Abstract
Objectives
The risk of venous thromboembolism (VTE) is highest during the initial months of oral contraceptive (OC) use. We sought to evaluate the extent of hemostatic variable changes during the initial OC cycle and if such changes are related to systemic ethinyl estradiol (EE2) exposure.
Study Design
Participants provided multiple blood samples during a 21-day OC cycle (30 mcg EE2; 150 mcg levonorgestrel) and after a single dose following a wash-out period. Analytes included D-dimer, factor VIII activity, protein C total antigen and the hepatic proteins corticosteroid- and sex-hormone-binding globulins (CBG and SHBG). EE2 pharmacokinetic analyses related to the 24 hours after the first OC tablet (OC1) and at steady state (OC21).
Results
Seventeen women completed the study. D-dimer more than doubled by OC6 (p = 0.013) and remained elevated at OC21 (p=0.012). D-dimer levels within women varied widely from day-to-day. Factor VIII increased 27% by OC2 (p < 0.001), but declined to a 9% increase by OC21. Protein C increased only 6%. EE2 steady-state area-under-the-curve ranged from 488 to 1103 pg·h/mL; higher levels were not correlated with greater increases in clotting variables. CBG and SHBG increased significantly, but were not significantly correlated with levels of EE2 or with the hemostatic variables.
Conclusions
D-dimer increases during the first OC cycle were at least as great as increases seen with longer OC use. These results provide support for the increased VTE risk during initial OC use. The extreme variability in D-dimer levels may be an important component of this risk.
Keywords: oral contraceptives, venous thromboembolism, D-dimer, factor VIII, protein C
1. Introduction
The risk of VTE within the first 3 months of oral contraceptive (OC) use may be more than double the risk after the first year, with the risk gradually decreasing between the first 3 months and 1 year [1–3], although this has not been invariably found [4]. Despite this, hemostatic variable changes before 3 months of use have not been reported. We therefore designed the study reported here to measure hemostatic changes during the first OC treatment cycle.
Numerous studies have assessed the effects of OC use on the coagulation system [5–7]. The large ‘Seven OC Study’, measured 24 hemostatic variables after 3 and 6 OC cycles in 707 women [6]. D-dimer concentration, a global marker of fibrinolysis associated with future venous thromboembolism (VTE) risk [8, 9], increased approximately 50% after 3 and 6 cycles of all OC regimens [6]. Factor VIII activity, another independent risk factor for VTE [10–15], increased approximately 20% after 3 and 6 cycles [6]. Neither EE2 dose or progestin type had a clear effect on these increases. The significance of the observed changes in D-dimer and factor VIII to the increased VTE risk among healthy OC users has not been studied. We chose to measure D-dimer concentration and factor VIII activity levels due to their association with risk of future VTE and their change with OC use.
OCs may also dis-equilibrate the coagulation system through increased synthesis of hepatic proteins. Protein C, a hepatic clotting factor, increased ~15% after 3 and 6 OC cycles [6]. We chose to measure protein C total antigen as our representative hepatic clotting factor, even though it is an anti-coagulant, as its short half-life (6–7 hours) [16, 17] may facilitate detection of short-term changes. We also studied how changes in these measures correlated with corticosteroid-binding globulin (CBG) and sex-hormone-binding globulin (SHBG) [18, 19].
Epidemiological studies show that higher OC doses of EE2 are associated with a greater increase in VTE risk [20–22]. We, therefore, also explored whether a woman’s systemic EE2 level during the first OC cycle was related to the magnitude of her clotting system changes.
2. Materials and methods
This single-arm, open-label pilot study took place at Columbia University Medical Center (CUMC) after Institutional Review Board approval. Participants provided written informed consent prior to enrolment. Women were eligible if aged 18–35 years and self-identified as white. We excluded women with any medical contraindication to use of OCs [23]. Additional exclusion criteria included: use of medications known to affect the CYP450 system; use of injectable contraception in the past 6 months or use of other hormonal contraceptives within the past month; pregnancy within the past six weeks; smoking; and a body mass index ≥ 30.0 kg/m2. We instructed participants to abstain from ibuprofen, aspirin and grapefruit juice throughout the study, alcohol within 24 hours, and caffeine within 1 hour of study visits as suggested by the European Concerted Action on Thrombosis Manual [24].
The study OC contained 30 mcg EE2 and 150 mcg levonorgestrel (LNG) packaged with 21 active and 7 placebo tablets (Portia®, Teva Pharmaceuticals, Philadelphia, PA). Treatment began within 7 days of the start of menses [25]. Participants selected a particular time to take her daily OC, and we directly observed OC intake at this particular time on study visit days. Participants underwent multiple blood draws to measure hemostatic variables over 4 weeks immediately before each OC was taken on days 1 (OC10), 2 (OC124), 3 (OC224), 4 (OC324), 7 (OC624) and 21 (OC210); and at the same time on day 22 (OC2124) and day 28. After completing the OC pack, each participant returned for a single OC pill at her next spontaneous menses and we collected blood samples over the following 4 days (noted as days 60–63). Participants sat quietly for 30 minutes prior to each blood draw using a 21 gauge butterfly needle in the antecubital vein. Each participant was admitted for 24 hours on days 1 and 21 to collect 14 timed samples for pharmacokinetic (PK) analyses, as previously described [25]. At each visit, participants answered questions about use of concomitant medications, caffeine/alcohol intake, and adverse events since the last visit. All study visits were conducted in winter 2012–2013, to minimize seasonal variation in hemostatic variables [26].
