Abstract
A recent genome–wide association study of IgA nephropathy (IgAN) identified 1q32, which contains multiple complement regulatory genes, including the complement factor H (CFH) gene and the complement factor H–related (CFHRs) genes, as an IgAN susceptibility locus. Abnormal complement activation caused by a mutation in CFHR5 was shown to cause CFHR5 nephropathy, which shares many characteristics with IgAN. To explore the genetic effect of variants in CFHR5 on IgAN susceptibility, we recruited 500 patients with IgAN and 576 healthy controls for genetic analysis. We sequenced all exons and their intronic flanking regions as well as the untranslated regions of CFHR5 and compared the frequencies of identified variants using the sequence kernel association test. We identified 32 variants in CFHR5, including 28 rare and four common variants. The distribution of rare variants in CFHR5 in patients with IgAN differed significantly from that in controls (P=0.002). Among the rare variants, in silico programs predicted nine as potential functional variants, which we then assessed in functional assays. Compared with wild-type CFHR5, three recombinant CFHR5 proteins, CFHR5-M (c.508G>A/p.Val170Met), CFHR5-S (c.533A>G/p.Asn178Ser), and CFHR5-D (c.822A>T/p.Glu274Asp), showed significantly higher C3b binding capacity (CFHR5-M: 109.67%±3.54%; P=0.02; CFHR5-S: 174.27%±9.78%; P<0.001; CFHR5-D: 127.25%±1.75%; P<0.001), whereas another recombinant CFHR5 (c.776T>A/p.Leu259Termination) showed less C3b binding (56.89%±0.57%; P<0.001). Our study found that rare variants in CFHR5 may contribute to the genetic susceptibility to IgAN, which suggests that CFHR5 is an IgAN susceptibility gene.
Keywords: CFHR5, IgA nephropathy, genetic analysis
IgA nephropathy (IgAN) is the most common primary GN worldwide.1 Recently, important discoveries have been made regarding the pathogenesis of IgAN,2–5 such as the aberrant glycosylation of IgA1 and the presence of antiglycan antibodies in circulation as well as the role of transferrin receptors in glomeruli. However, there are still many unknown aspects in IgAN. Although complement activation has been widely accepted for a long time to occur in IgAN,6 the exact pathways and regulatory mechanisms are still unclear.
Meanwhile, from racial and ethnic variations in disease incidence to familial aggregation of IgAN, increasing evidence implied a genetic effect on IgAN. A recent genome–wide association study (GWAS)7 and a GWAS replication study8 identified and confirmed 1q32 as an IgAN susceptibility locus, which suggested the involvement of genetic abnormalities in the complement factor H (CFH) and/or the complement factor H–related (CFHRs) genes in IgAN. This region contains several complement regulatory genes, CFH-CFHRs. CFHRs, which include CFHR3, CFHR1, CFHR4, CFHR2, and CFHR5, share high sequence homology with CFH. Accordingly, they have similar structures, which are built on a motif of distinct functional domains called short consensus repeats (SCRs), and similar functions in complement regulation,9 although their precise biologic roles are not completely identical.
Recently, a novel disease named CFHR5 nephropathy was identified after genetic analysis of a pedigree of Cypriot ancestry with primary GN.10 Patients with CFHR5 nephropathy presented with many characteristics, including young age onset, tendency in men, repeated episodes of synpharyngitic hematuria, mesangial matrix expansion, increased glomerular cellularity, glomerular staining for C3, mesangial electron–dense deposits, and high recurrence rate after renal transplantation, which shared many similarities with IgAN.11 Patients with CFHR5 nephropathy bear the same heterozygous internal duplication of the CFHR5 gene, which is likely to be the cause of their glomerular lesions.
The CFHR5 gene is located at 1q31.3 and spans 42 kb of the human genome. Its encoded product is a 62-kD protein named CFHR5. The CFHR5 gene has 10 exons encoded in nine SCRs, in which SCR3–7 and SCR8–9 showed a high homology with SCR10–14 and SCR19–20 in CFH, respectively. CFHR5 is the most recently discovered CFHR protein, and it is also the one that showed the most similarity with CFH among the five CFHRs.12 As with CFH and other CFHRs, CFHR5 is produced in the liver, circulates in plasma, and functions as a complement regulatory protein. Until today, the precise mechanism for CFHR5 in complement regulation is still unknown.13
However, with respect to IgAN, the disease presents complement activation in glomeruli and has clinical features similar to CFHR5 nephropathy, but whether some patients with IgAN have genetic changes in the CFHR5 gene and whether these variants contribute to IgAN susceptibility are still unknown. In this study, using a large population of patients with IgAN, we performed an intensive genetic analysis to explore the genetic effect of variants in the CFHR5 gene on IgAN susceptibility.
Results
Baseline Clinical, Laboratory, and Pathologic Characteristics
In total, 1076 individuals were recruited, including 500 patients with IgAN (258 men and 242 women) and 576 healthy controls (310 men and 266 women). The average ages for patients with IgAN and controls were 34.23±11.09 and 33.50±7.66 years old, respectively. For patients with IgAN, detailed clinical, laboratory, and pathologic characteristics were also available. Their proteinuria and eGFR levels were 1.42 (0.81–2.66) g/d and 84.19±27.03 ml/min per 1.73 m2, respectively. In total, 13.60% (68 of 500) had hypertension. Regarding pathologic features, 47.60% of patients with IgAN had a strong intensity of C3 deposition (3+ or 4+). Haas 1–5 were found in 8.60%, 0.80%, 45.20%, 33.20%, and 12.20% of patients, respectively. All patients were regularly followed for at least 1 year, with a mean follow-up time of 56.26 months. Patients’ treatment and follow-up were conducted at an IgAN-specific clinic in Peking University First Hospital and consistent with the Kidney Disease Improving Global Outcomes guideline.14 During follow-up, 16.80% (84 patients) of these patients reached a composited end point (ESRD, 50% eGFR decline, or death). The clinical, laboratory, and pathologic characteristics of the recruited patients were similar to those of previously reported IgAN cohorts.15
Identification of Genetic Variants in CFHR5
All exons in the CFHR5 gene were amplified and screened for genetic variants in recruited individuals. In total, 32 variants were identified (Figure 1, Table 1), including 10 in exons, 18 in introns, two in promoter, and two in 3′ untranslated region (UTR). Of 10 exonic variants, eight were nonsynonymous coding (including one nonsynonymous stop gain and nonsynonymous stop loss), and two were synonymous changes. Among 32 variants, 16 were found in the dbSNP database, whereas the other 16 variants were novel.
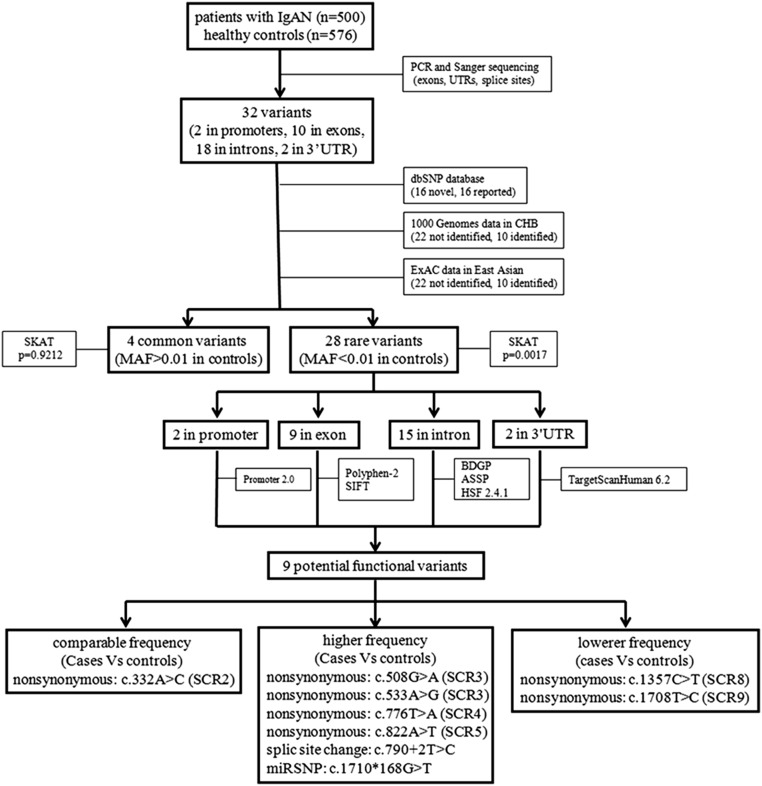
Figure 1.
Flowchart of the genetic analysis of CFHR5 variants in IgAN. After screening for variants in CFHR5 in 500 patients with IgAN and 576 healthy controls, 32 variants were identified. After checking against the dbSNP database, 1000 Genome data, and ExAC data to see whether these variants were reported before, 32 variants in CFHR5 were divided into two groups, common variants and rare variants, on the basis of the MAF in controls (cutoff =0.01). Then, variant distribution between patients with IgAN and controls was compared in each group using the SKAT. After the identification of rare variants in CFHR5 associated with IgAN, the functional meaning of these rare variants was predicted by in silico programs. At last, nine potential functional variants were identified, which were located in different SCRs of the CFHR5 protein. CHB, Chinese Han Beijing; dbSNP, single nucleotide polymorphism database; ExAC, exome aggregation consortium; HSF, human splicing finder; SIFT, sorting intolerant from tolerant.
