Abstract
Kidney retransplantation is a risk factor for decreased allograft survival. Repeated mismatched HLA antigens between first and second transplant may be a stimulus for immune memory responses and increased risk of alloimmune damage to the second allograft. Historical data identified a role of repeated HLA mismatches in allograft loss. However, evolution of HLA testing methods and a modern transplant era necessitate re-examination of this role to more accurately risk-stratify recipients. We conducted a contemporary registry analysis of data from 13,789 patients who received a second kidney transplant from 1995 to 2011, of which 3868 had one or more repeated mismatches. Multivariable Cox proportional hazards modeling revealed no effect of repeated mismatches on all–cause or death–censored graft loss. Analysis of predefined subgroups, however, showed that any class 2 repeated mismatch increased the hazard of death–censored graft loss, particularly in patients with detectable panel–reactive antibody before second transplant (hazard ratio [HR], 1.15; 95% confidence interval [95% CI], 1.02 to 1.29). Furthermore, in those who had nephrectomy of the first allograft, class 2 repeated mismatches specifically associated with all–cause (HR, 1.30; 95% CI, 1.07 to 1.58) and death–censored graft loss (HR, 1.41; 95% CI, 1.12 to 1.78). These updated data redefine the effect of repeated mismatches in retransplantation and challenge the paradigm that repeated mismatches in isolation confer increased immunologic risk. We also defined clear recipient categories for which repeated mismatches may be of greater concern in a contemporary cohort. Additional studies are needed to determine appropriate interventions for these recipients.
Keywords: transplant nephrectomy, chronic allograft failure, transplant outcomes, kidney transplantation, renal transplantation, transplantation
Kidney retransplantation is generally viewed to increase risk for immunologic damage and graft loss. The increased risk is attributed to the formation of T and B memory cells to mismatched HLA antigens in the original donors. Re-exposure to a repeated HLA antigen mismatched (RMM) in a subsequent donor potentially invokes alloimmune memory responses, resulting in earlier allograft damage and loss.
The nature and magnitude of this potential risk have changed with reanalysis over time. Whereas a number of studies have described worse allograft survival when repeated antigens are mismatched at the HLA-DR locus,1–3 with RMM at class 1 (HLA-A or -B) not influencing outcomes, others have shown a beneficial effect of RMM on graft survival4,5 or a neutral effect.6,7 A more recent analysis showed an effect of class 1 RMM on graft survival, with no differences seen with class 2 RMM.8
Reasons for varying conclusions between studies and over time may be related to smaller study subject numbers, differing patient population characteristics, and different eras of immunosuppression and immunologic testing. It is possible that current improvements in immunosuppression may reduce the effect of RMM on transplant outcome. Similarly, there have been improvements in the sensitivity and specificity of crossmatching and HLA antibody screening, such that low–level donor–specific HLA antibodies may now be avoided in repeat transplants, not having been as readily detected in previous eras. Results of smaller single–center studies may be influenced by local practices in ways that are systematically difficult to document.
Despite evolving and sometimes contradictory conclusions, the effect of RMM on clinical practice on the basis of these data may be substantial. Avoiding RMM can reduce donor opportunities, with consequent implications for wait times. Augmenting immunosuppression with potential attendant side effects in an endeavor to reduce early and late alloimmune damage in the presence of RMM may also have deleterious consequences.
We felt it was, therefore, important to re-examine the role of RMM in a more contemporary and larger multicenter registry cohort. We studied the effect of RMM on all–cause graft loss (ACGL) and death–censored graft loss (DCGL) in a US Renal Data System (USRDS) Cohort of 13,789 repeat renal transplant recipients from 1995 to 2011, transcending the era of more modern immunosuppression and improved HLA testing techniques. We hypothesized that, in the context of therapeutic and diagnostic advances, the effect of RMM on graft loss would be attenuated compared with historical cohorts. We further hypothesized that the effect of RMM may be greater for class 2 RMM (given increasing evidence of the deleterious effect of class 2 mismatch on transplant outcomes)9–11 and modified by predefined panel–reactive antibody (PRA), immunosuppression, and nephrectomy of the first transplant. Exploratory analyses for interactions between RMM and these covariates were also performed.
Results
There were 13,789 recipients of a second transplant identified in the USRDS Registry from 1995 to 2011. Of these, 3868 (28%) had an RMM between the first and second donor. There were 1764 (13%) graft losses and 3858 (28%) deaths (total of 5622 reaching the combined end point) during the study period. Median follow-up with a functioning graft was 4.61 years (25%, 75% percentiles, 2.19, 7.96).
Patient Characteristics
Patient characteristics are shown in Table 1. Patients with one or more RMM in their second transplant were more likely to be nonblack and have ESRD attributed to diabetes mellitus. At the time of first transplant, there was no difference in peak pretransplant PRA; however, they did have more HLA mismatches (potentially increasing the likelihood of an RMM in a subsequent transplant). At second transplant, patients with one or more RMM had lower peak pretransplant PRA, had more donor HLA mismatches, and were more likely to have a living donor, with no differences in choice of initial calcineurin inhibitor.
Table 1.
