Abstract
The ribonuclease angiogenin is a component of the mammalian stress response, and functions in both cell-autonomous and non-cell-autonomous ways to promote tissue adaptation to injury. We recently showed that angiogenin regulates tissue homeostasis during AKI associated with endoplasmic reticulum (ER) stress through the production of transfer RNA fragments that interfere with translation initiation and thereby alleviate ER stress. However, whether the paracrine signaling mediated by angiogenin secretion is a genuine component of the ER stress response to kidney injury is unknown. Here, we explored the molecular mechanisms by which angiogenin is secreted upon ER stress, and determined how it modulates the inflammatory microenvironment. In cultured renal epithelial cells, ER stress specifically induced angiogenin secretion under the selective control of inositol-requiring enzyme 1α, a key activator of the unfolded protein response. The transcription factors spliced X-box–binding protein 1 and p65, which are activated by inositol-requiring enzyme 1α upon ER stress, each bound the angiogenin promoter and controlled the amount of angiogenin secreted. Furthermore, p65 promoted angiogenin transcription in an ER stress-dependent manner. Similar to secretion of the ER stress-induced proinflammatory cytokine IL-6, secretion of angiogenin required the ER-Golgi pathway. Notably, incubation of human macrophages with angiogenin promoted macrophage reprogramming toward an activated and proinflammatory phenotype. In patients, angiogenin expression increased upon renal inflammation, and the urinary concentration of angiogenin correlated with the extent of immune-mediated kidney injury. Collectively, our data identify angiogenin as a mediator of the ER stress-dependent inflammatory response and as a potential noninvasive biomarker of AKI.
Keywords: acute rejection, cytokines, macrophages, renal tubular epithelial cells, cell activation
The characterization of the molecular basis of cellular responses to stress and their consequences at the tissue level are crucial for the development of preventive and therapeutic strategies in renal medicine. In general, adaptive responses to stress are bimodal, as these mechanisms regulate both intrinsic and extrinsic cell functions.1 Cell intrinsic (or cell-autonomous) responses promote adaptation at the individual cellular level. In parallel, cell extrinsic (or non-cell-autonomous) responses are likely involved in paracrine communication and produce signals that alert neighboring or even distant cells of the presence of a stress. A prototypical example of such a stress is endoplasmic reticulum (ER) stress, which often occurs in glomerular or tubulointerstitial cells in an injured microenvironment, and is involved in the pathophysiology of various renal diseases.2–5 Upon ER stress, the unfolded protein response (UPR) is activated, engaging transcriptional, post-transcriptional and translational programs to reduce the amount of nascent proteins translocated in the ER, and increase ER protein folding capacity. The UPR is transduced by three ER stress sensors: activated transcription factor 6 (ATF6), inositol-requiring enzyme 1α (IRE1α) and protein kinase RNA (PKR)-like ER kinase (PERK). In addition to cell-autonomous functions, the UPR controls non-cell-autonomous responses, including angiogenesis6,7 and inflammation,8,9 which are critical mediators of human renal diseases progression.
The ribonuclease angiogenin (ANG) acts both intracellularly and extracellularly to promote tissue adaptation.10–14 Under stressful conditions, such as heat shock or oxidative stress, cytoplasmic ANG contributes to stress-induced translational repression through the promotion of transfer RNA (tRNA) cleavage.10,15–17 In addition, ANG is secreted (in general by tumor cells) and induces angiogenesis through increase of ribosomal RNA transcription and endothelial cell proliferation.18 Interestingly, ANG has also been described as an acute-phase protein induced during inflammation19,20 with antimicrobicidal properties against bacterial and fungal pathogens.21 Recent evidence indicates that ANG signaling is implicated in the response to AKI. tRNA cleavage by ANG under cellular stress is a regulated process that implies initiation of native tRNA conformational modifications, leading to the loss of the tertiary structure of the molecule, allowing the ribonuclease activity of ANG to access the anticodon loop of the tRNA and promote its cleavage. Unfolded, and therefore precleaved, tRNAs accumulate in the proximal tubules of rat kidneys soon after ischemia-reperfusion injury.22 To monitor tRNA conformational changes under cellular stress, as a marker of tRNA catabolism, antibodies have been raised against the methylated adenosine in position 58 that specifically recognize unfolded tRNA.22 In addition, ANG controls a physiologically relevant ER stress-mediated adaptive translational control mechanism in the kidney in promoting stress-induced tRNA fragments production. ANG is a critical regulator of protection against ER stress-induced kidney injury in mice because the severity of the renal lesions induced by ER stress was dramatically increased in Ang−/− mice.23
In addition to its intracellular functions, ANG is secreted, and whether this paracrine signaling is a genuine component of the ER stress response to kidney injury is currently unknown. In the present study, we have explored the molecular basis of ANG secretion and non-cell-autonomous functions elicited upon ER stress. Our results indicate that ANG is conventionally secreted by the tubular epithelium under the control of the transcription factors NF-κB and spliced X-box binding protein 1 (sXBP1), both of which are necessary for ANG secretion. In addition, ANG may serve as an alarmin produced by epithelial cells to promote activation of macrophages. Lastly, we demonstrate that urinary ANG could be used as a noninvasive marker of immune-mediated tubular injury.
Results
ER Stress Induces ANG Secretion by Renal Epithelial Cells
To test whether ANG is secreted under ER stress, we cultured human renal epithelial cells (HREC), and performed a 24-hour time course of incubation with the ER stressors thapsigargin and tunicamycin. Etoposide, which promotes DNA damage and apoptosis without ER stress in our experimental system (Supplemental Figure 1) was used as control. Eight hours of ER stress induced ANG release in the extracellular milieu (Figure 1A). At this time point, cell death was undetectable, and propidium iodine uptake, indicative of cell membrane permeabilization (and therefore of late apoptosis or necrosis) was similar in cells incubated with etoposide, tunicamycin, thapsigargin or vehicle (Supplemental Figure 2). These results indicate that ANG release is not a nonspecific consequence of cell death and membrane permeabilization, and might rather be selectively controlled by ER stress.
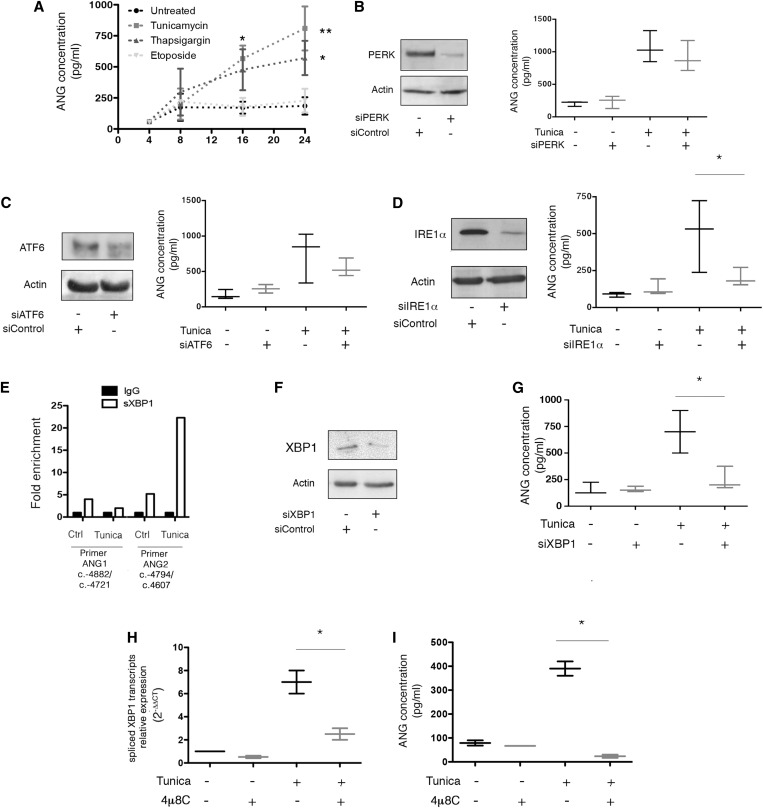
Figure 1.
