Abstract
Studies have revealed many analogies between podocytes and neurons, and these analogies may be key to elucidating the pathogenesis of podocyte injury. Cathepsin D (CD) is a representative aspartic proteinase in lysosomes. Central nervous system neurons in CD-deficient mice exhibit a form of lysosomal storage disease with a phenotype resembling neuronal ceroid lipofuscinoses. In the kidney, the role of CD in podocytes has not been fully explored. Herein, we generated podocyte–specific CD–knockout mice that developed proteinuria at 5 months of age and ESRD by 20–22 months of age. Immunohistochemical analysis of these mice showed apoptotic podocyte death followed by proteinuria and glomerulosclerosis with aging. Using electron microscopy, we identified, in podocytes, granular osmiophilic deposits (GRODs), autophagosome/autolysosome-like bodies, and fingerprint profiles, typical hallmarks of CD-deficient neurons. CD deficiency in podocytes also led to the cessation of autolysosomal degradation and accumulation of proteins indicative of autophagy impairment and the mitochondrial ATP synthase subunit c accumulation in the GRODs, again similar to changes reported in CD-deficient neurons. Furthermore, both podocin and nephrin, two essential components of the slit diaphragm, translocated to Rab7– and lysosome–associated membrane glycoprotein 1–positive amphisomes/autolysosomes that accumulated in podocyte cell bodies in podocyte–specific CD–knockout mice. We hypothesize that defective lysosomal activity resulting in foot process effacement caused this accumulation of podocin and nephrin. Overall, our results suggest that loss of CD in podocytes causes autophagy impairment, triggering the accumulation of toxic subunit c–positive lipofuscins as well as slit diaphragm proteins followed by apoptotic cell death.
Keywords: podocyte, Cathepsin D, Autophagy, Lysosome, subunit c of mitochondrial ATP synthase, glomerulosclerosis
CKD is marked by a progressive loss in renal function over a period of months or years and causes ESRD. It has become a worldwide problem in public health, because the prevalence is growing. Proteinuria is an early sign and a prognostic marker for patients with CKD. In addition, proteinuria is an independent risk factor for cardiovascular morbidity and mortality.1 Numerous studies have shown that reducing proteinuria is associated with an improved renal outcome, regardless of the underlying disease process. Glomerular podocytes with their foot processes (FPs) and interposed slit diaphragms serve as a final filtration barrier to urinary protein loss. The majority of nephrotic syndrome is characterized by FP effacement and/or molecular reorganization of the slit diaphragm.2 Defects in podocyte structure, function, or number can lead to pathologic lesions known as glomerulosclerosis.3–5 Accordingly, a better understanding of the mechanism of podocyte injury will provide potential therapeutic steps for the management of CKD.
Lysosomes participate in the turnover of cytoplasmic constituents transported via autophagy as well as the degradation of extracellular materials incorporated via endocytosis or phagocytosis, thus contributing essentially to maintaining cellular homeostasis; >20 lysosomal proteinases, including cathepsin L (CL), cathepsin B (CB), and cathepsin D (CD), play principal roles in lysosomal degradation. Most lysosomal proteinases are capable of efficiently cleaving a wide variety of substrates. However, recent investigations have shown that individual lysosomal proteinase is distributed differently in diverse tissues and involved in tissue–specific protein degradation.6
Recent studies have shown that CL upregulation in podocytes is important for the acceleration of proteinuria, because the induction of a cytoplasmic variant of CL in podocytes precedes FP effacement and proteinuria in mice.7,8 In fact, an increased expression of CL in podocytes has been observed in a variety of human proteinuric kidney diseases, including minimal change disease (MCD), FSGS, membranous nephropathy, and diabetic nephropathy.7 Cytosolic CL cleaves the large GTPase dynamin, actin–binding protein synaptopodin, and slit diaphragm–associated CD2–associated protein (CD2AP). These events result in a disorganization of the podocyte actin cytoskeleton and FP effacement. Thus, many glomerular diseases can be regarded as podocyte enzymatic disorders.7–10
CD, a major lysosomal asparatic endopeptidase that is widely distributed in various mammalian tissues and cells,11 has unique functions. In many cases, the absence of one single cathepsin in mice does not show a fatal course, because it is thought that a functional overlap among multiple cathepsins ensures the proper degree of protein degradation and turnover. However, CD-deficient mice die at approximately postnatal day 26 from a combination of morbidities, including intestinal necrosis, thromboembolia, and lymphopenia.12 Subsequent studies have indicated that mouse CD deficiency induces a neuropathology that is strikingly similar to that observed in human neuronal ceroid lipofuscinosis (or Batten disease). The pathologic symptoms include an accumulation of autofluorescent storage material that resembles granular osmiophilic deposits (GRODs) and the subunit c of mitochondrial ATP synthase.13 CD-deficient mice also exhibit seizures and blindness characteristic of patients with terminal–stage neuronal ceroid lipofuscinosis. Furthermore, an accumulation of abundant autophagosomes/autolysosomes has been noted in central nervous system neurons, which indicates that CD deficiency elicits a strong inhibition of autolysosomal degradation.14 In the kidney, CD is notably present in the glomerulus as well as the cortical collecting tubule cells.15 It has been suggested that CD-like activity in glomeruli isolated from puromycin aminonucleoside–treated rats increased significantly compared with that in control rats.16 However, it is unclear whether CD plays a role in podocytes under pathologic conditions.
Podocytes are terminally differentiated cells and process-bearing cells just like neurons; they also express many neuron-specific proteins, such as synaptopodin, nephrin, podocin, and NEPH1.17,18 In addition, recent studies have reported that podocytes exert a higher level of autophagy under basal conditions and that autophagy significantly contributes to maintaining the functional integrity of podocytes.19–24 Considering the importance of CD in neuronal autophagy and the resemblance between neurons and podocytes, we hypothesized that it is of particular importance to elucidate the role of CD in podocytes. In this study, we investigated the effects of CD deficiency on podocyte structure and function using podocyte–specific, CD–deficient mice.
Results
CD Deficiency in Podocytes Resulted in an Age–Dependent, Late–Onset Form of Glomerulosclerosis
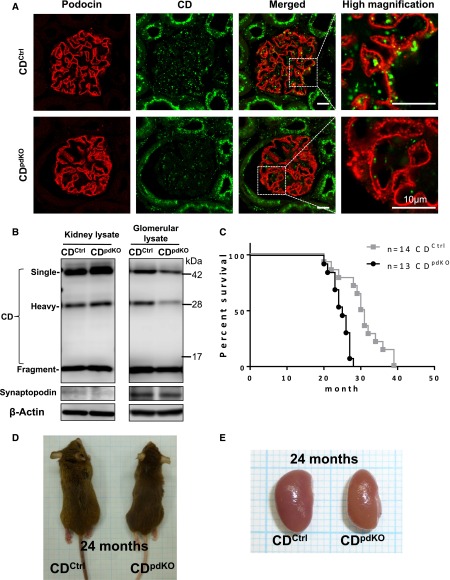
To determine the potential roles of the CD in podocytes, CD-floxed mice (CDflox/flox) were crossed with Podocin-Cre mice to generate podocyte–specific CD–knockout (CDpdKO) mice. Immunofluorescence staining confirmed the markedly diminished expression of CD protein in the glomeruli (Figure 1A). Western blot analysis from isolated glomeruli showed a significant reduction in CD protein in CDpdKO mice compared with that in their control (CDCtrl) littermates (Figure 1B). Because podocyte protein accounts for approximately 30%–40% of total glomerular protein, the data shown here are consistent with CD deficiency in podocytes.
Figure 1.
Podocyte-specific CD-deficiency causes lower survival ratio. (A and B) Effective deletion of CD protein caused by Podocin-Cre recombinase confirmed by (A) immunofluorescence staining and (B) Western blot analysis of isolated glomerular homogenates in 3-month-old mice. (C) Results from a 39-month follow-up of CDpdKO mice for survival of data. Kaplan–Meier survival curves showed that CDpdKO mice had a significantly lower survival ratio than their CDCtrl littermates. The hazard ratio (Mantel–Haenszel test) is 7.28, 95% CI, 2.524–21.00. P<0.001 by Mantel–Cox log-rank test. Median survival time: CDCtrl littermates, 30.5 months; CDpdKO, 25 months. (D and E) At 24 months, CDpdKO mice (D) became thin and (E) showed atrophic kidneys with a rough surface and a slightly yellowish appearance.
The resultant CDpdKO mice were born at the expected Mendelian frequency with no gross renal anomalies noted. Kaplan–Meier survival curves for a total of 39 months showed that the CDpdKO mice had a significantly lower survival ratio than their CDCtrl littermates (P<0.001) (Figure 1C). With regard to body weight, there were no significant differences between the CDpdKO mice and their CDCtrl littermates (data not shown). However, at around 24 months, the CDpdKO mice became thin (Figure 1D) and showed atrophic kidneys with a rough surface and a slightly yellowish appearance (Figure 1E).
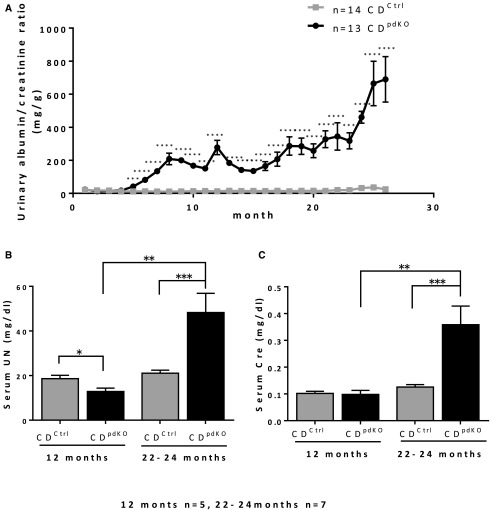
CDpdKO mice developed significantly higher levels of albuminuria compared with their CDCtrl littermates starting at around 5 months of age (Figure 2A). The levels of albuminuria were increased in the CDpdKO mice with aging. Blood biochemical analyses on 22- to 24-month-old mice showed that, compared with their CDCtrl littermates, the CDpdKO mice had significantly higher serum urea nitrogen (UN) levels (21.07±1.33 versus 48.30±8.58 mg/dl; P<0.001; n=7 per group) and higher serum creatinine levels (0.13±0.01 versus 0.36±0.07 mg/dl; P<0.001; n=7 per group) (Figure 2, B and C). These results might indicate that the CDpdKO mice were suffering from CKD and consequently, had a shorter life expectancy compared with their CDCtrl littermates.