Table 1.
Identified genetic variants in CFHR5
| Position | Location | Nucleotide | Amino Acid | dbSNP (Build142) | MAF | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Enrolled Individuals | 1000 Genomes Phase 3 | ExAC Data Version 0.3 | ||||||||
| IgAN, n=500 | HC, n=576 | Total | CHB | Total | East Asian | |||||
| Chr1:196946445 | Promoter | c.-350G>T | — | — | 0.001 (1/1000) | 0.000 (0/1152) | 0.000 | 0.000 | 0.000 | 0.000 |
| Chr1:196946448 | Promoter | c.-347G>A | — | rs9427942 | 0.000 (0/1000) | 0.0009 (1/1152) | 0.177 (886/5008) | 0.000 (0/206) | 0.000 | 0.000 |
| Chr1:196946869 | Intron 1 | c.58+17T>A | — | rs3748557 | 0.140 (140/1000) | 0.141 (163/1152) | 0.158 (790/5008) | 0.209 (43/206) | 0.208 (25,245/121,354) | 0.17 (1467/8644) |
| Chr1:196946881 | Intron 1 | c.58+29A>C | — | — | 0.000 (0/1000) | 0.0009 (1/1152) | 0.000 | 0.000 | 0.000 | 0.000 |
| Chr1:196951903 | Intron 1 | c.59–112G>A | — | — | 0.001 (1/1000) | 0.000 (0/1152) | 0.000 | 0.000 | 0.000 | 0.000 |
| Chr1:196953025 | Intron 2 | c.254–66A>T | — | rs570596954 | 0.002 (2/1000) | 0.000 (0/1152) | 0.0006 (3/5008) | 0.000 (0/206) | 0.000 | 0.000 |
| Chr1:196953169 | Exon 3 | c.332A>C | p.Gln111Pro | — | 0.001 (1/1000) | 0.0009 (1/1152) | 0.000 | 0.000 | <0.0001 (1/121,218) | 0.0001 (1/8646) |
| Chr1:196953379 | Intron 3 | c.430+112T>C | — | — | 0.001 (1/1000) | 0.000 (0/1152) | 0.000 | 0.000 | 0.000 | 0.000 |
| Chr1:196963130 | Intron 3 | c.431–80G>A | — | rs117310366 | 0.013 (13/1000) | 0.013 (15/1152) | 0.004 (18/5008) | 0.029 (6/206) | 0.000 | 0.000 |
| Chr1:196963213 | Exon 4 | c.434G>A | p.Gly145Glu | rs57960694 | 0.013 (13/1000) | 0.013 (15/1152) | 0.041 (204/5008) | 0.029 (6/206) | 0.013 (1496/119,312) | 0.016 (139/8546) |
| Chr1:196963287 | Exon 4 | c.508G>A | p.Val170Met | rs201073457 | 0.007 (7/1000) | 0.004 (5/1152) | 0.001 (6/5008) | 0.010 (2/206) | 0.0006 (77/120,992) | 0.008 (70/8644) |
| Chr1:196963312 | Exon 4 | c.533A>G | p.Asn178Ser | rs200427185 | 0.006 (6/1000) | 0.000 (0/1152) | 0.008 (4/5008) | 0.005 (1/206) | 0.0003 (37/121,072) | 0.004 (37/8646) |
| Chr1:196965015 | Exon 5 | c.776T>A | p.Leu259Termination | — | 0.001 (1/1000) | 0.000 (0/1152) | 0.000 | 0.000 | 0.000 | 0.000 |
| Chr1:196965031 | Intron 5 | c.790+2T>C | — | — | 0.001 (1/1000) | 0.000 (0/1152) | 0.000 | 0.000 | <0.0001 (1/120,052) | 0 (0/8581) |
| Chr1:196965076 | Intron 5 | c.790+47A>G | — | — | 0.001 (1/1000) | 0.000 (0/1152) | 0.000 | 0.000 | 0.000 | 0.000 |
| Chr1:196965183 | Exon 6 | c.822A>T | p.Glu274Asp | — | 0.001 (1/1000) | 0.000 (0/1152) | 0.000 | 0.000 | 0.000 | 0.000 |
| Chr1:196967458 | Intron 7 | c.1147+24T>G | — | rs1332664 | 0.332 (332/1000) | 0.316 (364/1152) | 0.694 (3475/5008) | 0.374 (77/206) | 0.752 (87,237/116,050) | 0.302 (2560/8467) |
| Chr1:196967528 | Intron 7 | c.1147+94G>T | — | rs116891819 | 0.013 (13/1000) | 0.008 (9/1152) | 0.004 (18/5008) | 0.029 (6/206) | 0.000 | 0.000 |
| Chr1:196971491 | Intron 7 | c.1148–121G>A | — | — | 0.001 (1/1000) | 0.000 (0/1152) | 0.000 | 0.000 | 0.000 | 0.000 |
| Chr1:196971504 | Intron 7 | c.1148–108A>G | — | rs147231103 | 0.000 (0/1000) | 0.009 (10/1152) | 0.004 (18/5008) | 0.029 (6/206) | 0.000 | 0.000 |
| Chr1:196971544 | Intron 7 | c.1148–68C>T | — | rs181464041 | 0.000 (0/1000) | 0.0009 (1/1152) | 0.010 (50/5008) | 0.000 (0/206) | 0.000 | 0.000 |
| Chr1:196971637 | Exon 8 | c.1173G>A | p.Pro391Pro | rs200148491 | 0.000 (0/1000) | 0.0009 (1/1152) | 0.0002 (1/5008) | 0.000 (0/206) | <0.0001 (5/120,578) | 0.0002 (2/8624) |
| Chr1:196971814 | Intron 8 | c.1330+20G>A | — | — | 0.000 (0/1000) | 0.0009 (1/1152) | 0.000 | 0.000 | <0.0001 (1/111,288) | 0.000 (0/7946) |
| Chr1:196971834 | Intron 8 | c.1330+40G>A | — | rs116937944 | 0.000 (0/1000) | 0.0009 (1/1152) | 0.0002 (1/5008) | 0.005 (1/206) | <0.0001 (2/104,778) | 0.000 (0/7380) |
| Chr1:196971872 | Intron 8 | c.1330+78A>G | — | — | 0.001 (1/1000) | 0.003 (3/1152) | 0.000 | 0.000 | 0.000 | 0.000 |
| Chr1:196973817 | Exon 9 | c.1357C>T | p.Pro453Ser | rs184883943 | 0.000 (0/1000) | 0.005 (6/1152) | 0.0002 (1/5008) | 0.005 (1/206) | 0.0001 (13/121,390) | 0.002 (13/8654) |
| Chr1:196974101 | Intron 9 | c.1513+126A>G | — | — | 0.003 (3/1000) | 0.000 (0/1152) | 0.000 | 0.000 | 0.000 | 0.000 |
| Chr1:196974110 | Intron 9 | c.1513+137G>A | — | rs142743076 | 0.000 (0/1000) | 0.0009 (1/1152) | 0.0002 (1/5008) | 0.000 (0/206) | 0.000 | 0.000 |
| Chr1:196977744 | Exon 10 | c.1641G>A | p.Ala547Ala | rs74323799 | 0.001 (1/1000) | 0.000 (0/1152) | 0.001 (3/5008) | 0.000 (0/206) | 0.0002 (29/121,322) | 0.003 (25/8640) |
| Chr1:196977811 | Exon 10 (stop codon) | c.1708T>C | p.Termination570Arg | — | 0.001 (1/1000) | 0.003 (3/1152) | 0.000 | 0.000 | <0.0001 (5/121,104) | 0.0005 (5/8634) |
| Chr1:196977816 | 3′ UTR | c.1710*3C>G | — | — | 0.001 (1/1000) | 0.000 (0/1152) | 0.000 | 0.000 | <0.0001 (9/120,944) | 0.000 (0/8626) |
| Chr1:196977981 | 3′ UTR | c.1710*168G>T | — | — | 0.001 (1/1000) | 0.000 (0/1152) | 0.000 | 0.000 | 0.000 | 0.000 |
dbSNP, single nucleotide polymorphism database; ExAC, exome aggregation consortium; HC, healthy controls; CHB, Chinese Han Beijing; —, inapplicable.
Genetic Association Analyses of CFHR5 with IgAN
Among the identified 32 variants, four were common variants (c.58+17T>A, c.431–80G>A, c.434G>A, and c.1147+24T>G; minor allele frequency [MAF] >0.01 in controls), whereas the other 28 were rare. We only observed individuals with homozygous minor alleles in two common variants (c.58+17T>A and c.1147+24T>G) and not in the other two common variants (c.431–80G>A [MAF=0.013] and c.434G>A [MAF=0.013]) and 28 rare variants. Of 28 rare variants, 14 were identified only in patients with IgAN (who exhibited a total of 22 rare variants), nine were identified only in healthy controls (with a total of 23 rare variants), and the other five variants were identified in both patients (23 total rare variants) and controls (21 total rare variants).
