Abstract
The mechanisms by which the glomerular filtration barrier prevents the loss of large macromolecules and simultaneously, maintains the filter remain poorly understood. Recent studies proposed that podocytes have an active role in both the endocytosis of filtered macromolecules and the maintenance of the filtration barrier. Deletion of a key endosomal trafficking regulator, the class 3 phosphatidylinositol (PtdIns) 3-kinase vacuolar protein sorting 34 (Vps34), in podocytes results in aberrant endosomal membrane morphology and podocyte dysfunction. We recently showed that the vacuolation phenotype in cultured Vps34–deficient podocytes is caused by the absence of a substrate for the Vps34 downstream effector PtdIns 3-phosphate 5-kinase (PIKfyve), which phosphorylates Vps34-generated PtdIns(3)P to produce PtdIns (3,5)P2. PIKfyve perturbation and PtdIns(3,5)P2 reduction result in massive membrane vacuolation along the endosomal system, but the cell-specific functions of PIKfyve in vivo remain unclear. We show here that the genetic deletion of PIKfyve in endocytically active proximal tubular cells resulted in the development of large cytoplasmic vacuoles caused by arrested endocytic traffic progression at a late-endosome stage. In contrast, deletion of PIKfyve in glomerular podocytes did not significantly alter the endosomal morphology, even in age 18-month-old mice. However, on culturing, the PIKfyve-deleted podocytes developed massive cytoplasmic vacuoles. In summary, these data suggest that glomerular podocytes and proximal tubules have different requirements for PIKfyve function, likely related to distinct in vivo needs for endocytic flux.
Keywords: podocyte, glomerulus, proteinuria
Albuminuria (proteinuria) is one of the hallmarks of proteinuric kidney disease and strongly and independently associated with kidney disease progression and cardiovascular events.1–3 The amount of albumin that gets across the healthy filtration barrier remains controversial.4–6 Studies have shown that proximal tubular cells can reabsorb albumin through receptor-mediated endocytosis.5–10 The neonatal Fc receptor (FcRn) located at the apical brush border of proximal tubular cells has been implicated in the reabsorption of albumin and Igs (IgG).7–9 Podocytes also express the FcRn receptor; glomeruli of mice lacking the FcRn receptor in the podocytes had delayed clearance and accumulation of IgG in tracer studies.9 More recently, podocyte uptake of Evans Blue–labeled albumin was observed in a puromycin aminonucleoside injury model by transcytosis, a well characterized process for moving materials from one cell membrane domain to another.10 However, many of the studies showing the ability of podocytes to endocytose labeled albumin or IgG have been performed in vitro11–14 (i.e., in isolated cultured podocytes) and may not truly mirror the events occurring in vivo. Human diseases and mouse models with disruption of the filtration barrier resulting in large amounts of protein leak are not ideally suited to elucidate the role of endocytosis in podocyte homeostasis, because the behavior of podocytes is likely altered.
Cargo internalization is an early endocytic event that occurs at the plasma membrane where extracellular material along with fragments of the plasma membrane form membrane-bound vesicles. Endocytosis is important for the cell to acquire nutrients, mount a response to antigens and pathogens, and regulate signaling that originates at the cell membrane. Similar to other cell types, endocytic membrane trafficking is a critical cellular mechanism that is required for podocyte homeostasis. Two recent studies investigated the consequence of perturbing endosomal trafficking in podocytes by deleting the phosphatidylinositol (PtdIns) 3-kinase, vacuolar protein sorting 34 (Vps34), in a podocyte-specific manner.15,16 Podocytes lacking Vps34 developed large cytoplasmic vacuoles and proteinuria, resulting in death at 3–9 weeks of age. Vps34 is a highly conserved class 3 PtdIns 3-kinase that phosphorylates the 3-OH inositol-head group to generate phosphatidylinositol-3-phosphate [PtdIns(3)P]. As part of distinct multiprotein complexes, Vps34-generated PtdIns(3)P is necessary for transition of early endosomes to late endosomes as well as autophagosome maturation.17–20
In our recent study, we showed that the Vps34 downstream effector phosphatidylinositol 3-phosphate 5-kinase (PIKfyve) is responsible for the vacuolation phenotype observed in Vps34-deleted podocytes in culture.21 Replenishment of Vps34 in cultured Vps34–deficient cells did rescue the vacuolation phenotype but only in the presence of intact PIKfyve activity.21 PIKfyve is an Fyve (Fab1p, YOTB, Vac1p, and early endosome antigen 1 [EEA1]) finger-containing phosphoinositide (PI) kinase that phosphorylates PtdIns and Vps34-generated PtdIns(3)P on the 5-hyrdroxyl position to make the low–abundance signaling lipids phosphatidylinositol-5-phosphate [PtdIns(5)P]and phosphatidylinositol-3,5-phosphate 2 [PtdIns(3,5)P2], respectively.22–24 In contrast to PtdIns(3)P, which is found in early endosomal compartments, PtdIns(3,5)P2 is thought to predominate in late endosomes and/or lysosomes.25–28 Similar to Vps34, perturbations in PIKfyve–catalyzed PtdIns(3,5)P2 synthesis lead to several defects, including a dramatic enlargement of endosomal/lysosomal membranes.23,29–31 To study the role of PIKfyve in vivo, we generated two mouse models, where PIKfyve is deleted in either podocytes or proximal tubular epithelial cells. To our surprise, deletion of PIKfyve in podocytes resulted in a normal phenotype, even when mice were ages ≤18 months old. In contrast, deletion of PIKfyve in highly endocytically active proximal tubular cells resulted in the development of large vacuoles. We also compared the podocyte-specific deletion of Vps34 and PIKfyve to better understand the dramatically different phenotypes. The data presented in this study suggest that the observed phenotype of Vps34 deletion in glomerular podocytes cannot be explained solely by a primary defect in endosomal membrane trafficking. Furthermore, lack of a marked morphologic phenotype in PIKfyve–deleted glomerular podocytes compared with proximal tubular cells suggests that healthy podocytes may have low rates of endocytic flux.
Results
Conditional Deletion of PIKfyve in Podocytes Results in a Normal Renal Phenotype
To study the podocyte-specific function of PIKfyve in vivo, we crossed the previously described PIKfyvefl/fl homozygous mice with Nphs2-Cre mice and generated mice that have PIKfyve deletion in podocytes (PIKfyvepodko).32,33 PIKfyvepodko mice were born in a normal Mendelian distribution and normal at birth. However, to our surprise, glomeruli of PIKfyvepodko mice were structurally similar to their littermate controls at both birth and 18 months of age. Transmission electron microscopy (EM) images showed a normal foot process structure and glomerular basement membrane and only the occasional presence of vacuoles (Figure 1A). The foot process width measured by the slit diaphragm frequency was also similar between PIKfyvepodko mice and their littermate controls (Figure 1B). Likewise, urine albumin excretion was similar in PIKfyvepodko mice compared with littermate controls (Figure 1, C and D).
Figure 1.
Conditional PIKfyve deletion in mouse podocytes results in normal phenotype. (A, top panel) Hematoxylin and eosin staining of paraffin–embedded mouse kidney sections from PIKfyvefl/fl,Nphs2-Cre− (control) and PIKfyvefl/fl,Nphs2-Cre+ (PIKfyvepodko) mice. (A, middle and bottom panels) Transmission electron micrographs of kidneys from control and PIKfyvepodko mice show a normal podocyte foot process architecture and the absence of vacuoles in the PIKfyvepodko podocytes. (B) Number of junctions per micrometer of glomerular basement membrane (GBM) surface as seen by transmission EM. Data are means±SEMs.(C) Urine collected from PIKfyvepodkomice and their age–matched littermate controls was resolved using SDS-PAGE. BSA standards were run for comparison. Results shown are representative of five or more 12-month-old mice per group. (D) Urine albumin-to-creatinine ratio in PIKfyvepodko and age–matched littermate control mice as measured by ELISA. Error bars are SEMs.
Confirmation of PIKfyve Gene Deletion in Glomerular Podocytes
Because of the lack of a phenotype, several additional studies were performed to confirm deletion of PIKfyve in podocytes. We first used the mTmG Cre-reporter mouse34 to visually confirm expression of Cre-recombinase. Nphs2-Cre mice were bred with the mTmGfl/fl mouse to generate mTmGfl/fl/Nphs2-Cre mice. Successful recombination was confirmed by the robust green fluorescence in podocytes (Figure 2A). Nphs2-Cre, mTmGfl/fl mice were subsequently bred with PIKfyvefl/fl mice to generate podocyte-specific deletion of PIKfyve. In addition, deletion of PIKfyve was confirmed at both the message (mRNA) and protein levels, and green fluorescent protein (GFP) -expressing podocytes were isolated from mTmG/PIKfyvefl/fl,Nphs2-Cre+ and non–Cre-expressing littermate mice using flow cytometry. cDNA generated by reverse transcription shows the change in size of the PCR product using primers on either side of exon 6 (Figure 2B). The PCR product was further sequenced to confirm the deletion of the floxed segment (Supplemental Figure 1). The deletion of PIKfyve mRNA transcript was further confirmed by insitu hybridization using fluorescence–labeled RNA probes complimentary to exon 6 of PIKfyve. Kidney sections were stained with podocin antibody to visualize the glomerular tuft. The cells on the periphery of the glomerular tuft in the wild–type kidney sections show presence of fluorescent dots (Figure 2C, upper panel). Similar dots are not seen in PIKfyve-deleted podocytes (Figure 2C, lower panel), indicating successful deletion of exon 6 after Cre-mediated recombination. We also confirmed the absence of PIKfyve protein by Western blot analyses with a C–terminal PIKfyve antibody subsequent to cell sorting from isolated glomeruli (Figure 2D). Podocytes transfected with a construct encoding PIKfyve were used as a positive control. We repeatedly documented approximately 90% reduction in the PIKfyve immunoreactive band in podocytes collected from PIKfyvefl/fl,Nphs2-Cre+ versus PIKfyvefl/fl,Nphs2-Cre− glomeruli (Figure 2D).
Figure 2.