Figure 2.
CD deficiency results in age–dependent, late–onset glomerulosclerosis. (A) Results from a 26-month follow-up of CDpdKO mice for urinary albumin-to-creatinine ratios. CDpdKO mice developed significantly higher levels of albuminuria compared with CDCtrl littermates starting at around the fifth month. The level of albuminuria was increased in aging CDpdKO mice. (B and C) Blood chemistry analysis on 22- to 24-month-old mice showed that, compared with CDCtrl littermates, CDpdKO mice had significantly higher serum UN levels and higher serum creatinine levels, indicating that the CDpdKO mice had a kidney insufficiency. The data are the means±SEMs. *P<0.05 by two–tailed Mann–Whitney U test; **P<0.01 by two–tailed Mann–Whitney U test; ***P<0.001 by two–tailed Mann–Whitney U test; ****P<0.001 by two–tailed Mann–Whitney U test.
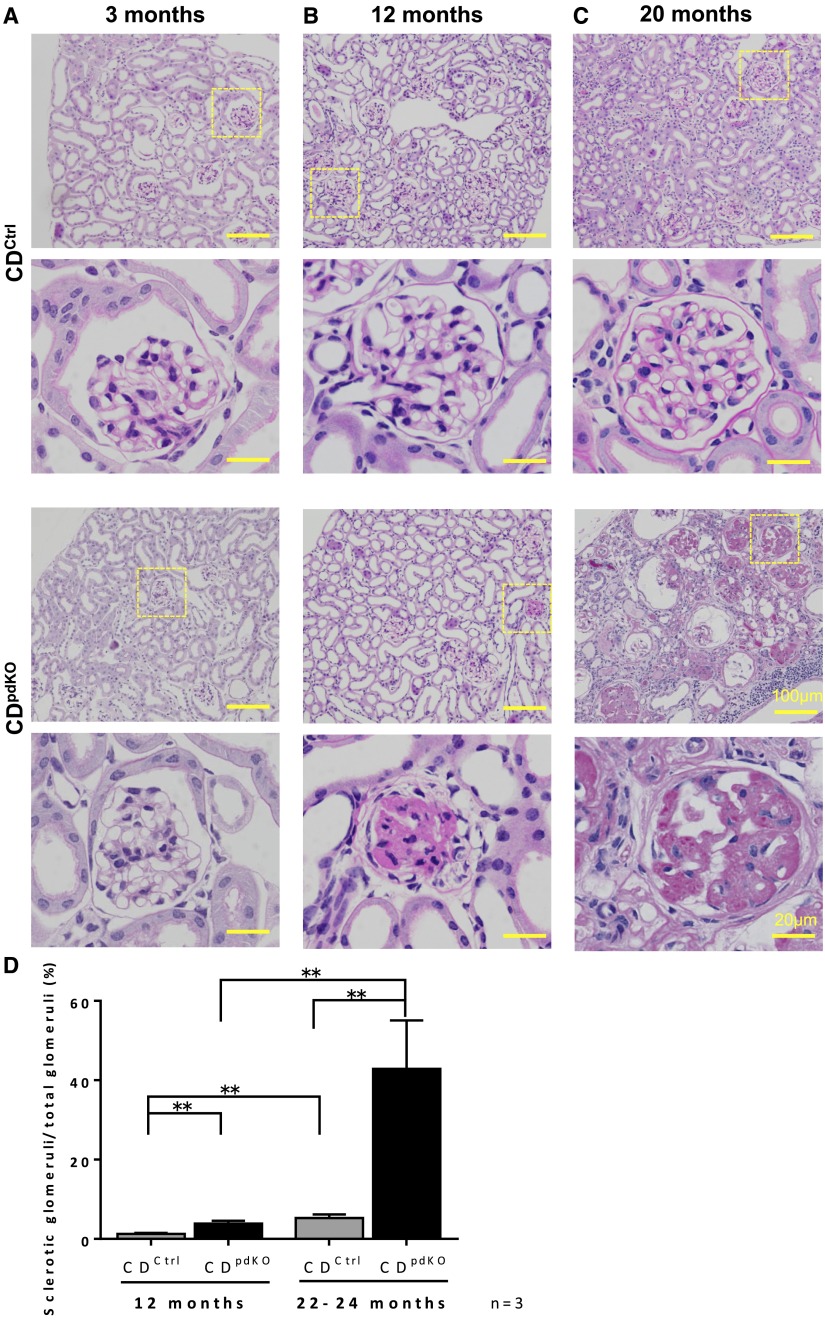
We next examined the kidney histology. For as long as 3 months of age, the CDpdKO mice were indistinguishable from their CDCtrl littermates via analysis by kidney histology and albuminuria (Figures 2A and 3A). The number of sclerotic glomeruli in the CDpdKO mice was significantly greater than that in their CDCtrl littermates at 12 months (Figure 3, B and D). At 20 months of age, many sclerotic glomeruli and severe tubulointerstitial lesions with cystic dilation of tubules were detected in the CDpdKO mice (Figure 3, C and D). The percentage ratio of sclerotic glomeruli at 12 months was 1.25±0.27 in the CDCtrl littermates and 3.89±0.67 in the CDpdKO mice (P<0.01; n=3), and at 20 months, it was 5.27±0.91 in the CDCtrl littermates and 42.9±12.17 in the CDpdKO mice (P<0.01; n=3). These results strongly suggest that CD deficiency in podocytes led to an age–dependent, late–onset form of glomerulosclerosis.
Figure 3.
Podocyte-specific loss of CD causes severe glomerulosclerosis and renal fibrosis. (A–C) Periodic acid–Schiff staining images of kidney sections showed that CDpdKO mice (A) had normal renal histologic findings at 3 months of age but (B and C) developed severe kidney lesions (focal sclerosis) by 12 months of age, including sclerotic glomeruli, severe tubulointerstitial lesions with cystic dilation of tubules, tubular atrophy, and interstitial fibrosis. (D) Quantification of sclerotic glomeruli in CDCtrl and CDpdKO mice at 12 and 20 months showed a significantly higher rate in CDpdKO mice than in their CDCtrl littermates. The sclerotic glomeruli per section were assessed by a total number count; ≥240 glomeruli were randomly selected for the determination of glomerulosclerosis. The data are the means±SEMs. **P<0.01 by a two–tailed Mann–Whitney U test.
Electron Microscopy Analysis of CD-Deficient Podocytes Revealed Characteristic Morphologic Changes
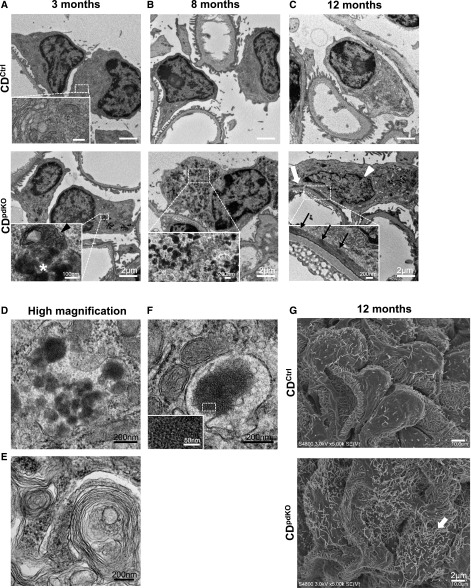
We analyzed the glomerular ultrastructure via electron microscopy. The newborn CDpdKO mice were indistinguishable from their CDCtrl littermates (data not shown). In podocytes of 3-month-old CDpdKO mice that did not exhibit albuminuria (Figure 2A), the obvious histologic phenotype was not detected (Figure 3A). However, significant changes, including an accumulation of electron-dense materials, lesions resembling GRODs (Figure 4, A, asterisk in lower panel inset and D), and autophagosome/autolysosome-like bodies, were identified (Figure 4A, arrowhead in lower panel inset). These granular structures, which varied in size, content, and electron density, were further increased in CDpdKO mice with aging. At 8 months, the CDpdKO mouse podocytes were completely filled with GRODs (Figure 4B); furthermore, autophagosome/autolysosome-like bodies were observed containing portions of the cytoplasm and encircled with multilayered membranes (Figure 4E). In addition, this portion of GRODs contained fingerprint profiles with tightly and concentrically arrayed compact membranes, such as in the structures observed in juvenile neuronal ceroid lipofuscinosis (Figure 4F). These characteristic morphologic changes were strikingly similar to those seen in CD–deficient mouse neurons.13
Figure 4.
Abundant GRODs accumulate in CD-deficient podocytes. (A) Up to 3 months after birth, CDpdKO mice were indistinguishable from CDCtrl littermates as analyzed by proteinuria levels and kidney histology. However, electron microscopy analysis showed significant changes, including GRODs (asterisk in A, lower panel inset) and autophagosome/autolysosome-like bodies (arrowhead in A, lower panel inset) in the podocytes of 3-month-old CDpdKO mice. (B) At 8 months of age, electron micrographs in CDpdKO mice showed that podocytes were filled with GRODs. (C) At 12 months of age, electron micrographs in CDpdKO mice showed FP effacement (large arrow) containing a dense band of actin filaments running parallel to the GBM (small arrows in C, lower panel inset). Irregularly shaped nuclei (arrowhead) also appeared. (D–F) Representative high–magnified images of (D) GRODs, (E) autophagosome/autolysosome-like bodies with multilayered membranes, and (F) a fingerprint profile from CDpdKO mouse podocytes obtained at 8 months of age are shown. To show the fingerprint pattern clearly, a boxed area in F is enlarged in the inset. (G) Scanning electron microscopy analysis also showed microvillus transformations (arrow).