Samples for clotting factor analyses were collected in a citrated vacutainer and centrifuged at 3000 rpm at 4°C for 10 minutes; plasma was transferred and frozen in 1 mL aliquots at −80°C until analysis in batches. The ARUP National Reference Laboratories (Salt Lake City, UT) measured D-dimer concentration, factor VIII activity and protein C total antigen. D-dimer was measured by immunoturbidimetric assay using the STA Compact analyzer (Diagnostica Stago Inc., Parsippany, NJ); factor VIII activity by a clotting assay using the STA-R analyzer (Diagnostica Stago Inc., Parsippany, NJ) and protein C total antigen by an enzyme-linked immunosorbent assay (ELISA) using EIA Reader 520 (ARUP, Salt Lake City, UT). The within-run precision for each assay was 1.9% for D-dimer at levels around 2 mcg/mL, and 5.8% and 3% for factor VIII and protein C, respectively. The between-run precision for each assay was 0.9% for D-dimer at levels around 2 mcg/mL, and 4.6% and 5.0% for factor VIII and protein C, respectively. The lower limits of detection were 0.2 mcg/mL, 1% and 10% respectively. We set D-dimer results that were below the detection limit as 0.2 mcg/mL for analysis; this produces a conservative bias in the measurement of increases in low level D-dimer concentration with OC use.
The CUMC Biomarkers Core Laboratory performed CBG radioimmunoassays (IBL-America, Minneapolis, MN) and SHBG chemiluminescence immunoassays on an automated immunochemistry analyzer (Immulite 1000, Siemens Healthcare Diagnostics Inc., Deerfield, IL) from serum collected at baseline and on days 21 and 28 and after the wash-out period. We measured EE2 serum concentrations using liquid chromatography-tandem mass spectrometry and conducted standard PK analyses [25].
The D-dimer, factor VIII and protein C measures were log-transformed for analysis, but all results are presented in the original units for ease of interpretation. We normalized factor VIII activity and protein C total antigen measurements to mean baseline values of 100% [6]. To reduce random variation at steady state we averaged the values of the hemostatic variables immediately before and 24 hours after OC21, except in Figures 1 and 2 where we show these values separately. We summarized the levels of hemostatic variables and binding globulins using descriptive statistics. We conducted matched-pairs t-tests to evaluate changes over time in D-dimer, factor VIII and protein C. We used linear regression to assess the relationship between (untransformed) steady-state 24-hour EE2 area-under-the-curve (EE2-AUC21) and the change in hemostatic variables from baseline to OC21 (note: absolute changes in log transformed values are equivalent to ratios of untransformed values). Confidence intervals for Pearson correlation coefficients (r values) were calculated using Fisher’s z transformation. Statistical analyses were conducted using Stata 12 (StataCorp, College Station, TX). All significance levels (p values) quoted are 2-sided.
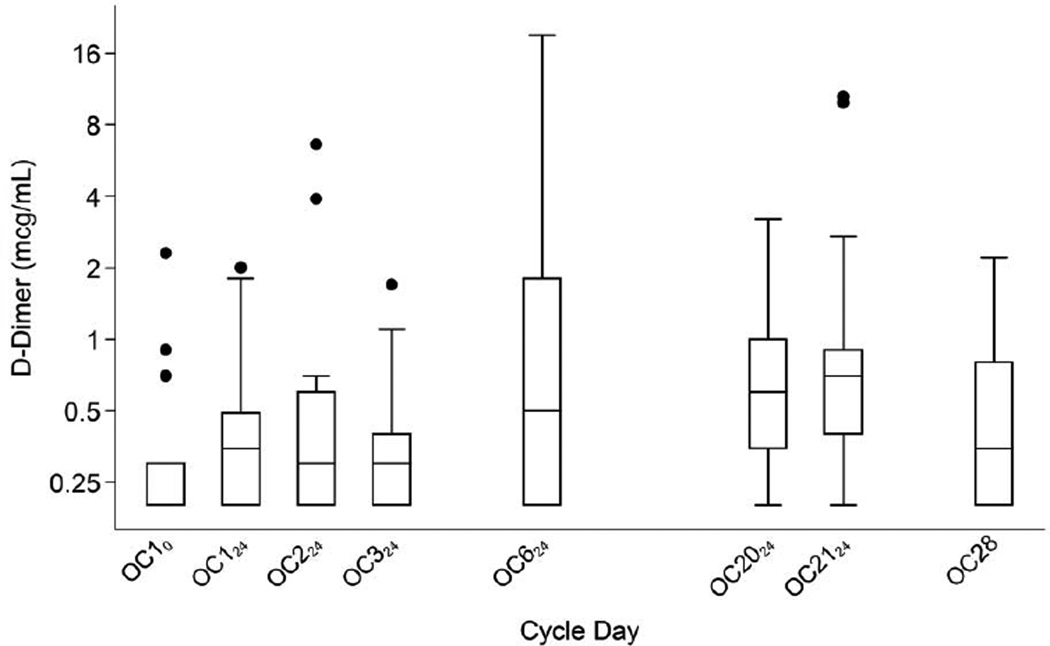
Fig. 1.
D-dimer levels during the OC cycle. Boxes show medians and interquartile ranges (IQR); lower whiskers denote the smallest values ≥ (25th percentile − 1.5 × IQR); upper whiskers denote the largest values ≤ (75th percentile + 1.5 × IQR); and individual points denote D-dimer values outside the whiskers [35]. Detection limit of the assay was 0.2 mcg/mL.