ER stress induces ANG expression in renal epithelial cells. (A) Graph representing the mean±SEM of ANG concentration measured by ELISA in the extracellular medium during a time course experiment on HREC incubated with 2.5 μg/ml tunicamycin, 0.25 μM thapsigargin, 100 μM etoposide or vehicle. Data are representative of four independent experiments, *P<0.05; **P<0.01; one-way ANOVA, with Dunnett’s test for multiple comparisons to compare treated samples with a single control. (B) (Left) Immunoblot representing PERK and actin expression at the protein level in HREC 24 hours after transfection of a siRNA targeting PERK mRNA. The immunoblot shown is representative of three independent experiments. (Right) Box-and-whisker plots representing ANG concentration measured by ELISA in the extracellular medium of HREC transfected with a siRNA targeting PERK, or a scrambled siRNA, and incubated with 2.5 μg/ml tunicamycin or vehicle for 24 hours. Data are from three independent experiments. (C) (Left) Immunoblot representing ATF6 and actin expression at the protein level in HREC 24 hours after transfection of a siRNA targeting ATF6 mRNA. The immunoblot shown is representative of three independent experiments. (Right) Box-and-whisker plots representing ANG concentration measured by ELISA in the extracellular medium of HREC transfected with a siRNA targeting ATF6, or a scrambled siRNA, and incubated with 2.5 μg/ml tunicamycin or vehicle for 24 hours. Data are from three independent experiments. (D) (Left) Immunoblot representing IRE1α and actin expression at the protein level in HREC 24 hours after transfection of a siRNA targeting IRE1α mRNA. The immunoblot shown is representative of three independent experiments. (Right) Box-and-whisker plots representing ANG concentration measured by ELISA in the extracellular medium of HREC transfected with an siRNA targeting IRE1α, or a scrambled siRNA, and incubated with 2.5 μg/ml tunicamycin or vehicle for 24 hours. Data are from three independent experiments. Mann–Whitney U test: *P<0.05. (E) Histograms representing the results of a ChIP assay of sXBP1, followed by amplification through the RT-qPCR of ANG promoter using primers targeting the promoter. HREC were incubated with 2 μg/ml tunicamycin or vehicle for 2 hours. The graph is representative of two experiments. (F) Immunoblot representing XBP1 and actin expression at the protein level in HREC 24 hours after transfection of an siRNA targeting IRE1α mRNA. The immunoblot shown is representative of three independent experiments. (G) Box-and-whisker plots representing ANG concentration measured by ELISA in the extracellular medium of HREC transfected with an siRNA targeting XBP1, or a scrambled siRNA, and incubated with 2.5 μg/ml tunicamycin or vehicle for 24 hours. Data are from three independent experiments. Mann–Whitney U test: *P<0.05. (H) Box-and-whisker plots representing sXBP1 transcripts relative expression in HREC incubated with 10 μM 4μ8C, or vehicle, and with 2 μg/ml tunicamycin or vehicle, for 24 hours, measured using RT-qPCR. Data are from three independent experiments. Mann–Whitney U test: *P<0.05. (I) Box-and-whisker plots representing ANG concentration in the HREC culture medium, measured by ELISA, after incubation with 10 μM 4μ8C, or vehicle, and with 2.5 μg/ml tunicamycin or vehicle, for 24 hours, measured using RT-qPCR. Data are from three independent experiments. Mann–Whitney U test: *P<0.05.
To determine if and how the UPR regulates ANG expression and release, we knocked-down IRE1α, PERK and ATF6 using small interfering RNA (siRNA)-mediated RNA interference, and monitored ANG release by ER-stressed HREC. We observed that only IRE1α was necessary to ensure ANG release in the extracellular milieu upon ER stress (Figure 1, B–D). Because sXBP1, a transcription factor generated by IRE1α after unconventional splicing, regulates the expression of a set of adaptive UPR genes,24 we tested whether sXBP1 is involved in ANG secretion. sXBP1-mediated chromatin immunoprecipitation (ChIP) assay showed a significant enrichment of the ANG promoter region (Figure 1E), indicating the presence of sXBP1 binding sites in the ANG promoter upon ER stress. To further dissect how sXBP1 regulates ANG expression, we silenced the expression of XBP1, which led to reduced ANG release upon ER stress (Figure 1, F and G), suggesting that the IRE1α-XBP1 axis is involved in ANG release by HREC in the extracellular milieu upon ER stress. In line with these findings, inhibition of the ribonuclease activity of IRE1α with 4μ8C,25 which reduces sXBP1 expression under ER stress in HREC (Figure 1H), is associated with a strong inhibition of the release of ANG (Figure 1I). Together, these results indicate that ANG is released by HREC exposed to ER stress, and that the IRE1α-XBP1 axis is instrumental in this process.
NF-κB Regulates ANG Expression under ER Stress
Because ANG expression is induced upon inflammatory conditions,19 and NF-κB is a signaling node activated by IRE1α through a mechanism that involves TNF Receptor Associated Factor 2 (TRAF2) recruitment and IκB kinase (IKK) activation,26 we tested whether NF-κB could regulate ANG expression. In HREC under ER stress, the cytosolic expression of the inhibitor of NF-κB, IκB, was reduced, and the transcription factor p65/RelA was enriched in nuclei, indicating NF-κB signaling activation by ER stress in our model (Figure 2A). Supporting this, inhibition of IRE1α expression by siRNA-mediated RNA interference reduced NF-κB activation (Figure 2B) and transcription of genes encoding the inflammatory cytokine IL-6, which is controlled by NF-κB (Figure 2C). The expression of the proinflammatory cytokine IL-6 under ER stress was inhibited by the IKK inhibitor BMS345541 (Figure 2D), thus confirming that NF-κB is activated upon ER stress in HREC.
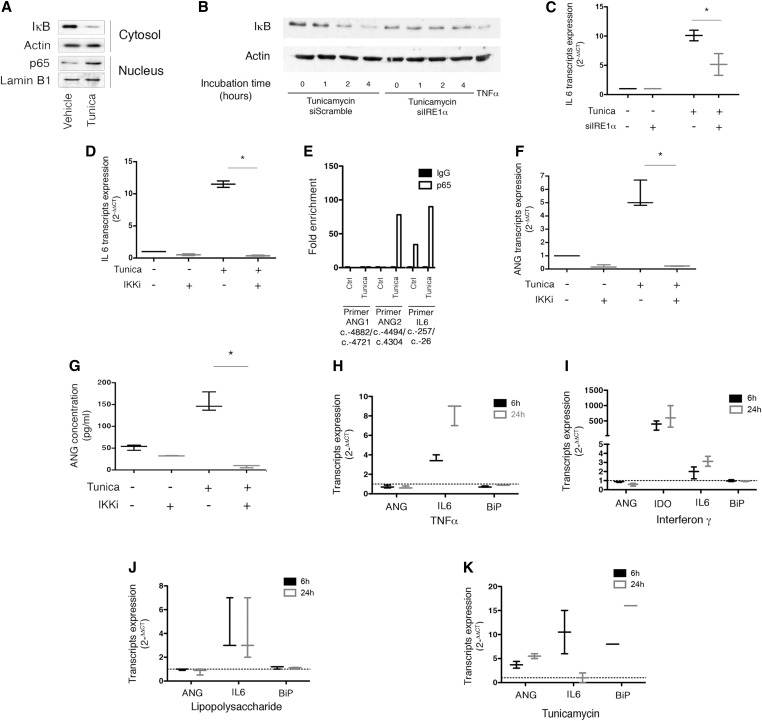
Figure 2.