By 12 months of age, when the glomerulosclerosis could be partially recognized by kidney histology (Figure 3), the FPs of CDpdKO mouse podocytes had effaced along the glomerular basement membrane (GBM) to form a continuous band of cytoplasm (Figure 4C, large arrow in lower panel). A dense band of actin filaments (Figure 4C, small arrows in lower panel inset) was seen along the effaced FPs running parallel to the GBM. These morphologic changes in the structure of podocyte FPs were characterized as a development of nephrotic syndrome.2 Moreover, irregularly shaped nuclei also appeared, with a small and dispersed heterochromatin and an unclear nucleolus, similar to the nuclei in dying central nervous system neurons13 (Figure 4C, arrowhead in lower panel). Scanning electron microscopy analysis showed prominent microvillus transformations, which were characterized as severe proteinuria (Figure 4G, arrow in lower panel).
Electron Micrographs of Irregularly Shaped Nuclei Show an Association with Apoptotic Podocyte Death in Aging CDpdKO Mice
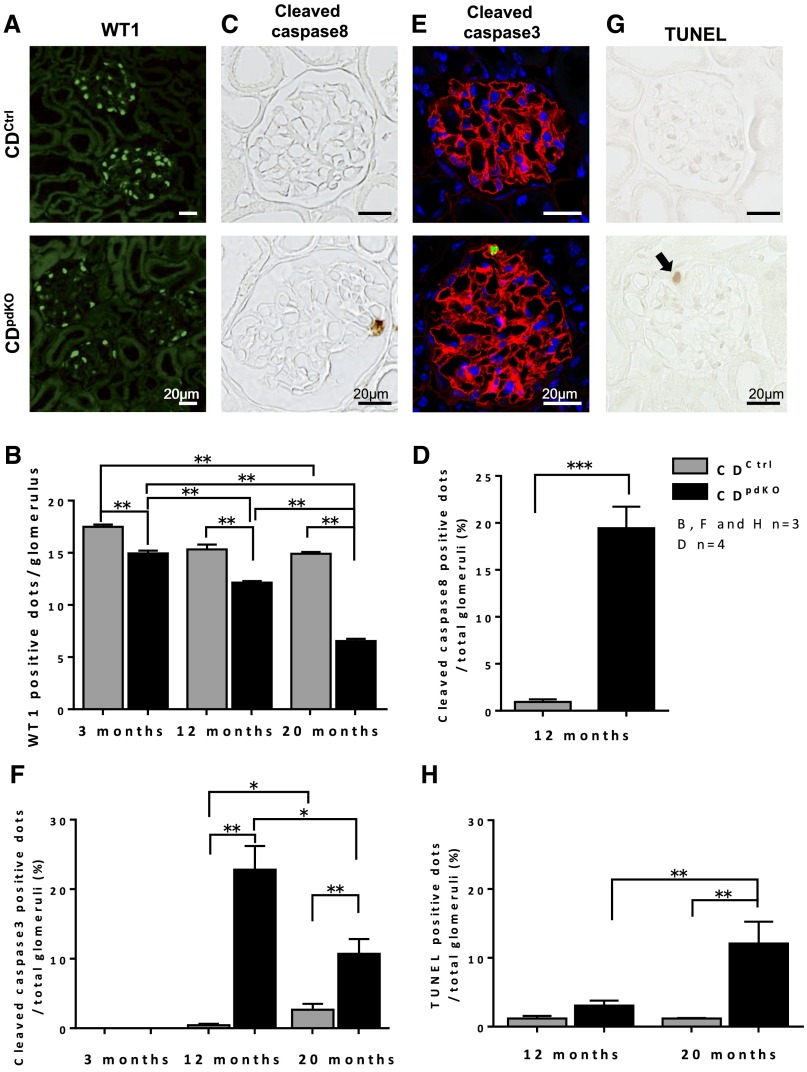
Many studies have shown that a reduction in the number of podocytes correlates with proteinuria and leads to glomerulosclerosis.25–28 To count the number of podocytes in the glomeruli, WT1, a nuclear marker for podocytes, staining was performed at 3, 12, and 20 months (Figure 5A). The number of WT1-positive cells was significantly decreased in CDpdKO mice compared with their CDCtrl littermates. The numbers of WT1-positive cells in the CDCtrl littermates and the CDpdKO mice were 17.49±0.21 versus 14.94±0.26 per glomerulus at 3 months, 15.33±0.47 versus 12.14±0.15 per glomerulus at 12 months, and 14.90±0.16 versus 6.54±0.22 per glomerulus at 20 months, respectively (n=3; P<0.01) (Figure 5B). These observations imply either an increase in podocyte cell death or a detachment from the GBM in CDpdKO mice.
Figure 5.
Irregularly shaped nuclei in electron micrographs are associated with apoptotic podocyte death in aging CDpdKO mice. (A) Immunofluorescence staining showed that WT1-positive dots (green) in glomeruli from CDpdKO mice were decreased at 15 months of age. (B) WT1-positive cells in CDpdKO mice were significantly decreased compared with those in their CDCtrl littermates at 3, 12, and 20 months of age. Analysis was performed on 50–60 glomeruli of each mouse. (C) Immunohistochemical staining with anticleaved caspase-8 showed cleaved caspase-8–positive podocytes of 12-month-old CDpdKO mice. (D) Quantification of the number of cleaved caspse-8–positive dots in 12-month-old mice showed a significantly higher rate in CDpdKO mice than in their CDCtrl littermates; ≥350 glomeruli of each mouse were analyzed. (E) Immunofluorescence staining showed that cleaved caspase-3–positive dots (green) in the glomeruli from CDpdKO mice were increased at 12 months of age (red, synaptopodin; green, cleaved caspase-3; 4′,6-diamidine-2-phenylindole staining). (F) Quantification of the number of cleaved caspase-3 dots in 12- and 20-month-old mice showed a significantly higher percentage ratio in CDpdKO mice than in their CDCtrl littermates; ≥200 glomeruli of each mouse were analyzed. (G) TUNEL staining showed that TUNEL-positive dots (arrow) in the glomeruli from CDpdKO mice were increased at 20 months of age. (H) Quantification of the number of TUNEL-positive dots in 20-month-old mice showed a significantly higher rate in CDpdKO mice than in their CDCtrl littermates; ≥200 glomeruli of each mouse were analyzed. The data are the means±SEMs. *P<0.05; **P<0.01 two–tailed Mann–Whitney U test; ***P<0.001 by two–tailed Mann–Whitney U test.
It is well known that podocyte injury causes either podocyte apoptosis or detachment.28,29 First, we assessed the podocyte apoptosis by analyzing cleaved caspase-3, which is classified as an effecter caspase. We counted cleaved caspase-3 dots in the glomeruli at 3, 12, and 20 months (Figure 5, E and F). At 3 months, cleaved caspase-3 dots were not detected in either the CDpdKO mice or their CDCtrl littermates. Quantification of the number of cleaved caspase-3–positive cells in 12- and 20-month-old mice showed a significantly higher rate in the CDpdKO mice than in their CDCtrl littermates (CDCtrl littermates versus CDpdKO mice: 0.45±0.20% versus 22.82±3.38% at 12 months and 2.67±0.86% versus 10.72±2.11% at 20 months, respectively; n=3; P<0.01). Second, we assessed the podocyte apoptosis by analyzing cleaved caspase-8, which is classified as an initiator caspase. Quantification of the number of cleaved caspase-8–positive dots in 12-month-old mice showed a significantly higher rate in CDpdKO mice than in their CDCtrl littermates (CDCtrl littermates versus CDpdKO mice: 0.94±0.27% versus 19.44±2.29%, respectively; n=4; P<0.001) (Figure 5, C and D). Finally, we performed terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick–end labeling (TUNEL) staining at 12 and 20 months (Figure 5, G and H). As shown in Figure 5H, the quantification of TUNEL-positive cells in 20-month-old mice revealed a significantly higher rate in CDpdKO mice than in their CDCtrl littermates (CDCtrl littermates versus CDpdKO mice: 1.18±0.38% versus 3.06±0.73% at 12 months and 1.19±0.08% versus 12.09±3.16% at 20 months, respectively; n=3; P<0.01). These findings indicate that podocyte cell death in CDpdKO mice, which is characterized by typical apoptotic features, such as positive staining for cleaved caspase-3 and cleaved caspase-8 and TUNEL staining, was increased with aging >12 months.