Deletion of PIKfyve in mouse podocytes. (A) Immunofluorescence images of frozen kidney section showing green fluorescence exclusively in the glomerulus in a podocyte-specific distribution when the mTmG Cre-reporter mouse was bred with the Nphs2-Cre mouse. (B) Deletion of the floxed segment was confirmed by reverse transcription of mRNA extracted from podocytes isolated by flow cytometry. PCR amplification with primers (arrows) flanking the floxed segment shows a change in the size of the PCR product by the predicted 172 bp. Depicted is the position of the primers along the PIKfyve gene (arrows). The box shows the amplified section of the gene. The length of the sequence between the loxP sites is 150 bp. (C) In situ hybridization using RNA scope probes against exon 6 of PIKfyve. Each individual dot indicates an mRNA strand. (C, upper panel) There are fluorescent dots in the cells on the periphery of the glomerular tuft in wild-type mouse (arrowheads). C, lower panel shows absence of dots in the cell periphery. Podocin was used to label the podocytes. (D) Lysates from wild-type podocytes (lane 1) and podocytes lacking PIKfyve (lane 2) were resolved using SDS-PAGE and blotted with antibody against the C terminus of PIKfyve. Lysates from HEK293 cells transfected with GFP-tagged PIKfyve were used as positive control. Quantification of band densities using Unscan presented next to the blot. Error bars are SEMs (n=4). Twelve-month-old mice were used in these experiments.
Pikfyve-Deleted Podocytes in Culture Readily Develop Translucent Vacuoles
In a second approach for confirming functional deletion of PIKfyve, we examined if both isolated and immortalized PIKfyve–deleted podocytes derived from the mTmGfl/fl, PIKfyvefl/fl,Cre+ mice could form cytoplasmic vacuoles in vitro. Reportedly, in rare cases, there might be a discrepancy between data obtained by the Cre reporter and the strength of the gene deletion.35 Formation of translucent cytoplasmic vacuoles is a proven sensitive assay for perturbed PIKfyve function and hence, reduced PtdIns(3,5)P2 levels as observed in a number of dividing cells, including podocytes in culture.21,25 Isolated glomeruli from the mTmGfl/fl, PIKfyvefl/fl,Cre+ mice were plated on culture dishes. At day 0, there was expression of both red and green fluorescence in the glomeruli (Figure 3A). Intriguingly, however, the GFP-expressing cells that spread outward from the glomeruli show development of large vacuoles by days 5–8. In contrast, endothelial and mesangial cells that did not undergo Cre-mediated recombination and express red fluorescence lacked such vacuolation morphology. We also observed vacuolization after PIKfyve deletion in nondividing immortalized podocyte cells that were allowed to differentiate at 37°C for 14 days (Supplemental Figure 2). These results are consistent with the conclusion that PIKfyve deletion has occurred in the glomeruli in vivo.
Figure 3.
PIKfyve-deleted podocytes develop vacuoles in vitro. (A) Glomeruli isolated from 6-month-old mTmG/PIKfyvefl/fl,Nphs2-Cre mouse kidneys develop large vacuoles by days 6–8 in culture. (B) Quantitation by HPLC from four independent experiments calculated as a percentage of the total PI radioactivity and presented as means±SEMs. The total PI radioactivity was obtained by summing the counts within the elution times corresponding to the individual [3H]GroPIns peaks as detailed in Concise Methods. The changes in PtdIns(3,5)P2, PtdIns(5)P, and PtdIns(3)P in PIKfyvefl/fl,Cre− versus PIKfyvefl/fl,Cre+ were statistically significant. *P<0.01 using paired t test analysis.
Levels of PtdIns(3,5)P2, PtdIns(5)P, and PtdIns(3)P in Cultured PIKfyve Knockout Podocytes
Cell studies have previously revealed that aberrant cytoplasmic vacuoles are formed after significant reduction of PtdIns(3,5)P2 levels (>60%)32 and only in dividing but not in terminally differentiated cells.26,36 Measurement of PIsusing HPLC in cultured cells expressing green fluorescence (mTmG/PIKfyvefl/fl,Nphs2-Cre+) after cell sorting by FACS is shown in Figure 3B. Although both PtdIns(3,5)P2 and PtdIns(5)P lipid products significantly decreased in PIKfyve-deleted cells, they were not completely absent. There was a statistically significant increase in total PtdIns(3)P levels in PIKfyve-deleted podocytes, suggesting accumulation of PtdIns(3)P in the absence of PIKfyve. These findings argue for a possible persistence of small amounts of PtdIns(3,5)P2 in the podocytes in vivo.
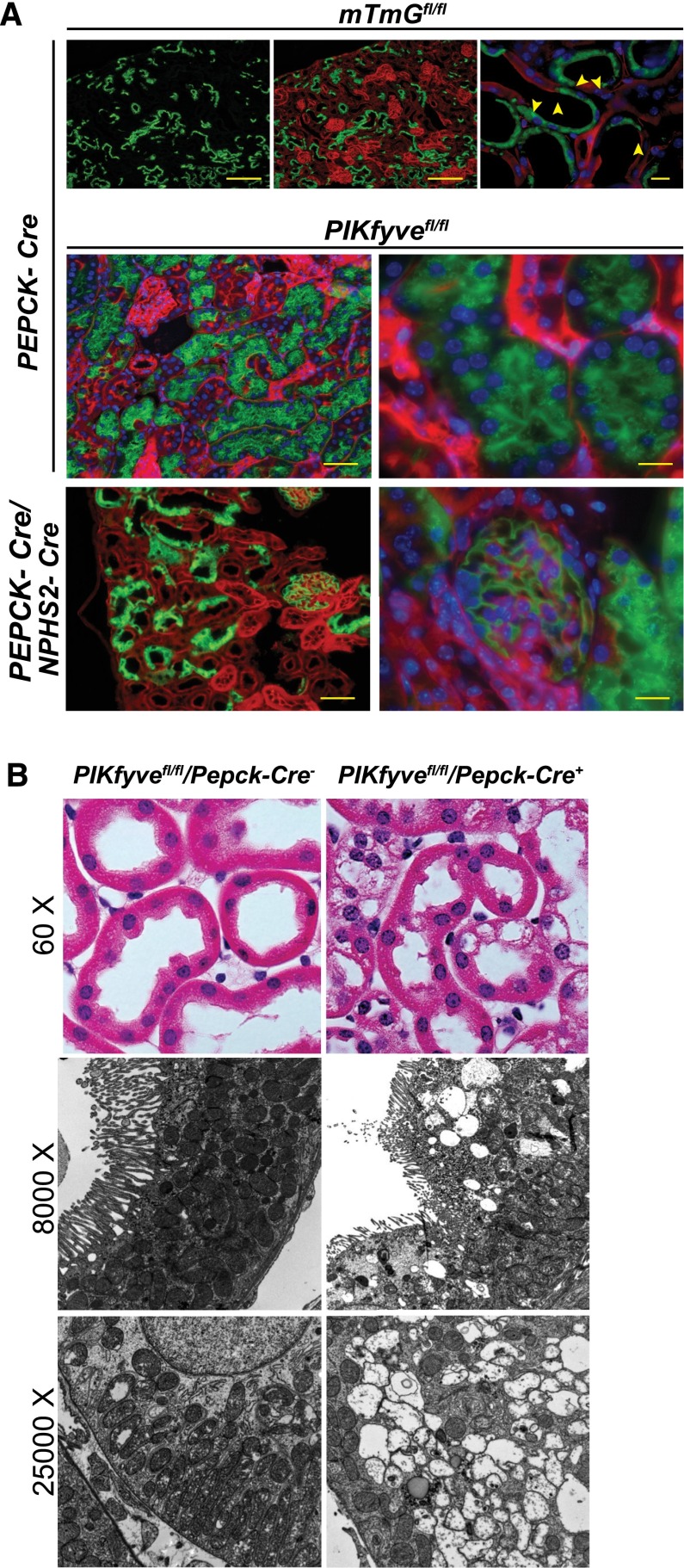
Proximal Tubular Cells Deleted of PIKfyve Robustly Develop Large Translucent Vacuoles
It is possible that persistence of low levels of PIKfyve gene function after deletion is sufficient to sustain podocyte homeostasis. We hypothesized that deletion of PIKfyve in a cell that is highly endocytically active would result in development of vacuoles, even if low levels of PIKfyve activity persisted. Kidney proximal tubular cells are highly active in reabsorption of proteins, electrolytes, and glucose from the ultrafiltrate generated by the glomerulus. To delete PIKfyve in the proximal tubules, we initially crossed PEPCK-Cre mice with the mTmG Cre-reporter mouse to generate PEPCK-Cre, mTmG mice. PEPCK-Cre–driven recombination resulted in a mosaic pattern (Figure 4A, top panel), because the PEPCK promoter is inserted as a single-copy transgene on the X chromosome.37 PEPCK-Cre, mTmG mice were then crossed with PIKfyvefl/fl mice to generate proximal tubule–specific PIKfyve deletion. The mice were born in normal Mendelian distribution and normal at birth. However, we observed large vacuoles in the enhanced green fluorescent protein (EGFP) –expressing proximal epithelial cells indicative of successful Cre–driven recombination (Figure 4A, middle panel). Presence of vacuoles was also confirmed by histology and transmission electron microscopy (Figure 4B). The absence of vacuolation in the podocytes is illustrated by simultaneous deletion of PIKfyve in glomeruli and proximal tubules (Figure 4A, bottom panel). Insitu hybridization using fluorescence–labeled cRNA probes for PIKfyve exon 6 shows an absence of the PIKfyve mRNA in proximal tubular cells with high expression of Lamp1 (Supplemental Figure 3A). Tissue processing for insitu hybridization resulted in loss of the GFP-emitted fluorescence. To circumvent this obstacle, we used Lamp1 as a marker of PIKfyve deletion. In a separate experiment, we were able to show increased Lamp1 staining in proximal tubular cells that have GFP expression in frozen kidney tissue (Supplemental Figure 3B).
Figure 4.