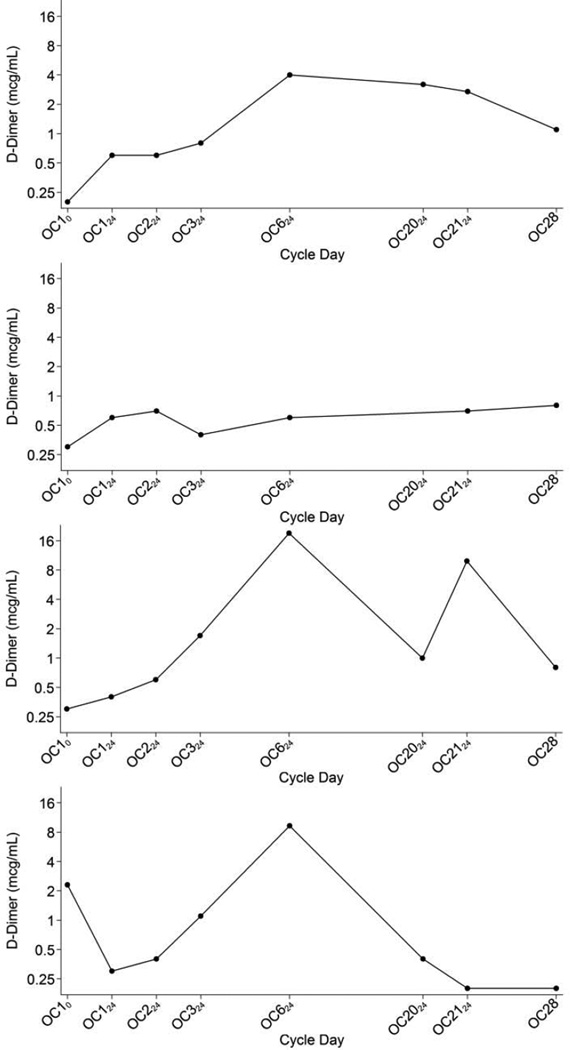
Fig. 2.
D-dimer levels of four representative individuals.
3. Results
Of 24 eligible women who consented to participate, we withdrew 3 due to scheduling conflicts. An additional 3 women withdrew after consent but prior to receiving study treatment: one due to fear of needles, one from use of an exclusionary medication and one withdrew consent. Another participant withdrew due to poor venous access at her first study visit. Thus, 17 women participated in this study completing 163 of 170 scheduled visits. Three participants missed the day 28 visit, and one participant missed the last four visits after the OC free period. Table 1 shows their baseline characteristics. The 7 participants who withdrew were similar in baseline characteristics to those who continued in the study.
Table 1.
Baseline characteristics of study participants (n=17).
| Variable | Study Participants |
|---|---|
| Age | 24.9 (±3.9) |
| Height (cm) | 168.1 (±7.7) |
| Weight (kg) | 63.9 (±10.5) |
| BMI (kg/m2) | 22.6 (±3.1) |
| Ever been pregnant | 2 (11.8) |
| Ever given birth | 0 (0.0) |
| Previously used OCs | 11 (64.7) |
Values are shown as mean (±SD) or n (%).
All 17 participants took the first study OC within 7 days of the start of menses. Compliant OC use during the study was confirmed by questioning and observing that the CBG changes from baseline to day 21 were consistent with good compliance [27].
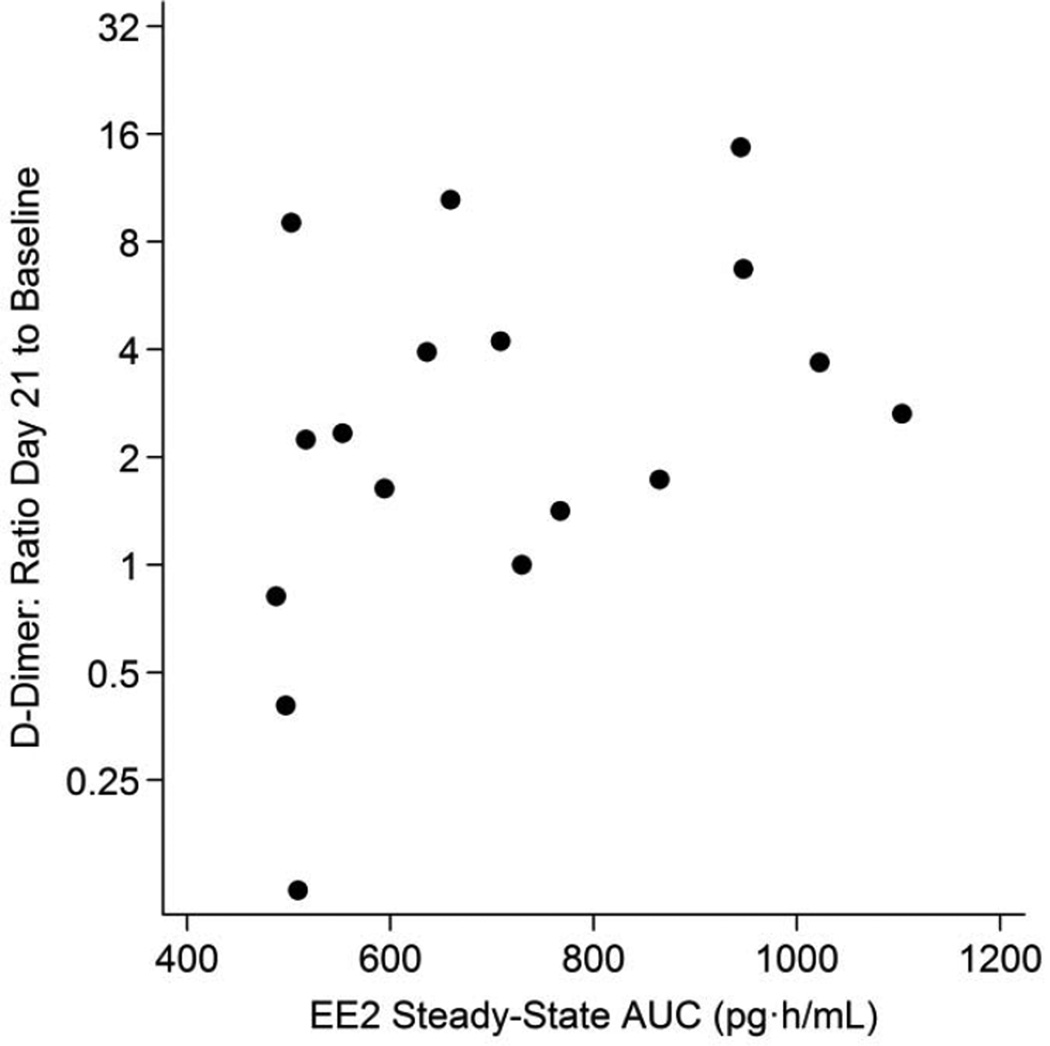
Table 2 shows measurements of the hemostatic variables over the study period. Mean (geometric mean of untransformed values) D-dimer concentration more than doubled from 0.31 mcg/mL at baseline (OC10) to 0.81 mcg/mL at day 6 (OC6; p = 0.013) and remained elevated at 0.72 mcg/mL at the last active pill (OC21; p = 0.012). Figures 1 and 2 show the increase and the substantial intra-individual variability in D-dimer levels. At OC21, 12 of the 17 participants had D-dimer values ≥ 0.4 mcg/mL, which exceeds the normal range of D-dimer for this laboratory and 10 had values ≥ 0.5 mcg/mL compared to only 3 of the 17 exceeding these values at baseline (10/17 vs 3/17; p = 0.012). D-dimer levels measured during OC use were poorly correlated with baseline D-dimer levels (Table 2). By 7 days after the last active pill was taken, mean D-dimer decreased to 0.45 mcg/mL (95% CI: 0.27, 0.75) not significantly different from the baseline value (p = 0.28). After the one-month wash-out, mean D-dimer concentration