Recipient characteristics
| Covariate | All Patients, n=13,789 | No RMM, n=9921; 72% | One or More RMM, n=3868; 28% | P Value |
|---|---|---|---|---|
| Age, yr | <0.001 | |||
| 18–39 | 5529 (40) | 4116 (42) | 1413 (37) | |
| 40–49 | 3698 (27) | 2614 (26) | 1084 (28) | |
| 50–59 | 2916 (21) | 2033 (20) | 883 (23) | |
| ≥60 | 1646 (12) | 1158 (12) | 488 (12) | |
| Women | 5572 (40) | 3980 (40) | 1592 (41) | 0.26 |
| Race | <0.001 | |||
| Black | 3631 (26) | 2736 (28) | 895 (23) | |
| Nonblack | 10,158 (74) | 7185 (72) | 2973 (77) | |
| ESRD cause | <0.001 | |||
| DM | 1961 (14) | 1307 (13) | 654 (16) | |
| GN | 5059 (37) | 3642 (37) | 1417 (37) | |
| Other | 6769 (49) | 4972 (50) | 1797 (46) | |
| First transplant characteristics | ||||
| Living donor | 5241 (38) | 3747 (38) | 1494 (39) | 0.35 |
| Deceased donor | 8548 (62) | 6174 (62) | 2374 (61) | |
| Peak PRA % | 0.30 | |||
| 0 | 5216 (49) | 3786 (50) | 1430 (48) | |
| 1–10 | 3311 (31) | 2343 (31) | 968 (33) | |
| 11–30 | 1118 (11) | 805 (10) | 313 (11) | |
| >30 | 947 (9) | 689 (9) | 258 (9) | |
| HLA mismatch | <0.001 | |||
| 1 | 771 (6) | 660 (7) | 111 (3) | |
| 2 | 2404 (18) | 1906 (19) | 498 (13) | |
| 3 | 3995 (29) | 2952 (30) | 1043 (27) | |
| 4 | 3050 (22) | 2128 (22) | 922 (24) | |
| 5 | 2356 (17) | 1513 (15) | 843 (22) | |
| 6 | 1056 (8) | 644 (7) | 412 (11) | |
| CSA | 9611 (70) | 6955 (70) | 2656 (69) | 0.26 |
| TAC | 3182 (23) | 2258 (23) | 924 (24) | |
| Neither CSA nor TAC | 996 (7) | 708 (7) | 288 (7) | |
| Aza | 6784 (49) | 4926 (50) | 1858 (48) | 0.23 |
| MMF | 4828 (35) | 3,445 (35) | 1383 (36) | |
| Neither Aza nor MMF | 2177 (16) | 1550 (16) | 627 (16) | |
| Induction | 0.43 | |||
| Depleting Ab | 5543 (40) | 3946 (40) | 1597 (41) | |
| Nondepleting Ab | 1301 (10) | 946 (10) | 355 (9) | |
| Both | 191 (1) | 137 (1) | 54 (2) | |
| Neither | 6754 (49) | 4892 (49) | 1862 (48) | |
| Second transplant characteristics | ||||
| Living donor | 4521 (33) | 2934 (30) | 1587 (41) | <0.001 |
| Deceased donor | 9268 (67) | 6987 (70) | 2281(59) | |
| Peak PRA % | <0.001 | |||
| 0 | 1678 (15) | 1087 (13) | 591 (20) | |
| 1–10 | 1541 (14) | 985 (12) | 556 (19) | |
| 11–30 | 1506 (13) | 1055 (13) | 451 (15) | |
| >30 | 6562 (58) | 5186 (62) | 1376 (46) | |
| HLA mismatch | <0.001 | |||
| 1 | 676 (5) | 574 (6) | 102 (3) | |
| 2 | 1749 (13) | 1414 (14) | 335 (9) | |
| 3 | 3130 (23) | 2371 (24) | 759 (19) | |
| 4 | 3521 (25) | 2517 (25) | 1004 (26) | |
| 5 | 3337 (24) | 2241 (23) | 1096 (28) | |
| 6 | 1376 (10) | 804 (8) | 572 (15) | |
| CSA | 2693 (20) | 1947 (0) | 746 (19) | 0.44 |
| TAC | 9208 (67) | 6596 (56) | 2612 (68) | |
| Neither CSA nor TAC | 1888 (13) | 1378 (14) | 510 (13) | |
| Aza | 941 (7) | 698 (7) | 243 (6) | 0.03 |
| MMF | 10,193 (74) | 736 (74) | 2831 (73) | |
| Neither Aza nor MMF | 2655 (19) | 1861 (19) | 794 (21) | |
| Induction | 0.73 | |||
| Depleting Ab | 7371 (54) | 5327 (54) | 2044 (53) | |
| Nondepleting Ab | 2070 (15) | 1,470 (15) | 600 (15) | |
| Both | 424 (3) | 305 (3) | 119 (3) | |
| Neither | 3924 (28) | 2819 (28) | 1105 (29) |
Missing: PRA first transplant (23%) and PRA second transplant (23%). DM, diabetes mellitus; CSA, cyclosporin A; TAC, tacrolimus; Aza, azathioprine; MMF, mycophenolic mofetil; Ab, antibody.
Of 3868 recipients with one or more RMM, 2164 (56%) had only class 1 RMM, 1092 (28%) had only class 2 RMM, and 612 (16%) had both classes 1 and 2 RMM. Only 916 (24%) had two or more RMM; therefore, RMM was classified as zero RMM versus one or more RMM for the purposes of analysis (Table 2).
Table 2.
Repeated HLA mismatches distribution
| No. of RMM | No. (%) with RMM | Class 1 Only | Class 2 Only | Classes 1 and 2 |
|---|---|---|---|---|
| 0 | 9921 (72) | — | — | — |
| 1 | 2952 (21) | 1881 | 1071 | — |
| 2 | 686 (5) | 272 | 21 | 393 |
| 3 | 211 (2) | 10 | — | 201 |
| 4 | 14 (0) | 1 | — | 13 |
| 5 | 3 (0) | — | — | 3 |
| 6 | 2 (0) | — | — | 2 |
—, no subject in category.
