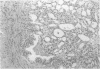
Abstract
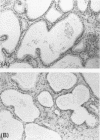
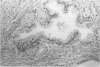
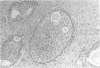
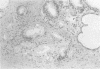
AIMS: To describe the prostatic adenectomy specimens of six patients with symptomatic benign prostatic hyperplasia (BPH) who failed to respond to long term treatment with a 5-alpha-reductase inhibitor, finasteride. METHODS: Histological sections from six cases of BPH who had been treated with finasteride were investigated. Five patients were prescribed 5 mg finasteride daily for six months and one patient 5 mg daily for 12 months. The patients underwent adenectomy as their urethral obstruction failed to resolve. Twenty cases of untreated BPH served as controls. RESULTS: In patients taking finasteride for six months the prostatic adenectomy specimens showed a reduction in the size of the prostate and an increase in the stroma:epithelial and stroma:lumen ratios compared with controls. The size of the ducts and acini was not as uniform as in the controls. In particular, some ducts and acini were still lined by a bistratified epithelium similar to that found in controls but lacked undulations at the epithelial border; other ducts/acini were atrophic. Some scattered clusters of small acini with a focally fragmented basal cell layer were observed in two of the five treated cases. One prostatic adenectomy specimen, from the patient treated for one year, showed extensive lobular atrophy and diffuse squamous and transitional cell metaplasia. At the periphery of the transition zone, there was a complex intra-acinar papillary-cribriform proliferation of clear cells without nuclear atypia, similar to clear cell papillary hyperplasia. The periurethral region showed stromal nodules in both patients and controls. CONCLUSIONS: Morphological evaluation of finasteride treated BPH showed changes in the lobules of the transition zone, but not in the periurethral stroma.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayala A. G., Srigley J. R., Ro J. Y., Abdul-Karim F. W., Johnson D. E. Clear cell cribriform hyperplasia of prostate. Report of 10 cases. Am J Surg Pathol. 1986 Oct;10(10):665–671. doi: 10.1097/00000478-198610000-00001. [DOI] [PubMed] [Google Scholar]
- BAINBOROUGH A. R. Squamous metaplasia of prostate following estrogen therapy. J Urol. 1952 Jul;68(1):329–336. doi: 10.1016/S0022-5347(17)68202-8. [DOI] [PubMed] [Google Scholar]
- Bologna M., Muzi P., Biordi L., Festuccia C., Vicentini C. Finasteride dose-dependently reduces the proliferation rate of the LnCap human prostatic cancer cell line in vitro. Urology. 1995 Feb;45(2):282–290. doi: 10.1016/0090-4295(95)80019-0. [DOI] [PubMed] [Google Scholar]
- Cheville J. C., Bostwick D. G. Postatrophic hyperplasia of the prostate. A histologic mimic of prostatic adenocarcinoma. Am J Surg Pathol. 1995 Sep;19(9):1068–1076. doi: 10.1097/00000478-199509000-00011. [DOI] [PubMed] [Google Scholar]
- Cohen S. M., Werrmann J. G., Rasmusson G. H., Tanaka W. K., Malatesta P. F., Prahalada S., Jacobs J. G., Harris G., Nett T. M. Comparison of the effects of new specific azasteroid inhibitors of steroid 5 alpha-reductase on canine hyperplastic prostate: suppression of prostatic DHT correlated with prostate regression. Prostate. 1995 Feb;26(2):55–71. doi: 10.1002/pros.2990260202. [DOI] [PubMed] [Google Scholar]
- Gormley G. J., Stoner E., Bruskewitz R. C., Imperato-McGinley J., Walsh P. C., McConnell J. D., Andriole G. L., Geller J., Bracken B. R., Tenover J. S. The effect of finasteride in men with benign prostatic hyperplasia. The Finasteride Study Group. N Engl J Med. 1992 Oct 22;327(17):1185–1191. doi: 10.1056/NEJM199210223271701. [DOI] [PubMed] [Google Scholar]