Because analysis of rare variants requires statistical methods that are fundamentally different than testing common variants, we applied Sequence Kernel Association Test (SKAT), which can evaluate not only rare variants’ effect but also, the cumulative effect of rare and common variants. The results of SKAT showed that the combined effect of rare and common variants in CFHR5 had a significant association with IgAN (P=0.03) (Table 2). Furthermore, we investigated common and rare variants separately. Rare variants in CFHR5 presented a significant association with IgAN (P=0.002) (Table 2), but common variants did not (P=0.92) (Table 2).
Table 2.
Genetic association analysis to common and rare variants in CFHR5 with IgAN susceptibility using SKAT
| Test Type | N of Variants | Q Value | P Value |
|---|---|---|---|
| Joint | 32 | 4.80 | 0.03 |
| Common only | 4 | 17.31 | 0.92 |
| Rare only | 28 | 15,240.54 | 0.002 |
Q value, test statistic of SKAT.
Regarding the four common variants, we also performed single variant–level analysis. In accordance with the results from SKAT, no significant difference was observed regarding the allele or genotype frequencies of each single common variant (Table 3).
Table 3.
Allele and genotype frequencies of common variants of CFHR5 in patients with IgAN and healthy controls
| Variants | Allele Frequencies | Genotype Frequencies | ||
|---|---|---|---|---|
| c.58+17 T>A | A | T | AA + AT | TT |
| IgAN | 140 (14.00%) | 860 (86.00%) | 15+110 (25.00%) | 375 (75.00%) |
| HC | 163 (14.10%) | 989 (85.90%) | 7+149 (27.10%) | 420 (72.90%) |
| P value | 0.92 | 0.44 | ||
| c.431–80 G>A | A | G | AA + AG | GG |
| IgAN | 13 (1.30%) | 987 (98.70%) | 0+13 (2.60%) | 487 (97.40%) |
| HC | 15 (1.30%) | 1137 (98.70%) | 0+15 (2.60%) | 561 (97.40%) |
| P value | >0.99 | >0.99 | ||
| c.434 G>A | A | G | AA + AG | GG |
| IgAN | 13 (1.30%) | 987 (98.70%) | 0+13 (2.60%) | 487 (97.40%) |
| HC | 15 (1.30%) | 1137 (98.70%) | 0+15 (2.60%) | 561 (97.40%) |
| P value | >0.99 | >0.99 | ||
| c.1147+24 T>G | G | T | GG + GT | TT |
| IgAN | 332 (33.20%) | 668 (66.80%) | 61+210 (54.20%) | 229 (45.80%) |
| HC | 364 (31.60%) | 788 (68.40%) | 49+266 (54.70%) | 261 (45.30%) |
| P value | 0.43 | 0.87 | ||
HC, healthy controls.
Clinical and Pathologic Manifestations of Patients with IgAN
Among our recruited 500 patients, 43 had rare variants in CFHR5. Of these patients, only two patients carried two rare variants (one patient had c.59–112G>A and c.822A>T, whereas the other patient had c.1147+94G>T and c.1641G>A), and the remaining 41 patients each carried only one rare variant (including c.1147+94G>T in 12 patients; c.508G>A in seven patients; c.533A>G in six patients; c.1513+126A>G in three patients; c.254–66A>T in two patients; and the following 11 variants, c.-350G>T, c.332A>C, c.430+112T>C, c.776T>A, c.790+2T>C, c.790+47A>G, c.1148–121G>A, c.1330+78A>G, c.1708T>C, c.1710*3C>G, and c.1710*168G>T, in one patient each).
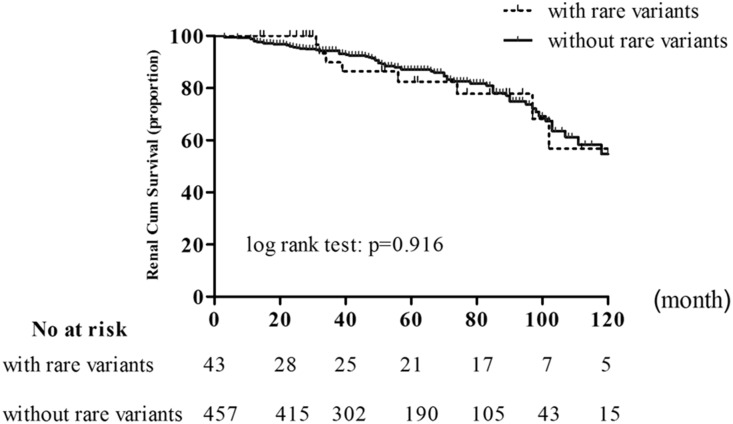
According to these rare variants in CFHR5, patients with IgAN were divided into two groups: those with rare variants in CFHR5 (43 patients) and those without (457 patients). However, clinical and pathologic manifestations showed no significant differences between the two groups (Table 4). For long–term renal survival, no significant difference was observed between patients in the two groups (Figure 2).
Table 4.
Comparison of clinical and pathologic manifestations in patients with IgAN according to rare variants in CFHR5
| Characteristics | Total, n=500 | Potential Pathogenic Variants | P Valuea | |
|---|---|---|---|---|
| With, n=43 | Without, n=457 | |||
| Baseline | ||||
| Age, yr, mean±SD | 34.23±11.09 | 33.72±11.71 | 34.27±11.05 | 0.75 |
| Men | 258 (51.60%) | 23 (53.50%) | 235 (51.40%) | 0.79 |
| Hypertension | 0.94 | |||
| With hypertension | 68 (13.62%) | 6 (14.00%) | 62 (13.60%) | |
| Without hypertension | 432 (86.38%) | 37 (86.00%) | 395 (86.40%) | |
| Initial proteinuria, g/d, median (IQR) | 1.42 (0.81, 2.66) | 1.40 (0.83, 2.51) | 1.43 (0.80, 2.72) | 0.92 |
| <1 | 168 (33.60%) | 14 (32.60%) | 154 (33.70%) | |
| 1–3.5 | 253 (50.60%) | 22 (51.20%) | 231 (50.50%) | |
| ≥3.5 | 79 (15.80%) | 7 (16.20%) | 72 (15.80%) | |
| eGFR, ml/min per 1.73 m2, mean±SD | 84.19±27.03 | 82.13±22.40 | 84.39±27.43 | 0.60 |
| Haas classification | 0.43b | |||
| 1 | 43 (8.60%) | 3 (7.00%) | 40 (8.80%) | |
| 2 | 4 (0.80%) | 0 (0.00%) | 4 (0.90%) | |
| 3 | 226 (45.20%) | 18 (41.90%) | 208 (45.50%) | |
| 4 | 166 (33.20%) | 16 (37.10%) | 150 (32.80%) | |
| 5 | 61 (12.20%) | 6 (14.00%) | 55 (12.00%) | |
| Serum IgA level, g/L, mean±SD | 3.34±1.22 | 3.29±1.53 | 3.35±1.19 | 0.77 |
| Serum C3 level, g/L, mean±SD | 1.04±0.28 | 1.11±0.39 | 1.04±0.27 | 0.14 |
| IgA deposition (1+/2+/3+–4+) | 14 (2.80%)/78 (15.60%)/408 (81.60%) | 1 (2.30%)/9 (20.90%)/33 (76.70%) | 13 (2.80%)/69 (15.10%)/375 (82.10%) | 0.52c |
| C3 deposition (−/1+/2+/3+–4+) | 41 (8.20%)/57 (11.40%)/164 (32.80%)/238 (47.60%) | 5 (11.60%)/5 (11.60%)/19 (44.20%)/14 (32.60%) | 36 (7.90%)/52 (11.40%)/145 (31.70%)/224 (49.00%) | 0.11c |
| Ratio of glomeruli with crescents (IQR) | 0.05 (0.00, 0.15) | 0.04 (0.00, 0.12) | 0.05 (0.00, 0.16) | 0.39 |
| Follow-up | ||||
| Follow-up time, mo, mean±SD | 56.26±36.61 | 62.51±37.35 | 55.67±31.00 | 0.17 |
| Prednisone/immunosuppressive agents | 208 (41.60%) | 19 (44.20%) | 189 (41.40%) | 0.72 |
| ARBs/ACE inhibitors | 483 (96.60%) | 43 (100%) | 440 (96.30%) | 0.40 |
| Time average proteinuria, g/d, median (IQR) | 0.75 (0.44, 1.41) | 0.71 (0.37, 1.70) | 0.77 (0.44, 1.38) | 0.95 |
| Slope, ml/min per mo, median (IQR) | −0.20 (−0.51, 0.00) | −0.17 (−0.53, −0.10) | −0.20 (−0.51, 0.00) | 0.80 |
| Composite events, no. | 84 (16.80%) | 10 (23.30%) | 74 (16.20%) | 0.24 |
IQR, interquartile range; ARB, angiotensin receptor blocker; ACE, angiotensin-converting enzyme.
The P value was used to indicate the difference between with and without potential pathogenic variants group.
The P value was calculated between Haas 1–3 and Haas 4–5.
The P value was calculated by linear-by-linear association.
Figure 2.
Kaplan–Meier renal survival curves of patients with IgAN according to whether patients were with or without rare variants in CFHR5. In our population of patients with IgAN, event-free survival of composite outcomes was comparable between patients with rare variants in CFHR5 (n=43) and those without rare variants (n=457; P value for log-rank test was P=0.92).