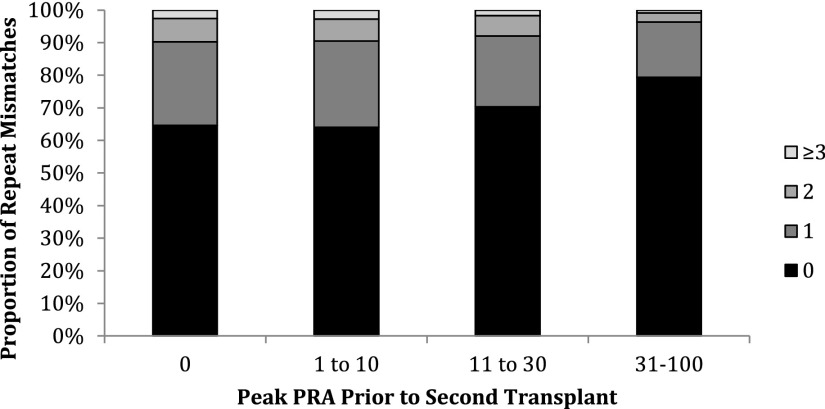
For second transplants, those recipients with higher peak pretransplant PRA received a greater proportion of zero RMM kidneys compared with unsensitized recipients (Figure 1).
Figure 1.
Sensitized recipients received fewer transplants overall with RMM. As the second transplant peak PRA increases, the number of RMM decreases, suggesting that crossmatching and/or antibody screening and identification may be screening out donors with RMM to whom recipients have developed donor–specific HLA antibody.
Effect of Any RMM on ACGL and DCGL
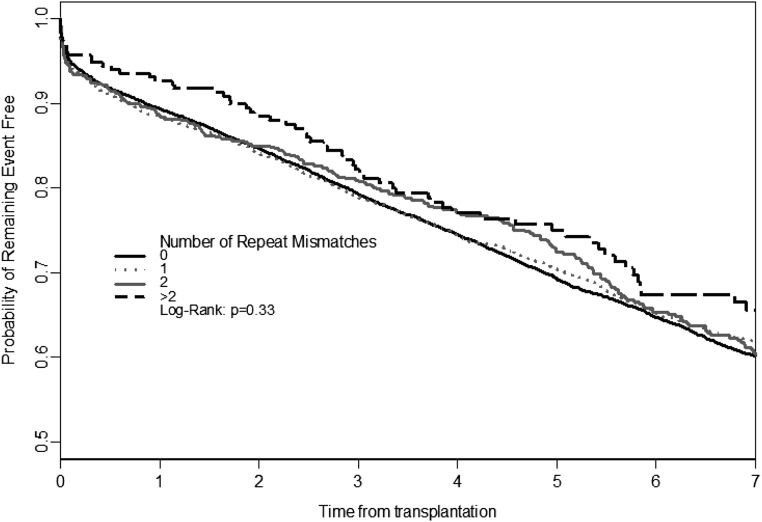
Figure 2 shows the Kaplan–Meier univariate survival curves for ACGL for recipients grouped by RMM. There was no univariate association between RMM and ACGL, and there was no association seen for level of peak PRA before second transplant (data not shown). Similar results were seen for DCGL (data not shown).
Figure 2.
Univariate Kaplan–Meier survival analyses for RMM and ACGL. There is no difference in univariate ACGL between RMM groups. There were also no differences seen when stratified by calculated panel reactive antibody category or considering DCGL (data not shown).
In the multivariable model, RMM is not independently associated with either ACGL or DCGL in the overall cohort (Table 3). This was consistent in analyses stratified by peak PRA before second transplant (data not shown). The CNI used for second transplant did not alter the effect of RMM on ACGL or DCGL, regardless of peak PRA (Table 4).
Table 3.
Cox proportional hazards model for ACGL and DCGL
| Covariate | HR (95% CI) | |
|---|---|---|
| ACGL | DCGL | |
| One or more RMM | 1.03 (0.96 to 1.09) | 1.03 (0.96 to 1.11) |
| Age, yr | ||
| 18–39 | 1.00 | 1.00 |
| 40–49 | 0.96 (0.89 to 1.02) | 0.80 (0.74 to 0.86) |
| 50–59 | 1.08 (1.01 to 1.16)a | 0.71 (0.65 to 0.78) |
| ≥60 | 1.55 (1.43 to 1.69)a | 0.76 (0.67 to 0.86) |
| Men | 1.09 (1.03 to 1.15)a | 1.08 (1.01 to 1.15)a |
| Race | ||
| Black | 1.33 (1.25 to 1.41)a | 1.43 (1.33 to 1.53)a |
| Nonblack | 1.00 | 1.00 |
| ESRD cause | ||
| DM | 1.32 (1.23 to 1.43)a | 1.02 (0.92 to 1.13) |
| GN | 1.00 | 1.00 |
| Other | 1.03 (0.97 to 1.09) | 1.05 (0.98 to 1.12) |
| Duration of first allograft survival, yr | ||
| <1 | 1.00 | 1.00 |
| 1–2.9 | 1.24 (1.14 to 1.34)a | 1.23 (1.12 to 1.35)a |
| ≥3 | 0.93 (0.87 to 0.99)a | 0.85 (0.78 to 0.92)a |
| Deceased donor age, yr | ||
| <40 | 1.00 | 1.00 |
| 40–59 | 1.09 (0.72 to 1.65) | 0.81 (0.49 to 1.36) |
| ≥60 | 1.24 (0.99 to 1.56) | 1.04 (0.82 to 1.33) |
| Second transplant characteristics | ||
| Peak PRA % | ||
| 0 | 1.00 | 1.00 |
| 1–10 | 1.04 (0.95 to 1.15) | 1.08 (0.95 to 1.22) |
| 11–30 | 1.06 (0.96 to 1.18) | 1.09 (0.96 to 1.23) |
| >30 | 1.22 (1.13 to 1.23)a | 1.33 (1.20 to 1.48)a |
| HLA mismatch | ||
| 1 | 1.00 | 1.00 |
| 2 | 1.16 (1.01 to 1.33)a | 1.07 (0.91 to 1.26) |
| 3 | 1.13 (1.00 to 1.29)a | 1.10 (0.95 to 1.29) |
| 4 | 1.18 (1.04 to 1.34)a | 1.18 (1.02 to 1.38)a |
| 5 | 1.23 (1.08 to 1.40)a | 1.18 (1.01 to 1.38)a |
| 6 | 1.29 (1.11 to 1.49)a | 1.21 (1.02 to 1.44)a |
| CSA | 1.00 | 1.00 |
| TAC | 0.94 (0.88 to 1.01) | 0.98 (0.90 to 1.07) |
| Neither CSA nor TAC | 1.38 (1.26 to 1.52)a | 1.41 (1.25 to 1.58)a |
| Aza | 1.00 | 1.00 |