- Imperato-McGinley J. 5 alpha-reductase deficiency: human and animal models. Eur Urol. 1994;25 (Suppl 1):20–23. doi: 10.1159/000475327. [DOI] [PubMed] [Google Scholar]
- Isaacs J. T. Etiology of benign prostatic hyperplasia. Eur Urol. 1994;25 (Suppl 1):6–9. doi: 10.1159/000475324. [DOI] [PubMed] [Google Scholar]
- Juniewicz P. E., Hoekstra S. J., Lemp B. M., Barbolt T. A., Devin J. A., Gauthier E., Frenette G., Dube J. Y., Tremblay R. R. Effect of combination treatment with zanoterone (WIN 49596), a steroidal androgen receptor antagonist, and finasteride (MK-906), a steroidal 5 alpha-reductase inhibitor, on the prostate and testes of beagle dogs. Endocrinology. 1993 Aug;133(2):904–913. doi: 10.1210/endo.133.2.8393778. [DOI] [PubMed] [Google Scholar]
- Lamb J. C., English H., Levandoski P. L., Rhodes G. R., Johnson R. K., Isaacs J. T. Prostatic involution in rats induced by a novel 5 alpha-reductase inhibitor, SK&F 105657: role for testosterone in the androgenic response. Endocrinology. 1992 Feb;130(2):685–694. doi: 10.1210/endo.130.2.1733716. [DOI] [PubMed] [Google Scholar]
- Montironi R., Magi Galluzzi C. M., Marina S., Diamanti L. Quantitative characterization of the frequency and location of cell proliferation and death in prostate pathology. J Cell Biochem Suppl. 1994;19:238–245. [PubMed] [Google Scholar]
- Montironi R., Magi-Galluzzi C., Muzzonigro G., Prete E., Polito M., Fabris G. Effects of combination endocrine treatment on normal prostate, prostatic intraepithelial neoplasia, and prostatic adenocarcinoma. J Clin Pathol. 1994 Oct;47(10):906–913. doi: 10.1136/jcp.47.10.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters C. A., Walsh P. C. The effect of nafarelin acetate, a luteinizing-hormone-releasing hormone agonist, on benign prostatic hyperplasia. N Engl J Med. 1987 Sep 3;317(10):599–604. doi: 10.1056/NEJM198709033171004. [DOI] [PubMed] [Google Scholar]
- Prahalada S. R., Keenan K. P., Hertzog P. R., Gordon L. R., Peter C. P., Soper K. A., van Zwieten M. J., Bokelman D. L. Qualitative and quantitative evaluation of prostatic histomorphology in rats following chronic treatment with finasteride, a 5-alpha reductase inhibitor. Urology. 1994 May;43(5):680–685. doi: 10.1016/0090-4295(94)90184-8. [DOI] [PubMed] [Google Scholar]
- Russo P., Warner J. A., Huryk R., Perez G., Heston W. D. TRPM-2 gene expression in normal rat ventral prostate following castration and exposure to diethylstilbestrol, flutamide, MK-906 (finasteride), and coumarin. Prostate. 1994 May;24(5):237–243. doi: 10.1002/pros.2990240504. [DOI] [PubMed] [Google Scholar]
- Shapiro E., Hartanto V., Lepor H. The response to alpha blockade in benign prostatic hyperplasia is related to the percent area density of prostate smooth muscle. Prostate. 1992;21(4):297–307. doi: 10.1002/pros.2990210406. [DOI] [PubMed] [Google Scholar]
- Tewari A., Shinohara K., Narayan P. Transition zone volume and transition zone ratio: predictor of uroflow response to finasteride therapy in benign prostatic hyperplasia patients. Urology. 1995 Feb;45(2):258–265. doi: 10.1016/0090-4295(95)80015-8. [DOI] [PubMed] [Google Scholar]
- Tuttle R. M., Loop S., Jones R. E., Meikle A. W., Ostenson R. C., Plymate S. R. Effect of 5-alpha-reductase inhibition and dexamethasone administration on the growth characteristics and intratumor androgen levels of the human prostate cancer cell line PC-3. Prostate. 1994 May;24(5):229–236. doi: 10.1002/pros.2990240503. [DOI] [PubMed] [Google Scholar]