PIKfyve deletion in mouse proximal tubular epithelial cells triggers formation of large cytoplasmic vacuoles. (A, top panel) Immunofluorescence images of frozen kidney sections showing expression of green fluorescence in proximal tubules when the mTmGfl/fl mouse was bred with the PEPCK-Cre mouse. There is a mosaic pattern of GFP expression in the proximal tubules (arrowheads). (A, middle panel) Numerous vacuoles are seen in the cytoplasm of proximal tubular cells expressing GFP. Frozen kidney sections of a 2-month-old mTmG/PIKfyvefl/fl mouse carrying both Nphs2-Cre and PEPCK-Cre (double knockout) show expression of GFP in both podocytes and proximal tubular cells. (A, bottom panel) There are numerous vacuoles in the proximal epithelial cells adjacent to a glomerulus with normal-appearing podocytes. (B, top panel) Hematoxylin and eosin–stained paraffin–embedded kidney section showing presence of large vacuoles in the PIKfyve–deleted proximal epithelial cells. (B, middle and bottom panels) Transmission EM images confirm the presence of large vacuoles in the cytoplasm of the proximal epithelial cells deleted of PIKfyve.
Membrane Localization of Podocyte Junctional Protein Nephrin Is Aberrant in Vps34–Deleted Glomerular Podocytes
As an initial characterization of the Vps34- and PIKfyve-deleted podocytes, we compared the targeting of junctional proteins, like nephrin and zona occludens-1 (ZO-1), with the podocyte-intercellular junction. Staining of kidney sections from 4-week-old Vps34podko mice revealed loss of the linear staining for nephrin compared with both PIKfyve–deleted and age–matched littermate control wild–type mice (Figure 5A). Interestingly, at 4 weeks of age, staining for ZO-1 and synaptopodin in Vps34-deleted podocytes was similar to controls. Electron microscopy using immunogold labeling showed nephrin in vesicles in Vps34–deleted mouse podocytes (Figure 5B), suggesting either improper targeting of newly synthesized nephrin from the trans-Golginetwork (TGN) or nephrin removed from the membrane by endocytosis. PIKfyve–deleted mouse podocytes showed a decrease in ZO-1 staining compared with age–matched littermate controls (Figure 5A). After calcium switch, in vitro return of tight junction proteins, like claudin and ZO-1, is affected by the presence of PIKfyve inhibitor, suggesting a role of PIKfyve in trafficking of these proteins to the plasma membrane.38 However, decreases in ZO-1 levels did not seem to affect the integrity of the podocyte intercellular junction as evidenced by the fact that PIKfyvepodko mice did not become proteinuric with age.
Figure 5.
Vesicular localization of nephrin in Vps34–deleted mouse podocytes. (A) Indirect immunofluorescence staining of paraffin–embedded 4-week-old mouse kidney sections from Vps34podko shows that nephrin is present largely in vesicles (arrowheads). Nephrin staining in PIKfyvepodko mice is similar to that of the littermate controls. Staining for synaptopodin and ZO-1 is similar in both Vps34podko and PIKfyvepodko and their littermate control kidneys. Scale bars, 50μm. (B) Immunogold transmission EM shows presence of nephrin in vesicles (arrowheads) in Vps34podko mouse podocytes. Images were photographed at ×5800 and cropped to emphasize the presence of immunogold particles in vesicles.
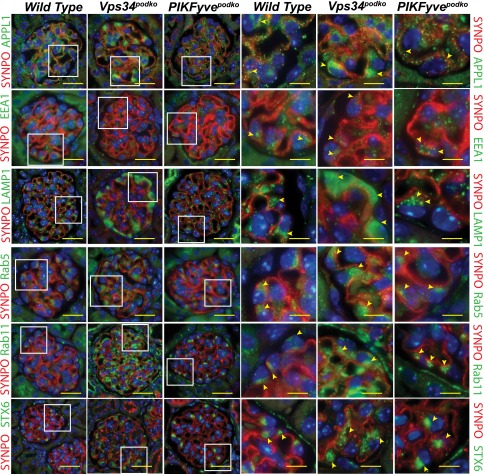
Endosome and TGN Morphology of Glomerular Podocytes Is Markedly Altered in Vps34-Deleted Podocytes but Not Markedly Altered in PIKfyve-Deleted Podocytes
To compare the consequences of the absence of PIKfyve or Vps34, we stained kidney sections from adult mice with a battery of vesicular markers. A schematic diagram of the endocytic compartments, their characteristic markers, and the direction of the vesicle trafficking is shown in Supplemental Figure 4A.39 Staining for APPL1 adaptor protein shows increased staining in podocytes deleted of Vps34 (Figure 6, row 1). APPL endosomes represent an early endocytic intermediate and are precursors of the classic PtdIns(3)P-containing endosomes, and Vps34 plays a critical role in this conversion.40 Staining for EEA1 showed a reduction in EEA1 staining in podocytes lacking Vps34 at the earlier time point of 4-week-old mice (Figure 6, row 2). EEA1 contains an Fyve finger domain that interacts with PtdIns(3)P, resulting in enrichment of EEA1 at the vesicular membrane. Lysosomal–associated membrane protein Lamp1 has been traditionally used as a lysosomal marker, but it is also found in late endosomes and lysosomal precursor vesicles being trafficked from the TGN.41–43 Lamp1 staining was robust and diffuse in podocytes lacking Vps34 (Figure 6, row 3). In wild-type and PIKfyve-deleted podocytes, the staining is similar and punctate, suggesting its presence in vesicles or lysosomes.
Figure 6.
Membrane trafficking and endocytic flux from Golgi is impaired in Vps34–deleted mouse podocytes. Immunofluorescence staining of kidney sections from 4-week-old Vps34–deleted and 6-month-old PIKfyve–deleted mouse podocytes for various vesicular markers. There is increased staining for Lamp1, APPL1 Rab5, Rab11, and Syntaxin-6. Rab11 and Syntaxin-6are markers of vesicles that originate from the Golgi. Staining in PIKfyve-deleted podocytes for these markers is similar to that in wild-type podocytes. Rows 4–6 are enlarged images of the boxed areas in rows 1–3. Arrowheads point to the podocytes at the periphery of the glomerulus. Scale bars, 20 μmin rows 1–3; 5 μm in rows 4–6.
Other than its role in endosome fusion, small GTPase Rab5 orchestrates early or sorting endosome dynamics. Switch of expression from Rab5 to Rab7 marks the transition to late endosomes and attainment of the capability to fuse with lysosomes.44 Rab5 expression is increased in the podocytes that lack Vps34 compared with wild-type and PIKfyve-deleted podocytes (Figure 6, row 4). The increase in expression of Lamp1 when there is failure of maturation to late endosomes would argue for a different source of Lamp1-positive vesicles, like the TGN. To examine the TGN, we monitored two proteins that are enriched in the Golgi apparatus (namely, Rab11 and Syntaxin-6). Rab11 has been localized at the TGN, post-Golgi vesicles, and recycling endosomes, placing it at the intersection between the endocytic and exocytic trafficking pathways.45,46 Rab11 localizes to the apical recycling endosomes and plays a role in apicobasal polarity, facilitating the delivery of appropriate proteins to the apical surface from the TGN.47 Vps34-deleted podocytes showed an increase in Rab11 staining (Figure 6, row 5), suggesting an arrest of trafficking of Rab11-positive vesicles. TGN marker Syntaxin-6 has an SNARE motif that drives the engine required for vesicular membrane fusion. Syntaxin-6 is implicated in TGN-endosome trafficking of lysosomal precursors.48 In wild-type and PIKfyve-deleted podocytes, Syntaxin-6 expression is limited to a small area adjacent to the nucleus in a pattern expected for the Golgi apparatus. In Vps34-deleted podocytes, Syntaxin-6 staining is diffuse within the cytoplasm (Figure 6, row 6), suggesting the possibility of aberrant trafficking from the Golgi apparatus in the absence of Vps34-generated PtdIns(3)P. Quantification of immunofluorescence using ImageJ (National Institutes of Health, Bethesda, MD) is shown in Supplemental 4B. Western blot analysis using cell lysates from isolated PIKfyve and Vps34-deleted podocytes confirms the observations made using immunohistochemistry on kidney sections (Supplemental Figure 5). Glomerular lysates were not used for this analysis, because there is contribution from mesangial as well as endothelial cells that made it difficult to assess changes occurring specifically in podocytes after deletion. In summary, these data suggest that there is significant contribution of the TGN to the accumulation of endocytic marker proteins in the Vps34-deleted podocytes. Consistent with the observed lack of gross morphologic abnormalities, our inspection of the endocytic structures with various markers in PIKfyve-deleted podocytes revealed that vesicular trafficking from the TGN is not significantly perturbed, likely related to the preserved and even augmented PtdIns(3)P levels.
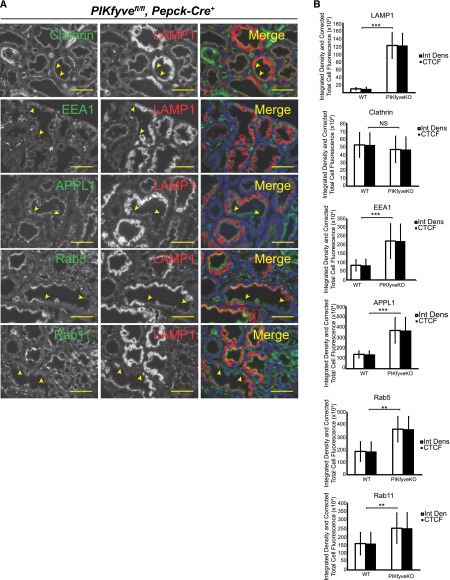
Accumulation of Late Endosomal Markers in PIKfyve–Deleted Proximal Tubular Cells
Because of the mosaic pattern of PEPCK-Cre–mediated deletion within the same tubules, there are regions where the cells do not have PIKfyve deletion (Figure 7A, regions between the arrowheads). Whereas the mosaic pattern prevented development of a phenotype in terms of tubular proteinuria or renal failure, it offered us control cells within the same kidney section. Staining for clathrin showed a linear pattern along the apical cell surface of the tubular cells in both normal and PIKfyve–deleted tubular cells (Figure 7A, row 1). This suggests the normal process of clathrin-mediated endocytosis as revealed in our previous cell studies with PIKfyve perturbation.49 By contrast, we observed accumulation of EEA1-, APPL1-, Rab5-, and Rab11-positive vesicles in the PIKfyve-deleted cells compared with normal cells (Figure 7A). Quantification of the immunofluorescence using ImageJ software (National Institutes of Health) confirms significant increase in accumulation of early, late, and recycling endosomes (Figure 7B). These data suggest that, in proximal tubular cells, the absence of PIKfyve results in the anticipated phenotype, where endosomal membrane trafficking is altered and resembles Vps34-deleted podocytes.