| MMF | 0.97 (0.88 to 1.06) | 0.98 (0.87 to 1.10) |
| Neither Aza nor MMF | 1.08 (0.97 to 1.20) | 1.12 (0.99 to 1.28) |
| Induction | ||
| Depleting Ab | 1.10 (1.02 to 1.20)a | 1.21 (1.10 to 1.34)a |
| Nondepleting Ab | 1.00 | 1.00 |
| Both | 1.39 (1.20 to 1.63)a | 1.54 (1.28 to 1.55)a |
| Neither | 1.00 (0.92 to 1.09) | 1.01 (0.91 to 1.13) |
| Living donor | 1.00 | 1.00 |
| Deceased donor | 1.21 (1.14 to 1.29)a | 1.18 (1.09 to 1.28)a |
| Year of transplant | ||
| 1995–1999 | 2.16 (1.92 to 2.43) | 2.50 (2.17 to 2.79) |
| 2000–2003 | 1.94 (1.74 to 2.16) | 2.11 (1.85 to 2.41) |
| 2004–2007 | 1.51 (1.37 to 1.67) | 1.64 (1.45 to 1.86) |
| 2008–2011 | 1.00 | 1.00 |
DM, diabetes mellitus; CSA, cyclosporin A; TAC, tacrolimus; Aza, azathioprine; MMF, mycophenolic mofetil; Ab, antibody.
Table 4.
No difference in effect of RMM on the basis of calcineurin inhibitor used, regardless of peak PRA before second transplant
| Peak PRA on Second Transplant | HR (95% CI) | |
|---|---|---|
| ACGL | DCGL | |
| Tacrolimus | ||
| Overall | 0.99 (0.92 to 1.08) | 0.99 (0.90 to 1.10) |
| PRA=0 | 0.96 (0.78 to 1.18) | 1.03 (0.80 to 1.32) |
| PRA>0 | 0.94 (0.85 to 1.05) | 0.94 (0.84 to 1.07) |
| Cyclosporin | ||
| Overall | 1.04 (0.93 to 1.17) | 1.10 (0.95 to 1.27) |
| PRA=0 | 0.97 (0.76 to 1.24) | 1.13 (0.83 to 1.54) |
| PRA>0 | 1.13 (0.98 to 1.29) | 1.14 (0.97 to 1.34) |
Of recipients defined as RMM, 440 (11%) met the RMM criteria on the basis of the definition that split antigens in one transplant were a RMM to a corresponding broad parent antigen in the other transplant. An additional 157 (2%) were classified as no RMM, where the broad antigen in the donor(s) was also present in the recipient (Supplemental Table 1). When we repeated the above analyses, excluding these recipients, the overall effect estimates and 95% confidence intervals (95% CIs) were not significantly changed (Supplemental Table 2).
Effect of Class 2 Versus Class 1 RMM in Sensitized Versus Unsensitized Recipients
Presence of any class 2 RMM (compared with no RMM or class 1 only RMM) was independently associated with ACGL and DCGL. Indeed, this effect was, in fact, modified by the presence of presecond transplant PRA; class 2 RMM was associated with DCGL only in those recipients with detectable PRA before second transplant (hazard ratio [HR], 1.15; 95% CI, 1.02 to 1.29) (Table 5).
Table 5.
Class 2 RMM increase risk for DCGL only in sensitized recipients before second transplant
| Covariate | HR (95% CI) | |
|---|---|---|
| ACGL | DCGL | |
| No RMM | 1.00 | 1.00 |
| Class 1 only | 0.98 (0.91 to 1.06) | 0.97 (0.88 to 1.06) |
| Any class 2 | 1.08 (1.00 to 1.17) | 1.11 (1.00 to 1.22) |
| Peak PRA at second transplant | ||
| PRA=0 | ||
| No RMM | 1.00 | 1.00 |
| Class 1 only | 0.91 (0.76 to 1.09) | 0.98 (0.78 to 1.23) |
| Any class 2 | 0.99 (0.82 to 1.20) | 1.07 (0.84 to 1.35) |
| PRA>0 | ||
| No RMM | 1.00 | 1.00 |
| Class 1 only | 0.97 (0.88 to 1.07) | 0.91 (0.81 to 1.03) |
| Any class 2 | 1.11 (1.00 to 1.22) | 1.15 (1.02 to 1.29) |
Models are adjusted for age, sex, race, cause of ESRD, donor age, donor type, duration of first graft survival, HLA match for second graft, PRA for second graft, induction, immunosuppression, and year of transplant.