NF-κB regulates ANG expression and secretion under ER stress. (A) Immunoblot representing IκB and actin expression at the protein level in HREC cytosols, and p65 and lamin B1 in HREC nuclei after 2 hours of incubation with 2.5 μg/ml tunicamycin or vehicle. The immunoblot shown is representative of three independent experiments. (B) Immunoblot representing IκB and actin expression at the protein level in HREC transfected with an siRNA targeting IRE1α, or a scrambled siRNA, and incubated with 2.5 μg/ml tunicamycin or vehicle. The immunoblot shown is representative of three independent experiments. (C) Box-and-whisker plots representing IL6 transcripts relative expression analyzed by RT-qPCR in HREC transfected with an siRNA targeting IRE1α, or a scrambled siRNA, and incubated with 2.5 μg/ml tunicamycin or vehicle for 24 hours. Data are from three independent experiments. Mann–Whitney U test: *P<0.05. (D) Box-and-whisker plots representing IL6 transcripts relative expression analyzed by RT-qPCR in HREC incubated 24 hours with 2.5 μg/mL tunicamycin or vehicle, and coincubated with 1 μM inhibitor of IKK (IKK2 inhibitor, BMS345541). Data are from three independent experiments. Mann–Whitney U test: *P<0.05. (E) Histograms representing the results of a ChIP assay of p65 followed by the amplification by RT-qPCR of ANG promoter using two different primers targeting two different regions of the promoter, and IL6 promoter as a positive control. HREC were incubated with 2.5 μg/ml tunicamycin or vehicle for 2 hours. The histogram is representative of two independent experiments. (F) Box-and-whisker plots representing ANG transcripts relative expression analyzed by RT-qPCR in HREC incubated for 16 hours with 2.5 μg/ml tunicamycin or vehicle, and coincubated with 1 μM BMS345541. Data are from three independent experiments. Mann–Whitney U test: *P<0.05. (G) Box-and-whisker plots representing ANG concentrations measured by ELISA in the extracellular medium of HREC incubated with 2.5 μg/mL tunicamycin or vehicle and coincubated with 1 μM BMS345541 for 24 hours. Data are from three independent experiments. Mann–Whitney U test: *P<0.05. (H) Box-and-whisker plots representing ANG, IL6, and BiP transcripts relative expression analyzed by RT-qPCR in HREC incubated for 6 or 24 hours with 10 ng/mL TNFα or vehicle. Data are from three independent experiments. The dotted line represents 1, the expression level of transcripts in the control condition when measured by the 2(–∆∆CT) method. (I) Box-and-whisker plots representing ANG, indoleamine 2–3 dioxygenase, IL6, and BiP transcripts relative expression analyzed by RT-qPCR in HREC incubated for 6 or 24 hours with 10 ng/mL IFNγ or vehicle. Data are from three independent experiments. The dotted line represents 1, the expression level of transcripts in the control condition when measured by the 2(–∆∆CT) method. (J) Box-and-whisker plots representing ANG, IL6, and BiP transcripts relative expression analyzed by RT-qPCR in HREC incubated for 6 or 24 hours with 10 μg/mL LPS or vehicle. Data are from three independent experiments. The dotted line represents 1, the expression level of transcripts in the control condition when measured by the 2(–∆∆CT) method. (K) Box-and-whisker plots representing ANG, IL6, and BiP transcripts relative expression analyzed by RT-qPCR in HREC incubated for 6 or 24 hours with 2.5 μg/ml tunicamycin or vehicle. The dotted line represents 1, the expression level of transcripts in the control condition when measured by the 2(–∆∆CT) method. Data are from three independent experiments.
The p65-mediated ChIP assay showed a significant enrichment of ANG promoter region, as well as IL-6 promoter (Figure 2E), suggesting that the NF-κB transcription factor p65 could be directly involved in ANG gene transcription. Similar to IL-6, ANG expression was induced by ER stress, and inhibited by BMS345541 (Figure 2F), indicating that during ER stress, NF-κB signaling is involved in the expression of ANG transcripts. Finally, ER stress-induced ANG release was blunted by BMS345541 (most likely through the attenuation of its expression) (Figure 2G). Collectively, these results indicate that NF-κB is involved in the regulation ANG expression and release under ER stress.
Because NF-κB is required for ANG expression under ER stress, we tested whether inflammation mediators that activate NF-κB without ER stress, including the proinflammatory cytokines TNFα and IFNγ, and the TLR4 ligand LPS, could induce ANG expression. Unlike tunicamycin, TNFα, IFNγ and LPS failed to induce ANG expression, despite the fact that they exerted their biologic functions in HREC, and did not induce ER stress (Figure 2, H–K). These results indicate that both p65 and sXBP1 converge to the ANG promoter to induce ANG expression; p65 alone cannot promote ANG expression; and p65 and sXBP1 likely interact to promote ANG expression upon ER stress, but the exact nature of the interaction remains to be clarified. Overall, the activation of NF-κB is necessary, but not sufficient alone, to promote ANG expression, and the activation of both NF-κB and sXBP1 is required for ANG expression and release upon ER stress.
ANG is Conventionally Secreted under ER Stress
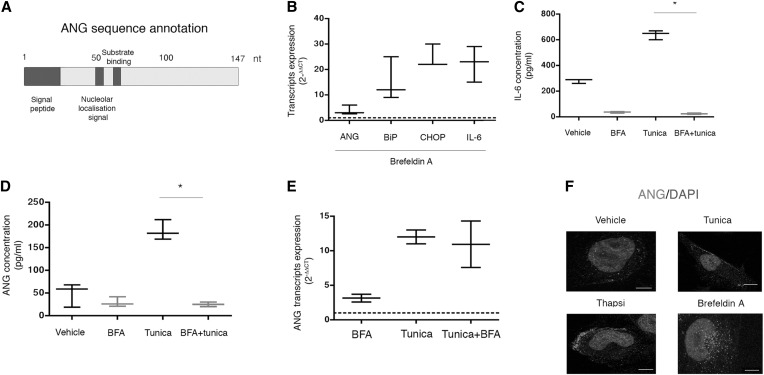
The mechanisms by which ANG is secreted are as yet unknown, but ANG carries a signal sequence for translocation in the ER lumen (Figure 3A), thus suggesting that it might follow the conventional secretory pathway. The hallmark of conventional protein secretion is the trafficking through the ER-Golgi network, a process inhibited by Brefeldin A (BFA).27 Because of the disruption of the secretory pathway, BFA is also an ER stress inducer. Therefore, we monitored ANG secretion by HREC incubated with BFA, using IL-6 secretion as a positive control because IL-6 expression is regulated by sXBP1,28 and is secreted through the conventional secretory pathway.29 As expected, BFA induced the expression of IL-6 and ANG transcripts (Figure 3B), but IL-6 and ANG could not be detected in the extracellular medium (Figure 3, C and D), suggesting that their secretion is inhibited when ER stress is generated by BFA. Furthermore, tunicamycin-induced IL-6 and ANG secretion was blunted by BFA (Figure 3, C and D), and ANG secretion was inhibited by BFA despite the fact that ANG transcripts were still expressed under tunicamycin exposure (Figure 3E). Finally, an immunofluorescence analysis of the subcellular localization of ANG in HREC indicated that ANG is mostly found in the cytoplasm under ER stress induced by tunicamycin and thapsigargin, beneath the plasma membrane with an asymmetric aspect reminiscent of a polarized process of secretion, whereas in HREC incubated with BFA, ANG remained localized close to the nucleus (Figure 3F). Together, these results indicate that the intracellular trafficking of ANG during ER stress is altered by BFA, and that ANG is likely secreted through the canonical path under ER stress.
Figure 3.
ANG is conventionally secreted under ER stress. (A) Schematic representation of the ANG protein sequence. The functional domains are annotated in dark gray. (B) Box-and-whisker plots representing ANG, Binding immunoglobulin protein (BiP), CCAAT-enhancer-binding protein homologous protein (CHOP) and IL-6 transcripts relative expression analyzed by RT-qPCR in HREC incubated for 16 hours with 5 μg/ml BFA or vehicle. Data are from three independent experiments. The dotted line represents 1, the expression level of transcripts in the control condition when measured by the 2(–∆∆CT) method. (C,D) Box-and-whisker plots representing (C) IL-6 and(D) ANG concentrations measured by ELISA in the extracellular medium of HREC incubated with 2.5 μg/ml tunicamycin or vehicle and coincubated with 5 μg/ml BFA for 24 hours. Data are from three independent experiments. Mann–Whitney U test: *P<0.05. (E) Box-and-whisker plots representing ANG transcripts relative expression analyzed by RT-qPCR in HREC incubated for 16 hours with 5 μg/ml BFA, 2.5 μg/ml tunicamycin, both, or vehicle. Data are from three independent experiments. The dotted line represents 1, the expression level of transcripts in the control condition when measured by the 2(–∆∆CT) method. (F) Immunofluorescence analysis by confocal microscopy of ANG expression in HREC incubated for 8 hours with 2.5 μg/ml tunicamycin, 0.25 μM thapsigargin, 5 μg/ml BFA, or vehicle. The bar represents 10 μm.