Figure 7.
Aberrant accumulation of endosomal markers in PIKfyve–deleted proximal tubular cells. (A) PIKfyve–deleted proximal tubular cells show increased staining for vesicular markers APPL1, EEA1, Rab5, Rab11, and Lamp1. Lamp1 staining is used to identify cells that have undergone Cre–mediated PIKfyve deletion because of the mosaic pattern of PEPCK promoter–driven Creexpression. Two-month-old mice were used for these experiments. Scale bars, 20 μm. (B) Quantification of the immunofluorescence in PIKfyve–deleted proximal tubular cells processed by ImageJ software. Data show significant accumulation of Lamp1 in proximal tubular epithelial cells. Lamp1 staining was used to double-stain cells that have deletion of PIKfyve. There is accumulation of Clathrin, EEA1, Rab5, and Rab11 in PIKfyve–deleted proximal tubular epithelial cells. Results are expressed as integrated density and corrected total cell fluorescence. Error bars are SEMs. WT, wild type. Scale bars, 20 μm. **P<0.01; ***P<0.001.
Autophagy Is Not Affected in PIKfyve-Deleted Podocytes and Proximal Tubular Cells
Autophagy is an important cellular process that has been shown to be important for podocyte homeostasis.50 Both Vps34 and PIKfyve have been shown to play a role in autophagic pathways.19,51–54 Kidney sections from the wild type, Vps34podko, and PIKfyve knockout (both podocyte and tubular) were immunostained for autophagy markers LC3 and p62 (Figure 8, A and C). Reportedly, the inhibition of autophagy correlates with increased level of p62, suggesting that steady-state levels of p62 reflect the autophagic status.55,56 Expression levels of both LC3 and p62 are higher in Vps34-deleted podocytes as revealed by quantification with ImageJ software (National Institutes of Health) (Figure 8, B and D). Western blot analysis using cell lysates from isolated Vps34– and PIKfyve–deleted podocytes shows similar increases in both LC3 and p62 after Vps34 deletion (Supplemental Figure 5). Interestingly, PIKfyve deletion in both podocytes and tubular cells does not affect expression of either LC3 or p62 (Figure 8D). These results suggest that a change in autophagic flux is likely to contribute toward the severe phenotype seen in Vps34podko mice.
Figure 8.
Deletion of PIKfyve in mouse podocytes and tubular cells does not affect autophagy markers LC3 and p62. (A) There is accumulation of autophagy marker LC3 in Vps34–deleted mouse podocytes indicative of altered autophagic flux. (B) Quantification of LC3 staining using ImageJ software in Vps34- and PIKfyve-deleted podocytes. (C) p62 Staining in wild-type podocytes, Vps34-deleted podocytes, PIKfyve-deleted podocytes, and PIKfyve–deleted proximal tubular cells. Note the increase of the p62 staining in Vps34-deleted podocytes (arrowheads in row 5). (D) Quantification of p62 staining using ImageJ software. Results are expressed as integrated density and corrected total cell fluorescence; 4-week-old Vps34 knockout mice and 3-month-old PIKfyve knockout mice were used in these experiments. Error bars are SEMs. KO, knockout; WT, wild type. Scale bars, 20 μm in A, upper panel and B; 5 μm in A, lower panel. ***P<0.001.
FITC–Labeled Albumin Uptake In Vivo
To assess the integrity of the filter in Vps34podko and PIKfyvepodko mice or the mice with simultaneous deletion of PIKfyve in podocytes and proximal tubular epithelial cells (i.e., PIKfyvefl/fl,Nphs2-Cre+/PEPCK-Cre+), we perfused the animals with FITC–labeled human albumin (FITC-halb). We used both 3- and 6-week-old Vps34podko mice for these experiments. The 3-week-old Vps34podko mice are not overtly proteinuric. Few glomeruli in the Vps34-deleted mouse that were vacuolated and unhealthy had significant amounts of FITC-halb leak, whereas there was no visible leak in the glomeruli that were completely healthy in appearance. Furthermore, the glomeruli from the Vps34podko mouse kidneys that had large amounts of leak did not show evidence of FITC-halb within the podocytes (Figure 9A). Rarely, a glomerulus from the 6-week-old Vps34podkomouse showed green droplets within the podocytes (Figure 9A, row 4 and inset). After perfusion at various time points, green fluorescence was visible along the glomerular basement membrane that was well demarcated by the synaptopodin staining (red in Figure 9B) on the urinary side (Figure 9B). Despite saturation with FITC-halb on the vascular side, we were unable to visualize FITC-halb uptake by the podocytes in kidney sections from the PIKfyvepodko mice. Quantification of the immunofluorescence in highly proteinuric 6-week-old Vps34podko and 6-month-old PIKfyvefl/fl,Nphs2-Cre+/PEPCK-Cre+ kidneys showed an increase in tubular uptake of the FITC-halb (Supplemental Figure 6). The PIKfyvefl/fl,Nphs2-Cre+/PEPCK-Cre+ dual–knockout mice that also have abnormality in membrane trafficking in tubular cells show accumulation of small amounts of FITC-halb that are present in the ultrafiltrate.
Figure 9.
Podocytes fail to accumulate FITC-halb. (A) Perfusion of 3-week-old kidneys of Vps34fl/fl,Nphs2-Cre+ mice and their littermate controls shows leak of FITC albumin in a few severely affected glomeruli (arrowheads). Accumulation of FITC-halb is not seen in wild–type or Vps34–deleted mouse podocytes. Even at 6 weeks of age, the Vps34–deleted mouse podocytes show only minimal accumulation of FITC-halb in rare glomeruli (row 4). Scale bars, 20 μm. (B) Perfusion time course of 12-month-old kidneys of PIKfyvefl/fl,Nphs2-Cre+mice and their littermate controls shows absence of FITC-halb in podocytes. B, right panel shows enlarged images of boxed area in B, left panel. Scale bars, 20 μm in B, left panel; 5 μm in B, right panel.
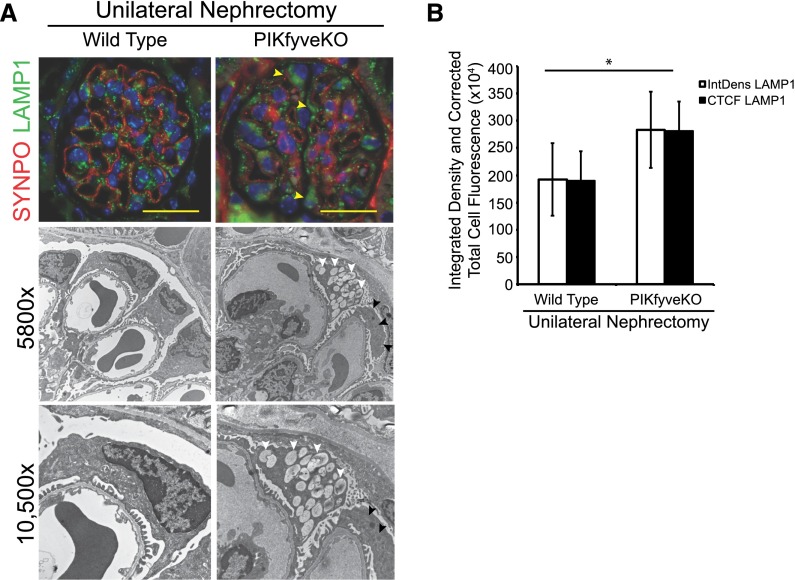
Some PIKfyve–Deleted Glomerular Podocytes Develop Vacuoles after Unilateral Nephrectomy
Numerous podocytes–specific gene deletion models develop a phenotype after stress. We hypothesized that PIKfyve-deleted podocytes under duress would develop vacuoles. We used the unilateral nephrectomy model, because in this model, the podocytes undergo hypertrophy and membrane expansion that require endosomal trafficking. After unilateral nephrectomy, the remnant kidney undergoes rapid enlargement in size, almost doubling in 4–5 days; 10-month-old PIKfyvepodko and age–matched littermate PIKfyvefl/fl,Nphs2Cre−mice were used in the experiment. Animals were euthanized at 5 and 10 days postsurgery, and analysis revealed similar findings at both time points. There is uniform increase in immunostaining for Lamp1 in podocytes postsurgery in PIKfyvepodko mice compared with the wild type (Figure 10A, top panel). Quantification of Lamp1 immunofluorescence by ImageJ software (National Institutes of Health) shows a statistically significant increase in Lamp1 staining in the PIKfyve-deleted podocytes (Figure 10B). Transmission EM images showed the presence of large vacuoles in some PIKfyvepodko glomerular podocytes (Figure 10A). Although only occasional PIKfyve-deleted podocytes developed vacuoles after nephrectomy, it is feasible that PIKfyvepodko nephrectomized mice could develop podocyte dysfunction on aging and/or additional insults, which remain to be tested.
Figure 10.
Occasional vacuole formation in PIKfyve–deleted mouse podocytes after unilateral nephrectomy. (A) Immunofluorescence images showing increased expression of Lamp1 (arrowheads) in PIKfyve-deleted podocytes 5 days after unilateral nephrectomy (A, top panel). Transmission EM images show vacuoles in PIKfyve-deleted podocytes. Large vacuoles were evident only in a few podocytes. The adjoining podocyte in the image does not have any vacuoles. (B) Quantification of immunofluorescence using ImageJ software showing significantly elevated Lamp1 expression in PIKfyve-deleted podocytes. Scale bars, 20 μm. *P<0.01.