Nephrectomy of First Allograft Influences the Effect of RMM on the Second Allograft Outcome
Where nephrectomy of the first allograft occurred before the second transplant, then the presence of any RMM was significantly associated with both ACGL (HR, 1.13; 95% CI, 1.01 to 1.26) and DCGL (HR, 1.13; 95% CI, 1.00 to 1.29). Additional analysis reveals that this effect is found only in those patients with nephrectomy and class 2 RMM (HR, 1.30; 95% CI, 1.07 to 1.58) (Table 6) (Supplemental Figure 1).
Table 6.
RMM increased risk of ACGL and DCGL in those recipients with nephrectomy that occurred before second transplant, and class 2 RMM increased risk of ACGL and DCGL only in recipients with nephrectomy of first allograft
| Covariate | HR (95% CI) | |
|---|---|---|
| ACGL | DCGL | |
| Any RMM | ||
| Nephrectomy of first allograft before second transplant, n=1719 | 1.13 (1.01 to 1.26) | 1.13 (1.00 to 1.29) |
| No nephrectomy, n=4454 | 0.94 (0.83 to 1.06) | 0.97 (0.82 to 1.15) |
| Nephrectomy | ||
| No RMM, n=1369 | 1.00 | 1.00 |
| Class 1 RMM alone, n=175 | 1.13 (0.92 to 1.38) | 1.20 (0.94 to 1.54) |
| Any class 2 RMM, n=175 | 1.30 (1.07 to 1.58) | 1.41 (1.12 to 1.78) |
| No nephrectomy | ||
| No RMM, n=3198 | 1.00 | 1.00 |
| Class 1 RMM alone, n=701 | 0.91 (0.81 to 1.03) | 0.90 (0.76 to 1.05) |
| Any class 2 RMM, n=555 | 1.04 (0.92 to 1.18) | 1.06 (0.90 to 1.25) |
Any RMM restricted to Medicare only and first graft failure before 2010 (because claims only go to 2009 and because most nephrectomies happen early after failure).
Discussion
Solid-organ transplantation is a significant risk factor for sensitization to HLA antigens and has potential for long–term B memory cell formation. Alloimmune damage in a second allograft may occur more readily when the recipient immune system is rechallenged, with RMM HLA antigen already recognized from the first transplant. Earlier studies have consistently found that classes 1 and/or 2 RMM are associated with rejection and graft survival adverse outcomes,1–3,8
In comparison, the major finding in this large registry analysis is that RMM is no longer categorically associated with adverse transplant outcomes as had been previously reported. Instead, we show that, in a more modern era (of both immunosuppression and histocompatibility testing), RMM has a deleterious effect on ACGL and DCGL in only biologically well defined subgroups (patients who were sensitized, or nephrectomized with class 2 RMM). As such, RMM alone is no longer sufficient to confer a label of immunologic risk in the majority of retransplanted kidney recipients; a more granular examination of recipient features is required to interpret RMM risk.
The new findings in this study are underscored by the intersection of improved modern era immunosuppression and HLA antibody detection methods on transplant outcomes, and the necessity to re-examine prior paradigms as these contributing variables continue to evolve. In particular, in many earlier RMM studies, detection of low–level HLA antibody was limited by the less–sensitive complement–dependent cytotoxicity crossmatching and PRA technology.12 Indeed, when HLA antibody detection methods were systematically less sensitive, the identification of the RMM was, in fact, one of the only ways (outside of detectable high–level cytotoxic antibodies to an RMM antigen precluding a subsequent transplant) of defining the potential increased risk in humoral immune response. Low-titer antibodies, even if present, may have gone undetected by older crossmatch techniques, permitting more transplants with RMM antigens. However, these low-titer antibodies could still result in negative transplant outcomes from memory responses.13–17 RMM was potentially the best surrogate marker for low-titer (undetected) antibodies in previous era studies.
In our contemporary cohort with potentially improved histocompatibility testing, we hypothesize that lower-level DSA to a RMM is more likely to be identified before transplant, leading to avoidance of the immune-stimulating RMM in the second transplant. An examination of our data shows that sensitized patients in this cohort received fewer second transplants with RMM. This may indicate that RMM antigens, where low-level DSAs are now detectable by newer testing methods, are being avoided in the subsequent transplant. However, we cannot rule out that there was an active clinical decision to avoid RMM, irrespective of DSA detection, and we cannot confirm the sensitivity of antibody detection methodology in a registry analysis. Other potential reasons for differing outcomes in this analysis include more modern immunosuppression, the size of the study population, and the inherent limitations of data and subjects in registry cohorts. In this analysis, the first CNI inhibitor used for the second transplant, regardless of peak PRA, does not alter RMM effect. Interpretation of this observation is limited, however, because we cannot identify factors influencing CNI choices, changes to immunosuppression over time, or centers actively considering RMM in their choice of immunosuppressive protocols. Most importantly, we cannot identify whether recipients were adherent to the prescribed regimen, with nonadherence being a major factor in dnDSA development and consequent poor outcomes.