was 0.44 mcg/mL (95% CI: 0.27, 0.73; p = 0.27). D-dimer did not increase 24, 48 and 72 hours after taking the single pill after the 1-month washout period (data not shown). EE2-AUC21 varied from 488 to 1103 pg·h/Ml (data not shown, [25]); change in D-dimer concentration from baseline to OC21 was somewhat correlated with the individual systemic EE2-AUC21 (r = 0.36; 95% CI: −0.21, 0.75; p = 0.21). Figure 3 shows that this association was, however, largely due to the 2 participants whose D-dimer levels decreased substantially after entry to the study; removing these individuals reduced this correlation substantially (r = 0.15; 95% CI: −0.46, 0.67; p = 0.65).
Table 2.
Clotting factor values during the first OC cycle
| Cycle Day | OC10 | OC124 | OC224 | OC324 | OC624 | OC21a | OC28 |
|---|---|---|---|---|---|---|---|
| D-dimer (mcg/mL) | |||||||
| Geometric mean (±95% CI)b |
0.31 (0.22, 0.44) |
0.37 (0.25, 0.55) |
0.42 (0.24, 0.71) |
0.37 (0.26, 0.53) |
0.81 (0.39, 1.71) |
0.72 (0.46, 1.11) |
0.45 (0.27, 0.75) |
| p-valuec | - | 0.47 | 0.31 | 0.36 | 0.013 | 0.012 | 0.28 |
| Correlation coefficientd (±95% CI)b,d |
- | 0.24 (−0.29, 0.66) |
0.18 (−0.33, 0.63) |
0.36 (−0.15, 0.72) |
0.30 (−0.21, 0.68) |
−0.23 (−0.64, 0.28) |
−0.16 (−0.64, 0.40) |
| p-valued | 0.38 | 0.50 | 0.16 | 0.25 | 0.52 | 0.59 | |
| Factor VIII activity (%) | |||||||
| Geometric mean (±95% CI)b |
100 (81, 124) |
109 (85, 141) |
126 (105, 153) |
127 (107, 149) |
117 (94, 146) |
109 (88, 135) |
103 (82, 129) |
| p-valuec | - | 0.36 | <0.001 | 0.011 | 0.076 | 0.24 | 0.92 |
| Correlation coefficientd (±95% CI)b,d |
- | 0.66 (0.26, 0.87) |
0.87 (0.67, 0.95) |
0.61 (0.18, 0.84) |
0.66 (0.26, 0.87) |
0.74 (0.40, 0.90) |
0.62 (0.13, 0.87) |
| p-valued | 0.003 | <0.001 | 0.008 | 0.003 | <0.001 | 0.016 | |
| Protein C total antigen (%) | |||||||
| Geometric mean (±95% CI)b |
100 (93, 107) |
101 (94, 108) |
101 (94, 108) |
101 (94, 108) |
102 (95, 108) |
106 (99, 114) |
105 (98, 113) |
| p-valuec | - | 0.83 | 0.69 | 0.66 | 0.52 | 0.48 | 0.22 |
| Correlation coefficientd (±95% CI)b,d |
- | 0.57 (0.12, 0.82) |
0.67 (0.28, 0.87) |
0.82 (0.56, 0.93) |
0.70 (0.33, 0.88) |
0.51 (0.04, 0.80) |
0.56 (0.04, 0.84) |
| p-valued | 0.015 | 0.002 | <0.001 | 0.001 | 0.035 | 0.036 |
OC21, average of values at t=0 and t=24 hrs;
CI, confidence interval;
Paired t-test against OC10;
Correlation with OC10; CI and p-value calculated with Fisher’s z-transformation of correlation coefficient.
Fig. 3.