ANG Promotes Activation of Macrophages
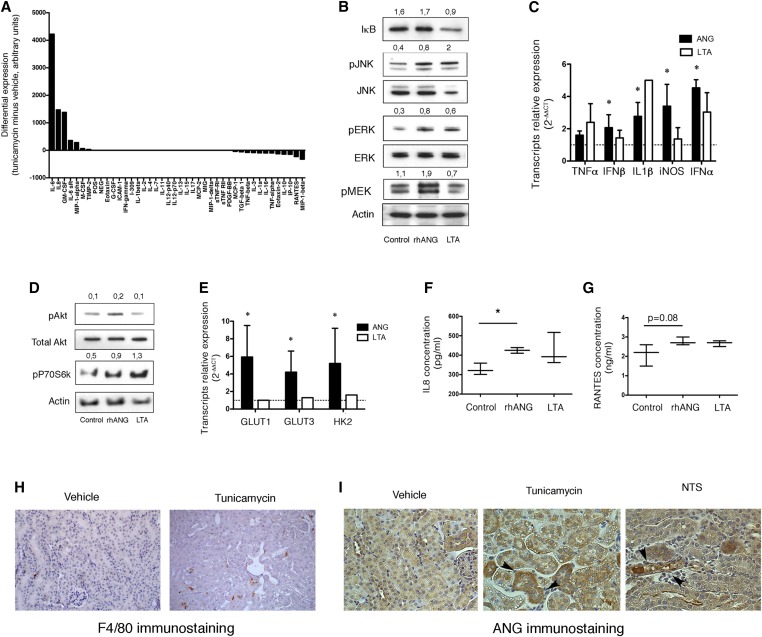
Based on the fact that ER stress can elicit a paracrine response though the secretion of soluble mediators,30–32 we considered that ANG could act as a mediator produced by HREC under ER stress that would transmit alarm signals on neighboring cells. Given that the paracrine crosstalk between renal epithelial cells and macrophages, which reside in the interstitium, is critical in kidney diseases,33 we tested whether ANG, as part of the ER stress-associated secretome, would shape the phenotype of macrophages. Supporting this, the proinflammatory secretome (assessed with a multiplexed cytokine detection array, which does not contain ANG) produced by HREC incubated with tunicamycin contains soluble mediators that directly target macrophages, like GM-CSF and MIP-1α (Figure 4A). To test whether ANG promotes macrophages activation, we incubated human macrophages with recombinant human ANG (rhANG), and monitored mitogen activated protein kinases signaling (including c-Jun N-terminal kinase [JNK], stress-activated protein kinase [SAPK], extracellular signal-regulated kinase [ERK], and Mitogen/Extracellular signal-regulated Kinase (MEK)), and NF-κB signaling, which are critical for macrophage activation.34 rhANG induced the phosphorylation of ERK, JNK, and MEK after 2 hours of incubation, but did not activate the NF-κB pathway, using IκB degradation as a primary readout (Figure 4B). Notably, ERK, JNK, and MEK activation was transient, and disappeared after 4 hours of stimulation with rhANG (not shown). Consistent with this, rhANG induced the expression of class I interferons, TNFα, IL1β and inducible nitric oxide synthase (Figure 4C). In addition, the Akt-mTOR pathway was activated by rhANG (Figure 4D), associated with the expression of genes involved in glycolysis (Figure 4E), suggesting that rhANG promotes metabolic reprogramming in macrophages, consistent with an activated phenotype.35,36 Of note, we did not detect HIF-1α and Myc expression in this model (not shown). In line with an activating effect of rhANG on macrophages, the secretion of the proinflammatory chemokines IL8 (CX-CL8) and regulated upon activation, normal T cell expressed and secreted (RANTES; CCL5) was increased upon rhANG exposure (Figure 4, F and G). Together, these results indicate that ANG can activate macrophages toward a proinflammatory phenotype, at least in vitro. In mice treated with tunicamycin, macrophages accumulate in the renal interstitium in a greater extent compared with nontreated mice (Figure 4H). Nevertheless, the precise contribution of ANG versus the other alarm signals, cytokines and chemokines, produced upon ER stress-induced tubular injury and necrosis, to the macrophage accumulation or proliferation remains to be clarified. Supporting a role for ANG in inflammatory cell accumulation upon ER-stress associated AKI (tunicamycin injection and nephrotoxic serum-induced glomerulonephritis, which is associated with tubular ER stress23), tubules that strongly express ANG, as assessed by immunohistochemistry, were often surrounded by inflammatory infiltrates (Figure 4I). Interestingly, these tubules are also less injured compared with those weakly expressing ANG, supporting the cytoprotective role of ANG.23
Figure 4.
ANG activates human macrophages. (A) Histogram representing the expression of inflammation mediators secreted by HREC using the DotBlot cytokine array (RayBio Human Inflammation Antibody Array 3). Data are representative of two independent experiments. (B) Immunoblots representing IκB, phospho JNK and total JNK, phospho ERK and total ERK, phospho MEK and actin at the protein level in human macrophages incubated for 2 hours with 1 μg/ml recombinant human ANG, 2 μg/ml Lipoteichoic acid (LTA), or vehicle. Results of the densitometric analysis are showed. The immunoblots shown are representative of three independent experiments. (C) Histograms representing TNFα, IFNβ, IL1β, IFNβ, iNOS, and IFNα transcripts relative expression analyzed by RT-qPCR in human macrophages incubated for 4 hours with 2 μg/ml LTA, 1 μg/ml recombinant human ANG, or vehicle. Data are from four independent experiments. *P<0.05, Mann–Whitney test. The dotted line represents 1, the expression level of transcripts in the control condition when measured by the 2(–∆∆CT) method. (D) Immunoblots representing phospho Akt, total Akt, phospho P70S6Kinase and actin at the protein level in human macrophages incubated for 2 hours with 1 μg/ml recombinant human ANG, 2 μg/ml LTA, or vehicle. Results of the densitometric analysis are showed. The immunoblots shown are representative of three independent experiments. (E) Histograms representing GLUT1, GLUT3, and Hexokinase 2 transcripts relative expression analyzed by RT-qPCR in human macrophages incubated for 4 hours with 2 μg/ml LTA, 1 μg/ml recombinant human ANG, or vehicle. Data are from four independent experiments. *P<0.05, Mann–Whitney test. The dotted line represents 1, the expression level of transcripts in the control condition when measured by the 2(–∆∆CT) method. (F) Box-and-whisker plots representing IL8 (CX-CL8) concentrations in the culture medium of human macrophages incubated for 4 hours with 2 μg/ml LTA, 1 μg/ml recombinant human ANG, or vehicle. Data are from three independent experiments. *P<0.05, Mann–Whitney test. (G) Box-and-whisker plots representing RANTES (CCL5) concentrations in the culture medium of human macrophages incubated for 4 hours with 2 μg/ml LTA, 1 μg/ml recombinant human ANG, or vehicle. Data are from three independent experiments. Mann–Whitney test. (H) Representative photomicrographs of F4/80 (macrophage marker) expression in kidneys of 5 mice 96 hours after injection of 1 mg/kg tunicamycin, evaluated using immunohistochemistry. Original magnification ×200. (I) Representative photomicrographs of ANG expression in kidneys of 5 mice 96 hours after injection of 1 mg/kg tunicamycin, or 14 days after anti- glomerular basement membrane nephrotoxic serum injection, all being evaluated using immunohistochemistry. Original magnification ×200.
ANG is a Noninvasive Marker of Tubular Injury
Given that ANG is secreted by the renal epithelium under stressful conditions in vitro, we wondered if it could be detected in the urine of individuals with a renal tubular epithelial injury. Supporting this possibility, we found that ANG expression was increased only in epithelial cells and not in glomerular, or infiltrating immune cells, in kidney allografts with tissue injury, and was accumulated beneath the apical membrane of the cells, a feature suggestive of a secretion process (Figure 5A).
Figure 5.