Discussion
Podocytes are highly complex terminally differentiated epithelial cells that are in constant contact with large amounts of ultrafiltrate that is available for pinocytosis. Although vesicles have been observed in podocytes,57 their origin remains unclear. Studies have shown accumulation of aberrant cytoplasmic vacuoles in podocytes with Vps34 deletion, which resulted in glomerular scarring and proteinuria.15,16 Although these studies highlighted the importance of endocytosis and autophagy, they did not address the issue of endocytic flux in healthy podocytes. Our recent studies have shown that cultured Vps34–deficient podocytes cannot be rescued by re-expression of Vps34 when PIKfyve activity is chemically inhibited.21 These data suggest that the abnormal vacuolation phenotype observed in Vps34–deleted cultured podocytes is caused by failed PtdIns(3,5)P2 synthesis. This study was undertaken to determine the role of PIKfyve in glomerular podocytes and proximal tubular cells in mouse models with the respective tissue–specific PIKfyve gene deletions. The key finding that we presented here is the conspicuous absence of a vacuolation phenotype in glomerular podocytes with the PIKfyve knockout, which persisted in aged animals. By contrast, deletion of PIKfyve in proximal tubular cells, which are known to actively proliferate and endocytose,58–60 produced massive cytoplasmic vacuolation along with an aberrant morphology of the endosomal system. Whereas our results might be explained in several ways as discussed below, we favor the interpretation that healthy podocytes exhibit low levels of basal endocytosis.
Our study provides several lines of evidence that support the contention that the lack of aberrant cytoplasmic vacuolation in glomerular podocytes in the PIKfyvepodko mouse model is associated with a low rate of endosomal flux in the glomeruli. Thus, we observed formation of vacuoles in cultured podocytes isolated from the PIKfyvepodko mouse glomeruli (Figure 3). The podocytes that spread outward from isolated glomeruli could have developed vacuoles partly as a consequence of increased PtdIns(3,5)P2 requirements for cell division. We also observed similar vacuolization phenotypes in immortalized podocytes that were grown under conditions that favor differentiation but not cell division (Supplemental Figure 2). Thus, it is conceivable that the demand for PtdIns(3,5)P2-dependent processes increases as cultured cells undergo adhesion, growth, migration, and division. Concordantly, PIKfyve–deleted proximal tubular cells that, in addition to being highly endocytically active, are under constant cell turnover readily developed vacuoles in vivo (Figure 4). The role of PIKfyve in cell division is substantiated by the facts that global PIKfyve gene knockout results in mouse embryo lethality before the 32- to 64-cell stage, and the knockout MEFs display reduced DNA synthesis and impaired cell division.32 Furthermore, in FITC-albumin perfusion studies, although we observed accumulation of FITC-albumin in PIKfyve–deleted proximal tubular cells (Supplemental Figure 6), we were unable to find albumin droplets in both PIKfyve- and Vps34-deleted podocytes, even when the glomeruli showed large amounts of protein leak as seen in the Vps34podko mouse kidneys (Figure 9). Importantly, our notion for reduced endocytosis in podocytes versus proximal tubular cells is also corroborated by findings in the PIKfyve hypomorphic mouse model, where cytoplasmic vacuolation is readily seen in renal tubular cells but not in glomeruli derived from young pups.61 A definitive proof of our claim that, in situ, glomerular podocytes have relatively low levels of basal endocytosis would require additional investigation by more advanced and powerful in vivo approaches, such as intravital multiphoton microcopy.
Alternatively, the contrast in morphologic phenotypes after PIKfyve deletion in podocytes and proximal tubular cells in vivo could suggest either that PIKfyve is dispensable for podocyte homeostasis or that the residual levels of PtdIns(3,5)P2 are sufficient to handle the demands of endosomal trafficking in unchallenged podocytes. Indeed, findings by both PIKfyve knockdown and knockout approaches in different cell types have established that≥10% of the normal levels of PIKfyve could provide significant amounts of PtdIns(3,5)P2 and thus, that they may not trigger cytoplasmic vacuolation and endomembrane dysregulation.32,61–63 In fact, depending on the cell type and status (cycling or differentiated), ≤60%–88% diminution of PtdIns(3,5)P2 may not produce aberrant endomembrane vacuolation as revealed in differentiated adipocytes in culture, yeast cells, muscle, fat, or other tissues.27,32,61–65 Although our data with the mTmG reporter and Western blotting are consistent with a complete gene/protein loss (Figure 2), a potential insufficiency of the antibody sensitivity and/or discrepancies occurring by the mTmG reporter35 could not rule out residual amounts of the PIKfyve protein. Concordantly, lack of a complete loss of PtdIns(3,5)P2 in PIKfyve-deleted podocytes is suggested by detecting approximately 15% residual levels of the PIKfyve lipid products PtdIns(3,5)P2 and PtdIns(5)P by HPLC (Figure 3B). Because the glomerular sieving coefficient for albumin in healthy mouse glomeruli is quite low, the residual low levels of PIKfyve activity might have been able to handle the basal demands of healthy podocytes.66,67
Typically, all epithelial cells endocytose cargo from the apical surface, which is then transported to the vascular space through the basolateral membrane. Unlike conventional epithelial cells, podocytes might be unable to easily move cargo back to the vascular space across the glomerular basement membrane on their basolateral aspect. One might, thus, postulate that the unique location of podocytes underlines low basal endocytic flux. It is feasible that podocytes are also unique in that they rely on autophagic pathways to process proteins and cellular debris. Several studies have shown high levels of autophagy in podocytes,50,68,69 which are beneficial to podocytes, whereas perturbation of autophagy is detrimental.70,71 Our immunofluorescence data showing unaltered endogenous LC3 and p62 levels on deletion of PIKfyve in glomeruli and proximal tubule are consistent with intact autophagy (Figure 8, B and D, Supplemental Figure 5). By contrast, aberrant autophagic flux is evident in the Vps34-deleted podocytes that have higher levels of LC3 and p62 compared with wild-type and PIKfyve-deleted podocytes (Figure 8, B and D, Supplemental Figure 5). We also observed perturbed staining of TGN marker Syntaxin 6 as well as slit diaphragm junctional protein nephrin after deletion of Vps34. These observations suggest that the severe phenotype observed in Vps34-deleted podocytes could be caused by perturbation of multiple cellular processes that are regulated by PtdIns(3)P. Our study highlights that there are fundamental differences in the endocytic flux on cell culturing compared with the same cell type in its natural in vivo environment. We cannot exclude the possibility that, in humans, prolonged proteinuria and injury to the podocytes in proteinuric kidney diseases might result in accelerated endocytosis of proteins and further damage of podocytes and glomerular integrity. Additional studies are needed to unravel the unique nature of podocytes and how different cell types modify the need of critical cellular mechanisms to adapt to their environment.
Concise Methods
Antibodies
Affinity–purified rabbit polyclonal antibodies against nephrin were previously described.72–74 Purified rabbit mAbs against APPL1 (D83H4), Clathrin (D3C6), EEA1 (C45B10), LC3, p62, Rab5 (CDB1), Syntaxin-6 (C34B2), and Rab11 (D4F5) were obtained from Cell Signaling Technology (Danvers, MA). Rabbit polyclonal C–terminal PIKfyve antibodies were described elsewhere.32 The Lamp1 (1D4B) mAb, developed by August J. Thomas, was obtained from the Developmental Studies Hybridoma Bank created by the NICHD of the National Institutes of Health and maintained at the Department of Biology, University of Iowa (Iowa City, IA).75 Mouse mAb against synaptopodin (D1B4) was purchased from American Research Products (Waltham, MA).
Plasmids
Control and Cre–encoding adenoviral constructs were obtained from the viral core at the University of Michigan. GFP–tagged PIKfyve construct was generating by subcloning mouse PIKfyve into pTag-GFP-C (Evrogen, Moscow, Russia) vector using standard PCR–based cloning techniques. Construct was sequenced at the University of Michigan Sequencing Core to confirm the incorporation of PIKfyve gene.
Cell Culture
Cells were maintained in RPMI 1640 with glutamax (Life Technologies, Carlsbad, CA) and supplemented with 10% FBS (Life Technologies) and 200 U/ml penicillin and streptomycin (Roche, Basel, Switzerland) along with insulin, transferrin, and selenium (Life Technologies). Transient transfections were carried out using Lipofectamine 2000 (Life Technologies) and Amaxa Nucleofactor II (Lonza Corporation). Stable transfections were carried out using packaged adenoviral vectors along with polybrene as previously described.76
Generation of the Podocyte– and Proximal Tubule–Specific PIKfyve Knockout Mouse
The PIKfyvefl/fl mouse has been previously described.32 Briefly, two loxP sites were inserted to flank exon 6 that encodes the FYVE finger domain. The excision of exon 6 by Cre recombinase results in a frameshift and early stop codon at residue 170, with the first 159 amino acids from the mouse pikfyve sequence. Double–fluorescent Cre-reporter mouse [STOCK Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J; obtained from The Jackson Laboratory, Bar Harbor, ME] expressing red fluorescence before and green fluorescence after Cre-mediated recombination has been described previously.34 This Cre-reporter mouse line contains an loxP–flanked membrane–targeted Tomato Red (mT) followed by membrane–targeted EGFP (mG). The targeted vector was designed with a CMV enhancer/chicken β–actin core promoter (pCA) driving expression of the loxP–flanked, N–terminal membraned–tagged tdTomato protein (mT) followed by a polyadenylation signal. Immediately distal to the second loxP site is an N–terminal membrane–tagged EGFP sequence (mG) itself followed by a polyadenylation site. Cre–mediated recombination results in deletion of the mT cassette, allowing expression of GFP located downstream in Cre-expressing cells or tissues. Nphs2-Cre and PEPCK-Cre mice were initially bred with the double–fluorescent Cre-reporter mice (referred to as the mTmG mice in the text) to generate mTmGfl/fl,Nphs2-Cre and mTmGfl/fl,PEPCK-Cre mice. A subset of the mTmGfl/fl,Nphs2-Cre and mTmGfl/fl,PEPCK-Cre mice was bred to generate mTmG mice with both Nphs2-Cre and PEPCK-Cre. These three mice strains were then bred with the PIKfyvefl/fl mice to generate deletion of PIKfyve in both podocytes and proximal tubules individually and together.