D-dimer changes from baseline to Day 21 versus ethinyl estradiol steady-state AUC.
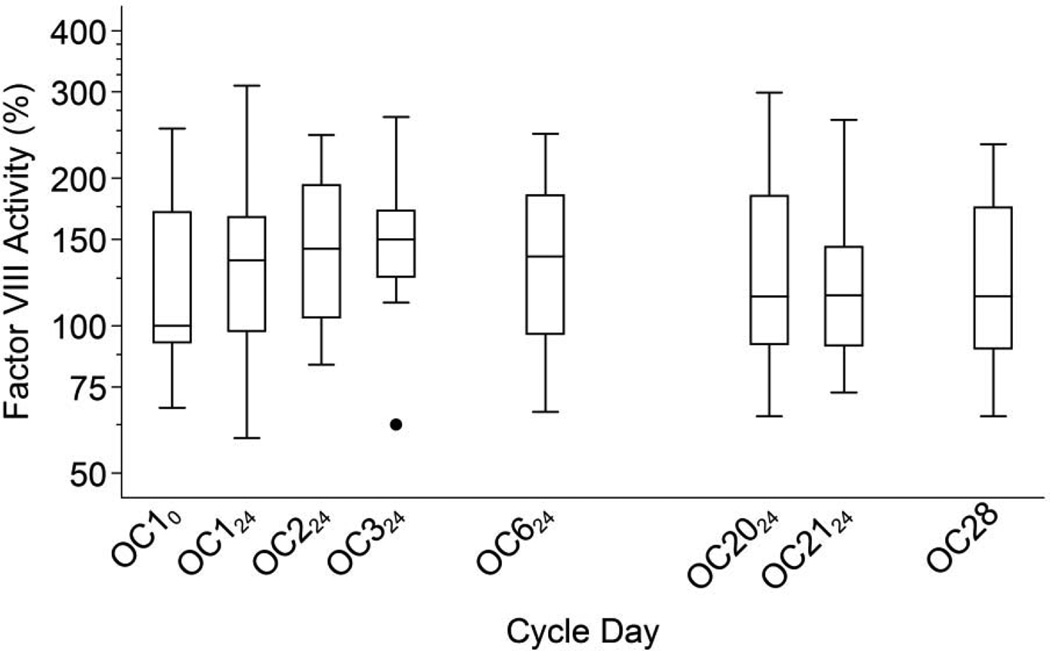
Factor VIII activity varied widely between individuals, but increased little from baseline to OC21 (p = 0.24) (Figure 4). Factor VIII did, however, increase briefly after the second and third OC tablets; at OC224 the mean increased to 126 (p < 0.001) and at OC324 to 127 (p = 0.011). Factor VIII levels during OC use were strongly correlated with baseline levels (Table 2). Seven days after the last active OC, mean factor VIII was 103. Following the single OC after the 1-month washout, factor VIII activity at 24 hours was 109, and then decreased to 107 and 103 at 48 and 72 hours (p = 0.065, 0.040 and 0.19, respectively). Systemic exposure to EE2 from the first OC (EE2-AUC1) was not correlated with the change in factor VIII activity from baseline to OC324 (r = 0.22; 95% CI: –0.29, 0.63; p = 0.40); analysis of factor VIII levels at OC124 and OC224 yielded similar results. Greater EE2-AUC21 was not associated with increases in factor VIII from baseline to OC21, rather the reverse (r = −0.57; 95% CI: −0.85, −0.06; p = 0.032).
Fig. 4.
Factor VIII activity levels during the OC cycle. Boxes show medians and interquartile ranges (IQR); lower whiskers denote the smallest values ≥ (25th percentile − 1.5 × IQR); upper whiskers denote the largest values ≤ (75th percentile + 1.5 × IQR); and individual points denote D-dimer values outside the whiskers [35].
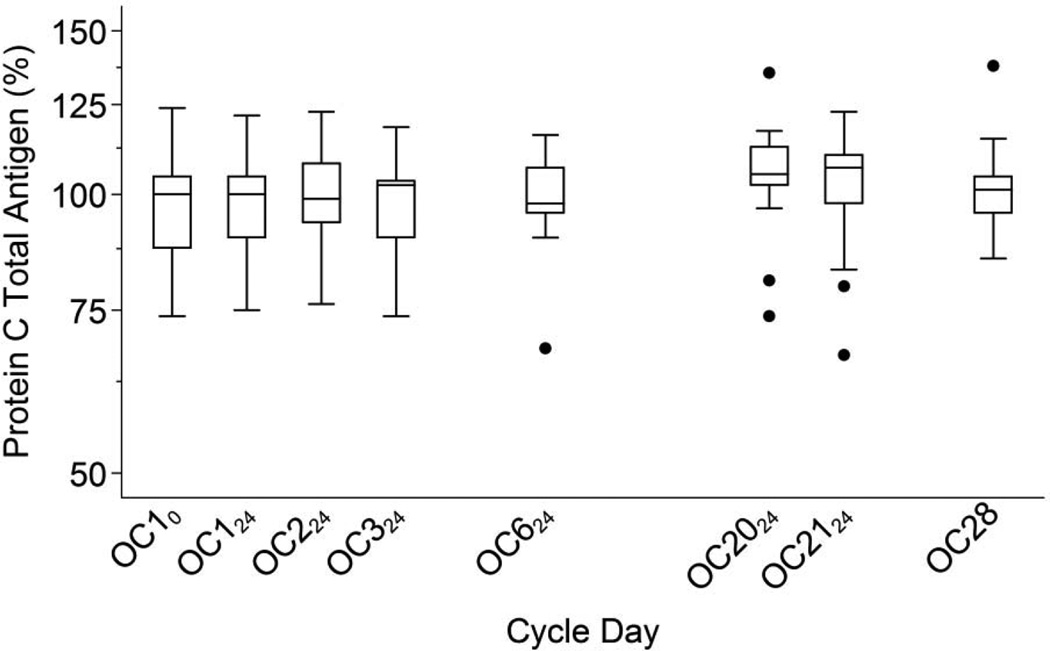
We found little average change in protein C total antigen levels at any point in the OC cycle and little variability (Figure 5). Protein C levels during OC use were moderately to strongly correlated with baseline levels (Table 2). We found no association between EE2-AUC21 and the individual changes in protein C at OC21 (r = −0.13; 95% CI: −0.62, 0.43; p= 0.67).