In Silico Functional Prediction of Rare Variants in CFHR5
Because rare variants in CFHR5 showed significant association with IgAN, we next explored their effect on CFHR5 expression, structure, and function. Several types of software were used according to the position of the variants. In total, nine of 28 rare variants were predicted to influence function or expression of CFHR5 (Figure 3, Table 5). Of these nine, five variants might result in potential pathogenic amino acid changes, including c.332A>C/p.Gln111Pro (identified in patients and controls) in exon 3, c.508G>A/p.Val170Met (identified in patients and controls) and c.533A>G/p.Asn178Ser (identified in patients) in exon 4, c.822A>T/p.Glu274Asp (identified in patients) in exon 6, and c.1357C>T/p.Pro453Ser (identified in controls) in exon 9. Three variants would lead to protein length change, and they included c.776T>A/p.Leu259Termination (identified in patients), a stop-gain variant that produced a shortened CFHR5 protein (258 amino acids only); c.1708T>C/p.Termination570Arg (identified in patients and controls), a stop-loss variant that added 37 amino acids to the C terminus of the wild–type CFHR5 protein; and c.790+2T>C (identified in patients), a variant located 2 bp away from an exon-intron boundary (exon 5 to intron 5) that, thus, appeared to break the GT-AG splicing rule; c.790+2T>C was also predicted to be a splicing site change by the Alternative Splice Site Predictor (ASSP) and software available from the Berkeley Drosophila Genome Project (BDGP). Moreover, c.1710*168G>T (identified in patients) in the 3′ UTR, located 168 bp away from the stop codon, was in the microRNA binding region. Therefore, TargetScanHuman 6.2 was used to see whether the variant would interfere with microRNA binding efficiency. The results showed that c.1710*168G>T might also be predicted as damaging for its potential influence on the binding of hsa-miR-2117 and hsa-miR-4273 to CFHR5 mRNA.
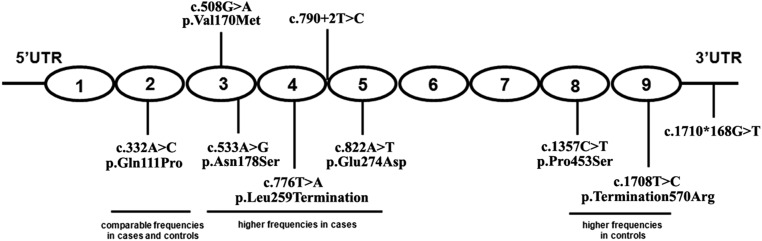
Figure 3.
Schematic illustration for the location of nine potential pathogenic variants in CFHR5. The CFHR5 protein contains nine functional domains called SCRs, which are drawn as ovals. Variant c.332A>C/p.Gln111Pro is located in SCR2, c.508G>A/p.Val170Met and c.533A>G/p.Asn178Ser are in SCR3, c.776T>A/p.Leu259Termination is in SCR4, c.822A>T/p.Glu274Asp in SCR5, and c.1357C>T/p.Pro453Ser and c.1708T>C/p.Ter570Arg are located in SCR8 and SCR9, respectively. Splicing variant c.790+2T>C is 2 bp downstream of the exon 5–intron 5 boundary. Variant c.1710*168G>T is positioned at 3′ UTR.
Table 5.
Predicted functional rare variants in CFHR5
| Position | Location | Nucleotide | Amino Acid | Identified in Population | dbSNP (Build142) | Function Change Prediction | Software |
|---|---|---|---|---|---|---|---|
| Chr1:196946445 | Promoter | c.-350G>T | — | Patients | — | Not transcript elements binding sites | Promoter 2.0 |
| Chr1:196946448 | Promoter | c.-347G>A | — | Controls | rs9427942 | Not transcript elements binding sites | Promoter 2.0 |
| Chr1:196946881 | Intron 1 | c.58+29A>C | — | Controls | — | Not branch-point mutation | HSF 2.4.1 |
| Chr1:196951903 | Intron 1 | c.59–112G>A | — | Patients | — | Not branch-point mutation | HSF 2.4.1 |
| Chr1:196953025 | Intron 2 | c.254–66A>T | — | Patients | rs570596954 | Not branch-point mutation | HSF 2.4.1 |
| Chr1:196953169 | Exon 3 | c.332A>C | p.Gln111Pro | Patients and controls | — | Tolerated/benign | SIFT-Polyphen-2 |
| Chr1:196953379 | Intron 3 | c.430+112T>C | — | Patients | — | Not branch-point mutation | HSF 2.4.1 |
| Chr1:196963287 | Exon 4 | c.508G>A | p.Val170Met | Patients and controls | rs201073457 | Tolerated/probably damaging | SIFT-Polyphen-2 |
| Chr1:196963312 | Exon 4 | c.533A>G | p.Asn178Ser | Patients | rs200427185 | Damaging/benign | SIFT-Polyphen-2 |
| Chr1:196965015 | Exon 5 | c.776T>A | p.Leu259Termination | Patients | — | Stop gain | — |
| Chr1:196965031 | Intron 5 | c.790+2T>C | — | Patients | — | Splicing site | BDGP, ASSP |
| Chr1:196965076 | Intron 5 | c.790+47A>G | — | Patients | — | Not branch-point mutation | HSF 2.4.1 |
| Chr1:196965183 | Exon 6 | c.822A>T | p.Glu274Asp | Patients | — | Tolerated/benign | SIFT-Polyphen-2 |
| Chr1:196967528 | Intron 7 | c.1147+94G>T | — | Patients and controls | rs116891819 | Not branch-point mutation | HSF 2.4.1 |
| Chr1:196971491 | Intron 7 | c.1148–121G>A | — | Patients | — | Not branch-point mutation | HSF 2.4.1 |
| Chr1:196971504 | Intron 7 | c.1148–108A>G | — | Controls | rs147231103 | Not branch-point mutation | HSF 2.4.1 |
| Chr1:196971544 | Intron 7 | c.1148–68C>T | — | Controls | rs181464041 | Not branch-point mutation | HSF 2.4.1 |
| Chr1:196971637 | Exon 8 | c.1173G>A | p.Pro391Pro | Controls | rs200148491 | Synonymous mutation | — |
| Chr1:196971814 | Intron 8 | c.1330+20G>A | — | Controls | — | Not branch-point mutation | HSF 2.4.1 |
| Chr1:196971834 | Intron 8 | c.1330+40G>A | — | Controls | rs116937944 | Not branch-point mutation | HSF 2.4.1 |
| Chr1:196971872 | Intron 8 | c.1330+78A>G | — | Patients and controls | — | Not branch-point mutation | HSF 2.4.1 |
| Chr1:196973817 | Exon 9 | c.1357C>T | p.Pro453Ser | Controls | rs184883943 | Tolerated/probably damaging | SIFT-Polyphen-2 |
| Chr1:196974101 | Intron 9 | c.1513+126A>G | — | Patients | — | Not branch-point mutation | HSF 2.4.1 |
| Chr1:196974110 | Intron 9 | c.1513+137G>A | — | Controls | rs142743076 | Not branch-point mutation | HSF |
| Chr1:196977744 | Exon 10 | c.1641G>A | p.Ala547Ala | Patients | rs74323799 | Synonymous mutation | — |
| Chr1:196977811 | Exon 10 (stop codon) | c.1708T>C | p.Termination570Arg | Patients and controls | — | Stop loss | — |
| Chr1:196977816 | 3′ UTR | c.1710*3C>G | — | Patients | — | Not microRNA binding site | TargetScanHuman 6.2 |
| Chr1:196977981 | 3′ UTR | c.1710*168G>T | — | Patients | — | hsa-mir-2117/hsa-mir-4273 binding site | TargetScanHuman 6.2 |
dbSNP, single nucleotide polymorphism database; —, inapplicable; HSF, Human Splicing Finder; SIFT, Sorting Intolerant from Tolerant; Polyphen-2, Polymorphism Phenotyping v2.