Generation of Immortalized Podocyte Cell Lines
Glomeruli were isolated from kidneys using the standard sieving method described previously.77,78 Isolated glomeruli were plated onto 10-cm culture dish. Cell outgrowths from the glomeruli are seen in 3 days and primarily podocytes.21,79 The cells are trypsinized and plated on new culture plates. When nearing confluence, cells were treated with adenovirus carrying SV40 Tantigen (titer 1.3×1011; Viral Core, University of Michigan, Ann Arbor, MI). Packaged viral particles were dissolved in complete media at MOI = 200 and minimal volume covering the cells; 4 hours later, medium was added to a volume optimal for the dish size. Medium was replaced 14 hours later with fresh complete media.
Fluorescent InSituRNA Hybridization
In situ RNA hybridization was performed using multiplex RNA scope technology (Advanced Cell Diagnostics, Hayward, CA) following the manufacturer’s protocol described previously.80 Briefly, formalin–fixed 10-μm-thick frozen mouse kidney sections were mounted on glass slides. The slides were subjected to RNAscope Multiplex Fluorescent Assay. The procedure began with 20 minutes of boiling in Pretreat 2 followed by Pretreat 4 (protease) for 30 minutes at room temperature. The slides were hybridized with RNAscope probes against PIKfyve exon 6 for 3 hours at 40°C, and the remainder of the assay protocol was implemented. The fluorescent signal was visualized and captured using a Leica Inverted SP5× Confocal Microscope (Leica Microsystems, Buffalo Grove, IL; University of Michigan Microscopy and Imaging Analysis Core Facility). Multiple images were stacked together to generate a composite image. According to Advanced Cell Diagnostics, each mRNA molecule hybridized to a probe appears as a separate small fluorescent dot.
HPLC Analysis of Cultured Mouse Podocytes
FACS–sorted green fluorescence–expressing mouse podocytes from the mTmGfl/fl, PIKfyvefl/fl,Nphs2-Cre+ mouse and age–matched control mouse were subjected to HPLC analysis. Cells from the first passage were grown in 100-mm dishes to 60%–70% confluence. Cultures were rinsed twice with PBS (pH 7.4) and incubated for 48 hours with inositol-labeling medium inositol (GE Healthcare, Waukesha, WI) containing 6 ml inositol-free DMEM (Chemicon International, Inc.), 10 μCi/ml myo-[2-3H] inositol (GE Healthcare), 10% (vol/vol) dialyzed FBS (26400; Invitrogen, Carlsbad, CA), 20mM Hepes (pH 7.2–7.5), 5μg/ml transferrin, and 5μg/ml insulin. Myo-[2-3H] inositol–labeled cells were washed with PBS and treated with 1 ml 4.5% perchloric acid for 15 minutes at room temperature. Cells were scraped off and spun at 12,000×g for 10 minutes at 4°C. To deacylate lipids, samples were mixed with 1 ml methanol/40% methylamine/l-butanol (45.7% methanol, 10.7% methylamine, and 11.4% l-butanol; vol/vol/vol) and incubated at 55°C for 1 hour. Samples were vacuum dried, resuspended in 0.5 ml water, and extracted with butanol:ethyl ether:ethyl formate (20:4:1; vol/vol/vol). The aqueous phase was vacuum dried and resuspended in 70μl water, and 50 μleach sample was analyzed by HPLC (UFLC CBM-20A Lite; Shimadzu, Tokyo, Japan) using an amino exchange 4.6×250-mm column (PartiSphere SAX; Whatman) as described previously.21,22 Radiolabeled eluate was detected by an inline scintillation analyzer (Beta-RAM Model 5 RHPLC Detector; LabLogic). Fractions were collected every 0.25 minutes and analyzed for radioactivity. For comparison of PtdIns polyphosphate levels, raw counts of each peak were expressed as a percentage of total PtdInscalculated from summation of the radioactivity from [3H]-GroPIns (PI; PI3P; PI4P; PI5P; PI3,5P2; and PI4,5P2) peaks.
Immunofluorescence Staining of Kidney Sections
Kidneys were perfused and fixed with periodate-lysine-paraformaldehyde (PLP) and immersed in 10% neutral–buffered formalin overnight (Fisher Scientific, Waltham, MA) followed by paraffin embedding. The paraffin-embedded tissue was sectioned at 3μm using a Leica Microtome (RM2255; Leica Microsystems). Before staining, the slides were deparaffinized in xylene and hydrated by a series of graded alcohol solutions followed by PBS. Epitopes were retrieved using retrieve all one from 92°C to 94°C for 3–4 hours in a water bath. Slides were immersed in 0.1% sodium borohydrate in Sorenson phosphate buffer for 15 minutes and permeabilized in 0.1% Triton X-100 for 5 minutes. Blocking was done with 10% host serum depending on the host animal of the primary antibody. Sections were incubated overnight at 4°C with primary antibody that was diluted in 1% BSA. After washes with PBS, sections were incubated with fluorescence–conjugated secondary antibody for 2 hours at room temperature. Confocal microscopy and acquisition of images were performed using a Leica Inverted SP5× Confocal Microscope (Leica Microsystems; University of Michigan Microscopy and Imaging Analysis Core Facility).
FITC-Albumin Perfusion Experiments
FITC-halb (Sigma-Aldrich, St. Louis, MO) was reconstituted in 1 ml PBS at a concentration of 100 mg/ml (10% solution). Mice were injected with 250 μl 10% FITC-halb through the tail vein. The animals were euthanized at various time points. Kidneys were perfused and fixed with PLP and immersed in 30% sucrose solution in PBS overnight. The kidney slices were embedded in Tissue-Tek O.C.T Compound (Sakura Finetek, Inc., Torrance, CA) and snap frozen in liquid nitrogen. The embedded tissue was sectioned at 3- to 4-μm thickness using a Leica Cryostat (Leica Microsystems). Confocal microscopy and acquisition of images were performed using a Leica Inverted SP5× Confocal Microscope (Leica Microsystems; University of Michigan Microscopy and Imaging Analysis Core Facility).
Immunogold Electron Microscopy
PLP–perfused and –fixed kidney tissue was sectioned using a vibrotome (Leica Microsystems) at 100-μm thickness and treated with 0.1% sodium borohydrate in Sorensen phosphate buffer for 15 minutes. Permeabilization was achieved with 0.05% Triton X-100 for 30 minutes. Sections were blocked using a combination of normal 5%–10% goat (host) serum, 0.1% cold water fish-skin gelatin, and 1%–5% BSA (Electron Microscopy Sciences, Hatfield, PA). After blocking sections were incubated in 0.1%–0.2% Aurion BSA-c Incubation Buffer (Electron Microscopy Sciences), sections were then incubated with primary antibody diluted (1:500 dilution) in incubation buffer overnight. After a rigorous wash, specimens were incubated with nanogold conjugate (ultra small immunogold 0.8-nmparticle diameter; Electron Microscopy Sciences) diluted (1:50 dilution) in incubation buffer (Electron Microscopy Sciences). Before silver enhancement, sections were postfixed in 2% glutraldehyde in Sorenson phosphate buffer for 2 hours. Silverenhancement of the sections was done with Aurion R-Gent SE-EM Reagent (Electron Microscopy Sciences). Sections were fixed with 0.5% osmium for 15 minutes followed by embedding in resin. Images were taken using a Philips CM-100 Transmission Electron Microscope at the Microscopy and Image Analysis Core Facility at the University of Michigan.
EM and Slit Diaphragm Frequency Analyses
Preparation of mouse kidneys for scanning and transmission EM was performed by standard methods. For scanning EM, 20 glomeruli of each sample were analyzed with the AMRAY 1910 Field Emission Scanning Electron Microscope at the University of Michigan Microscope and Image Analysis Core Facility. For transmission EM, samples were examined by the Philips CM-100 Transmission Electron Microscopeat the University of Michigan Microscope and Image Analysis Core Facility. Slit diaphragm frequency was assessed by counting the number of junctions per micrometer of the basement membrane; ≥10 samples were analyzed in each experimental condition.
ImageJ Quantitation and Statistical Analyses
Data are presented as means±SEMs throughout the text unless otherwise specified. The numbers of experiments performed for each experiment have been mentioned in the figures. All experiments were performed at least three times. ImageJ software (National Institutes of Health) was used to quantify the fluorescence in ≥10 cells in each glomerulus; ≥10 glomeruli in each experimental condition were examined to generate the data for statistical analysis. Using the ImageJ (National Institutes of Health) drawing tool, an outline was sketched around the cell to measure the fluorescence (for podocytes, cells on the periphery of the tuft were selected). A region next to each cell that had no fluorescence was selected for background fluorescence. Corrected total cell fluorescence was calculated using the formula corrected total cell fluorescence = integrated density−(area of selected cell × mean fluorescence of background reading). Statistical comparisons were performed using two–tailed t test or ANOVA where applicable. A value of P≤0.05 was considered to represent statistically significant difference.
Unilateral Nephrectomy Mouse Model of Podocyte Hypertrophy
Six-month-old PIKfyvefl/fl,Nphs2-Cre+ mice and their age–matched littermate controls (PIKfyvefl/fl,Nphs2-Cre−) underwent left unilateral nephrectomy under isofluorane anesthesia. At day 5 or 10, mice were euthanized, and kidneys were harvested for histologic analysis.
Study Approval
All animal studies were approved by the University Committee on the Use and Care of Animals, Institutional Review Board at the University of Michigan School of Medicine.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors thank Jeff Harrison at the University of Michigan Microscopy and Image Analysis Core Facility for his advice and help in electron microscopy and confocal imaging. We also thank Jeffery Hodgin and Yingbao Yang for their assistance in performance of the unilateral nephrectomy.
This work was supported by National Institutes of Health (NIH), National Institute of Diabetes and Digestive and Kidney Diseases Grants DK097027 (to P.G.) and DK096053 (to P.G.) and American Society of Nephrology Carl Gottshalk Research Scholar Award (to P.G.). The work was also supported by NIH and American Diabetes Association Grants DK082409 (to S.P.) and DK05058, 7-13-BS-161 (to A.S.). Support was also obtained from the University of Michigan’s George M. O’Brien Translational Research Core Center Grant P30 DK0801943 and Michigan Center for Diabetes Translational Research Core Grant P60 DK020572.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015050555/-/DCSupplemental.