We performed predefined stratified analyses to examine for the effect of RMM on outcomes considering certain biologically anticipated risk groups. Given the increasing evidence that class 2 antigen differences9–11 and class 2 HLA antibodies18–20 specifically affect graft survival, the effect of class 2 RMM versus only class 1 was explored, and we found that the presence of class 2 RMM was associated with graft loss, specifically in sensitized recipients. This information may be of value in further refining the classification of immunologic risk in recipients with RMM, such that unsensitized recipients with class 2 RMM may be considered at lower risk than their sensitized counterparts. The biologic mechanism underlying this observation cannot be determined from this analysis, but a hypothesis worthy of further investigation is that the peak PRA itself is a marker of the potential immunologic reactivity of the individual. It may be that the pathogenesis of injury incited by RMM is realized specifically in those individuals already shown to mount humoral immune responses.21 Alternatively, the higher peak PRA may simply be a marker of increased likelihood of low-level DSAs that went undetected without precluding transplant. Conversely, RMM seems to have limited effect in patients who have declared themselves, via their peak PRA, to be immunologically less active or have lower chance of DSA. The effect of RMM specifically in patients with DSA versus third party HLA antibody cannot be assessed in this registry dataset.
Nephrectomy can result in an increase in PRA22–25 as well as negatively affect subsequent graft survival.26 Therefore, we specifically examined the effect of RMM in patients with and without nephrectomy of the first allograft occurring before the second transplant and found that class 2 RMM carried increased hazard for DCGL and ACGL, specifically in patients who were nephrectomized. The potential mechanisms supporting this observation warrant further study but would be consistent with increases in measurable alloimmune activity.22–25 These data are difficult to interpret conclusively, because the reason for nephrectomy is not known, and we cannot surmise that avoiding nephrectomy would abrogate this risk. These observations must be weighed against the potential survival benefits that have been reported with other studies of allograft nephrectomy.27
This study has limitations common to all retrospective analyses of administrative databases, where errors and missing data cannot be fully verified. Additionally, HLA typing can only be reliably commented on at HLA-A, -B, and -DRB1 loci, where data were routinely collected during the period of study. Any effect of repeated mismatches at HLA-C, -DQA1, -DQB1, -DPA1, and -DPB1 loci cannot be assessed, however, because HLA-DRB1 and -DQB1 are inherited in strong linkage disequilibrium, DR RMM carries a higher likelihood of associated DQ RMM. We emphasize that this linkage is not absolute for any DR antigen, and DQ RMM cannot be confirmed. More thorough center–level studies with comprehensive HLA typing are necessary to investigate the specific roles of DR and DQ RMM.
Although anticipated to be more advanced than in historical cohorts, crossmatch and PRA methods cannot be verified and may differ in sensitivity between patients and centers. Higher PRA is only a surrogate marker for the presence of HLA DSA, which would be the expected mediator of RMM-induced damage, and the presence or absence of DSA cannot be confirmed. The cause of graft failure is not identified, and this may be an important consideration given the immunologic mechanisms of injury that are invoked for RMM. After censoring for death, however, similar findings remain, and it is likely that chronic alloimmune damage is a major cause of graft loss in this cohort as reported elsewhere.28–32 Finally, HLA typing methods have evolved, such that the HLA antigens identified in one donor may be more fully resolved than the other donor or in the recipient.33 Where this resolution discrepancy existed, we classified the antigens as equivalent. The broad antigen groups, by definition, share many of the same immunogenic epitopes as their derived splits and in an RMM circumstance, would be subject to similar alloimmune injury mechanisms.34,35 We acknowledge that the split RMM assignment may be incorrect in these groups; however, sensitivity analysis excluding these patients with potential broad/split discrepancy did not meaningfully alter the effect estimate. It remains essential that all registry analyses offering insights into new paradigms of risk assessment be interpreted in the context of these limitations and augmented with center/patient-level studies to enhance accurate application in the clinical setting.
Despite these limitations, this analysis remains the largest study of RMM to date. It is potentially more relevant in a contemporary transplant era, and the multivariable modeling approach accounts for more confounders than previously ever considered in other studies. These data are more generalizable to the current transplantation era of immunosuppression and immunologic testing and challenge the prior paradigm that RMM in isolation confers increased risk for graft loss. Application of these results refines immunologic risk assessment further and defines subgroups that may benefit from more detailed pretransplant evaluation. Additional studies are needed to determine if patients in these risk groups would benefit from modified allocation algorithms or immunosuppressive protocols and further characterize the effect of RMM in the setting of clearly defined DSA versus third party HLA antibody.
Concise Methods
Data Source and Study Population
The study population included all adult (≥18 years old) kidney–only transplant recipients captured in the Standard Analysis Files of the USRDS who received a second transplant between January 1, 1995 and October 31, 2011. Patients who received a zero HLA mismatched kidney for either their first or second transplant were excluded.
HLA Typing
During the study period, HLA typing methods have evolved from serologic to molecular platforms. Consequently, the number of recognized HLA antigens has increased, and many of which are now named as serologic split equivalents of previously identified broader antigens. For example, DR15 and DR16 are now recognized as unique antigens but may previously have been typed as the broader antigen DR2. In this analysis, where donors shared a broad/split (in either order) that differed from the recipient, an RMM was coded. Where the recipient also shared the corresponding broad typing, no RMM was coded (Supplemental Table 1). Antigen equivalents were assigned as described in the IMGT/HLA database33,36 and OPTN Policy 4.10 (previously policy 4.8).37 The number of RMM was calculated as a total out of the maximum of six (two each of A, B, and DR). RMM was further classified as class 1 (HLA-A and -B) or class 2 (HLA-DR). Subjects were considered in groups of zero RMM versus one or more RMM. Sensitivity analysis was performed, excluding those patients in whom the split/broad classification had been used to determine RMM status.