Subunit c of Mitochondrial ATP Synthase Accumulated in CDpdKO Mouse Podocytes
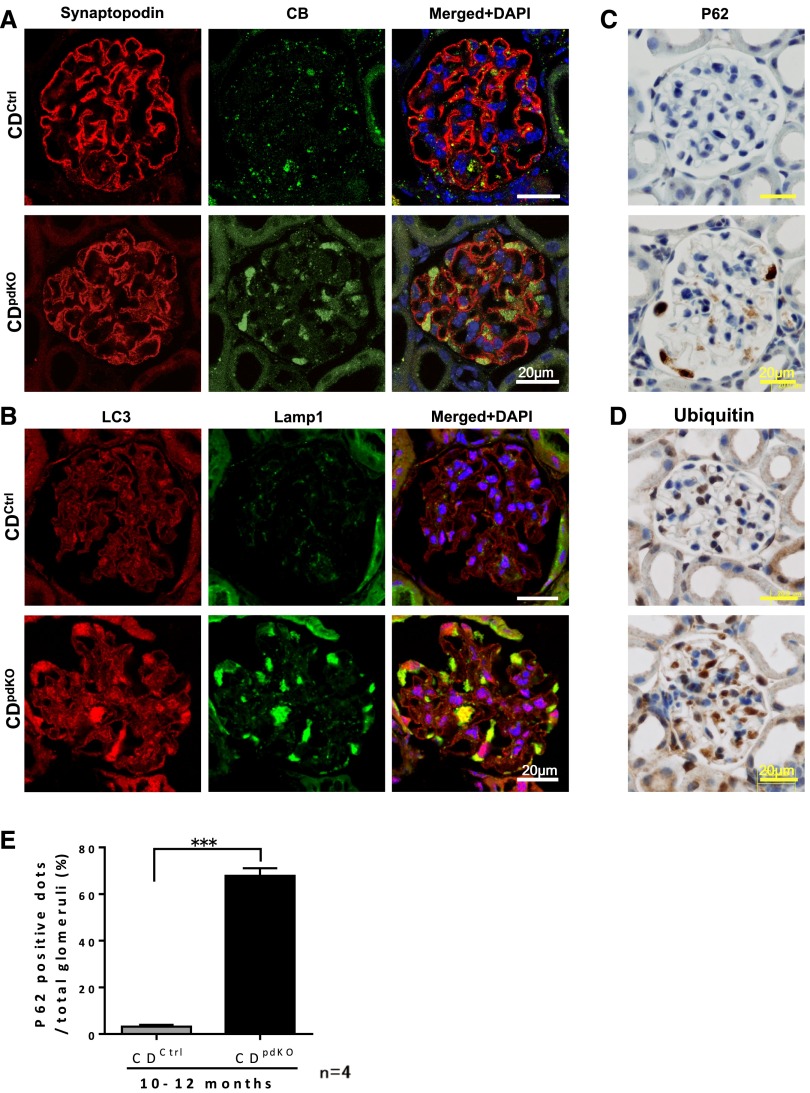
The CDpdKO mouse podocytes exhibited characteristic morphologic changes, like GRODs and autophagosome/autolysosome-like bodies, which are common in CD–deficient mouse neurons. Biochemical investigations have revealed that subunit c of mitochondrial ATP synthase is a major storage protein in the GRODs of CD–deficient mouse neurons13 and that, in particular, an accumulation of autophagosomes/autolysosomes is involved along with the GRODs.14 To assess the autophagy-lysosome pathways in the podocytes of CDpdKO mice, we performed immunohistochemical analysis. Lysosomal CB, lysosomal–associated membrane protein 1 (Lamp1), microtubule–associated protein 1 light chain 3 (LC3), p62, and ubiquitin all markedly accumulated in the podocytes of CDpdKO mice (Figure 6). These data were further supported by Western blots of glomerular lysates, which showed that Lamp1, polyubiquitin, and LC3-II had accumulated in CDpdKO mouse glomeruli (Supplemental Figure 1). Quantification of the number of p62-positive cells in 10- to 12-month-old mice showed a significantly higher rate in CDpdKO mice than in their CDCtrl littermates (Figure 6E). Strikingly, enlarged Lamp1 dots were colocalized with LC3 dots, indicating that autolysosomes accumulate in podocytes (Figure 6B). These data are consistent with the data published previously on CD-deficient neurons.14,30
Figure 6.
CB, Lamp1, LC3, ubiquitin, and p62 accumulate in CDpdKO mouse podocytes. (A and B) Immunofluorescence staining showed that (A) lysosomal CB and (B) Lamp1 and LC3 were increased in 10-month-old CDpdKO mouse podocytes. Most dots were enlarged to form aggregates in the podocytes. (B, right panel) LC3 dots were significantly colocalized with Lamp1. (C and D) Immunohistochemical staining with anti-p62 and antiubiquitin showed p62- and ubiquitin-positive podocytes of 10-month-old CDpdKO mice. (E) Quantification of the number of p62 dots in 10- to 12-month-old mice showed a significantly higher rate in CDpdKO mice than in their CDCtrl littermates; ≥150 glomeruli of each mouse were analyzed. The data are the means±SEMs. CDCtrl littermates versus CDpdKO mice: 3.23±0.75% versus 67.83±3.26%, respectively; n=4. ***P<0.001 by a two–tailed Mann–Whitney U test.
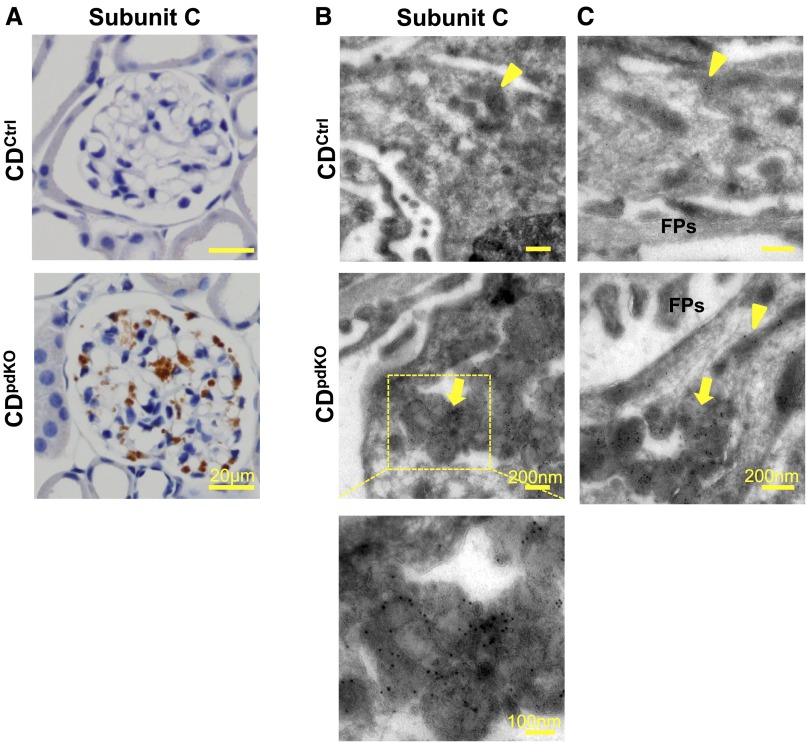
Interestingly, we confirmed that these CD-deficient podocytes emitted autofluorescence, particularly after 8 months of age (data not shown). Because these data are consistent with those because of the lysosome-like bodies containing ceroid lipofuscin,13 we further sought to determine whether subunit c of mitochondrial ATP synthase is present in these lysosomal structures. No immunoreactivity for subunit c was detected in any of the kidney sections obtained from the CDCtrl littermates. However, dotted immunoreactivity for subunit c was detected in CDpdKO mouse podocytes (Figure 7A).
Figure 7.
Subunit c of mitochondrial ATP synthase accumulate in lysosomal structures of podocytes as in CD-deficient neurons. (A) Immunohistochemical staining showed subunit c of mitochondrial ATP synthase accumulated in 10-month-old CDpdKO mouse podocytes. (B and C) Immunoelectron microscopy indicated that, in podocytes from CDpdKO mice, the labeling for subunit c was associated with membrane-bound structures containing GRODs. Immunocytochemical staining of subunit c of mitochondrial ATP synthase in podocytes from CDpdKO mice and their CDCtrl littermates at 10 months using the cryothin section immunogold method is shown. (B, top panel and C, upper panel) Subunit c gold particles label only the mitochondrial inner membrane in the CDCtrl littermates (arrowheads), whereas (B, middle panel and C, lower panel) they are associated with both the inner membrane of intact mitochondria (arrowhead) and the membrane-bound compartments with dense materials (GRODs; arrows) in the CDpdKO mice.
To identify the subcellular localization of subunit c in podocytes from CDpdKO mice and their CDCtrl littermates, immunoelectron microscopy using the cryothin section immunogold method was applied to the tissues. In the podocytes of the CDCtrl littermates, subunit c was detectable by immunogold labeling in the mitochondrial inner membrane (Figure 7, B, arrowhead in top panel and C, arrowhead in upper panel). In podocytes from CDpdKO mice, the labeling for subunit c was associated with both the inner membrane of intact mitochondria and the membrane-bound compartments with dense materials (GRODs) (Figure 7, B, arrow in middle panel and C, arrowhead and arrow in lower panel). These findings indicate that subunit c of mitochondrial ATP synthase is a major storage protein of GRODs in CDpdKO mouse podocytes.