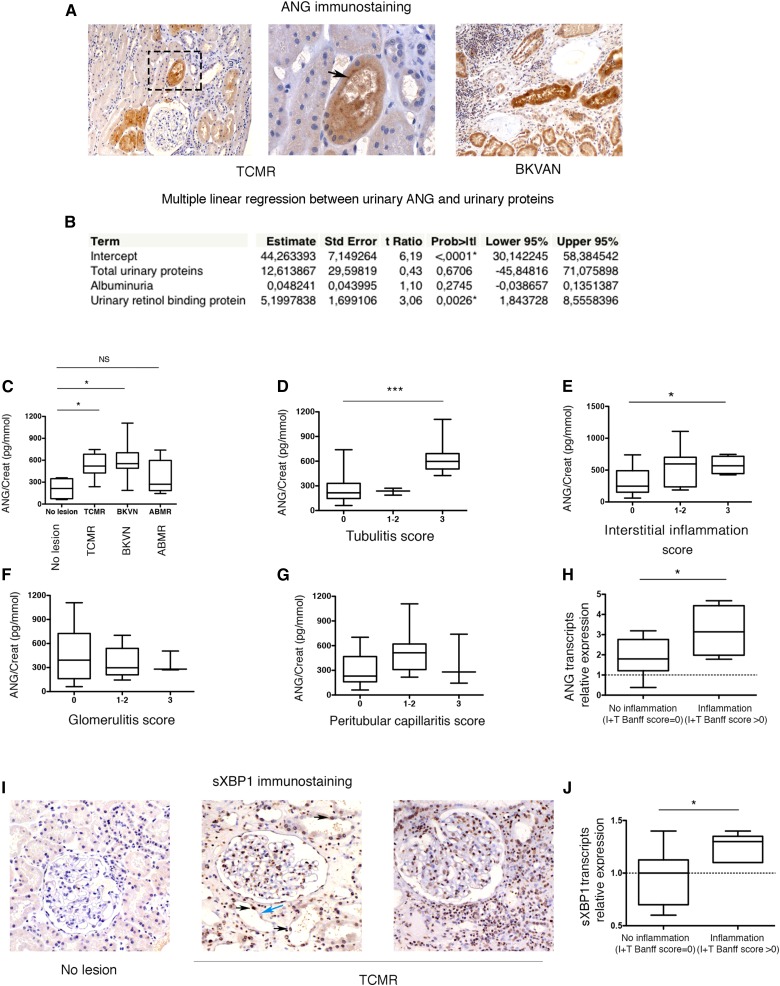
ANG is secreted in the urine of individuals with kidney injury. (A) Representative photomicrograph of ANG expression evaluated by immunohistochemistry in kidney allografts from an individual with TCMR (left) and an individual BKVAN (right). Original magnifications ×10 and ×100. The black arrow denotes ANG concentrated beneath the apical plasma membrane of the epithelial cell. (B) Multiple linear regression analysis between urinary ANG, total proteinuria, albuminuria, and urinary RBP as explanatory variables, in a cohort of 166 individuals under investigation for a kidney disease, using a partial least-squares model. All variables were reported by the value of creatininuria. (C) Box-and-whisker plots representing the ANG/creatinine ratio in the urine of 28 KTRs with or without AKI: TCMR (n=7), BKVN (n=7), ABMR (n=7), or no lesion (n=12). *P<0.05, one-way ANOVA with Dunnett’s correction test for multiple comparisons to a single control group (KTR without lesion). (D–G) Box-and-whisker plots representing the urinary ANG/creatinine ratio of the same cohort of 28 KTRs according to the semiquantification of tubulitis, interstitial inflammation, glomerulitis and peritubular capillaritis, using the Banff score. *P<0.05, ***P=0.001, one-way ANOVA with Dunnett’s correction test for multiple comparisons to a single control group (KTR without lesion). (H) Box-and-whisker plots representing the distribution of ANG transcripts according to T+I Banff scores in 16 kidney transplant biopsies using RT-qPCR. The ANG/RPL13A ratio was calculated compared with a normal kidney cDNA. *P<0.05, Mann–Whitney test. (I) Representative photomicrograph of sXBP1 expression evaluated by immunohistochemistry in kidney allografts from individuals with TCMR or no lesion. Original magnifications ×10 and ×40. The black arrows denote sXBP1-positive epithelial cells nuclei, and the blue arrow denotes an sXBP1-negative nuclei. (J) Box-and-whisker plots representing the distribution of sXBP1 transcripts according to T+I Banff scores in 16 kidney transplant biopsies using RT-qPCR. The sXBP1/RPL13A ratio was calculated compared with a normal kidney cDNA. *P<0.05, Mann–Whitney test.
Therefore, we measured the concentrations of ANG in the urine of individuals with kidney disease. We first aimed at providing insights into the specificity of urinary ANG with regard to the other components of proteinuria. To this end, we measured the concentrations of ANG in the urine of 166 consecutive patients referred to a nephrology department for a CKD (defined by presence of significant proteinuria and/or an estimated GFR<60 ml/min per 1.73 m2). ANG concentration was reported to the urinary concentrations of creatinine to avoid bias related to variations in urine concentration. We tested the correlation between the levels of urinary ANG and the low molecular weight protein retinol-binding protein (RBP, a marker of tubular injury37) and the high molecular weight protein albumin (a marker of glomerular injury), and total proteinuria, in a multiple linear regression analysis (partial least-squares fit model). Indeed, the presence of ANG in urine could be a nonspecific consequence of increased glomerular permeability, and therefore a component of total proteinuria. Moreover, albumin is a carrier for ANG, and the presence of ANG in urine could be a consequence of albuminuria. We found that the urinary concentrations of ANG did not correlate with total urinary proteins and albuminuria, but with RBP (Figure 5B). Because any increase in the urinary excretion of RBP is specific for tubular disease,37 these findings indicate that increased ANG concentration in urines likely reflects an ongoing tubular injury.
Because ANG is secreted by the tubular epithelium under inflammatory conditions, we next tested if urinary ANG would be increased in pathologic situations of immune insults targeting tubular cells. To this end, we measured the concentrations of ANG in urine of kidney transplant recipients (KTR) who underwent an indication biopsy (i.e., renal allograft dysfunction). We compared individuals with T cell mediated rejection (TCMR) and BK virus associated nephropathy (BKVN), two pathologic situations with strong immune tubular injury, exemplified by the so-called “tubulitis” lesion characterized by the presence of inflammatory cells in the tubular wall, and compared them with individuals with antibody-mediated rejection (ABMR), or no obvious histologic lesion, which are usually exempt of acute tubular injuries. The urinary ANG/creatinine ratio was significantly higher in the urine of individuals with TCMR or BKVN compared with those with ABMR or no lesion (Figure 5C). In line with these findings, the urinary concentration of ANG was significantly higher in individuals with high tubulitis scores (which semiquantitatively reflects the severity of tubulitis) and interstitial inflammation according to the Banff classification38 in this cohort (Figure 5, D and E), but not with in those with high glomerulitis or peritubular capillaritis scores (Figure 5, F and G). In line with an increased expression of ANG associated with renal inflammation, the relative expression of ANG transcripts in kidney allograft biopsies, systematically performed 3 months after transplantation, was significantly higher in tissue samples with inflammation (T+I score, according to the Banff classification) (Figure 5H). These results indicate that ANG expression levels, both at the transcripts level and at the secretion level, are positively associated with renal inflammation. Notably, the number of ANG-positive tubules, as estimated by the means of immunohistochemistry, did not actually correlate with the intensity of immune infiltrates on the KTR biopsies analyzed (not shown). Because ANG is mostly secreted, tubular staining is not a reliable marker for estimating the intensity of ANG expression and consequently for determining a potential link with inflammation.
sXBP1 regulates ANG expression in epithelial cells in culture, and we wondered whether it is expressed in allografts with immune-mediated injuries. To do this, we performed an immunohistochemical analysis of sXBP1 expression in biopsies from KTR whose urine was analyzed for ANG concentration measurements using a monoclonal antibody produced by immunizing mice with a Keyhole Limpet Hemocyanin-coupled synthetic peptide corresponding to residues localized in the C-terminus domain, and purified from ascites by Protein A chromatography. As a proof for specificity, a subset of lymphocytes circulating in glomerular capillaries or infiltrating tissues under TCMR were sXBP1-positive (Figure 5I), consistent with the critical role for sXBP1 in B cells homeostasis and maturation.39–41 Of note, the staining was nuclear, in line with the fact that sXBP1 is a transcription factor. We found that nuclei from numerous epithelial cells, but not all, were sXBP1-positive, suggesting that the IRE1α-XBP1 axis of the UPR is activated in immune-injured kidney allografts. Along similar lines, analysis of ANG and sXBP1 expression by immunohistochemistry on consecutive slices of kidney biopsies indicates that the same structure can express both ANG and sXBP1 (Supplemental Figure 3). Nonetheless, cells with sXBP1-positive nuclei can lack ANG accumulation, and vice versa, suggesting that, in vivo, sXBP1 nuclear expression is not systematically associated with ANG accumulation in the cell, and that ANG expression can be under the control of other factors independent of sXBP1. In line with the implication of sXBP1 in mediating renal inflammation, the expression of sXBP1 transcripts was significantly higher in biopsies with inflammatory lesions compared with those without lesions (Figure 5J).