Statistical Analyses
Patient characteristics were described using frequencies and proportions and compared between groups using the chi-squared test. Kaplan–Meier survival curves were used to identify differences in ACGL by the number of RMM stratified by PRA before the second transplant. Cox multivariate proportional hazards regression analyses were used to model time from second transplantation to ACGL or DCGL, with follow-up until October 31, 2012. Models were adjusted for age at second transplant, sex, race, cause of ESRD, duration of first graft survival, and characteristics at the time of second transplant (donor type, peak PRA, donor age, immunosuppression, induction, and year of transplant). Patients with missing covariate information were coded as missing for that covariate and included as such in the multivariable models.
Stratified Analyses
To examine for predefined interactions, additional Cox regression analyses were performed in strata defined by (1) PRA peak 0% versus >0% and (2) calcineurin inhibitor (tacrolimus versus cyclosporin). Finally, in the subset of patients insured by Medicare, first transplant nephrectomy was identified using ICD-9-CM Code 55.53 in Medicare claims data, and multivariate Cox proportional hazards models were performed in strata identified by nephrectomy (yes/no) and PRA before second transplant.
Disclosures
No financial support received.
Supplementary Material
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015060626/-/DCSupplemental.
References
- 1.Ting A, Morris PJ: Renal transplantation and B-cell cross-matches with autoantibodies and alloantibodies. Lancet 2: 1095–1097, 1977 [DOI] [PubMed] [Google Scholar]
- 2.Opelz G: Repeated HLA mismatches increase the failure rate of second kidney transplants. Collaborative Transplant Study. Transplant Proc 27: 658–659, 1995 [PubMed] [Google Scholar]
- 3.Cecka JM, Terasaki PI: Repeating HLA antigen mismatches in renal retransplants--a second class mistake? Transplantation 57: 515–519, 1994 [PubMed] [Google Scholar]
- 4.Mjörnstedt L, Konar J, Nyberg G, Olausson M, Sandberg L, Karlberg I: Renal retransplantation in patients with HLA-antibodies. Transpl Int 5[Suppl 1]: S32–S34, 1992 [DOI] [PubMed] [Google Scholar]
- 5.Gjertson DW: A multi-factor analysis of kidney regraft outcomes. Clin Transpl 2002: 335–349, 2002 [PubMed] [Google Scholar]
- 6.Opelz G, Terasaki PI: Absence of immunization effect in human-kidney retransplantation. N Engl J Med 299: 369–374, 1978 [DOI] [PubMed] [Google Scholar]
- 7.Farney AC, Matas AJ, Noreen HJ, Reinsmoen N, Segall M, Schmidt WJ, Gillingham K, Najarian JS, Sutherland DE: Does re-exposure to mismatched HLA antigens decrease renal re-transplant allograft survival? Clin Transplant 10: 147–156, 1996 [PubMed] [Google Scholar]
- 8.House AA, Chang PC, Luke PP, Leckie SH, Howson WT, Ball EJ, Tan AK, Rehman F, Muirhead N, Hollomby DJ, McAlister VC, Hodsman AB, Jevnikar AM: Re-exposure to mismatched HLA class I is a significant risk factor for graft loss: Multivariable analysis of 259 kidney retransplants. Transplantation 84: 722–728, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Wiebe C, Gibson IW, Blydt-Hansen TD, Karpinski M, Ho J, Storsley LJ, Goldberg A, Birk PE, Rush DN, Nickerson PW: Evolution and clinical pathologic correlations of de novo donor-specific HLA antibody post kidney transplant. Am J Transplant 12: 1157–1167, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Wiebe C, Pochinco D, Blydt-Hansen TD, Ho J, Birk PE, Karpinski M, Goldberg A, Storsley LJ, Gibson IW, Rush DN, Nickerson PW: Class II HLA epitope matching-A strategy to minimize de novo donor-specific antibody development and improve outcomes. Am J Transplant 13: 3114–3122, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Sapir-Pichhadze R, Tinckam K, Quach K, Logan AG, Laupacis A, John R, Beyene J, Kim SJ: HLA-DR and -DQ eplet mismatches and transplant glomerulopathy: A nested case-control study. Am J Transplant 15: 137–148, 2015 [DOI] [PubMed] [Google Scholar]
- 12.Gebel HM, Bray RA: Sensitization and sensitivity: Defining the unsensitized patient. Transplantation 69: 1370–1374, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Karpinski M, Rush D, Jeffery J, Exner M, Regele H, Dancea S, Pochinco D, Birk P, Nickerson P: Flow cytometric crossmatching in primary renal transplant recipients with a negative anti-human globulin enhanced cytotoxicity crossmatch. J Am Soc Nephrol 12: 2807–2814, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Kimball P, Rhodes C, King A, Fisher R, Ham J, Posner M: Flow cross-matching identifies patients at risk for postoperative elaboration of cytotoxic antibodies. Transplantation 65: 444–446, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Bray RA, Nickerson PW, Kerman RH, Gebel HM: Evolution of HLA antibody detection: Technology emulating biology. Immunol Res 29: 41–54, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Mahoney RJ, Norman DJ, Colombe BW, Garovoy MR, Leeber DA: Identification of high- and low-risk second kidney grafts. Transplantation 61: 1349–1355, 1996 [DOI] [PubMed] [Google Scholar]
- 17.Ogura K, Terasaki PI, Johnson C, Mendez R, Rosenthal JT, Ettenger R, Martin DC, Dainko E, Cohen L, Mackett T, Berne T, Barba L, Lieberman E: The significance of a positive flow cytometry crossmatch test in primary kidney transplantation. Transplantation 56: 294–298, 1993 [DOI] [PubMed] [Google Scholar]