Slit Diaphragm Proteins, Podocin and Nephrin, Accumulated in the Podocyte Cell Bodies of CDpdKO Mice
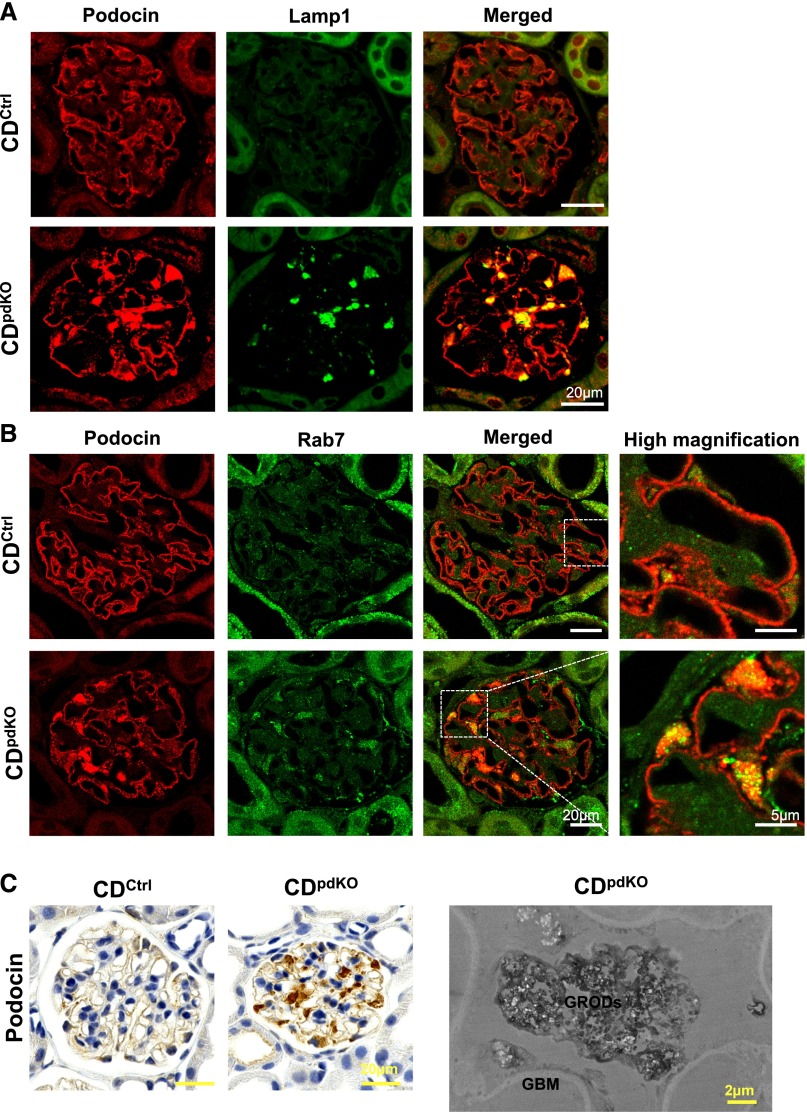
The majority of nephrotic syndrome is characterized by FP effacement resulting from the molecular reorganization of the slit diaphragm.2 We assessed the expression of podocin and nephrin, two essential proteins that help maintain slit diaphragm integrity, in CDpdKO mice. Immunofluorescence staining clearly showed the localization of podocin and nephrin in the GBM in CDCtrl littermates. By contrast, podocin and nephrin were found to be significantly distributed in the podocyte cell bodies in CDpdKO mice (Supplemental Figure 2A). Notably, the enlarged cytoplasmic staining of podocin and nephrin in podocyte cell bodies was found to be well colocalized with enlarged Lamp1 staining (Figure 8A, Supplemental Figure 3A).
Figure 8.
The accumulation of podocin in 10-month-old CDpdKO mouse podocyte cell bodies is particularly colocalized with Lamp1 and Rab7. (A and B) Immunofluorescence staining showed the localization of podocin in the GBM in CDCtrl littermates. By contrast, podocin was localized in both the GBM and podocyte cell bodies in CDpdKO mice. The late endosomal marker Rab7 was increased in CDpdKO mouse podocytes compared with CDCtrl littermate podocytes. Podocin localized in podocyte cell bodies was particularly colocalized with (A) Lamp1 and (B) Rab7 in CDpdKO mice. (C) For immunohistochemical staining, we detected a strong accumulation of podocin in CDpdKO mouse podocyte cell bodies. Immunoelectron microscopy using paraffin–embedded tissue sections indicated that the granular structures (GRODs) that accumulate in the podocyte cell bodies of CDpdKO mouse glomeruli are autophagosomes/autolysosomes containing degraded podocin.
Endocytosis and degradation of slit diaphragm proteins play an important role in the maintenance of slit diaphragm function.31 Neither the CDpdKO mouse podocytes nor the podocytes of their CDCtrl littermates displayed significant colocalization of podocin when tested with an early endosomal marker Rab5 (Supplemental Figure 2B). By contrast, a late endosomal marker for Rab7 was increased in CDpdKO mouse podocytes compared with CDCtrl littermate podocytes (Figure 8B, Supplemental Figures 2C and 3B). Furthermore, both podocin and nephrin were considerably colocalized with Rab7 and Lamp1 in CDpdKO mice compared with their CDCtrl littermates. These data indicated that podocin and nephrin were mainly localized in the late endosomes and lysosomes of CDpdKO mouse podocyte cell bodies.
To further identify the subcellular localization of podocin in CDpdKO mouse podocytes, immunoelectron microscopy using paraffin–embedded tissue sections was performed (Figure 8C). As clearly shown, podocin was localized in the GRODs accumulating in the podocyte cell bodies of CDpdKO mouse glomeruli. This finding indicates that cytoplasmic podocin is localized together with other storage proteins on the GRODs in CDpdKO mouse podocytes.
Podocyte CD Expression May Be Related to the Progression of Human Glomerular Diseases
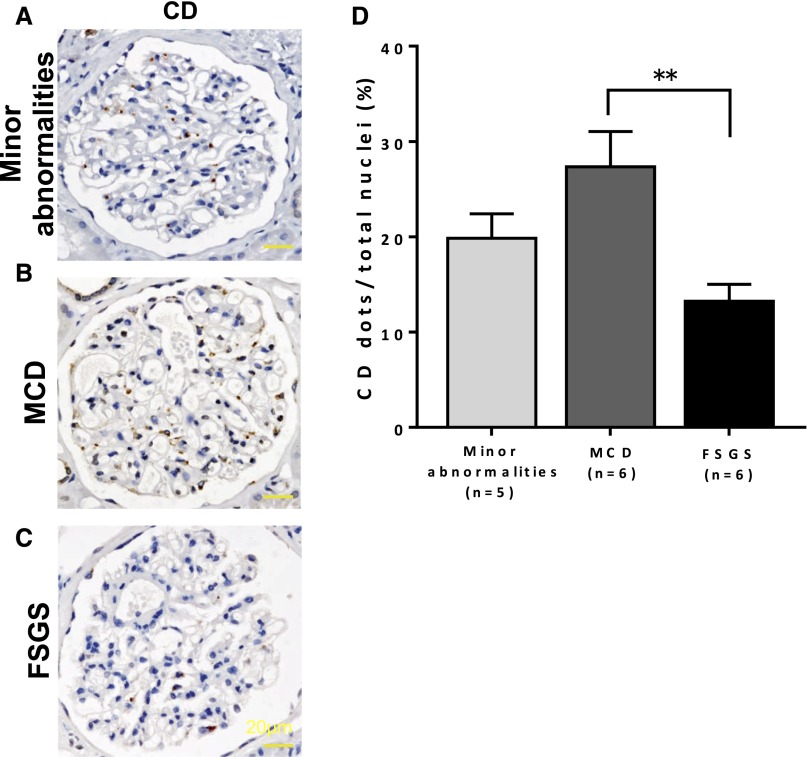
To examine the expression of CD in the podocytes of human glomerular diseases with nephrotic syndrome by immunohistochemical staining, we stained the CD in human kidney biopsy specimens of minor glomerular abnormalities, MCD, and FSGS. The expression of CD was significantly increased in the MCD samples (Figure 9B) compared with that in the FSGS samples (Figure 9C) (MCD versus FSGS: 27.34±3.72% versus 13.25±1.77%, respectively; P<0.01). These results indicated that the degree of podocyte injury is related to the expression levels of CD in the glomeruli.
Figure 9.
Kidney sections from FSGS show a reduction in the CD expression in podocytes. (A–C) Staining of CD in human kidney biopsy specimens. (D) The numbers of CD-positive cells per glomerulus are indicated as the means±SEMs of five minor glomerular abnormalities, six MCD samples, and six FSGS samples. The expression of CD was significantly decreased in (C) FSGS compared with that in (B) MCD. **P<0.01 by two–tailed Mann–Whitney U test.
Discussion
In addition to the physiologic role in lysosomal degradation, recent reports have linked CD to apoptosis32 as well as several other diseases, such as Alzheimer disease,33 atherosclerosis,34 cancer,35 and neuronal ceroid lipofuscinosis.36 In the kidney, CD is expressed in not only podocytes but also, tubular cells. Previous studies showed that the expression of CD is increased in the renal tubules of nephrotic rats and mice to decompose endocytosed proteins.16,37 However, the association between CD and CKD is not yet fully understood. This study provides the first evidence that the deletion of CD in podocytes impairs autophagy through an accumulation of toxic subunit c–positive lipofuscins and delocalization of slit diaphragm proteins, leading to podocyte apoptotic cell death.
In our study, we showed that aging CDpdKO mice developed age–dependent, late–onset glomerulosclerosis, which explains the shorter life expectancy of CDpdKO mice (Figure 1). The entire process of disorder, which finally leads to glomerulosclerosis, proceeded in a stepwise manner. CDpdKO mice exhibited spontaneous proteinuria as early as 5 months after birth, which gradually increased until 20 months (Figure 2). Then, the mice manifested remarkable podocyte loss when they grew older—between 12 and 20 months of age (Figure 5). Increases in the numbers of cleaved caspase-3–positive cells as well as TUNEL-positive cells strongly suggested that apoptosis had partially contributed to the podocyte loss. In addition, an increase in the number of cleaved caspase-8–positive cells indicated a contribution of the extrinsic apoptotic signaling pathway in CD-deficient podocytes, because caspase-8 activation functions as an upstream activator of caspase-3.38 The increase in podocyte loss in the aging CDpdKO mice, in turn, evoked the marked impairment of glomerular filtration typically shown by high levels of serum UN and serum creatinine (Figure 2), which eventually leads to obvious glomerulosclerosis, including FP effacement and the detachment of podocytes from the GBM (Figures 3–5).