Detection of C3b Binding Capacity
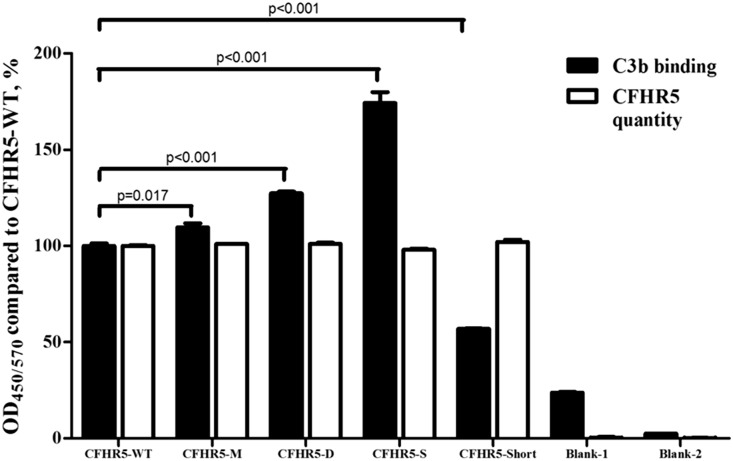
Within nine rare variants predicted to influence CFHR5 function, seven were nonsynonymous. Comparing these seven nonsynonymous variants between patients and controls, we found four variants that were located in SCR3–5 (c.508G>A/p.Val170Met, c.533A>G/p.Asn178Ser, c.776T>A/p.Leu259Termination, and c.822A>T/p.Glu274Asp) and showed higher frequencies in IgAN. Because SCR3–5 in CFHR5 had high amino acid identities similar to SCR10–12 in CFH and because SCR12 in CFH has the ability to bind to C3b,16 we further evaluated their influence on the recombinant CFHR5 proteins (Supplemental Figure 1) and its C3b binding capacity. We found that, when 1–4 μg/ml C3b was added, 1 μg/ml CFHR5 protein (including all of our detected allotypes) was within the linearity range for the C3b binding capacity (Supplemental Figure 2). Compared with wild-type CFHR5, CFHR5-M (c.508G>A/p.Val170Met), CFHR5-S (c.533A>G/p.Asn178Ser), and CFHR5-D (c.822A>T/p.Glu274Asp) showed significantly higher capacities for binding to 2 μg/ml C3b (CFHR5-M: 109.67%±3.54%; P=0.02; CFHR5-S: 174.27%±9.78%; P<0.001; CFHR5-D: 127.25%±1.75%; P<0.001) (Figure 4). However, CFHR5-Short (c.776T>A/p.Leu259Termination) showed significantly impaired C3b binding (CFHR5-Short: 56.89%±0.57%; P<0.001) (Figure 4).
Figure 4.
Rare coding variants induced a change in the C3b binding capacity of the CFHR5 protein. Compared with CFHR5-WT, CFHR5-M (c.508G>A/p.Val170Met), CFHR5-S (c.533A>G/p.Asn178Ser), and CFHR5-D (c.822A>T/p.Glu274Asp) showed significantly higher binding capacities for C3b (CFHR5-M: 109.67%±3.54%; P=0.02; CFHR5-S: 174.27%±9.78%; P<0.001; CFHR5-D: 127.25%±1.75%; P<0.001), whereas CFHR5-Short (c.776T>A/p.Leu259Termination) had a significantly lower C3b binding capacity (CFHR5-Short: 56.89%±0.57%; P<0.001). Experiments were performed in triplicate and repeated three times with similar results. White columns represent quantities of recombinant CFHR5 proteins coated on the plates. Black columns represent C3b binding capacity of the coated CFHR5 proteins compared with wild-type CFHR5. Blank-1, no CFHR5 + C3b + polyclonal anti–C3c/CFHR5 antibody; blank-2, no CFHR5 + no C3b + polyclonal anti–C3c/CFHR5 antibody.
Discussion
On the basis of the GWAS identification of CFH-CFHRs as factors associated with IgAN predisposition,7,17 the high similarity of IgAN with CFHR5 nephropathy, and the evidence of complement activation in IgAN, we suspected that variants in the CFHR5 gene might also play some part in influencing genetic predisposition to the complex disease IgAN. In this study, through genetic analysis of the CFHR5 gene, we found significant genetic association of rare variants in CFHR5 with IgAN, and we further proved an impaired C3b binding capacity of some rare variants in CFHR5.
In recent years, increasing evidence indicated that the allelic spectrum of disease-associated variants at a given locus might include novel, rare, and common variants. Reports from inflammatory bowel disease and rheumatoid arthritis supported the involvement of both rare and common variants from single genes or gene sets in common complex diseases.18,19 Recently, after the identification of membranous nephropathy (MN) –associated common variants in PLA2R1 by the GWAS, Coenen et al.20 convincingly excluded the possibility that rare variants in PLA2R1 are involved in MN pathogenesis.21 However, for IgAN, although the GWAS identified several disease–associated loci and common variants,7,17,22,23 few studies have focused on rare variants in candidate genes. Here, after screening for variants in the exons, intronic flanking regions, and UTRs of CFHR5, we applied a popular rare variant association test, SKAT, to evaluate the association between variants in CFHR5 and IgAN.24 SKAT has two distinguishing features among group–wise association tests. First, SKAT can evaluate not only the effect of rare variants but also, the cumulative effect of rare and common variants.25 Second, SKAT allows for rare variants to have opposite effects (both effective and deleterious) on the traits.26 Because we identified both rare and common variants as well as their gain-of-function or loss-of-function properties, we tested the genetic associations between IgAN and rare plus common variants, rare variants alone, and common variants alone in CFHR5 by SKAT. Different from the findings in MN, we found that it was rare variants and not common variants in CFHR5 that contribute to IgAN susceptibility.
In this study, the proportion of patients with IgAN bearing rare variants in CFHR5 reached 8.6% (43 of 500). Because IgAN is widely regarded as a polygenic disease, rare variants in the CFHR5 gene are likely to play some role in IgAN predisposition, but they are not, as in CFHR5 nephropathy, likely to be the only causal factor in the disease. Accordingly, we failed to observe significant differences regarding clinical findings, pathologic features, and renal outcomes between patients with or without rare variants in CFHR5. In monogenic diseases induced by abnormal complement activation, including aHUS and DDD, mutations in both CFH and CFHR5 were reported,27–30 but in complement–involved complex disease, such as SLE, age–related macular degeneration, and IgAN, although variants in CFH were widely investigated,15,31,32 limited studies focused on the CFHR5 gene. To the best of our knowledge, our study is the first to report the association of rare variants in CFHR5 with IgAN. The role of rare variants in CFHR5 still remains to be elucidated in other complement–involved complex diseases and validated in larger IgAN cohorts of other ethnicities.
By in silico prediction, we identified nine potential functional variants of 28 rare variants to influence the CFHR5 effect, although we cannot exclude the possibility of some others also having functional meaning. Except for c.1710*168G>T, which is located in 3′ UTR and predicted to change CFHR5 expression, the other eight variants were assumed to influence CFHR5 function. SCRs are the basic functional domains in CFH and CFHRs, including CFHR5.12 When we compared the allele frequencies of the eight potential functional variants in patients with IgAN and healthy controls, the interesting thing that we found was that variants that showed higher frequencies in IgAN (c.508G>A, c.533A>G, c.776T>A, c.790+2T>C, and c.822A>T) clustered in SCR3–5, whereas those with higher frequencies in controls (c.1357C>T and c.1708T>C) clustered in SCR8–9. The variant with comparable frequency between patients and controls (c.332A>C) was located in SCR2.
In the SCR3–5 region, we identified five potential pathogenic variants, with higher frequencies in IgAN. Variant c.790+2T>C is a splicing acceptor site variant. The minor allele C would affect the complex dynamics of splicing and can potentially lead to a functional change of CFHR5 because of erroneous exon skipping or the inclusion of a nonintronic sequence. For the other four variants in SCR3–5, we detected their influence on the CFHR5 protein with regard to C3b binding. Results showed that three variant–associated CFHR5 proteins, CFHR5-M (c.508G>A/p.Val170Met), CFHR5-S (c.533A>G/p.Asn178Ser), and CFHR5-D (c.822A>T/p.Glu274Asp), enhanced the binding of CFHR5 to C3b. Recently, CFHR5 was reported to deregulate complement activation by competing with CFH.33,34 Because a high degree of complement activation was associated with poor renal survival in IgAN, it is reasonable for us to suspect CFHR5-M, CFHR5-S, and CFHR5-D as gain of function for CFHR5; therefore, they exhibited stronger competition with CFH, resulted in a higher degree of complement activation, and contributed to IgAN susceptibility. Different from these, CFHR5-Short resulted from a stop-gain variant (c.776T>A/p.Leu259Termination) and exhibited significantly lower C3b binding capacity. This mutant CFHR5 (CFHR5-Short), as a half-truncated protein (258 amino acids only compared with 569 amino acids in wild-type CFHR5), was prematurely terminated at SCR4, and it, therefore, is missing SCR5–9 and part of SCR4. Because the accurate mechanism for SCR5–9 is still unknown, we suspected that it might change the biologic function of CFHR5 through some other mechanism harbored by SCR5–9, which overcame its influence on C3b binding capacity and contributed to IgAN susceptibility. Unfortunately, our study was unable to clarify the pathogenic mechanism of CFHR5-Short, and therefore, additional studies will be required in the future after the biologic effect of SCR5–9 is revealed.
The other two potential functional variants, which showed higher frequencies in controls, were located in SCR8–9 in CFHR5 and correspond to SCR19–20 in CFH.12 SCR19–20 in CFH is critical for binding to the cell surface. Most genetic mutations in CFH that induced aHUS,35 a disease with severe endothelial injury, are located in SCR19–20.36 Therefore, we assumed that variants in CFHR5 SCR8–9 would have a similar influence and might be associated with endothelial injury. Although we now have considerable evidence regarding endothelial injury in IgAN,37–39 the underlying mechanism is still unknown. Additional functional studies are required to clarify whether our identified variants in SCR8–9 of CFHR5 influence its binding to endothelial cells and what their genetic mechanisms are in IgAN.