Fig. 5.
Protein C total antigen levels during the OC cycle. Boxes show medians and interquartile ranges (IQR); lower whiskers denote the smallest values ≥ (25th percentile − 1.5 × IQR); upper whiskers denote the largest values ≤ (75th percentile + 1.5 × IQR); and individual points denote D-dimer values outside the whiskers [35].
The geometric mean CBG and SHBG increased from baseline to OC2124 (52.4 to 135.1 mcg/mL, p < 0.001; and 44.7 to 73.4 nmol/L, p < 0.001, respectively), and all participants showed values consistent with good OC compliance. These increases were closely mirrored when calculations were made with untransformed values (50.7 to 140.7, and 46.5 to 77.2, respectively). Changes in untransformed CBG and SHBG from baseline to day 21 were unrelated to baseline values, and all further calculations with were made with untransformed values. The changes in CBG and SHBG levels were not statistically significantly correlated with EE2-AUC21, or with the changes in D-dimer, factor VIII or protein C.
4. Discussion
In this pilot study D-dimer levels increased gradually during a single cycle of OC use at least as much as the increases reported after 3 and 6 cycles of OC [6]. Similarly, we found a statistically significant increase in factor VIII activity after as little as 1–2 days of OC use; these transient increases were similar in magnitude to those reported after 3 and 6 cycles of OC use. We chose to focus on these particular factors because prospective studies have shown that both D-dimer and factor VIII levels are related to future VTE risk [8, 14]. These results may provide a physiological explanation to support the epidemiological studies that have reported an increased VTE risk among OC users seen even during the very first months of use [3]. As shown in Figure 2, participants demonstrated great variability in day-to-day D-dimer levels and in their apparent response to the OC with several participants experiencing extreme increases in D-dimer; in this small study however we cannot assess whether a subset of women might be uniquely susceptible to an OC-mediated disequilibrium of hemostasis.
A number of epidemiological studies indicate that OC-related VTE risk is less in women using an OC with a lower dose of EE2 [20–22]. The level of EE2 in a tablet is, however, a poor indicator of a woman’s individual exposure due to individual differences in absorption and metabolism. Among the women in this study, all taking the same OC, we found the expected greater than two-fold range in steady state 24-hour EE2 exposure (488–1103 pg·h/mL) [25]. However, contrary to expectation, higher individual EE2 exposures were not associated with greater changes in either D-dimer or factor VIII activity. These results may indicate that changes in D-dimer and factor VIII may not be the most relevant markers of OC-associated VTE risk. Future, larger studies should further evaluate EE2 exposure and consider its effect on other aspects of the clotting system.
Previous studies report moderate changes in protein C during OC use; however, we found no significant increases in protein C during the first OC cycle. Protein C (an anticoagulant factor) was of interest in this study because it is a hepatic protein with a short half-life (thus a brief study might be able to identify changes), and because of the hypothesis that the OC primarily affects coagulation due to an effect on hepatic proteins. We did not study other hepatic coagulation factors because their longer half-lives could reduce the chances of identifying a change within 21 days, particularly in such a small study. We found the expected increases in the hepatic proteins SHBG and CBG. The OC effects on hepatic proteins are demonstrably variable rather than uniform -- i.e., we found a small not-significant change in protein C, a larger, but still modest increase in SHBG and a much larger increase in CBG.
The small number of participants and small number of hemostasis variables tested are a substantial limitation of this pilot study. However, even in this small study we demonstrated that OC-mediated coagulation changes begin and are readily detectable during the first OC cycle – a finding that may be useful in the design of future studies. This study used an immunoturbidometric D-dimer assay, which is less sensitive than an ELISA assay. The D-dimer changes found here, however, were large enough to detect even with this less sensitive assay. Strengths of the study included a homogeneous sample and careful adherence to guidelines for collection of specimens for studying hemostasis, as well as deliberate minimization of menstrual cycle or seasonal variation.
SHBG increases have been suggested as a marker of VTE risk, an unsettled association [28–34]. In the present study, we found little association between SHBG or CBG increases and changes in the hemostatic variables or the systemic EE2 exposure. This limits our enthusiasm for SHBG as a surrogate variable in explaining aspects of OC-mediated VTE risk.
Short-term effects of the OC on hemostatic variables found here support reports of early increases in VTE risk from OC use. A short-term study such as this is relatively easier to carry out than 3- or 6-month studies. This approach may thus be useful for the study of additional hemostatic variables, and for making comparisons among different OCs.
Implications.
This study showed that increases in D-dimer are clearly evident in the first cycle of OC use and may be larger than are seen after a longer duration of use, and thus provide biological support for the increased VTE risk during initial OC use found in epidemiological studies.
Acknowledgments
Dr. Westhoff receives honoraria from Merck and Bayer as a data safety and monitoring board member, both of which produce oral contraceptives; however, not the oral contraceptive studied here.