In view of the gradual increase in proteinuria (5–20 months) as well as no obvious latent postnatal phenotype at 5 months, the accumulation of detrimental effects directly caused by CD deficiency seemed to play an important role. When considering the direct effect of CD deficiency, it should be noted that CD-deficient podocytes share many common characteristics with CD-deficient neurons. In electron microscopic analysis, accumulations of GROD–like electron–dense materials, fingerprint profiles, and autophagosome/autolysosome-like bodies (Figure 4) also are often reported as the most characteristic features in the neuronal tissues of CD-deficient mice.14 In addition, the subunit c of mitochondrial ATP synthase had accumulated in the GRODs in CDpdKO mouse podocytes (Figure 7). This characteristic is reminiscent of the neuronal ceroid lipofuscinosis that is caused by CD deficiency in neuronal cells as reported by Koike et al.14 and other groups.39–41 Thus, the loss of CD evoked disorders that are common to both neurons and podocytes, confirming that the two cell systems resemble each other, even under pathologic conditions in addition to normal physiologic conditions as reported previously.42
It should be emphasized that these characteristics are closely connected to the autophagic inhibition of CDpdKO mouse podocytes. Both immunohistochemical and Western blot analyses clearly showed that CB, Lamp1, LC3-II, p62, and ubiquitin were markedly accumulated in the podocytes of CDpdKO mice (Figure 6, Supplemental Figure 1). The accumulation of p62 is a hallmark of autophagy impairment. Furthermore, enlarged Lamp1 dots that were colocalized with LC3 dots were noted, indicating abundant autolysosome accumulation (Figure 6). Using Western blotting, we confirmed that S6 kinase and ribosomal S6 were dephospholyrated in the isolated glomeruli of both CDpdKO and CDCtrl mice (data not shown), indicating mammalian target of rapamycin inactivation. Taken together, these characteristics clearly showed that podocyte autophagy was severely inhibited during autolysosomal degradation because of the absence of CD.
Unexpectedly, we found that podocin and nephrin were abnormally distributed in the podocyte cell bodies and colocalized with Rab7 and Lamp1 in CDpdKO mice (Figure 8, Supplemental Figures 2 and 3). Because Rab7 is a late endosomal marker and Lamp1 is also an endosomal-lysosomal marker, our finding strongly suggests that the two slit diaphragm proteins must shift their localization to remain on accumulated autolysosomes. In slit diaphragm, podocin and nephrin play an essential role in maintaining the glomerular filtration barrier. In this role, their homeostatic secretion to slit diaphragm and the subsequent endocytic trafficking are important.43 In CDpdKO mouse podocytes, autophagosome formation proceeded normally, but autolysosomal turnover was severely hindered. This resulted in more late endosomes and lysosomes that were recruited to form autolysosomes in a compensatory manner. As a result, endocytosed nephrin and podocin become confined in Rab7- and/or Lamp1-positive autolysosomes. The disproportionate endosomal-lysosomal localization of podocin and nephrin must affect the integrity of slit diaphragm, which directly connects to the vulnerability of the slit diaphragm.
In recent reports, podocyte–specific autophagy–related 5 (Atg5) –knockout mice developed significantly higher levels of proteinuria compared with their control littermates by 8–12 months of age, and the level of proteinuria was increased with aging. Furthermore, 20- to 24-month-old podocyte–specific Atg5–knockout mice displayed significantly increased glomerulosclerosis compared with their control littermates.19 Thus, autophagy impairment in podocytes, whether it is elicited directly by the loss of an autophagy-essential gene (Atg5) or indirectly by the loss of lysosomal CD, compromises the normal ability of podocytes to sustain a glomerular filtration barrier. It is curious that CDpdKO mice developed proteinuria and glomerulosclerosis at an earlier age compared with podocyte–specific Atg5–knockout mice (CDpdKO mice versus podocyte–specific Atg5–knockout mice: 5 versus 8–12 months for the onset of proteinuria and 12 versus 20–24 months for the onset of glomerulosclerosis). The reasons for this difference may have been the presence of toxic subunit c–positive lipofuscins, podocin, and nephrin accumulating in the podocytes of the CDpdKO mice.
To determine if a compensatory increase in podocyte CL might explain the development of podocyte injury in the CDpdKO mice,7,8 we examined CL expression by immunofluorescence (Supplemental Figure 4A). Although there was a suggestion of increased podocyte staining for CL, the expressions of synaptopodin and CD2AP, which are considered the substrates for CL, displayed no significant differences between CDpdKO mice and CDCtrl littermates (Supplemental Figure 4). These findings suggest that the effect of CD deficiency is independent of CL; however, this question needs additional study.
Immunohistochemical staining of human kidney biopsy specimens indicated that the expression of CD was significantly increased in MCD compared with that in FSGS (Figure 9). Podocyte injury is more severe in FSGS, which leads to irreversible damage in many patients compared with MCD. Studies have found that podocytes from patients with MCD had higher levels of beclin 1–mediated autophagic activity than those from patients with FSGS, suggesting that maintaining a relatively high level of autophagic activity may prevent the progression of podocyte injury in MCD.44 High CD expression in patients with MCD may be because of a high level of autophagic activity. The detailed mechanisms underlying these observations will require additional study.
Many lysosomal disorders cause neurodegenerative problems, but very few lysosomal defects are known to cause renal disease. Lysosomal dysfunction in Fabry disease impairs podocyte function by disrupting podocyte FPs, but the mechanism by which lysosomes accumulate glycosphingolipids in podocytes and trigger renal failure is not yet fully understood.45 Mutations in the CD gene cause a congenital neuronal ceroid lipofuscinosis classified as CLN10 in humans. CLN10 manifests severe symptoms, such as early blindness and psychomotor disability.36,46 Because of the ephemerality of patients with fatal neurodegeneration, whether the infants of these patients suffer renal failure has not been investigated.
In conclusion, this study indicates that CD plays a principal role in autophagic degradation and contributes to maintaining podocyte homeostasis. The results together with a previous report by Hartleben et al.47 underscore the importance of autophagy in podocyte homeostasis and health. The regulation of slit diaphragm proteins through the membrane trafficking system is also considered to contribute greatly to the maintenance of podocyte FPs. The clinical relevance of CD in human podocyte injury requires additional investigation in future studies.
Concise Methods
Generation of CDpdKO Mice
CDflox/flox were crossed with Podocin-Cre mice to generate CDpdKO. CDflox/WT;Podocin-Cre+ and CDflox/flox;Podocin-Cre− littermates served as control animals (CDCtrl) in this study. These animals showed no pathologic phenotypes when examined by histologic, immunohistochemical, and biochemical methods. CDflox/flox mice are described elsewhere. Podocin-Cre mice have been previously reported.48 All mice were kept in specific pathogen–free facilities. All animal experiments were guided and approved by the Committee for Animal Experiments of Juntendo University (Tokyo, Japan).
Biochemical Measurements
Body weight and urinary albumin-to-creatinine ratios were measured in the mice once a month. Urinary albumin and creatinine levels were measured by immunoassay (DCA 2000 System; Siemens Healthcare, Erlangen, Germany).49 Blood samples were obtained from anesthetized mice before death and then, centrifuged at 5000 rpm for 30 minutes at 4°C to obtain serum. Serum UN levels were measured by ultraviolet-visible spectroscopy, and creatinine levels were determined using enzymatic methods.
Antibodies
Commercially available antibodies were obtained from the following sources: polyclonal goat anti–mouse CD antibody (R&D Systems, Minneapolis, MN) and polyclonal goat anti–mouse CL antibody (R&D Systems) for immunofluorescence study; polyclonal rabbit anti–human CD antibody (Cell Signaling Technology, Danvers, MA); polyclonal rabbit anti–WT1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA); polyclonal rabbit anticleaved caspase-3 (Asp175) antibody (Cell Signaling Technology); monoclonal rabbit anticleaved caspase-8 (Asp387) antibody (Cell Signaling Technology); monoclonal rat anti–Lamp1 antibody (1D4B; Santa Cruz Biotechnology) for immunofluorescence study; monoclonal rat anti–mouse Lamp1 antibody (1D4B; BD Biosciences Pharmingen, San Diego, CA) for Western blotting; polyclonal rabbit anti–Rab5 antibody (Cell Signaling Technology); monoclonal rabbit anti–Rab7 antibody (Cell Signaling Technology) for Western blotting; monoclonal mouse antisynaptopodin antibody (Progen, Heidelberg, Germany); polyclonal guinea pig antinephrin antibody (Progen); monoclonal mouse anti–β-Actin antibody (Sigma-Aldrich, St. Louis, MO); polyclonal rabbit anti–LC3 antibody (Abcam, Inc., Cambridge, MA) for immunofluorescence study; polyclonal rabbit anti–SQSTM1/A170/P62 antibody (Wako Pure Chemicals, Tokyo, Japan); polyclonal rabbit antiubiquitin antibody (Dako); and monoclonal mouse antimultiubiquitin antibody (FK2; Medical & Biologic Laboratories Co., Ltd.). Rabbit polyclonal antibodies against podocin50 and synaptopodin17 were prepared as previously described. The antibodies against CD, CL, and LC3 for Western blotting and CB and Rab7 for immunofluorescence study were donated by Eiki Kominami (Juntendo University), and the antibodies against subunit c of mitochondrial ATP synthase were donated by Junji Ezaki (Fukushima Medical University).
Histologic Analyses and Immunohistochemistry
Kidneys were fixed via the cardiac ventricular perfusion of 4% paraformaldehyde and 20% sucrose in PBS and embedded in paraffin. The 3-μm-thick kidney sections were stained with either a periodic acid–Schiff method or primary antibodies followed by the respective secondary antibodies. Nuclei were stained with Mayer Hematoxylin Solution in immunohistochemical staining. All histologic and immunohistochemical specimens were observed via a light microscope (Olympus BX41; Olympus, Tokyo, Japan).
To detect podocyte apoptotic cells, the TUNEL assay was performed using the Apoptag Plus Peroxidase In Situ Apoptosis Detection Kit (Chemicon International Inc., Temecula, CA). The kit was used according to the manufacturer’s instructions.
The results of the immunofluorescence study are shown in Figures 5 and 6 and Supplemental Figure 4, and the fixed kidneys were frozen in optimal cutting temperature compound. Frozen sections (3 μm) were incubated with primary antibodies and developed with secondary antibodies. Nuclei were stained with 4′,6-diamidine-2-phenylindole. For the immunofluorescence study in Figures 1 and 8 and Supplemental Figures 2 and 3, paraffin–embedded tissue sections (3 μm) were deparaffinized and hydrated according to standard procedures. Sections were subjected to antigen retrieval by boiling in citrate buffer (10 mM, pH 6.0) for 5 minutes and treated with 0.3% H2O2 in methanol for 10 minutes. They were then stained in the same manner as mentioned above.