Discussion
In the present study, we describe a non-cell-autonomous response mediated through ANG during ER stress in the kidney. Together with our recent study demonstrating that ANG engages a cell-autonomous adaptive response, which implicates translation inhibition under ER stress,23 the present work provides a prototypical example of the dichotomy in cellular stress and defense responses which is a the origin of cell-autonomous (cell-intrinsic) mechanisms of stressor elimination and stress adaptation, and equally important non–cell-autonomous (cell-extrinsic) responses containing gene products that are likely involved in paracrine communication to neighboring cells.1 Overall, our results indicate that stress-induced ANG is integrated to the UPR and mediates the original biologic pathways involved in protein synthesis inhibition through RNA interference-based mechanisms, and a paracrine signaling leading to activation of macrophages. There are only few examples of proteins regulated under ER stress that act in both intracellular and extracellular mechanisms to promote cellular adaptation and modulate extracellular homeostasis. For example, ERdj3 is a cochaperone that influences the trafficking of ER client proteins and mediates extracellular proteostasis through cosecretion with misfolding-prone proteins.32 Another example comes from studies of prostate apoptosis response 4, a pro-apoptotic protein that resides in both the cytoplasm and the nucleus, and when secreted under ER stress, promotes the apoptosis of cancer cells.30
Our findings indicate that ANG might modulate innate immunity in injured kidneys, at least under ER stress, and are reminiscent of those obtained by Mahadevan and colleagues who demonstrated that tumor cells under ER stress transmit paracrine signals (not yet identified) to macrophages which direct them toward a proinflammatory phenotype.42 Resident macrophages can detect disruptions of tissue homeostasis and secrete signals that orchestrate tissue-level defense and adaptation. Following kidney injury, activated macrophages play a critical role in tissue remodeling, eventually leading to scarring and CKD.33 A further characterization of the biologic consequences of ANG on tissue-resident innate immune cells, in terms of activation and polarization, in an inflammatory milieu, will specify whether ANG triggers or participates in tissue inflammation mediated through ER stress under sterile or septic conditions following AKIs such as ischemia-reperfusion injury, and eventually participate in the occurrence of CKD following AKI. The role of the secretion of ANG in vivo on macrophage activation and proliferation is difficult to assess because ANG has intracellular and extracellular functions. Ang−/− mice with ER stress-related AKI have more severe renal lesions than their wildtype counterparts.23 Consequently, tubular cell necrosis observed in Ang−/− will generate damage-associated molecular patterns that would favor macrophage activation and proliferation. On the other hand, secreted ANG can also impact macrophage phenotypes upon ER stress, but the phenotypic consequences of the lack of ANG secretion on macrophages in Ang−/− mice will be blurred by increased tubular necrosis. In addition, numerous mediators of inflammation are produced by renal epithelial cells upon ER stress-induced AKI. Therefore, the specific contribution of ANG versus the other alarm signals, cytokines and chemokines, produced upon ER stress during AKI, to the macrophage phenotypic changes is difficult to delineate precisely. This drawback could be adequately addressed using engineered mutant mice that express ANG in tubular cells but cannot secrete it.
Our findings indicate that ANG may serve as an alarm signal generated by injured tissues: once secreted, ANG would promote interstitial macrophage activation through an as yet unidentified mechanism. In this prospect, the fact that ANG is rapidly secreted using the ER-Golgi secretion pathway, but not released by apoptotic cells, and may activate cells of the innate immune system corresponds to the properties of an alarmin. Nonetheless, the identification of the ANG receptor will greatly improve our understanding of the molecular biology of the paracrine functions of ANG, and the mechanism by which ANG activates macrophages. One can speculate that ANG can activate macrophages through a receptor-mediated PI3K-Akt signaling.43 Another possibility would be that tRNA fragments produced by ANG after endocytosis would activate intracellular pattern recognition receptors that recognize RNA, such as Retinol Inducible Gene-1, leading to an inflammatory reaction.
The clinical relevance of the secretion of ANG for diagnostic purposes is underscored by our findings suggesting that ANG could be a noninvasive marker of acute tubular injury. Consistent with the secretion of ANG by epithelial cells, ANG might serve as a diagnostic marker of tissue injury, including TCMR and BKVN. The findings of the present study suggest that factors contributing to acute tubular injury under an inflammatory microenvironment might promote ER stress in the renal epithelium, and the secretion of molecules under the control of the UPR, such as ANG, can be detected and quantified in urine samples. Consequently, a detailed analysis of the secretome generated in renal epithelial cells under ER stress will be a source for potential noninvasive biomarkers of kidney injury. The factors that promote ER stress in epithelial cells under immune injury have not yet been identified, but the evidence indicates that there are multiple mechanisms for modulating the UPR during immune response.8,9 Numerous pathogen-derived molecular patterns activate ER stress or selectively activate UPR arms,8 including virus44; therefore, BK virus or its related pathogen-derived molecular patterns, such as ribonucleic acids, could activate the UPR; in addition, microenvironmental metabolic disturbances operating under renal inflammation, including ischemia, oxidative stress, or competition between epithelial cells and immune cells for nutrients, could also theoretically induce ER stress. Finally, because ER stress promotes the secretion of inflammatory cytokines and chemokines, an auto-amplification loop could occur during immune-mediated tubular injury between the epithelium and the infiltrating immune cells.
In conclusion, we have demonstrated that ANG, a stress-induced ribonuclease, is directly and specifically secreted under ER stress by the renal epithelium. The most significant finding of our study comes from the integration to the UPR of an original biologic pathway involved in paracrine signaling: our results provide mechanistic insights into how ANG is secreted by the renal epithelium under ER stress and activates macrophages. In addition, our findings might contribute to the development of new diagnostic tools: the detection of ANG in urine could be used to monitor active kidney injury in its early stage, and to identify individuals at high risk for disease evolution.
Concise Methods
Cell Culture and Chemicals
Normal HREC of proximal origin (HK-2) were purchased from ATCC/LGC Standards, lot No. 710257641. Detailed methods are available in the Supplemental Material. The human monocytic cell line THP-1 was purchased from ATCC/LGC Standards. Macrophages were derived from these monocytes using 200 nM phorbol 12-myristate 13-acetate (Sigma-Aldrich, St. Louis, MO) for 3 days, and enhanced by removing the phorbol 12-myristate 13-acetate–containing media and then incubating cells in fresh RPMI 1640 (10% FCS, 1% l-glutamine) for a further 5 days. Tunicamycin, thapsigargin, BFA, BMS345541, lipoteichoic acid, LPS and 4μ8C were from Sigma-Aldrich. IFNγ and TNFα were purchased from Life Technologies. Recombinant human ANG (carrier-free) was from purchased from RD Systems and resuspended in PBS.
RNA Extraction and Real-time Quantitative PCR (RT-qPCR)
Total RNA was extracted using the RNeasy Mini Kit (Qiagen, Germantown, MD) according to the manufacturer’s protocol. Transcript expression levels were quantified through SYBR green RT-qPCR using an ABI PRISM 7900 sequence detector system (Applied Biosystems). Vehicle-treated samples were used as controls, and the fold-changes for each tested gene were normalized to the Ribosomal Protein L13A (RPL13A) housekeeping gene. The relative expression levels were calculated using the 2–∆∆CT method.45 By definition, the expression level of a given gene in control sample, using the 2–∆∆CT method to calculate relative expression levels, is 1. Primers sequences are listed in Supplemental Table 1A.
Fluorescent Microscopy
Detailed methods are available in the Supplemental Material.
Chromatin Immunoprecipitation Assay
ChIP was performed according to the manufacturer’s protocol (EZ-Magna CHIP G; EMD Millipore, Fontenay sous Bois, France). Detailed methods are available in the Supplemental Material.
ELISA
ELISA In Vitro
Subconfluent cells were grown in 6- or 12-well plates for the indicated times under the indicated conditions. The secretion of ANG and IL-6, IL8 and RANTES was quantified in the cell culture supernatant using the Quantikine human ANG, IL6, IL8, and RANTES immunoassays (RD Systems, Lille, France), respectively, according to the manufacturer’s protocol.
Urinary ANG quantification by ELISA
ANG was quantified using the Quantikine human ANG immunoassay (RD Systems), respectively, according to the manufacturer’s protocol. ANG concentrations were corrected for urinary creatinine levels. At the time of biopsy, urine samples were collected and stored at –80°C.
