc Disord. 2013;13:29. doi: 10.1186/1471-2261-13-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alosco ML, Brickman AM, Spitznagel MB, Griffith EY, Narkhede A, Raz N, Cohen R, Sweet LH, Colbert LH, Jospheson R, Hughes J, Rosneck J, Gunstad J. Poorer physical fitness is associated with reduced structural brain integrity in heart failure. J Neurol Sci. 2013;328:51–57. doi: 10.1016/j.jns.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ainslie PN, Cotter JD, George KP, Lucas S, Murrell C, Shave R, Thoomas KN, Williams MJ, Atkinson G. Elevation in cerebral blood flow velocity with aerobic fitness throughout healthy human ageing. J Physiol. 2008;586:4005–4010. doi: 10.1113/jphysiol.2008.158279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wendell CR, Gunstad J, Waldstein SR, Wright JG, Ferrucci L, Zonderman AB. Cardiorespiratory fitness and accelerated cognitive decline with aging. J Gerontol A Biol Sci Med Sci. 2013 doi: 10.1093/gerona/glt144. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mackay-Lyons M. Aerobic treadmill training effectively enhances cardiovascular fitness and gait function for older persons with chronic stroke. J Physiother. 2012;58:271. doi: 10.1016/S1836-9553(12)70131-5. [DOI] [PubMed] [Google Scholar]
- 20.Davidson PM, Cockburn J, Newton PJ, Webster JK, Betihavas V, Howes L, Owensby DO. Can a heart failure-specific cardiac rehabilitation program decrease hospitalizations and improve outcomes in high-risk patients? Eur J Cardiovasc Prev Rehabil. 2010;17:393–402. doi: 10.1097/HJR.0b013e328334ea56. [DOI] [PubMed] [Google Scholar]
- 21.Tanne D, Freimark D, Poreh A, Merzeliak O, Bruck B, Schwammenthal Y, Schwammenthal E, Motroo M, Adler Y. Cognitive functions in severe congestive heart failure before and after an exercise training program. Int J Cardiol. 2005;103:145–149. doi: 10.1016/j.ijcard.2004.08.044. [DOI] [PubMed] [Google Scholar]
- 22.Stanek KM, Gunstad J, Spitznagel MB, Waechter D, Hughes JW, Luyster F, Josephson R, Rosneck J. Improvements in cognitive function following cardiac rehabilitation for older adults with cardiovascular disease. Int J Neurosci. 2011;121:86–93. doi: 10.3109/00207454.2010.531893. [DOI] [PubMed] [Google Scholar]
- 23.Gunstad J, Macgregor KL, Paul RH, Poppas A, Jefferson AL, Todaro JF, Cohen RA. Cardiac rehabilitation improves cognitive performance in older adults with cardiovascular disease. J Cardiopulm Rehabil. 2005;25:173–176. doi: 10.1097/00008483-200505000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones CJ, Rikli RE. Measuring functional fitness of older adults. The Journal on Active Aging. 2002 March April;:24–30. [Google Scholar]
- 25.Alosco ML, Spitznagel MB, Raz N, Cohen R, Sweet LH, Colbert LH, Josephson R, Waechter D, Hughes J, Rosneck J, Gunstad J. The 2-minute step test is independently associated with cognitive function in older adults. Aging Clin Exp Res. 2012;24:468–474. doi: 10.3275/8186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Folstein MF, Folstein SE, McHugh PR. "Mini-Mental State". A practical method for grading the cognitive state of patients for the clinician. J Psychiatric Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 27.Reitan R. Validity of the Trail Making Test as an indicator of organic brain damage. Percept Motor Skills. 1958;8:271–276. [Google Scholar]
- 28.Smith A. Clinical psychological practice and principals of neuropsychological assessment. In: Walker C, editor. Handbook of clinical psychology: Theory, Research, and practice. Homewood, IL: Dorsey Press; 1983. [Google Scholar]
- 29.Delis D, Kramer J, Kaplan E, Ober B. California Verbal Learning Test-Second Edition: Adult Version. Manual. San Antonio (TX): Psychological Corporation; 2000. [Google Scholar]