References
- 1.van der Velde M, Matsushita K, Coresh J, Astor BC, Woodward M, Levey A, de Jong P, Gansevoort RT, van der Velde M, Matsushita K, Coresh J, Astor BC, Woodward M, Levey AS, de Jong PE, Gansevoort RT, Levey A, El-Nahas M, Eckardt KU, Kasiske BL, Ninomiya T, Chalmers J, Macmahon S, Tonelli M, Hemmelgarn B, Sacks F, Curhan G, Collins AJ, Li S, Chen SC, Hawaii Cohort KP, Lee BJ, Ishani A, Neaton J, Svendsen K, Mann JF, Yusuf S, Teo KK, Gao P, Nelson RG, Knowler WC, Bilo HJ, Joosten H, Kleefstra N, Groenier KH, Auguste P, Veldhuis K, Wang Y, Camarata L, Thomas B, Manley T Chronic Kidney Disease Prognosis Consortium : Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int 79: 1341–1352, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Ninomiya T, Perkovic V, de Galan BE, Zoungas S, Pillai A, Jardine M, Patel A, Cass A, Neal B, Poulter N, Mogensen CE, Cooper M, Marre M, Williams B, Hamet P, Mancia G, Woodward M, Macmahon S, Chalmers J ADVANCE Collaborative Group : Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol 20: 1813–1821, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruggenenti P, Perna A, Mosconi L, Pisoni R, Remuzzi G: Urinary protein excretion rate is the best independent predictor of ESRF in non-diabetic proteinuric chronic nephropathies. “Gruppo Italiano di Studi Epidemiologici in Nefrologia” (GISEN). Kidney Int 53: 1209–1216, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Russo LM, Sandoval RM, Brown D, Molitoris BA, Comper WD: Controversies in nephrology: Response to ‘renal albumin handling, facts, and artifacts’. Kidney Int 72: 1195–1197, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Russo LM, Sandoval RM, McKee M, Osicka TM, Collins AB, Brown D, Molitoris BA, Comper WD: The normal kidney filters nephrotic levels of albumin retrieved by proximal tubule cells: Retrieval is disrupted in nephrotic states. Kidney Int 71: 504–513, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Klinghoffer RA, Sachsenmaier C, Cooper JA, Soriano P: Src family kinases are required for integrin but not PDGFR signal transduction. EMBO J 18: 2459–2471, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dickson LE, Wagner MC, Sandoval RM, Molitoris BA: The proximal tubule and albuminuria: Really! J Am Soc Nephrol 25: 443–453, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roopenian DC, Akilesh S: FcRn: The neonatal Fc receptor comes of age. Nat Rev Immunol 7: 715–725, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Akilesh S, Huber TB, Wu H, Wang G, Hartleben B, Kopp JB, Miner JH, Roopenian DC, Unanue ER, Shaw AS: Podocytes use FcRn to clear IgG from the glomerular basement membrane. Proc Natl Acad Sci U S A 105: 967–972, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kinugasa S, Tojo A, Sakai T, Tsumura H, Takahashi M, Hirata Y, Fujita T: Selective albuminuria via podocyte albumin transport in puromycin nephrotic rats is attenuated by an inhibitor of NADPH oxidase. Kidney Int 80: 1328–1338, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Okamura K, Dummer P, Kopp J, Qiu L, Levi M, Faubel S, Blaine J: Endocytosis of albumin by podocytes elicits an inflammatory response and induces apoptotic cell death. PLoS One 8: e54817, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dobrinskikh E, Okamura K, Kopp JB, Doctor RB, Blaine J: Human podocytes perform polarized, caveolae-dependent albumin endocytosis. Am J Physiol Renal Physiol 306: F941–F951, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eyre J, Ioannou K, Grubb BD, Saleem MA, Mathieson PW, Brunskill NJ, Christensen EI, Topham PS: Statin-sensitive endocytosis of albumin by glomerular podocytes. Am J Physiol Renal Physiol 292: F674–F681, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Carson JM, Okamura K, Wakashin H, McFann K, Dobrinskikh E, Kopp JB, Blaine J: Podocytes degrade endocytosed albumin primarily in lysosomes. PLoS One 9: e99771, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bechtel W, Helmstädter M, Balica J, Hartleben B, Kiefer B, Hrnjic F, Schell C, Kretz O, Liu S, Geist F, Kerjaschki D, Walz G, Huber TB: Vps34 deficiency reveals the importance of endocytosis for podocyte homeostasis. J Am Soc Nephrol 24: 727–743, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen J, Chen MX, Fogo AB, Harris RC, Chen JK: mVps34 deletion in podocytes causes glomerulosclerosis by disrupting intracellular vesicle trafficking. J Am Soc Nephrol 24: 198–207, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Backer JM: The regulation and function of Class III PI3Ks: Novel roles for Vps34. Biochem J 410: 1–17, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Funderburk SF, Wang QJ, Yue Z: The Beclin 1-VPS34 complex--at the crossroads of autophagy and beyond. Trends Cell Biol 20: 355–362, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurihara H, Anderson JM, Farquhar MG: Increased Tyr phosphorylation of ZO-1 during modification of tight junctions between glomerular foot processes. Am J Physiol 268: F514–F524, 1995 [DOI] [PubMed] [Google Scholar]
- 20.Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B: The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol 11: 329–341, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Ikonomov OC, Sbrissa D, Venkatareddy M, Tisdale E, Garg P, Shisheva A: Class III PI 3-kinase is the main source of PtdIns3P substrate and membrane recruitment signal for PIKfyve constitutive function in podocyte endomembrane homeostasis. Biochim Biophys Acta 1853: 1240–1250, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shisheva A: PIKfyve: The road to PtdIns 5-P and PtdIns 3,5-P(2). Cell Biol Int 25: 1201–1206, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Shisheva A: PIKfyve: Partners, significance, debates and paradoxes. Cell Biol Int 32: 591–604, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shisheva A, Sbrissa D, Ikonomov O: Plentiful PtdIns5P from scanty PtdIns(3,5)P2 or from ample PtdIns? PIKfyve-dependent models: Evidence and speculation (response to: DOI 10.1002/bies.201300012). BioEssays 37: 267–277, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ikonomov OC, Sbrissa D, Shisheva A: Mammalian cell morphology and endocytic membrane homeostasis require enzymatically active phosphoinositide 5-kinase PIKfyve. J Biol Chem 276: 26141–26147, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Shisheva A, Rusin B, Ikonomov OC, DeMarco C, Sbrissa D: Localization and insulin-regulated relocation of phosphoinositide 5-kinase PIKfyve in 3T3-L1 adipocytes. J Biol Chem 276: 11859–11869, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Gary JD, Wurmser AE, Bonangelino CJ, Weisman LS, Emr SD: Fab1p is essential for PtdIns(3)P 5-kinase activity and the maintenance of vacuolar size and membrane homeostasis. J Cell Biol 143: 65–79, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nicot AS, Fares H, Payrastre B, Chisholm AD, Labouesse M, Laporte J: The phosphoinositide kinase PIKfyve/Fab1p regulates terminal lysosome maturation in Caenorhabditis elegans. Mol Biol Cell 17: 3062–3074, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ikonomov OC, Sbrissa D, Mlak K, Deeb R, Fligger J, Soans A, Finley RL Jr., Shisheva A: Active PIKfyve associates with and promotes the membrane attachment of the late endosome-to-trans-Golgi network transport factor Rab9 effector p40. J Biol Chem 278: 50863–50871, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Colognato H, Ramachandrappa S, Olsen IM, ffrench-Constant C: Integrins direct Src family kinases to regulate distinct phases of oligodendrocyte development. J Cell Biol 167: 365–375, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pozzi A, Jarad G, Moeckel GW, Coffa S, Zhang X, Gewin L, Eremina V, Hudson BG, Borza DB, Harris RC, Holzman LB, Phillips CL, Fassler R, Quaggin SE, Miner JH, Zent R: Beta1 integrin expression by podocytes is required to maintain glomerular structural integrity. Dev Biol 316: 288–301, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ikonomov OC, Sbrissa D, Delvecchio K, Xie Y, Jin JP, Rappolee D, Shisheva A: The phosphoinositide kinase PIKfyve is vital in early embryonic development: preimplantation lethality of PIKfyve−/− embryos but normality of PIKfyve+/− mice. J Biol Chem 286(15): 13404–13413, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moeller MJ, Sanden SK, Soofi A, Wiggins RC, Holzman LB: Two gene fragments that direct podocyte-specific expression in transgenic mice. J Am Soc Nephrol 13: 1561–1567, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L: A global double-fluorescent Cre reporter mouse. Genesis 45: 593–605, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Vooijs M, Jonkers J, Berns A: A highly efficient ligand-regulated Cre recombinase mouse line shows that LoxP recombination is position dependent. EMBO Rep 2: 292–297, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arias-Salgado EG, Lizano S, Sarkar S, Brugge JS, Ginsberg MH, Shattil SJ: Src kinase activation by direct interaction with the integrin beta cytoplasmic domain. Proc Natl Acad Sci U S A 100: 13298–13302, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rankin EB, Tomaszewski JE, Haase VH: Renal cyst development in mice with conditional inactivation of the von Hippel-Lindau tumor suppressor. Cancer Res 66: 2576–2583, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dukes JD, Whitley P, Chalmers AD: The PIKfyve inhibitor YM201636 blocks the continuous recycling of the tight junction proteins claudin-1 and claudin-2 in MDCK cells. PLoS One 7: e28659, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huotari J, Helenius A: Endosome maturation. EMBO J 30: 3481–3500, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zoncu R, Perera RM, Balkin DM, Pirruccello M, Toomre D, De Camilli P: A phosphoinositide switch controls the maturation and signaling properties of APPL endosomes. Cell 136: 1110–1121, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Green SA, Zimmer KP, Griffiths G, Mellman I: Kinetics of intracellular transport and sorting of lysosomal membrane and plasma membrane proteins. J Cell Biol 105: 1227–1240, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harter C, Mellman I: Transport of the lysosomal membrane glycoprotein lgp120 (lgp-A) to lysosomes does not require appearance on the plasma membrane. J Cell Biol 117: 311–325, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Höning S, Hunziker W: Cytoplasmic determinants involved in direct lysosomal sorting, endocytosis, and basolateral targeting of rat lgp120 (lamp-I) in MDCK cells. J Cell Biol 128: 321–332, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poteryaev D, Datta S, Ackema K, Zerial M, Spang A: Identification of the switch in early-to-late endosome transition. Cell 141: 497–508, 2010 [DOI] [PubMed] [Google Scholar]