- 18.Everly MJ, Everly JJ, Arend LJ, Brailey P, Susskind B, Govil A, Rike A, Roy-Chaudhury P, Mogilishetty G, Alloway RR, Tevar A, Woodle ES: Reducing de novo donor-specific antibody levels during acute rejection diminishes renal allograft loss. Am J Transplant 9: 1063–1071, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Walsh RC, Brailey P, Girnita A, Alloway RR, Shields AR, Wall GE, Sadaka BH, Cardi M, Tevar A, Govil A, Mogilishetty G, Roy-Chaudhury P, Woodle ES: Early and late acute antibody-mediated rejection differ immunologically and in response to proteasome inhibition. Transplantation 91: 1218–1226, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Freitas MC, Rebellato LM, Ozawa M, Nguyen A, Sasaki N, Everly M, Briley KP, Haisch CE, Bolin P, Parker K, Kendrick WT, Kendrick SA, Harland RC, Terasaki PI: The role of immunoglobulin-G subclasses and C1q in de novo HLA-DQ donor-specific antibody kidney transplantation outcomes. Transplantation 95: 1113–1119, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Otten HG, Verhaar MC, Borst HP, Hené RJ, van Zuilen AD: Pretransplant donor-specific HLA class-I and -II antibodies are associated with an increased risk for kidney graft failure. Am J Transplant 12: 1618–1623, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Bocrie O, Hussein Aly AA, Guignier F, Funes de la Vega M, Rifle G, Mousson C, Martin L: Distribution of donor-specific antibodies in the cortex and the medulla of renal transplants with chronic allograft nephropathy. Transpl Immunol 17: 227–229, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Martin L, Guignier F, Mousson C, Rageot D, Justrabo E, Rifle G: Detection of donor-specific anti-HLA antibodies with flow cytometry in eluates and sera from renal transplant recipients with chronic allograft nephropathy. Transplantation 76: 395–400, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Lair D, Coupel S, Giral M, Hourmant M, Karam G, Usal C, Bignon JD, Brouard S, Soulillou JP: The effect of a first kidney transplant on a subsequent transplant outcome: An experimental and clinical study. Kidney Int 67: 2368–2375, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Sumrani N, Delaney V, Hong JH, Daskalakis P, Sommer BG: The influence of nephrectomy of the primary allograft on retransplant graft outcome in the cyclosporine era. Transplantation 53: 52–55, 1992 [DOI] [PubMed] [Google Scholar]
- 26.Johnston O, Rose C, Landsberg D, Gourlay WA, Gill JS: Nephrectomy after transplant failure: Current practice and outcomes. Am J Transplant 7: 1961–1967, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Ayus JC, Achinger SG, Lee S, Sayegh MH, Go AS: Transplant nephrectomy improves survival following a failed renal allograft. J Am Soc Nephrol 21: 374–380, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mengel M, Reeve J, Bunnag S, Einecke G, Jhangri GS, Sis B, Famulski K, Guembes-Hidalgo L, Halloran PF: Scoring total inflammation is superior to the current Banff inflammation score in predicting outcome and the degree of molecular disturbance in renal allografts. Am J Transplant 9: 1859–1867, 2009 [DOI] [PubMed] [Google Scholar]
- 29.El-Zoghby ZM, Stegall MD, Lager DJ, Kremers WK, Amer H, Gloor JM, Cosio FG: Identifying specific causes of kidney allograft loss. Am J Transplant 9: 527–535, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Gaston RS, Cecka JM, Kasiske BL, Fieberg AM, Leduc R, Cosio FC, Gourishankar S, Grande J, Halloran P, Hunsicker L, Mannon R, Rush D, Matas AJ: Evidence for antibody-mediated injury as a major determinant of late kidney allograft failure. Transplantation 90: 68–74, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Mannon RB, Matas AJ, Grande J, Leduc R, Connett J, Kasiske B, Cecka JM, Gaston RS, Cosio F, Gourishankar S, Halloran PF, Hunsicker L, Rush D DeKAF Investigators : Inflammation in areas of tubular atrophy in kidney allograft biopsies: A potent predictor of allograft failure. Am J Transplant 10: 2066–2073, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sellarés J, de Freitas DG, Mengel M, Reeve J, Einecke G, Sis B, Hidalgo LG, Famulski K, Matas A, Halloran PF: Understanding the causes of kidney transplant failure: The dominant role of antibody-mediated rejection and nonadherence. Am J Transplant 12: 388–399, 2012 [DOI] [PubMed] [Google Scholar]
- 33.Robinson J, Mistry K, McWilliam H, Lopez R, Parham P, Marsh SG: The IMGT/HLA database. Nucleic Acids Res 39: D1171–D1176, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeevi A, Girnita A, Duquesnoy R: HLA antibody analysis: Sensitivity, specificity, and clinical significance in solid organ transplantation. Immunol Res 36: 255–264, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Duquesnoy RJ, Askar M: HLAMatchmaker: A molecularly based algorithm for histocompatibility determination. V. Eplet matching for HLA-DR, HLA-DQ, and HLA-DP. Hum Immunol 68: 12–25, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holdsworth R, Hurley CK, Marsh SG, Lau M, Noreen HJ, Kempenich JH, Setterholm M, Maiers M: The HLA dictionary 2008: A summary of HLA-A, -B, -C, -DRB1/3/4/5, and -DQB1 alleles and their association with serologically defined HLA-A, -B, -C, -DR, and -DQ antigens. Tissue Antigens 73: 95–170, 2009 [DOI] [PubMed] [Google Scholar]
- 37.OPTN Policy 4.10 Reference Tables of HLA Antigen Values and Split Equivalences. Available at: https://optn.transplant.hrsa.gov/ContentDocuments/OPTN_Policies.pdf. Accessed November 1, 2015
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.