Immunofluorescence specimens were analyzed using a confocal fluorescence microscope (Leica TCS SP8; Leica Microsystems, Buffalo Grove, IL).
Transmission Electron Microscopy
Kidney samples (approximately 2 mm3) were immersed in 2.5% glutaraldehyde with 0.1 M phosphate buffer (PB; pH 7.4) for 2 hours. Next, the samples were postfixed in 2% OsO4 with 0.1 M PB for 2 hours. Tissues were dehydrated in ethanol and embedded in epoxy resin (Epok 812; Epok, Okenshoji, Japan). Ultrathin sections (1 μm) were cut with an ultramicrotome (Leica Ultracut UC7; Leica Microsystems) and mounted on a copper grid. The sections were stained in uranyl acetate for 30 minutes and lead citrate for 4 minutes at room temperature. Grids were viewed with a transmission electron microscope (HT7700; Hitachi, Yokohama, Japan) at 100.0 kV.
Scanning Electron Microscopy
Kidney samples (approximately 2 mm3) were immersed in 2.5% glutaraldehyde with 0.1 M PB (pH 7.4) for 2 hours. Next, the samples were fixed in 2% OsO4 with 0.1 M PB for 2 hours. Tissues were dehydrated in ethanol and freeze dried with tert-butyl alcohol in a freeze dryer (ES-2030; Hitachi). After drying, the samples were coated with OsO4 and visualized with a scanning electron microscope (S-4800; Hitachi) at an accelerating voltage of 3.0 kV.
Immunoelectron Microscopy
Immunoelectron microscopy for subunit c using ultrathin cryosections was performed as previously described.13 Briefly, mice were deeply anesthetized with pentobarbital (25 mg/kg intraperitoneally) and fixed by cardiac perfusion with 4% paraformaldehyde buffered with 0.1 M PB (pH 7.4). Kidneys were quickly removed from the mice, cut into small pieces, further immersed in the same fixative at 4°C for 2 hours, washed thoroughly with 7.5% sucrose in 0.1 M PB (pH 7.4), embedded in 12% gelatin, and immersed in 2.3 M sucrose in 0.1 M PB overnight at 4°C. The samples were then placed on a specimen holder (Leica Microsystems) and quickly plunged into liquid nitrogen. Ultrathin sections were cut with a microtome using a cryoattachment (Ultracut UC7/FC7; Leica Microsystems) and mounted on Formvar Carbon–Coated Nickel Grids. The sections were rinsed with PBS, treated with 1% BSA in PBS, and incubated overnight with antisubunit c (10 mg/ml) in PBS and then, 1 hour with anti–goat IgG conjugated with 10-nm colloidal gold particles (1:40; British Biocell International). After the immunoreactions, the sections were embedded in 2% methyl cellulose containing 0.4% uranyl acetate and observed with a Hitachi H-7100 Electron Microscope. For control experiments, deparaffinized and ultrathin sections were incubated with nonimmunized rabbit serum diluted 1:1000 followed by respective second antibodies.
Immunoelectron microscopy for podocin was performed in the following way. The deparaffinized section that was immunostained with antipodocin antibody, as described above, was immersed in 1% OsO4 with 0.1 M PB for 1 hour. After being washed with distilled water, the sections were dehydrated with a graded series of ethanol and embedded in Epon 812. Ultrathin gold sections were processed with a diamond knife, transferred to copper grids (50 mesh) that were coated with Formvar Membrane, and observed with a JEM1230 Transmission Electron Microscope (JEOL, Tokyo, Japan).
Western Blotting
Mouse glomeruli were isolated as previously described.51 Isolated glomeruli were lysed in 0.5% CHAPS buffer. Samples were resolved on SDS-polyacrylamide gels. Proteins from the gels were transferred to membranes, and then, the membranes were blocked with 5% nonfat milk and incubated with the appropriate primary antibodies.
Statistical Analyses
All statistical analyses were performed using GraphPad Prism version 6.0 for Windows (GraphPad Software Inc., San Diego, CA). Data are represented as means±SEMs. Comparisons between groups were analyzed via a Mann–Whitney U test. Differences at P<0.05 were considered significant.
Human Kidney Specimens
Human kidney specimens were collected from kidney biopsies that were performed at the Juntendo University Hospital. This study was conducted according to the Declaration of Helsinki and approved by the Institutional Review Board of Juntendo University Hospital. Informed consent was obtained from all patients. We selected patients who developed nephrotic syndrome that was proven to be MCD (n=6) or FSGS (n=6). As control human samples, we used biopsy samples from the patients with minor glomerular abnormalities (n=5). The clinical data of the patients are presented in Supplemental Table 1.
Immunohistochemical studies were performed on formalin–fixed, paraffin–embedded human kidney specimens with the use of standard techniques as mentioned above. CD-positive dots were evaluated using ImageJ software by blind analysis.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Dr. Rin Asao, Dr. Eriko Tanaka, Dr. Yoshiko Hosoe-Nagai, Dr. Yu Sasaki, Ms. Terumi Shibata, Mr. Kanai Fumio, Mr. Junichi Nakamoto, Dr. Soichiro Kakuta, Ms. Kaori Takahashi, and Mr. Mitsutaka Yoshida (Juntendo University Graduate School of Medicine, Tokyo, Japan) for excellent technical assistance. We also thank the staff of the Laboratory of Molecular and Biochemical Research, Research Support Center, Juntendo University Graduate School of Medicine for excellent technical assistance.
This work was supported, in part, by Grant-in-Aid for Japan Society for the Promotion of Science (JSPS) Fellows 12268 (to K.Y.-N.); a project research grant from Juntendo University (to K.Y.-N.); the Ministry of Education, Culture, Sports, Science and Technology–Supported Program for the Strategic Research Foundation at Private Universities (to K.Y.-N.); Grant-in-Aid for Scientific Research (C) 23591201 (to K.A.); Challenging Exploratory Research Grant 26670431 (to K.A.); and Grant-in-Aid for Young Scientists (B) 24790856 (to M.T.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015040366/-/DCSupplemental.
References
- 1.Currie G, Delles C: Proteinuria and its relation to cardiovascular disease. Int J Nephrol Renovasc Dis 7: 13–24, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smoyer WE, Mundel P: Regulation of podocyte structure during the development of nephrotic syndrome. J Mol Med (Berl) 76: 172–183, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Wang W, Tzanidis A, Divjak M, Thomson NM, Stein-Oakley AN: Altered signaling and regulatory mechanisms of apoptosis in focal and segmental glomerulosclerosis. J Am Soc Nephrol 12: 1422–1433, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Kriz W, LeHir M: Pathways to nephron loss starting from glomerular diseases-insights from animal models. Kidney Int 67: 404–419, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Shankland SJ: The podocyte’s response to injury: Role in proteinuria and glomerulosclerosis. Kidney Int 69: 2131–2147, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Uchiyama Y, Waguri S, Sato N, Watanabe T, Ishido K, Kominami E: Cell and tissue distribution of lysosomal cysteine proteinases, cathepsins B, H, and L, and their biological roles. Acta Histochem Cytochem 27: 287–308, 1994 [Google Scholar]
- 7.Sever S, Altintas MM, Nankoe SR, Möller CC, Ko D, Wei C, Henderson J, del Re EC, Hsing L, Erickson A, Cohen CD, Kretzler M, Kerjaschki D, Rudensky A, Nikolic B, Reiser J: Proteolytic processing of dynamin by cytoplasmic cathepsin L is a mechanism for proteinuric kidney disease. J Clin Invest 117: 2095–2104, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reiser J, Oh J, Shirato I, Asanuma K, Hug A, Mundel TM, Honey K, Ishidoh K, Kominami E, Kreidberg JA, Tomino Y, Mundel P: Podocyte migration during nephrotic syndrome requires a coordinated interplay between cathepsin L and alpha3 integrin. J Biol Chem 279: 34827–34832, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Faul C, Donnelly M, Merscher-Gomez S, Chang YH, Franz S, Delfgaauw J, Chang JM, Choi HY, Campbell KN, Kim K, Reiser J, Mundel P: The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat Med 14: 931–938, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yaddanapudi S, Altintas MM, Kistler AD, Fernandez I, Möller CC, Wei C, Peev V, Flesche JB, Forst AL, Li J, Patrakka J, Xiao Z, Grahammer F, Schiffer M, Lohmüller T, Reinheckel T, Gu C, Huber TB, Ju W, Bitzer M, Rastaldi MP, Ruiz P, Tryggvason K, Shaw AS, Faul C, Sever S, Reiser J: CD2AP in mouse and human podocytes controls a proteolytic program that regulates cytoskeletal structure and cellular survival. J Clin Invest 121: 3965–3980, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barrett AJ, Kirschke H: Cathepsin B, cathepsin H, and cathepsin L. Methods Enzymol 80: 535–561, 1981 [DOI] [PubMed] [Google Scholar]
- 12.Saftig P, Hetman M, Schmahl W, Weber K, Heine L, Mossmann H, Köster A, Hess B, Evers M, von Figura K, Peters C: Mice deficient for the lysosomal proteinase cathepsin D exhibit progressive atrophy of the intestinal mucosa and profound destruction of lymphoid cells. EMBO J 14: 3599–3608, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koike M, Nakanishi H, Saftig P, Ezaki J, Isahara K, Ohsawa Y, Schulz-Schaeffer W, Watanabe T, Waguri S, Kametaka S, Shibata M, Yamamoto K, Kominami E, Peters C, von Figura K, Uchiyama Y: Cathepsin D deficiency induces lysosomal storage with ceroid lipofuscin in mouse CNS neurons. J Neurosci 20: 6898–6906, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koike M, Shibata M, Waguri S, Yoshimura K, Tanida I, Kominami E, Gotow T, Peters C, von Figura K, Mizushima N, Saftig P, Uchiyama Y: Participation of autophagy in storage of lysosomes in neurons from mouse models of neuronal ceroid-lipofuscinoses (Batten disease). Am J Pathol 167: 1713–1728, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yokota S, Tsuji H, Kato K: Immunocytochemical localization of cathepsin D in lysosomes of cortical collecting tubule cells of the rat kidney. J Histochem Cytochem 33: 191–200, 1985 [DOI] [PubMed] [Google Scholar]