Different from those variants that influenced CFHR5 function, c.1710*168G>T, located in the 3′ UTR, was predicted to influence the expression rather than the sequence of CFHR5. In recent years, increasing microRNA–associated single–nucleotide polymorphisms were found on microRNA target sites within the 3′ UTRs of mRNAs, such as hsa-miR-1207–5p in CFHR5 nephropathy,40 miR-148b in IgAN,41 and so on. The c.1710*168G>T variant in CFHR5 was at the target site for hsa-miR-2117/has-miR-4273 binding. The mutant T allele substituted for the G allele theoretically influences the binding of miR-2117/miR-4273 and therefore, would change CFHR5 levels. It was reported that CFHR5 colocalized with complement–containing glomerular immune deposits in a variety of glomerular pathologic states.42 Although it has an unclear biologic role, CFHR5 is postulated to have a physiologic role in complement processing within the kidney. We speculated that c.1710*168G>T might contribute to IgAN predisposition through its post-transcriptional regulation of CFHR5 expression.
Our study had several limitations. First, we identified several potential functional rare variants. However, C3b binding capacity was only detected in some of them. Additional functional studies are required to reveal their exact biologic roles in IgAN. Second, because we failed to harvest the secreted CFHR5 from an insect cell system, an Escherichia coli system, which lacks post-translational modifications, was used for recombinant CFHR5 protein expression. Whether post-translational modification would influence the C3b binding capacity of CFHR5 is still unknown. Third, diverse incidences of IgAN were reported in different racial and ethnic populations, and therefore, future replication studies are important to validate the genetic association of CFHR5 in IgAN. We failed to find any genotype-phenotype correlation for rare variants in CFHR5. Whether rare variants in CFHR5 contributed to clinical or pathologic phenotypes or the progression of IgAN is a question awaiting further convincing evidence.
In conclusion, through screening the genetic variants in CFHR5, we found that rare variants in CFHR5 contributed to genetic susceptibility to IgAN, which suggested that CFHR5 is an IgAN susceptibility gene.
Concise Methods
Study Population
Here, we recruited 1076 northern Chinese individuals, including 500 patients with sporadic IgAN (diagnosed from 2003 to 2009) and 576 age-, sex-, and geographically matched unrelated healthy controls during this time interval. The diagnosis of IgAN was proven by renal biopsy on the basis of granular deposition of IgA in the glomerular mesangium by immunofluorescence detection and the deposition of electron-dense material in the mesangium by ultrastructural examination. Patients with Henoch–Schonlein purpura, SLE, and chronic hepatic diseases were excluded by detailed clinical and laboratory examinations.
The study protocol was approved by the Medical Ethics Committee of Peking University, and informed written consent was obtained from all patients. Figure 1 shows the flow chart of genetic analysis applied in our study.
Screening for Genetic Variants in CFHR5
Genomic DNA was extracted from EDTA anticoagulated whole–blood samples by a salting out procedure.43 The genomic DNA reference sequence (version NC_000001.11) and mRNA reference sequence (version NM_030787.3) for the CFHR5 gene were obtained from the Entrez gene database (http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=gene). For the enrolled 500 patients with IgAN and 576 healthy controls, a coding sequence in each exon with the intronic flanking regions as well as the 5′ and 3′ UTRs of the CFHR5 gene were amplified by PCR. Primers used for PCR were designed by the Primer 3 program (http://bioinfo.ut.ee/primer3-0.4.0/) and are listed in Table 6 along with the PCR annealing temperatures. PCR products were sequenced by the ABI 3730 XL Genetic Analyzer (Applied Biosystems, Foster City, CA) and aligned to the reference sequence to identify genetic variants in CFHR5. The identified genetic variants were checked against the dbSNP database (build142) to see if they were reported before. Meanwhile, these variants were also screened against two publicly available databases of exome and genome sequences: the 1000 Genomes Project (phase 3; http://www.1000genomes.org) and ExAC browser (Beta; version 0.3; http://exac.broadinstitute.org/).
Table 6.
PCR conditions and primers used to amplify CFHR5 gene
| Exon | Forward (5′–3′) | Reverse (5′–3′) | Annealing Temperature, °C | Fragment, bp |
|---|---|---|---|---|
| 1 | CTTGCTTGCCTTTTGAAACA | CCCCTTCAAATTATCCTCAGC | 61.2 | 585 |
| 2 | CTGGGCAACAAGAGTAAAACCT | TCTCAAAATAGGAGGACTACATCTC | 61.2 | 582 |
| 3 | CGGTAGCATGACCCAAATTC | GGTAGGCAAACTATGTTATTGCAC | 61.2 | 488 |
| 4 | AGTTTCCCAATTTGCCTGAG | CTGCATCCTTTCTCCTTTGC | 61.2 | 501 |
| 5 and 6 | GAGGAAACGAATGCAGTCAA | TCCATTCATCATGCCAGAAA | 57 | 901 |
| 7 | TCAGTCAAAACTCCCACTAGGAA | CATCTTTACCAGAAAGCCAAGG | 57 | 637 |
| 8 | GGAGATACAAGAGAGCATCTGAAA | GTTTCTTCTATGAACACTGTTGGAG | 61.2 | 636 |
| 9 | AATTATTTGAATTTCCAGACACCTT | CGAATAGGCCCCATAAATAGC | 57 | 568 |
| 10 | CATATGTAGCCCATACACAGTGC | CCCCACCATCTTGGACTTCT | 61.2 | 690 |
Association Analyses of Variants in CFHR5 with IgAN
To evaluate the genetic contribution of variants in CFHR5 to IgAN susceptibility, a popular genetic association test, SKAT, was applied. The R package SKAT 1.0.7 was used for the analysis. At first, the combined effect of all variants in CFHR5 was analyzed. Then, variants in CFHR5 were grossly divided into common and rare variants according to their MAF in healthy controls (cutoff =1%). The effects of rare variants alone and common variants alone were evaluated by SKAT_CommonRare. Additionally, for common variants in CFHR5, comparisons of allele and genotype frequencies at the single-variant level were also performed between 500 patients with IgAN and 576 healthy controls.
Comparative Analyses between Patients with IgAN According to Rare Variants in CFHR5
For the enrolled 500 patients with sporadic IgAN, clinical parameters at the time of renal biopsy, including serum IgA level, serum C3 level, serum creatinine level, 24-hour urine protein excretion, and history of hypertension, were collected from the medical records. The eGFR was calculated using the Modified Glomerular Filtration Rate Estimating Equation for Chinese.44 The Haas classification was assessed in patients whose renal biopsy tissues contained more than eight glomeruli.45
According to the presence or absence of rare variants in CFHR5, patients with IgAN were divided into two groups: those with rare variants and those without rare variants. At first, baseline clinical manifestations and histologic classifications were compared between the two groups. Then, survival analysis was used to evaluate whether patients in the two groups had a significant difference in long–term renal outcomes. A composite end point, which was defined as 50% eGFR decline, ESRD, or death, whichever occurred first, was used in this study. ESRD was defined as eGFR<15 ml/min per 1.73 m2 or the need for RRT (such as hemodialysis, peritoneal dialysis, or renal transplantation) for the purpose of this study.
Computational Assessment of Rare Variants in CFHR5
A functional evaluation of the identified rare variants in CFHR5 was analyzed using several in silico prediction programs according to their position in the genome. For variants in the promoter and 5′ UTR, promoter 2.0 (http://www.cbs.dtu.dk/services/Promoter/) was used to predict whether the variants were in transcription element binding sites; for those in the coding region, their potential pathogenicity was analyzed by two independent programs: one was Sorting Intolerant from Tolerant (http://sift.jcvi.org/), with prediction that is on the basis of the degree of the conservation of amino acid residues in sequence alignments derived from closely related sequences, and the other was Polymorphism Phenotyping v2 (http://genetics.bwh.harvard.edu/pph2/), in which variants with scores of >2.0, 1.5–2.0, and <1.5 were defined as probably damaging, possibly damaging, and benign, respectively. For variants in intronic flanking region, splice donor sites or acceptor sites were checked by the splice site prediction software available by the BDGP and the ASSP, which is a sequence analysis tool for the prediction and classification of splice sites. Meanwhile, splicing branch point A was predicted by Human Splicing Finder software (version 2.4.1; http://www.umd.be/HSF/), which helps with the study of the pre-mRNA splicing; for variants in 3′ UTR, a microRNA target prediction tool TargetScanHuman 6.2 (http://www.targetscan.org/), which predicts biologic targets of microRNAs by searching for the presence of conserved 8mer and 7mer sites that match the seed region of each microRNA, was used to evaluate their influence on microRNA binding.