- 30.Hawkins KA, Sledge WH, Orlean JF, Quinlan DM, Rakfeldt J, Huffman RE. Normative implications of the relationship between reading vocabulary and Boston Naming Test performance. Arch Clin Neuropsychol. 1993;8:525–537. [PubMed] [Google Scholar]
- 31.Morris J, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, Mellits ED, Clark C. The consortium to establish a registry for Alzheimer’s disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimers disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 32.Jessup M, Abraham WT, Casey DE, Feldman AM, Francis GS, Ganiats TG, Konstam MA, Mancini DM, Rahko PS, Silver MA, Stevenson LW, Yancy CW. 2009 focused update: ACCF/AHA guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines: developed in collaboration with the international society for heart and lung transplantation. Circulation. 2009;119:1977–2016. doi: 10.1161/CIRCULATIONAHA.109.192064. [DOI] [PubMed] [Google Scholar]
- 33.Erbs S, Hollriegel, Linke A, Beck EB, Adams V, Gielen S, Mobius-Winkler S, Sandri M, Krankel N, Hambrecht R, Schuler G. Exercise training in patients with advanced chronic heart failure (NYHA IIIb) promotes restoration of perpheral vasomotor function, induction of endogenous regernation, and improvement of left ventricular function. Circulation: Heart Failure. 2010;3:486–494. doi: 10.1161/CIRCHEARTFAILURE.109.868992. [DOI] [PubMed] [Google Scholar]
- 34.Gruhn N, Larsen FS, Boesgaard S, Knudsen GM, Mortensen SA, Thomsen G, Aldershvile J. Cerebral blood flow in patients with chronic heart failure before and after heart transplantation. Stroke. 2001;32:2530–2533. doi: 10.1161/hs1101.098360. [DOI] [PubMed] [Google Scholar]
- 35.Chapman SB, Asian S, Spence JS, Defina LF, Keebler MW, Didehbani N, Lu H. Shorter term aerobic exercise improves brain, cognition, and cardiovascular fitness in aging. Front Aging Neurosci. 2013;5:75. doi: 10.3389/fnagi.2013.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fu TC, Wang CH, Lin PS, Hsu CC, Cherng WJ, Huang SC, Liu MH, Chiang CL, Wang JS. Aerobic interval training improves oxygen uptake efficiency by enhancing cerebral and muscular hemodynamics in patients with heart failure. Int J Cardiol. 2013;167:41–50. doi: 10.1016/j.ijcard.2011.11.086. [DOI] [PubMed] [Google Scholar]
- 37.Kumar R, Woo MA, Birrer BV, Macey PM, Fonarow GC, Hamilton MA, Harper RM. Mammillary bodies and fornix fibers are injured in heart failure. Neurobiol Dis. 2009;33:236–242. doi: 10.1016/j.nbd.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Almeida OP, Garrido GJ, Etherton-Beer C, Lautenschlager NT, Arnolda L, Alfonso H, Flicker L. Brain and mood changes over 2 years in healthy controls and adults with heart failure and ischaemic heart disease. Eur J Heart Fail. 2013;15:850–858. doi: 10.1093/eurjhf/hft029. [DOI] [PubMed] [Google Scholar]
- 39.Beckers PJ, Denollet J, Possemiers NM, Wuyts K, Vrints CJ, Conraads VM. Maintaining physical fitness of patients with chronic heart failure: a randomized controlled trial. Eur J Cardiovasc Prev Rehabil. 2010;17:660–667. doi: 10.1097/HJR.0b013e328339ccac. [DOI] [PubMed] [Google Scholar]
- 40.Savage PD, Antkowiak M, Ades PA. Failure to improve cardiopulmonary fitness in cardiac rehabilitation. J Cardiopulm Rehabil Prev. 2009;29:284–291. doi: 10.1097/HCR.0b013e3181b4c8bd. [DOI] [PubMed] [Google Scholar]
- 41.Alosco ML, Spitznagel MB, Raz N, Cohen R, Sweet LH, Colbert LH, Josephson R, van Dulmen M, Hughes J, Rosneck J, Gunstad J. Dietary habits moderate the association between heart failure and cognitive impairment. J Nutr Gerontol Geriatr. 2013;32:106–121. doi: 10.1080/21551197.2013.781408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kulcu DG, Kurtais Y, Tur BS, Gulec S, Seckin B. The effect of cardiac rehabilitation on quality of life, anxiety and depression in patients with congestive heart failure. A randomized controlled trail, short-term results. Eura Medicophys. 2007;43:489–497. [PubMed] [Google Scholar]