Protein Extraction and Western Blot Analysis
Total protein lysate from HREC was separated by SDS-PAGE under denaturing conditions and transferred to a polyvinylidene fluoride membrane (GE Healthcare, Waukesha, WI). Primary antibodies (listed in Supplemental Table 1B) were visualized using horseradish peroxidase-conjugated polyclonal secondary antibodies (Dako, Les Ulis, France) and detected by ECL reagent (GE Healthcare).
siRNA transfections
Two different siRNAs directed against the same target were transfected using HiPerfect transfection reagent according to the manufacturer’s protocol: PERK (Hs_EIF2AK3_5; Hs_EIF2AK3_6), IRE1α (Hs_ERN1_17; Hs_ERN1_17), ATF6 (Hs_ATF6_5; Hs_ATF6_5), XBP1 (Hs_XBP1_7; Hs_XBP1_10). Cells were incubated with siRNA for 24 hours before conducting the experiments. The control siRNA we used is a scrambled siRNA called AllStars Negative Control siRNA (Qiagen, Ref 1027281). AllStars Negative Control siRNA siRNA has no homology to any known mammalian gene.
Cytokine Arrays
Subconfluent cells were grown in 6-well plates. Cytokine expression was evaluated in the cell culture supernatant using the RayBio Human Inflammation Antibody Array 3 (AAH-IFN-3) according to the manufacturer’s protocol. The signal intensities were quantified by densitometry after background subtraction and positive controls were used to normalize the results from the different membranes being compared. Evaluations of the relative cytokine expression levels were made by comparing the signal intensities between the different conditions.
Experimental Animal Models
Tunicamycin (1 mg/kg) or vehicle (dimethylsulfoxide) was intraperitoneally injected at day 0, and C57/BL6 mice were sacrificed 2 and 4 days postinjection. Kidneys were then processed for F4/80 and ANG immunohistochemistry staining. Five mice per group were analyzed. Anti-glomerular basement membrane nephrotoxic serum was injected to C57Bl6/J mice through the retro-orbital venous sinus at 6 μl/g body wt for 3 days continuously. Animals were euthanized on day 14.
Human Studies
Urinary ANG Concentration Measurements in Individuals with CKD
One hundred sixty-six consecutive patients who were referred to the Nephrology Department at the Georges Pompidou European hospital (Paris, France) for kidney biopsy were included. Indications for biopsy were estimated GFR<60 ml/min and/or proteinuria >0.5 g/l. Kidney biopsies were not performed for the purpose of this noninterventional study, but only for patient care. At the time of biopsy, urine samples were collected for routine clinical chemistry analyses and stored at –80°C.
Urinary ANG Concentration Measurements in KTRs
Urinary ANG was quantified in the urine of 28 KTR who underwent a biopsy for cause at the Necker hospital with similar renal function and proteinuria <0.5 g/l. This group of KTRs belongs to a recently published cohort.46 For the present pilot study, 4 groups of 7 KTR were randomly selected according to the histologic diagnosis: no lesion (n=7), TCMR (n=7), BKVN (n=7), and ABMR (n=7). At the time of biopsy, urine and blood samples were collected for routine clinical chemistry analyses and stored at –80°C.
Immunohistochemistry of Human Kidney Biopsies with Acute Injury
Twelve kidney allograft biopsies from the group of KTR in which urinary ANG has been measured (3 normal, 3 with TCMR, 3 with BKVN, and 3 with ABMR) were retrospectively analyzed for ANG and sXBP1 immunohistochemistry studies.
RNA Isolation from Kidney Transplant Biopsies
Sixteen surplus kidney allograft protocol biopsies, performed 3 months after transplantation, were retrospectively analyzed for ANG and sXBP1 mRNA expression. Detailed methods are available in the Supplemental Material.
Approvals
Analyses were performed anonymously. Participants provided written consents, and the Paris Descartes University ethics committee (Comité de Protection des Personnes/Patients Protection Committee) approved this study.
Immunohistochemistry
Kidney biopsies were fixed in alcohol-formalin-acetic acid, dehydrated with ethanol and xylene, embedded in paraffin, and cut into 3 μm sections. Samples were then deparaffinized, rehydrated and heated for 20 minutes at 97°C in citrate buffer. Endogenous peroxidase was inactivated by incubation for 10 minutes at room temperature in 0.3% H2O2. Sections were incubated with PBS containing 1:20 anti-ANG (sc-9044), or 1:100 anti sXBP1 (clone 2G4–3E11–3E9). XBP1s 2G4–3E11–3E9 is a mouse monoclonal antibody of IgG1 isotype produced by immunizing animals with a synthetic peptide (Keyhole Limpet Hemocyanin-coupled) corresponding to residues localized in the C-terminus domain, and purified from ascites by Protein A chromatography. Next, sections were incubated with anti-goat or anti-rabbit antibody conjugated with peroxidase-labeled polymer (Dako), visualized with a peroxidase kit (Dako). Finally, the tissue sections were counterstained with hematoxylin.
Clinical Chemistry Analyses
Detailed methods are available in the Supplemental Material.
Statistical Analysis
The distribution of variables is represented using box-and-whiskers plots: the bottom and top of the box are the first and third quartiles, the band inside the box is the median, and the ends of the whiskers represent the minimum and maximum of all of the data. The proportions are represented using histograms. We used the Mann–Whitney U test for nonparametric data comparisons between two groups, and t test for the comparison of parametric data. One-way ANOVA followed by Dunnett’s post-test correction test were performed to compare more than two groups, and to correct for the effect of multiple comparisons to a single control. Multiple linear regression analysis was performed using a partial least-squares fit model. Statistical analyses were performed using JMP.10 (SAS software), and graphs using Prism-GraphPad software. P values <0.05 were considered significant.
Disclosure
None.
Supplementary Material
Acknowledgments
This work was funded by grants from the Institut National de la Santé et de la Recherche Médicale (INSERM), la Fondation du Rein, et la Fédération Nationale pour l’Aide aux Insuffisants Rénaux.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015060703/-/DCSupplemental
References
- 1.Chovatiya R, Medzhitov R: Stress, inflammation, and defense of homeostasis. Mol Cell 54: 281–288, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cybulsky AV: The intersecting roles of endoplasmic reticulum stress, ubiquitin-proteasome system, and autophagy in the pathogenesis of proteinuric kidney disease. Kidney Int 84: 25–33, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Inagi R: Endoplasmic reticulum stress as a progression factor for kidney injury. Curr Opin Pharmacol 10: 156–165, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Inagi R, Ishimoto Y, Nangaku M: Proteostasis in endoplasmic reticulum--new mechanisms in kidney disease. Nat Rev Nephrol 10: 369–378, 2014 [DOI] [PubMed] [Google Scholar]
- 5.Pallet N, Fougeray S, Beaune P, Legendre C, Thervet E, Anglicheau D: Endoplasmic reticulum stress: an unrecognized actor in solid organ transplantation. Transplantation 88: 605–613, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Ghosh R, Lipson KL, Sargent KE, Mercurio AM, Hunt JS, Ron D, Urano F: Transcriptional regulation of VEGF-A by the unfolded protein response pathway. PLoS One 5: e9575, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drogat B, Auguste P, Nguyen DT, Bouchecareilh M, Pineau R, Nalbantoglu J, Kaufman RJ, Chevet E, Bikfalvi A, Moenner M: IRE1 signaling is essential for ischemia-induced vascular endothelial growth factor-A expression and contributes to angiogenesis and tumor growth in vivo. Cancer Res 67: 6700–6707, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Janssens S, Pulendran B, Lambrecht BN: Emerging functions of the unfolded protein response in immunity. Nat Immunol 15: 910–919, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang K, Kaufman RJ: From endoplasmic-reticulum stress to the inflammatory response. Nature 454: 455–462, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamasaki S, Ivanov P, Hu GF, Anderson P: Angiogenin cleaves tRNA and promotes stress-induced translational repression. J Cell Biol 185: 35–42, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pereira ER, Liao N, Neale GA, Hendershot LM: Transcriptional and post-transcriptional regulation of proangiogenic factors by the unfolded protein response. PLoS One 5: e12521, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moenner M, Gusse M, Hatzi E, Badet J: The widespread expression of angiogenin in different human cells suggests a biological function not only related to angiogenesis. Eur J Biochem 226: 483–490, 1994 [DOI] [PubMed] [Google Scholar]