We wish to thank the CUMC Biomarkers Core Laboratory staff for serum analyses; in particular, May Huang, Susan Pollack, Roseann Zott, and Tiffany Thomas, PhD. We also wish to thank Mary-Jane McEneaney, DNP of the School of Nursing; Arielle Rodman, MD of the Department of Medicine; and Da Li of the Department of Obstetrics and Gynecology, all Columbia University, New York, NY for assistance with study visits. Our greatest thanks are due to the volunteers who took part in this study. This pilot study was funded by the Irving Institute for Clinical and Translational Research Collaborative and Multidisciplinary Pilot Research Award at Columbia University Medical Center (CUMC) and by the Howard Solomon Research Fund (CUMC). The Biomarkers Core Laboratory of the Irving Institute for Clinical and Translational Research (CUMC) supported the ethinyl estradiol assays. This work was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number UL1 TR000040, formerly the National Center for Research Resources, Grant Number UL1 RR024156. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: None of the other authors have any conflicts to report.
References
- 1.Suissa S, Blais L, Spitzer WO, Cusson J, Lewis M, Heinemann L. First-time use of newer oral contraceptives and the risk of venous thromboembolism. Contraception. 1997;56:141–146. doi: 10.1016/s0010-7824(97)00119-4. [DOI] [PubMed] [Google Scholar]
- 2.Farley TM, Meirik O, Marmot MG, Chang CL, Poulter NR. Oral contraceptives and risk of venous thromboembolism: impact of duration of use. Contraception. 1998;57:61–65. doi: 10.1016/s0010-7824(97)00209-6. [DOI] [PubMed] [Google Scholar]
- 3.van Hylckama Vlieg A, Helmerhorst FM, Vandenbroucke JP, Doggen CJ, Rosendaal FR. The venous thrombotic risk of oral contraceptives, effects of oestrogen dose and progestogen type: results of the MEGA case-control study. BMJ. 2009;339:b2921. doi: 10.1136/bmj.b2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vinogradova Y, Coupland C, Hippisley-Cox J. Use of combined oral contraceptives and risk of venous thromboembolism: nested case-control studies suing the QResearch and CPRD databases. BMJ. 2015;350:h2135. doi: 10.1136/bmj.h2135. coi:10.1136/bmj.h2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bloemenkamp KW, Rosendaal FR, Helmerhorst FM, Koster T, Bertina RM, Vandenbroucke JP. Hemostatic effects of oral contraceptives in women who developed deep-vein thrombosis while using oral contraceptives. Thromb Haemost. 1998;80:382–387. [PubMed] [Google Scholar]
- 6.The Oral Contraceptive and Hemostasis Study Group. The effects of seven monophasic oral contraceptive regimens on hemostatic variables: conclusions from a large randomized multicenter study. Contraception. 2003;67:173–185. doi: 10.1016/s0010-7824(02)00476-6. [DOI] [PubMed] [Google Scholar]
- 7.DeSancho MT, Dorff T, Rand JR. Thrombophilia and the risk of thromboembolic events in women on oral contraceptives and hormone replacement therapy. Blood Coagul Fibrinolysis. 2010;21:534–538. doi: 10.1097/MBC.0b013e32833b2b84. [DOI] [PubMed] [Google Scholar]
- 8.Cushman M, Folsom AR, Wang L, Aleksic N, Rosamond WD, Tracy RP, et al. Fibrin fragment D-dimer and the risk of future venous thrombosis. Blood. 2003;101:1243–1248. doi: 10.1182/blood-2002-05-1416. [DOI] [PubMed] [Google Scholar]
- 9.Verhovsek M, Douketis JD, Yi Q, Shrivastava S, Tait RC, Baglin T, et al. Systematic review: D-dimer to predict recurrent disease after stopping anticoagulant therapy for unprovoked venous thromboembolism. Ann Intern Med. 2008;149:481–490. W94. doi: 10.7326/0003-4819-149-7-200810070-00008. [DOI] [PubMed] [Google Scholar]
- 10.Bloemenkamp KWM, Helmerhorst FM, Rosendaal FR, Vandenbroucke JP. Venous thrombosis, oral contraceptives and high factor VIII levels. Thromb Haemost. 1999;82:1024–1027. [PubMed] [Google Scholar]
- 11.De Mitrio V, Marino R, Scaraggi FA, Di Bari L, Giannoccaro F, Petronelli M, et al. Influence of factor VIII/von Willebrand complex on the activated protein C-resistance phenotype and on the risk for venous thromboembolism in heterozygous carriers of the factor V Leiden mutation. Blood Coagul Fibrinolysis. 1999;10:409–416. [PubMed] [Google Scholar]
- 12.Kraaijenhagen RA, in’t Anker PS, Koopman MM, Reitsma PH, Prins MH, van den Ende A, et al. High plasma concentration of factor VIIIc is a major risk factor for venous thromboembolism. Thromb Haemost. 2000;83:5–9. [PubMed] [Google Scholar]
- 13.Sitruk-Ware R, Plu-Bureau G, Menard J, Conard J, Kumar S, Thalabard JC, et al. Effects of oral and transvaginal ethinyl estradiol on hemostatic factors and hepatic proteins in a randomized, crossover study. J Clin Endocrinol Metab. 2007;92:2074–2079. doi: 10.1210/jc.2007-0026. [DOI] [PubMed] [Google Scholar]