Detection of the Influence of Rare Variants on Binding Capacity of CFHR5 to C3b
Recombinant CFHR5 proteins (including the wild type [CFHR5-WT] and those containing p.Val170Met [CFHR5-M], p.Asn178Ser [CFHR5-S], p.Glu274Asp [CFHR5-D], and p.Leu259Termination [CFHR5-Short]) were supplied by GenScript Corporation through customization. In brief, codon–optimized DNA sequences coding for CFHR5 as well as eight His amino acids at the C terminus of CFHR5, were synthesized and ligated into a pUC57 vector and expressed in E. coli expression systems. Recombinant CFHR5 proteins were purified by His tag affinity chromatography from the culture medium of E. coli. For the detection of the binding capacity of recombinant CFHR5 proteins to C3b, recombinant CFHR5 (1 μg/ml) was coated onto MaxiSorp Plastic Plates (Nalge-Nunc, Rochester, NY) at 4°C overnight. After being blocked with 1% BSA (Sigma-Aldrich, St. Louis, MO), 2 μg/ml C3b (Calbiochem, San Diego, CA) was added, and the mixture was incubated for 1 hour at 37°C. The binding of C3b to CFHR5 was examined by Polyclonal Rabbit Anti-Human C3c Antibody (DAKO, Copenhagen, Denmark) and followed by horseradish peroxidase–labeled donkey anti–rabbit IgG (Santa Cruz Biotechnology, Santa Cruz, CA). The reaction was developed using 3,3′,5,5′-Tetramethylbenzidine Horseradish Peroxidase Color Development Solution for ELISA (Sigma-Aldrich) and stopped with 1 mol/L sulfuric acid before the absorbance was measured at 450/570 nm.
Meanwhile, 0.125 μg/ml recombinant CFHR5 was also coated and blocked as described above, and polyclonal anti–CFHR5 antibody (ab175254; Abcam, Inc., Cambridge, MA) and horseradish peroxidase–labeled goat anti–rabbit IgG (Santa Cruz Biotechnology) were sequentially added and incubated for 1 hour at 37°C. After reaction development and stopping with 1 mol/L sulfuric acid, the absorbance was measured at 450/570 nm.
Statistical Analyses
Statistical analyses were performed using SPSS software (version 16.0; SPSS Inc., Chicago, IL). Normally distributed quantitative variables were expressed as the means±SDs, whereas for those with a non-normal distribution, the medians and interquartile ranges were used. Categorical data were summarized as absolute frequencies and percentages. For continuous variables, the independent samples t test was used if the data had a normal distribution, and if not, the Mann–Whitney or Kruskal–Wallis test was performed. Categorical variables were compared using the chi-squared test. Cumulative kidney survival curves were derived using the Kaplan–Meier method, and differences between the curves were analyzed using the log-rank test. A two-tailed P<0.05 was considered statistically significant.
Disclosures
None.
Supplementary Material
Acknowledgments
This work was supported by Major State Basic Research Development Program of China 973 Program grant 2012CB517700, Natural Science Foundation for Innovation Research Group of China grant 81321064, Capital of Clinical Characteristics and Applied Research Fund grant Z141107002514037, National Science Foundation of China grant 81470945, and Beijing Natural Science Foundation grant 7131016.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Challenges in Rare Variant Association Studies for Complex Kidney Traits: CFHR5 and IgA Nephropathy,” on pages 2547–2551.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015010012/-/DCSupplemental.
References
- 1.D’Amico G: The commonest glomerulonephritis in the world: IgA nephropathy. Q J Med 64: 709–727, 1987 [PubMed] [Google Scholar]
- 2.Allen AC, Bailey EM, Brenchley PE, Buck KS, Barratt J, Feehally J: Mesangial IgA1 in IgA nephropathy exhibits aberrant O-glycosylation: Observations in three patients. Kidney Int 60: 969–973, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Suzuki H, Moldoveanu Z, Hall S, Brown R, Julian BA, Wyatt RJ, Tomana M, Tomino Y, Novak J, Mestecky J: IgA nephropathy: Characterization of IgG antibodies specific for galactose-deficient IgA1. Contrib Nephrol 157: 129–133, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Moura IC, Centelles MN, Arcos-Fajardo M, Malheiros DM, Collawn JF, Cooper MD, Monteiro RC: Identification of the transferrin receptor as a novel immunoglobulin (Ig)A1 receptor and its enhanced expression on mesangial cells in IgA nephropathy. J Exp Med 194: 417–425, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lai KN, Tang SC, Leung JC: Recent advances in IgA nephropathy--the glomerulopodocytic-tubular communication. Adv Otorhinolaryngol 72: 40–44, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Haas M: Histology and immunohistology of IgA nephropathy. J Nephrol 18: 676–680, 2005 [PubMed] [Google Scholar]
- 7.Gharavi AG, Kiryluk K, Choi M, Li Y, Hou P, Xie J, Sanna-Cherchi S, Men CJ, Julian BA, Wyatt RJ, Novak J, He JC, Wang H, Lv J, Zhu L, Wang W, Wang Z, Yasuno K, Gunel M, Mane S, Umlauf S, Tikhonova I, Beerman I, Savoldi S, Magistroni R, Ghiggeri GM, Bodria M, Lugani F, Ravani P, Ponticelli C, Allegri L, Boscutti G, Frasca G, Amore A, Peruzzi L, Coppo R, Izzi C, Viola BF, Prati E, Salvadori M, Mignani R, Gesualdo L, Bertinetto F, Mesiano P, Amoroso A, Scolari F, Chen N, Zhang H, Lifton RP: Genome-wide association study identifies susceptibility loci for IgA nephropathy. Nat Genet 43: 321–327, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kiryluk K, Li Y, Sanna-Cherchi S, Rohanizadegan M, Suzuki H, Eitner F, Snyder HJ, Choi M, Hou P, Scolari F, Izzi C, Gigante M, Gesualdo L, Savoldi S, Amoroso A, Cusi D, Zamboli P, Julian BA, Novak J, Wyatt RJ, Mucha K, Perola M, Kristiansson K, Viktorin A, Magnusson PK, Thorleifsson G, Thorsteinsdottir U, Stefansson K, Boland A, Metzger M, Thibaudin L, Wanner C, Jager KJ, Goto S, Maixnerova D, Karnib HH, Nagy J, Panzer U, Xie J, Chen N, Tesar V, Narita I, Berthoux F, Floege J, Stengel B, Zhang H, Lifton RP, Gharavi AG: Geographic differences in genetic susceptibility to IgA nephropathy: GWAS replication study and geospatial risk analysis. PLoS Genet 8: e1002765, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skerka C, Chen Q, Fremeaux-Bacchi V, Roumenina LT: Complement factor H related proteins (CFHRs). Mol Immunol 56: 170–180, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Gale DP, de Jorge EG, Cook HT, Martinez-Barricarte R, Hadjisavvas A, McLean AG, Pusey CD, Pierides A, Kyriacou K, Athanasiou Y, Voskarides K, Deltas C, Palmer A, Frémeaux-Bacchi V, de Cordoba SR, Maxwell PH, Pickering MC: Identification of a mutation in complement factor H-related protein 5 in patients of Cypriot origin with glomerulonephritis. Lancet 376: 794–801, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barbour TD, Pickering MC, Cook HT: Recent insights into C3 glomerulopathy. Nephrol Dial Transplant 28: 1685–1693, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McRae JL, Cowan PJ, Power DA, Mitchelhill KI, Kemp BE, Morgan BP, Murphy BF: Human factor H-related protein 5 (FHR-5). A new complement-associated protein. J Biol Chem 276: 6747–6754, 2001 [DOI] [PubMed] [Google Scholar]
- 13.McRae JL, Duthy TG, Griggs KM, Ormsby RJ, Cowan PJ, Cromer BA, McKinstry WJ, Parker MW, Murphy BF, Gordon DL: Human factor H-related protein 5 has cofactor activity, inhibits C3 convertase activity, binds heparin and C-reactive protein, and associates with lipoprotein. J Immunol 174: 6250–6256, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Kidney Disease: Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group: KDIGO Clinical Practice Guideline for Glomerulonephritis. Kidney Int Suppl 2: 209–217, 2012