- 13.Sköldenberg EG, Christiansson J, Sandstedt B, Larsson A, Läckgren G, Christofferson R: Angiogenesis and angiogenic growth factors in Wilms tumor. J Urol 165: 2274–2279, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Fett JW, Strydom DJ, Lobb RR, Alderman EM, Bethune JL, Riordan JF, Vallee BL: Isolation and characterization of angiogenin, an angiogenic protein from human carcinoma cells. Biochemistry 24: 5480–5486, 1985 [DOI] [PubMed] [Google Scholar]
- 15.Fu H, Feng J, Liu Q, Sun F, Tie Y, Zhu J, Xing R, Sun Z, Zheng X: Stress induces tRNA cleavage by angiogenin in mammalian cells. FEBS Lett 583: 437–442, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Ivanov P, Emara MM, Villen J, Gygi SP, Anderson P: Angiogenin-induced tRNA fragments inhibit translation initiation. Mol Cell 43: 613–623, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ivanov P, O’Day E, Emara MM, Wagner G, Lieberman J, Anderson P: G-quadruplex structures contribute to the neuroprotective effects of angiogenin-induced tRNA fragments. Proc Natl Acad Sci U S A 111: 18201–18206, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sheng J, Yu W, Gao X, Xu Z, Hu GF: Angiogenin stimulates ribosomal RNA transcription by epigenetic activation of the ribosomal DNA promoter. J Cell Physiol 229: 521–529, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olson KA, Verselis SJ, Fett JW: Angiogenin is regulated in vivo as an acute phase protein. Biochem Biophys Res Commun 242: 480–483, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Koutroubakis IE, Xidakis C, Karmiris K, Sfiridaki A, Kandidaki E, Kouroumalis EA: Serum angiogenin in inflammatory bowel disease. Dig Dis Sci 49: 1758–1762, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Hooper LV, Stappenbeck TS, Hong CV, Gordon JI: Angiogenins: a new class of microbicidal proteins involved in innate immunity. Nat Immunol 4: 269–273, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Mishima E, Inoue C, Saigusa D, Inoue R, Ito K, Suzuki Y, Jinno D, Tsukui Y, Akamatsu Y, Araki M, Araki K, Shimizu R, Shinke H, Suzuki T, Takeuchi Y, Shima H, Akiyama Y, Toyohara T, Suzuki C, Saiki Y, Tominaga T, Miyagi S, Kawagisihi N, Soga T, Ohkubo T, Yamamura K, Imai Y, Masuda S, Sabbisetti V, Ichimura T, Mount DB, Bonventre JV, Ito S, Tomioka Y, Itoh K, Abe T: Conformational change in transfer RNA is an early indicator of acute cellular damage. J Am Soc Nephrol 25: 2316–2326, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mami I, Bouvier N, El Karoui K, Gallazzini M, Rabant M, Laurent-Puig P, Li S, Tharaux PL, Beaune P, Thervet E, Chevet E, Hu GF, Pallet N: Angiogenin Mediates Cell-Autonomous Translational Control under Endoplasmic Reticulum Stress and Attenuates Kidney Injury. J Am Soc Nephrol: ASN.2015020196, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Acosta-Alvear D, Zhou Y, Blais A, Tsikitis M, Lents NH, Arias C, Lennon CJ, Kluger Y, Dynlacht BD: XBP1 controls diverse cell type- and condition-specific transcriptional regulatory networks. Mol Cell 27: 53–66, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Hetz C, Chevet E, Harding HP: Targeting the unfolded protein response in disease. Nat Rev Drug Discov 12: 703–719, 2013 [DOI] [PubMed] [Google Scholar]
- 26.Kaneko M, Niinuma Y, Nomura Y: Activation signal of nuclear factor-kappa B in response to endoplasmic reticulum stress is transduced via IRE1 and tumor necrosis factor receptor-associated factor 2. Biol Pharm Bull 26: 931–935, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Fujiwara T, Oda K, Yokota S, Takatsuki A, Ikehara Y: Brefeldin A causes disassembly of the Golgi complex and accumulation of secretory proteins in the endoplasmic reticulum. J Biol Chem 263: 18545–18552, 1988 [PubMed] [Google Scholar]
- 28.Martinon F, Chen X, Lee AH, Glimcher LH: TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nat Immunol 11: 411–418, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu FG, Gomi K, Marshall JS: Short-term and long-term cytokine release by mouse bone marrow mast cells and the differentiated KU-812 cell line are inhibited by brefeldin A. J Immunol 161: 2541–2551, 1998 [PubMed] [Google Scholar]
- 30.Burikhanov R, Zhao Y, Goswami A, Qiu S, Schwarze SR, Rangnekar VM: The tumor suppressor Par-4 activates an extrinsic pathway for apoptosis. Cell 138: 377–388, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mahadevan NR, Rodvold J, Sepulveda H, Rossi S, Drew AF, Zanetti M: Transmission of endoplasmic reticulum stress and pro-inflammation from tumor cells to myeloid cells. Proc Natl Acad Sci U S A 108: 6561–6566, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Genereux JC, Qu S, Zhou M, Ryno LM, Wang S, Shoulders MD, Kaufman RJ, Lasmezas CI, Kelly JW, Wiseman RL: Unfolded protein response-induced ERdj3 secretion links ER stress to extracellular proteostasis. EMBO J 34: 4–19, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meng XM, Nikolic-Paterson DJ, Lan HY: Inflammatory processes in renal fibrosis. Nat Rev Nephrol 10: 493–503, 2014 [DOI] [PubMed] [Google Scholar]
- 34.Ivashkiv LB: Inflammatory signaling in macrophages: transitions from acute to tolerant and alternative activation states. Eur J Immunol 41: 2477–2481, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kelly B, O’Neill LA: Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res 25: 771–784, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weichhart T, Hengstschläger M, Linke M: Regulation of innate immune cell function by mTOR. Nat Rev Immunol 15: 599–614, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amer H, Lieske JC, Rule AD, Kremers WK, Larson TS, Franco Palacios CR, Stegall MD, Cosio FG: Urine high and low molecular weight proteins one-year post-kidney transplant: relationship to histology and graft survival. Am J Transplant 13: 676–684, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Solez K, Colvin RB, Racusen LC, Sis B, Halloran PF, Birk PE, Campbell PM, Cascalho M, Collins AB, Demetris AJ, Drachenberg CB, Gibson IW, Grimm PC, Haas M, Lerut E, Liapis H, Mannon RB, Marcus PB, Mengel M, Mihatsch MJ, Nankivell BJ, Nickeleit V, Papadimitriou JC, Platt JL, Randhawa P, Roberts I, Salinas-Madriga L, Salomon DR, Seron D, Sheaff M, Weening JJ: Banff ’05 Meeting Report: differential diagnosis of chronic allograft injury and elimination of chronic allograft nephropathy (‘CAN’). Am J Transplant 7: 518–526, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Todd DJ, McHeyzer-Williams LJ, Kowal C, Lee AH, Volpe BT, Diamond B, McHeyzer-Williams MG, Glimcher LH: XBP1 governs late events in plasma cell differentiation and is not required for antigen-specific memory B cell development. J Exp Med 206: 2151–2159, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shaffer AL, Shapiro-Shelef M, Iwakoshi NN, Lee AH, Qian SB, Zhao H, Yu X, Yang L, Tan BK, Rosenwald A, Hurt EM, Petroulakis E, Sonenberg N, Yewdell JW, Calame K, Glimcher LH, Staudt LM: XBP1, downstream of Blimp-1, expands the secretory apparatus and other organelles, and increases protein synthesis in plasma cell differentiation. Immunity 21: 81–93, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Schebesta M, Heavey B, Busslinger M: Transcriptional control of B-cell development. Curr Opin Immunol 14: 216–223, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Mahadevan NR, Fernandez A, Rodvold JJ, Almanza G, Zanetti M: Prostate cancer cells undergoing ER stress in vitro and in vivo activate transcription of pro-inflammatory cytokines. J Inflamm Res 3: 99–103, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peng Y, Li L, Huang M, Duan C, Zhang L, Chen J: Angiogenin interacts with ribonuclease inhibitor regulating PI3K/AKT/mTOR signaling pathway in bladder cancer cells. Cell Signal 26: 2782–2792, 2014 [DOI] [PubMed] [Google Scholar]
- 44.He B: Viruses, endoplasmic reticulum stress, and interferon responses. Cell Death Differ 13: 393–403, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Livak KJ, Schmittgen TD: Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Rabant M, Amrouche L, Lebreton X, Aulagnon F, Benon A, Sauvaget V, Bonifay R, Morin L, Scemla A, Delville M, Martinez F, Timsit MO, Duong Van Huyen JP, Legendre C, Terzi F, Anglicheau D: Urinary C-X-C Motif Chemokine 10 Independently Improves the Noninvasive Diagnosis of Antibody-Mediated Kidney Allograft Rejection. J Am Soc Nephrol 26: 2840–2851, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.