- 16.Baricos WH, Shah SV: Increased cathepsin D-like activity in cortex, tubules, and glomeruli isolated from rats with experimental nephrotic syndrome. Biochem J 223: 393–399, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asanuma K, Kim K, Oh J, Giardino L, Chabanis S, Faul C, Reiser J, Mundel P: Synaptopodin regulates the actin-bundling activity of alpha-actinin in an isoform-specific manner. J Clin Invest 115: 1188–1198, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grahammer F, Schell C, Huber TB: The podocyte slit diaphragm--from a thin grey line to a complex signalling hub. Nat Rev Nephrol 9: 587–598, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Hartleben B, Gödel M, Meyer-Schwesinger C, Liu S, Ulrich T, Köbler S, Wiech T, Grahammer F, Arnold SJ, Lindenmeyer MT, Cohen CD, Pavenstädt H, Kerjaschki D, Mizushima N, Shaw AS, Walz G, Huber TB: Autophagy influences glomerular disease susceptibility and maintains podocyte homeostasis in aging mice. J Clin Invest 120: 1084–1096, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cinà DP, Onay T, Paltoo A, Li C, Maezawa Y, De Arteaga J, Jurisicova A, Quaggin SE: Inhibition of MTOR disrupts autophagic flux in podocytes. J Am Soc Nephrol 23: 412–420, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oshima Y, Kinouchi K, Ichihara A, Sakoda M, Kurauchi-Mito A, Bokuda K, Narita T, Kurosawa H, Sun-Wada GH, Wada Y, Yamada T, Takemoto M, Saleem MA, Quaggin SE, Itoh H: Prorenin receptor is essential for normal podocyte structure and function. J Am Soc Nephrol 22: 2203–2212, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riediger F, Quack I, Qadri F, Hartleben B, Park JK, Potthoff SA, Sohn D, Sihn G, Rousselle A, Fokuhl V, Maschke U, Purfürst B, Schneider W, Rump LC, Luft FC, Dechend R, Bader M, Huber TB, Nguyen G, Muller DN: Prorenin receptor is essential for podocyte autophagy and survival. J Am Soc Nephrol 22: 2193–2202, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen J, Chen MX, Fogo AB, Harris RC, Chen JK: mVps34 deletion in podocytes causes glomerulosclerosis by disrupting intracellular vesicle trafficking. J Am Soc Nephrol 24: 198–207, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bechtel W, Helmstädter M, Balica J, Hartleben B, Kiefer B, Hrnjic F, Schell C, Kretz O, Liu S, Geist F, Kerjaschki D, Walz G, Huber TB: Vps34 deficiency reveals the importance of endocytosis for podocyte homeostasis. J Am Soc Nephrol 24: 727–743, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gassler N, Elger M, Kränzlin B, Kriz W, Gretz N, Hähnel B, Hosser H, Hartmann I: Podocyte injury underlies the progression of focal segmental glomerulosclerosis in the fa/fa Zucker rat. Kidney Int 60: 106–116, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Kim YH, Goyal M, Kurnit D, Wharram B, Wiggins J, Holzman L, Kershaw D, Wiggins R: Podocyte depletion and glomerulosclerosis have a direct relationship in the PAN-treated rat. Kidney Int 60: 957–968, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Macconi D, Bonomelli M, Benigni A, Plati T, Sangalli F, Longaretti L, Conti S, Kawachi H, Hill P, Remuzzi G, Remuzzi A: Pathophysiologic implications of reduced podocyte number in a rat model of progressive glomerular injury. Am J Pathol 168: 42–54, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Asanuma K, Akiba-Takagi M, Kodama F, Asao R, Nagai Y, Lydia A, Fukuda H, Tanaka E, Shibata T, Takahara H, Hidaka T, Asanuma E, Kominami E, Ueno T, Tomino Y: Dendrin location in podocytes is associated with disease progression in animal and human glomerulopathy. Am J Nephrol 33: 537–549, 2011 [DOI] [PubMed] [Google Scholar]
- 29.Mundel P, Shankland SJ: Podocyte biology and response to injury. J Am Soc Nephrol 13: 3005–3015, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Shacka JJ: Mouse models of neuronal ceroid lipofuscinoses: Useful pre-clinical tools to delineate disease pathophysiology and validate therapeutics. Brain Res Bull 88: 43–57, 2012 [DOI] [PubMed] [Google Scholar]
- 31.Soda K, Balkin DM, Ferguson SM, Paradise S, Milosevic I, Giovedi S, Volpicelli-Daley L, Tian X, Wu Y, Ma H, Son SH, Zheng R, Moeckel G, Cremona O, Holzman LB, De Camilli P, Ishibe S: Role of dynamin, synaptojanin, and endophilin in podocyte foot processes. J Clin Invest 122: 4401–4411, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benes P, Vetvicka V, Fusek M: Cathepsin D--many functions of one aspartic protease. Crit Rev Oncol Hematol 68: 12–28, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou W, Scott SA, Shelton SB, Crutcher KA: Cathepsin D-mediated proteolysis of apolipoprotein E: Possible role in Alzheimer’s disease. Neuroscience 143: 689–701, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Haidar B, Kiss RS, Sarov-Blat L, Brunet R, Harder C, McPherson R, Marcel YL: Cathepsin D, a lysosomal protease, regulates ABCA1-mediated lipid efflux. J Biol Chem 281: 39971–39981, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Reid WA, Valler MJ, Kay J: Immunolocalization of cathepsin D in normal and neoplastic human tissues. J Clin Pathol 39: 1323–1330, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siintola E, Partanen S, Strömme P, Haapanen A, Haltia M, Maehlen J, Lehesjoki AE, Tyynelä J: Cathepsin D deficiency underlies congenital human neuronal ceroid-lipofuscinosis. Brain 129: 1438–1445, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Nielsen R, Mollet G, Esquivel EL, Weyer K, Nielsen PK, Antignac C, Christensen EI: Increased lysosomal proteolysis counteracts protein accumulation in the proximal tubule during focal segmental glomerulosclerosis. Kidney Int 84: 902–910, 2013 [DOI] [PubMed] [Google Scholar]
- 38.Twomey C, McCarthy JV: Pathways of apoptosis and importance in development. J Cell Mol Med 9: 345–359, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koike M, Shibata M, Ohsawa Y, Nakanishi H, Koga T, Kametaka S, Waguri S, Momoi T, Kominami E, Peters C, Figura K, Saftig P, Uchiyama Y: Involvement of two different cell death pathways in retinal atrophy of cathepsin D-deficient mice. Mol Cell Neurosci 22: 146–161, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Nakanishi H, Zhang J, Koike M, Nishioku T, Okamoto Y, Kominami E, von Figura K, Peters C, Yamamoto K, Saftig P, Uchiyama Y: Involvement of nitric oxide released from microglia-macrophages in pathological changes of cathepsin D-deficient mice. J Neurosci 21: 7526–7533, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shacka JJ, Klocke BJ, Young C, Shibata M, Olney JW, Uchiyama Y, Saftig P, Roth KA: Cathepsin D deficiency induces persistent neurodegeneration in the absence of Bax-dependent apoptosis. J Neurosci 27: 2081–2090, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pavenstädt H, Kriz W, Kretzler M: Cell biology of the glomerular podocyte. Physiol Rev 83: 253–307, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Soda K, Ishibe S: The function of endocytosis in podocytes. Curr Opin Nephrol Hypertens 22: 432–438, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zeng C, Fan Y, Wu J, Shi S, Chen Z, Zhong Y, Zhang C, Zen K, Liu Z: Podocyte autophagic activity plays a protective role in renal injury and delays the progression of podocytopathies. J Pathol 234: 203–213, 2014 [DOI] [PubMed] [Google Scholar]
- 45.Surendran K, Vitiello SP, Pearce DA: Lysosome dysfunction in the pathogenesis of kidney diseases. Pediatr Nephrol 29: 2253–2261, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steinfeld R, Reinhardt K, Schreiber K, Hillebrand M, Kraetzner R, Bruck W, Saftig P, Gartner J: Cathepsin D deficiency is associated with a human neurodegenerative disorder. Am J Hum Genet 78: 988–998, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hartleben B, Wanner N, Huber TB: Autophagy in glomerular health and disease. Semin Nephrol 34: 42–52, 2014 [DOI] [PubMed] [Google Scholar]
- 48.Moeller MJ, Sanden SK, Soofi A, Wiggins RC, Holzman LB: Podocyte-specific expression of cre recombinase in transgenic mice. Genesis 35: 39–42, 2003 [DOI] [PubMed] [Google Scholar]
- 49.Tanimoto M, Gohda T, Kaneko S, Hagiwara S, Murakoshi M, Aoki T, Yamada K, Ito T, Matsumoto M, Horikoshi S, Tomino Y: Effect of pyridoxamine (K-163), an inhibitor of advanced glycation end products, on type 2 diabetic nephropathy in KK-A(y)/Ta mice. Metabolism 56: 160–167, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Schwarz K, Simons M, Reiser J, Saleem MA, Faul C, Kriz W, Shaw AS, Holzman LB, Mundel P: Podocin, a raft-associated component of the glomerular slit diaphragm, interacts with CD2AP and nephrin. J Clin Invest 108: 1621–1629, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ueno T, Kobayashi N, Nakayama M, Takashima Y, Ohse T, Pastan I, Pippin JW, Shankland SJ, Uesugi N, Matsusaka T, Nagata M: Aberrant Notch1-dependent effects on glomerular parietal epithelial cells promotes collapsing focal segmental glomerulosclerosis with progressive podocyte loss. Kidney Int 83: 1065–1075, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.