- 14.Cushman M, O’Meara ES, Folsom AR, Heckbert SR. Coagulation factors IX through XIII and the risk of future venous thrombosis: the Longitudinal Investigation of Thromboembolism Etiology. Blood. 2009;114:2878–2883. doi: 10.1182/blood-2009-05-219915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ota S, Yamada N, Ogihara Y, Tsuji A, Ishikura K, Nakamura M, et al. High plasma level of factor VIII: an important risk factor for venous thromboembolism. Circ J. 2011;75:1472–1475. doi: 10.1253/circj.cj-10-1051. [DOI] [PubMed] [Google Scholar]
- 16.Kitchens CS, Kessler CM, Konkle BA. Consultative Hemostasis and Thrombosis. Philadelphia: Elsevier; 2013. [Google Scholar]
- 17.Saba HI, Roberts HR. Hemostasis and Thrombosis. Chichester: John Wiley & Sons; 2014. [Google Scholar]
- 18.van der Vange N, Blankenstein MA, Kloosterboer HJ, Haspels AA, Thijssen JH. Effects of seven low-dose combined oral contraceptives on sex hormone binding globulin, corticosteroid binding globulin, total and free testosterone. Contraception. 1990;41:345–352. doi: 10.1016/0010-7824(90)90034-s. [DOI] [PubMed] [Google Scholar]
- 19.Wiegratz I, Kutschera E, Lee JH, Moore C, Mellinger U, Winkler UH, et al. Effect of four different oral contraceptives on various sex hormones and serum-binding globulins. Contraception. 2003;67:25–32. doi: 10.1016/s0010-7824(02)00436-5. [DOI] [PubMed] [Google Scholar]
- 20.Piper JM, Kennedy DL. Oral contraceptives in the United States: trends in content and potency. Int J Epidemiol. 1987;16:215–221. doi: 10.1093/ije/16.2.215. [DOI] [PubMed] [Google Scholar]
- 21.Gerstman BB, Piper JM, Tomita DK, Ferguson WJ, Stadel BV, Lundin FE. Oral contraceptive estrogen dose and the risk of deep venous thromboembolic disease. Am J Epidemiol. 1991;133:32–37. doi: 10.1093/oxfordjournals.aje.a115799. [DOI] [PubMed] [Google Scholar]
- 22.Lidegaard O, Nielsen LH, Skovlund CW, Skjeldestad FE, Lokkegaard E. Risk of venous thromboembolism from use of oral contraceptives containing different progestogens and oestrogen doses: Danish cohort study, 2001–9. BMJ. 2011;343:d6423. doi: 10.1136/bmj.d6423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization. Medical eligibility criteria for contraceptive use. 4th. Geneva: World Health Organization; 2010. [PubMed] [Google Scholar]
- 24.Jespersen J, Bertina RM, Haverkate F. Laboratory Techniques in Thrombosis: A Manual. 2nd. Dordrecht: Kluwer Academic Publishers BV; 1999. [Google Scholar]
- 25.Westhoff CL, Pike MC, Tang R, DiNapoli MN, Sull M, Cremers S. Estimating systemic exposure to ethinyl estradiol from an oral contraceptive. Am J Obstet Gynecol. 2015;212:614.e1–614.e7. doi: 10.1016/j.ajog.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Montagnana M, Salvagno GL, Lippi G. Circadian variation within hemostasis: an underrecognized link between biology and disease? Sem Thromb Hemost. 2009;35:23–33. doi: 10.1055/s-0029-1214145. [DOI] [PubMed] [Google Scholar]
- 27.Westhoff CL, Petrie KA, Cremers S. Using changes in binding globulins to assess oral contraceptive compliance. Contraception. 2013;87:176–181. doi: 10.1016/j.contraception.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Odlind V, Milsom I, Persson I, Victor A. Can changes in sex hormone binding globulin predict the risk of venous thromboembolism with combined oral contraceptive pills? Acta Obstet Gynecol Scand. 2002;81:482–490. [PubMed] [Google Scholar]
- 29.van Rooijen M, Silveira A, Hamsten A, Bremme K. Sex hormone-binding globulin—A surrogate marker for the prothrombotic effects of combined oral contraceptives. AJOG. 2004;190:332–337. doi: 10.1016/s0002-9378(03)00950-5. [DOI] [PubMed] [Google Scholar]
- 30.Stanczyk FZ, Grimes DA. Sex hormone-binding globulin: not a surrogate marker for venous thromboembolism in women using oral contraceptives. Contraception. 2008;78:201–203. doi: 10.1016/j.contraception.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 31.van Vliet HA, Rosendaal FR, Rosing J, Helmerhorst FM. Sex hormone-binding globulin: an adequate surrogate marker for venous thromboembolism in women using new hormonal contraceptives. Contraception. 2009;79:328–330. doi: 10.1016/j.contraception.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 32.Raps M, Helmerhorst F, Fleischer K, Thomassen S, Rosendaal F, Rosing J, et al. Sex hormone-binding globulin as a marker for the thrombotic risk of hormonal contraceptives. J Thromb Haemost. 2012;10:992–997. doi: 10.1111/j.1538-7836.2012.04720.x. [DOI] [PubMed] [Google Scholar]
- 33.Stegeman BH, Helmerhorst FM, Vos HL, Rosendaal FR, van Hylckama Vlieg A. Sex hormone-binding globulin levels are not causally related to venous thrombosis risk in women not using hormonal contraceptives. J Thromb Haemost. 2012;10:2061–2067. doi: 10.1111/j.1538-7836.2012.04878.x. [DOI] [PubMed] [Google Scholar]
- 34.Kluft C, Skouby SO, Jespersen J, Burggraaf J. Sex hormone-binding globulin as a marker for the thrombotic risk of hormonal contraceptives: a rebuttal. J Thromb Haemost. 2013;11:394–395. doi: 10.1111/jth.12067. [DOI] [PubMed] [Google Scholar]
- 35.Tukey JW. Exploratory Data Analysis. Reading, Addison-Wesley Publishing Company; 1977. [Google Scholar]