- 15.Zhu L, Zhai YL, Wang FM, Hou P, Lv JC, Xu DM, Shi SF, Liu LJ, Yu F, Zhao MH, Novak J, Gharavi AG, Zhang H: Variants in complement factor H and complement factor H-related protein genes, CFHR3 and CFHR1, affect complement activation in IgA nephropathy. J Am Soc Nephrol 26: 1195–1204, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jokiranta TS, Hellwage J, Koistinen V, Zipfel PF, Meri S: Each of the three binding sites on complement factor H interacts with a distinct site on C3b. J Biol Chem 275: 27657–27662, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Yu XQ, Li M, Zhang H, Low HQ, Wei X, Wang JQ, Sun LD, Sim KS, Li Y, Foo JN, Wang W, Li ZJ, Yin XY, Tang XQ, Fan L, Chen J, Li RS, Wan JX, Liu ZS, Lou TQ, Zhu L, Huang XJ, Zhang XJ, Liu ZH, Liu JJ: A genome-wide association study in Han Chinese identifies multiple susceptibility loci for IgA nephropathy. Nat Genet 44: 178–182, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Diogo D, Kurreeman F, Stahl EA, Liao KP, Gupta N, Greenberg JD, Rivas MA, Hickey B, Flannick J, Thomson B, Guiducci C, Ripke S, Adzhubey I, Barton A, Kremer JM, Alfredsson L, Sunyaev S, Martin J, Zhernakova A, Bowes J, Eyre S, Siminovitch KA, Gregersen PK, Worthington J, Klareskog L, Padyukov L, Raychaudhuri S, Plenge RM Consortium of Rheumatology Researchers of North America Rheumatoid Arthritis Consortium International : Rare, low-frequency, and common variants in the protein-coding sequence of biological candidate genes from GWASs contribute to risk of rheumatoid arthritis. Am J Hum Genet 92: 15–27, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rivas MA, Beaudoin M, Gardet A, Stevens C, Sharma Y, Zhang CK, Boucher G, Ripke S, Ellinghaus D, Burtt N, Fennell T, Kirby A, Latiano A, Goyette P, Green T, Halfvarson J, Haritunians T, Korn JM, Kuruvilla F, Lagacé C, Neale B, Lo KS, Schumm P, Törkvist L, Dubinsky MC, Brant SR, Silverberg MS, Duerr RH, Altshuler D, Gabriel S, Lettre G, Franke A, D’Amato M, McGovern DP, Cho JH, Rioux JD, Xavier RJ, Daly MJ National Institute of Diabetes and Digestive Kidney Diseases Inflammatory Bowel Disease Genetics Consortium (NIDDK IBDGC) United Kingdom Inflammatory Bowel Disease Genetics Consortium International Inflammatory Bowel Disease Genetics Consortium : Deep resequencing of GWAS loci identifies independent rare variants associated with inflammatory bowel disease. Nat Genet 43: 1066–1073, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coenen MJ, Hofstra JM, Debiec H, Stanescu HC, Medlar AJ, Stengel B, Boland-Augé A, Groothuismink JM, Bockenhauer D, Powis SH, Mathieson PW, Brenchley PE, Kleta R, Wetzels JF, Ronco P: Phospholipase A2 receptor (PLA2R1) sequence variants in idiopathic membranous nephropathy. J Am Soc Nephrol 24: 677–683, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salant DJ: Genetic variants in membranous nephropathy: Perhaps a perfect storm rather than a straightforward conformeropathy? J Am Soc Nephrol 24: 525–528, 2013 [DOI] [PubMed] [Google Scholar]
- 22.Feehally J, Farrall M, Boland A, Gale DP, Gut I, Heath S, Kumar A, Peden JF, Maxwell PH, Morris DL, Padmanabhan S, Vyse TJ, Zawadzka A, Rees AJ, Lathrop M, Ratcliffe PJ: HLA has strongest association with IgA nephropathy in genome-wide analysis. J Am Soc Nephrol 21: 1791–1797, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kiryluk K, Li Y, Scolari F, Sanna-Cherchi S, Choi M, Verbitsky M, Fasel D, Lata S, Prakash S, Shapiro S, Fischman C, Snyder HJ, Appel G, Izzi C, Viola BF, Dallera N, Del Vecchio L, Barlassina C, Salvi E, Bertinetto FE, Amoroso A, Savoldi S, Rocchietti M, Amore A, Peruzzi L, Coppo R, Salvadori M, Ravani P, Magistroni R, Ghiggeri GM, Caridi G, Bodria M, Lugani F, Allegri L, Delsante M, Maiorana M, Magnano A, Frasca G, Boer E, Boscutti G, Ponticelli C, Mignani R, Marcantoni C, Di Landro D, Santoro D, Pani A, Polci R, Feriozzi S, Chicca S, Galliani M, Gigante M, Gesualdo L, Zamboli P, Battaglia GG, Garozzo M, Maixnerová D, Tesar V, Eitner F, Rauen T, Floege J, Kovacs T, Nagy J, Mucha K, Pączek L, Zaniew M, Mizerska-Wasiak M, Roszkowska-Blaim M, Pawlaczyk K, Gale D, Barratt J, Thibaudin L, Berthoux F, Canaud G, Boland A, Metzger M, Panzer U, Suzuki H, Goto S, Narita I, Caliskan Y, Xie J, Hou P, Chen N, Zhang H, Wyatt RJ, Novak J, Julian BA, Feehally J, Stengel B, Cusi D, Lifton RP, Gharavi AG: Discovery of new risk loci for IgA nephropathy implicates genes involved in immunity against intestinal pathogens. Nat Genet 46: 1187–1196, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu MC, Lee S, Cai T, Li Y, Boehnke M, Lin X: Rare-variant association testing for sequencing data with the sequence kernel association test. Am J Hum Genet 89: 82–93, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ionita-Laza I, Lee S, Makarov V, Buxbaum JD, Lin X: Sequence kernel association tests for the combined effect of rare and common variants. Am J Hum Genet 92: 841–853, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee S, Wu MC, Lin X: Optimal tests for rare variant effects in sequencing association studies. Biostatistics 13: 762–775, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monteferrante G, Brioschi S, Caprioli J, Pianetti G, Bettinaglio P, Bresin E, Remuzzi G, Noris M: Genetic analysis of the complement factor H related 5 gene in haemolytic uraemic syndrome. Mol Immunol 44: 1704–1708, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Abrera-Abeleda MA, Nishimura C, Smith JL, Sethi S, McRae JL, Murphy BF, Silvestri G, Skerka C, Józsi M, Zipfel PF, Hageman GS, Smith RJ: Variations in the complement regulatory genes factor H (CFH) and factor H related 5 (CFHR5) are associated with membranoproliferative glomerulonephritis type II (dense deposit disease). J Med Genet 43: 582–589, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Q, Wiesener M, Eberhardt HU, Hartmann A, Uzonyi B, Kirschfink M, Amann K, Buettner M, Goodship T, Hugo C, Skerka C, Zipfel PF: Complement factor H-related hybrid protein deregulates complement in dense deposit disease. J Clin Invest 124: 145–155, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Westra D, Vernon KA, Volokhina EB, Pickering MC, van de Kar NC, van den Heuvel LP: Atypical hemolytic uremic syndrome and genetic aberrations in the complement factor H-related 5 gene. J Hum Genet 57: 459–464, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jönsen A, Nilsson SC, Ahlqvist E, Svenungsson E, Gunnarsson I, Eriksson KG, Bengtsson A, Zickert A, Eloranta ML, Truedsson L, Rönnblom L, Nordmark G, Sturfelt G, Blom AM: Mutations in genes encoding complement inhibitors CD46 and CFH affect the age at nephritis onset in patients with systemic lupus erythematosus. Arthritis Res Ther 13: R206, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Narendra U, Pauer GJ, Hagstrom SA: Genetic analysis of complement factor H related 5, CFHR5, in patients with age-related macular degeneration. Mol Vis 15: 731–736, 2009 [PMC free article] [PubMed] [Google Scholar]
- 33.Goicoechea de Jorge E, Caesar JJ, Malik TH, Patel M, Colledge M, Johnson S, Hakobyan S, Morgan BP, Harris CL, Pickering MC, Lea SM: Dimerization of complement factor H-related proteins modulates complement activation in vivo. Proc Natl Acad Sci U S A 110: 4685–4690, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tortajada A, Yébenes H, Abarrategui-Garrido C, Anter J, García-Fernández JM, Martínez-Barricarte R, Alba-Domínguez M, Malik TH, Bedoya R, Cabrera Pérez R, López Trascasa M, Pickering MC, Harris CL, Sánchez-Corral P, Llorca O, Rodríguez de Córdoba S: C3 glomerulopathy-associated CFHR1 mutation alters FHR oligomerization and complement regulation. J Clin Invest 123: 2434–2446, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kavanagh D, Goodship T: Genetics and complement in atypical HUS. Pediatr Nephrol 25: 2431–2442, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mele C, Remuzzi G, Noris M: Hemolytic uremic syndrome. Semin Immunopathol 36: 399–420, 2014 [DOI] [PubMed] [Google Scholar]
- 37.Zhu L, Shi S, Liu L, Lv J, Zhang H: Increased plasma sVCAM-1 is associated with severity in IgA nephropathy. BMC Nephrol 14: 21, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhai YL, Zhu L, Shi SF, Liu LJ, Lv JC, Zhang H: Elevated soluble VEGF receptor sFlt-1 correlates with endothelial injury in IgA nephropathy. PLoS One 9: e101779, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang JJ, Jiang L, Liu G, Wang SX, Zou WZ, Zhang H, Zhao MH: Elevation of serum von Willebrand factor and anti-endothelial cell antibodies in patients with immunoglobulin A nephropathy are associated with intrarenal arterial lesions. Nephrology (Carlton) 13: 712–720, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Papagregoriou G, Erguler K, Dweep H, Voskarides K, Koupepidou P, Athanasiou Y, Pierides A, Gretz N, Felekkis KN, Deltas C: A miR-1207-5p binding site polymorphism abolishes regulation of HBEGF and is associated with disease severity in CFHR5 nephropathy. PLoS One 7: e31021, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Serino G, Sallustio F, Cox SN, Pesce F, Schena FP: Abnormal miR-148b expression promotes aberrant glycosylation of IgA1 in IgA nephropathy. J Am Soc Nephrol 23: 814–824, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murphy B, Georgiou T, Machet D, Hill P, McRae J: Factor H-related protein-5: A novel component of human glomerular immune deposits. Am J Kidney Dis 39: 24–27, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Miller SA, Dykes DD, Polesky HF: A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 16: 1215, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ma YC, Zuo L, Chen JH, Luo Q, Yu XQ, Li Y, Xu JS, Huang SM, Wang LN, Huang W, Wang M, Xu GB, Wang HY: Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol 17: 2937–2944, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Haas M: Histologic subclassification of IgA nephropathy: A clinicopathologic study of 244 cases. Am J Kidney Dis 29: 829–842, 1997 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.