- 45.Urbé S, Huber LA, Zerial M, Tooze SA, Parton RG: Rab11, a small GTPase associated with both constitutive and regulated secretory pathways in PC12 cells. FEBS Lett 334: 175–182, 1993 [DOI] [PubMed] [Google Scholar]
- 46.Welz T, Wellbourne-Wood J, Kerkhoff E: Orchestration of cell surface proteins by Rab11. Trends Cell Biol 24: 407–415, 2014 [DOI] [PubMed] [Google Scholar]
- 47.Apodaca G, Gallo LI, Bryant DM: Role of membrane traffic in the generation of epithelial cell asymmetry. Nat Cell Biol 14: 1235–1243, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bock JB, Klumperman J, Davanger S, Scheller RH: Syntaxin 6 functions in trans-Golgi network vesicle trafficking. Mol Biol Cell 8: 1261–1271, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ikonomov OC, Sbrissa D, Foti M, Carpentier JL, Shisheva A: PIKfyve controls fluid phase endocytosis but not recycling/degradation of endocytosed receptors or sorting of procathepsin D by regulating multivesicular body morphogenesis. Mol Biol Cell 14: 4581–4591, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hartleben B, Gödel M, Meyer-Schwesinger C, Liu S, Ulrich T, Köbler S, Wiech T, Grahammer F, Arnold SJ, Lindenmeyer MT, Cohen CD, Pavenstädt H, Kerjaschki D, Mizushima N, Shaw AS, Walz G, Huber TB: Autophagy influences glomerular disease susceptibility and maintains podocyte homeostasis in aging mice. J Clin Invest 120: 1084–1096, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vicinanza M, Korolchuk VI, Ashkenazi A, Puri C, Menzies FM, Clarke JH, Rubinsztein DC: PI(5)P regulates autophagosome biogenesis. Mol Cell 57: 219–234, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martin S, Harper CB, May LM, Coulson EJ, Meunier FA, Osborne SL: Inhibition of PIKfyve by YM-201636 dysregulates autophagy and leads to apoptosis-independent neuronal cell death. PLoS One 8: e60152, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ferguson CJ, Lenk GM, Meisler MH: Defective autophagy in neurons and astrocytes from mice deficient in PI(3,5)P2. Hum Mol Genet 18: 4868–4878, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen L, Sung SS, Yip ML, Lawrence HR, Ren Y, Guida WC, Sebti SM, Lawrence NJ, Wu J: Discovery of a novel shp2 protein tyrosine phosphatase inhibitor. Mol Pharmacol 70: 562–570, 2006 [DOI] [PubMed] [Google Scholar]
- 55.Komatsu M, Wang QJ, Holstein GR, Friedrich VL Jr., Iwata J, Kominami E, Chait BT, Tanaka K, Yue Z: Essential role for autophagy protein Atg7 in the maintenance of axonal homeostasis and the prevention of axonal degeneration. Proc Natl Acad Sci U S A 104: 14489–14494, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Komatsu M, Waguri S, Koike M, Sou YS, Ueno T, Hara T, Mizushima N, Iwata J, Ezaki J, Murata S, Hamazaki J, Nishito Y, Iemura S, Natsume T, Yanagawa T, Uwayama J, Warabi E, Yoshida H, Ishii T, Kobayashi A, Yamamoto M, Yue Z, Uchiyama Y, Kominami E, Tanaka K: Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell 131: 1149–1163, 2007 [DOI] [PubMed] [Google Scholar]
- 57.Farquhar MG, Vernier RL, Good RA: An electron microscope study of the glomerulus in nephrosis, glomerulonephritis, and lupus erythematosus. J Exp Med 106: 649–660, 1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vogetseder A, Picard N, Gaspert A, Walch M, Kaissling B, Le Hir M: Proliferation capacity of the renal proximal tubule involves the bulk of differentiated epithelial cells. Am J Physiol Cell Physiol 294: C22–C28, 2008 [DOI] [PubMed] [Google Scholar]
- 59.Brunskill NJ: Molecular interactions between albumin and proximal tubular cells. Exp Nephrol 6: 491–495, 1998 [DOI] [PubMed] [Google Scholar]
- 60.Brunskill NJ, Stuart J, Tobin AB, Walls J, Nahorski S: Receptor-mediated endocytosis of albumin by kidney proximal tubule cells is regulated by phosphatidylinositide 3-kinase. J Clin Invest 101: 2140–2150, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lowell CA, Fumagalli L, Berton G: Deficiency of Src family kinases p59/61hck and p58c-fgr results in defective adhesion-dependent neutrophil functions. J Cell Biol 133: 895–910, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sbrissa D, Ikonomov OC, Strakova J, Dondapati R, Mlak K, Deeb R, Silver R, Shisheva A: A mammalian ortholog of Saccharomyces cerevisiae Vac14 that associates with and up-regulates PIKfyve phosphoinositide 5-kinase activity. Mol Cell Biol 24: 10437–10447, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sbrissa D, Shisheva A: Acquisition of unprecedented phosphatidylinositol 3,5-bisphosphate rise in hyperosmotically stressed 3T3-L1 adipocytes, mediated by ArPIKfyve-PIKfyve pathway. J Biol Chem 280: 7883–7889, 2005 [DOI] [PubMed] [Google Scholar]
- 64.Ikonomov OC, Sbrissa D, Shisheva A: Fat cell-specific inactivation of one or both PIKfyve alleles in mice causes insulin [Abstract]. Available at: http://physiology.med.wayne.edu/pdfs/psl2012abstracts.pdf. Accessed August 24, 2012 [Google Scholar]
- 65.Ikonomov OC, Sbrissa D, Delvecchio K, Feng HZ, Cartee GD, Jin JP, Shisheva A: Muscle-specific Pikfyve gene disruption causes glucose intolerance, insulin resistance, adiposity, and hyperinsulinemia but not muscle fiber-type switching. Am J Physiol Endocrinol Metab 305: E119–E131, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Russo LM, Sandoval RM, Campos SB, Molitoris BA, Comper WD, Brown D: Impaired tubular uptake explains albuminuria in early diabetic nephropathy. J Am Soc Nephrol 20: 489–494, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sandoval RM, Wagner MC, Patel M, Campos-Bilderback SB, Rhodes GJ, Wang E, Wean SE, Clendenon SS, Molitoris BA: Multiple factors influence glomerular albumin permeability in rats. J Am Soc Nephrol 23: 447–457, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hartleben B, Wanner N, Huber TB: Autophagy in glomerular health and disease. Semin Nephrol 34: 42–52, 2014 [DOI] [PubMed] [Google Scholar]
- 69.Weide T, Huber TB: Implications of autophagy for glomerular aging and disease. Cell Tissue Res 343: 467–473, 2011 [DOI] [PubMed] [Google Scholar]
- 70.Liebau MC, Braun F, Höpker K, Weitbrecht C, Bartels V, Müller RU, Brodesser S, Saleem MA, Benzing T, Schermer B, Cybulla M, Kurschat CE: Dysregulated autophagy contributes to podocyte damage in Fabry’s disease. PLoS One 8: e63506, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu L, Feng Z, Cui S, Hou K, Tang L, Zhou J, Cai G, Xie Y, Hong Q, Fu B, Chen X: Rapamycin upregulates autophagy by inhibiting the mTOR-ULK1 pathway, resulting in reduced podocyte injury. PLoS One 8: e63799, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Holzman LB, St. John PL, Kovari IA, Verma R, Holthofer H, Abrahamson DR: Nephrin localizes to the slit pore of the glomerular epithelial cell. Kidney Int 56: 1481–1491, 1999 [DOI] [PubMed] [Google Scholar]
- 73.Verma R, Kovari I, Soofi A, Nihalani D, Patrie K, Holzman LB: Nephrin ectodomain engagement results in Src kinase activation, nephrin phosphorylation, Nck recruitment, and actin polymerization. J Clin Invest 116: 1346–1359, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Verma R, Wharram B, Kovari I, Kunkel R, Nihalani D, Wary KK, Wiggins RC, Killen P, Holzman LB: Fyn binds to and phosphorylates the kidney slit diaphragm component Nephrin. J Biol Chem 278: 20716–20723, 2003 [DOI] [PubMed] [Google Scholar]
- 75.Hughes EN, August JT: Characterization of plasma membrane proteins identified by monoclonal antibodies. J Biol Chem 256: 664–671, 1981 [PubMed] [Google Scholar]
- 76.Venkatareddy M, Cook L, Abuarquob K, Verma R, Garg P: Nephrin regulates lamellipodia formation by assembling a protein complex that includes Ship2, filamin and lamellipodin. PLoS One 6: e28710, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Salant DJ, Darby C, Couser WG: Experimental membranous glomerulonephritis in rats. Quantitative studies of glomerular immune deposit formation in isolated glomeruli and whole animals. J Clin Invest 66: 71–81, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Garg P, Verma R, Cook L, Soofi A, Venkatareddy M, George B, Mizuno K, Gurniak C, Witke W, Holzman LB: Actin-depolymerizing factor cofilin-1 is necessary in maintaining mature podocyte architecture. J Biol Chem 285: 22676–22688, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ni L, Saleem M, Mathieson PW: Podocyte culture: Tricks of the trade. Nephrology (Carlton) 17: 525–531, 2012 [DOI] [PubMed] [Google Scholar]
- 80.Gross-Thebing T, Paksa A, Raz E: Simultaneous high-resolution detection of multiple transcripts combined with localization of proteins in whole-mount embryos. BMC Biol 12: 55, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.