Abstract
The APOL1 high-risk genotype, present in approximately 13% of blacks in the United States, is a risk factor for kidney function decline in populations with CKD. It is unknown whether genetic screening is indicated in the general population. We evaluated the prognosis of APOL1 high-risk status in participants in the population-based Atherosclerosis Risk in Communities (ARIC) study, including associations with eGFR decline, variability in eGFR decline, and related adverse health events (AKI, ESRD, hypertension, diabetes, cardiovascular disease, pre-ESRD and total hospitalization rate, and mortality). Among 15,140 ARIC participants followed from 1987–1989 (baseline) to 2011–2013, 75.3% were white, 21.5% were black/APOL1 low-risk, and 3.2% were black/APOL1 high-risk. In a demographic-adjusted analysis, blacks had a higher risk for all assessed adverse health events; however, in analyses adjusted for comorbid conditions and socioeconomic status, blacks had a higher risk for hypertension, diabetes, and ESRD only. Among blacks, the APOL1 high-risk genotype associated only with higher risk of ESRD in a fully adjusted analysis. Black race and APOL1 high-risk status were associated with faster eGFR decline (P<0.001 for each). However, we detected substantial overlap among the groups: median (10th–90th percentile) unadjusted eGFR decline was 1.5 (1.0–2.2) ml/min per 1.73 m2 per year for whites, 2.1 (1.4–3.1) ml/min per 1.73 m2 per year for blacks with APOL1 low-risk status, and 2.3 (1.5–3.5) ml/min per 1.73 m2 per year for blacks with APOL1 high-risk status. The high variability in eGFR decline among blacks with and without the APOL1 high-risk genotype suggests that population-based screening is not yet justified.
Keywords: end-stage renal disease, glomerular filtration rate, ethnicity
In the United States, blacks have a significantly higher risk of advanced CKD than whites.1–3 At least some of the increased risk is explained by genetic factors: two copies of the APOL1 risk alleles, present in 13% of blacks but <1% of whites, confer 1.7–2-times higher risk of CKD progression and up to 30-fold higher risk for specific etiologies of ESRD.4–7 However, not all persons with the high-risk APOL1 genotype develop kidney disease.8 The variation in kidney function decline associated with APOL1 risk status has not been well characterized, particularly in the general population, nor has the risk of potentially mediating events, such as new hypertension, diabetes, cardiovascular disease, and intervening hospitalizations.
In light of the strong association between APOL1 high-risk status and ESRD, recent discussion has focused on optimal screening strategies, with some experts recommending screening for APOL1 risk alleles in specific populations, such as potential kidney donors.9–11 However, much of the evidence supporting such a program is derived from studies of populations with CKD.6 Patterns of eGFR decline may differ between general and CKD populations. A “second hit” may be needed to induce kidney function decline in those with an APOL1 high-risk genotype,8,12,13 and persons with APOL1 high-risk status in a CKD population may have already experienced the necessary instigating event. Long-term follow-up of APOL1 high-risk individuals in the general population is necessary to determine the natural history of kidney disease, as well as other meaningful adverse health events associated with APOL1 genetic risk variants.
Using the Atherosclerosis Risk in Communities (ARIC) study, a prospective cohort of 15,792 individuals followed at five study visits over 25 years, we sought to determine the natural history of APOL1 high-risk status with respect to eGFR decline. To fully characterize prognosis and evaluate for potential etiologies underlying differences in kidney function, we also quantified the incidence of new-onset hypertension, diabetes, cardiovascular disease, AKI, hospitalizations, ESRD, and all-cause mortality by race and APOL1 high-risk status.
Results
Baseline Characteristics, by Race–APOL1 Risk Status
There were 15,140 participants included: 75.7% were white, 21.2% black APOL1 low-risk, and 3.1% black APOL1 high-risk (Table 1). Compared with whites, blacks of both APOL1 risk groups were slightly younger, more often female, with a higher average body mass index, and higher prevalence of hypertension and diabetes. Interestingly, blacks had both higher average eGFR and a higher proportion with eGFR<60 ml/min per 1.73 m2 at baseline compared with whites. Blacks of both APOL1 risk groups had higher HDL cholesterol, lower triglycerides, and a lower prevalence of aspirin use than whites. Annual family income and education level were also significantly lower in both black APOL1 risk groups. There were no statistically significant differences in characteristics between blacks by APOL1 risk group at visit 1 (age 45–65 years).
Table 1.
Baseline characteristics of ARIC participants at visit 1 (1987–1989), by race/APOL1 status
| White Participants | Black APOL1 Low-Risk Participants | Black APOL1 High-Risk Participants | |
|---|---|---|---|
| N | 11,464 | 3206 | 470 |
| % | 75.7 | 21.2 | 3.1 |
| Age, yr (mean) | 54.9 | 54.0a | 53.5a |
| Female, % | 52.7 | 62.1a | 63.2a |
| Body mass index, kg/m2 (mean) | 27.0 | 29.6a | 29.6a |
| Systolic BP, mmHg (mean) | 118.5 | 128.3a | 128.9a |
| Diabetes, % | 7.2 | 17.2a | 16.6a |
| Hypertension, % | 27.3 | 55.0a | 57.1a |
| Prior CHD event, % | 5.3 | 3.9a | 4.8 |
| eGFRcr, ml/min per 1.73 m2 (mean) | 99.4 | 111.2a | 110.4a |
| eGFRcr<60 ml/min per 1.73 m2, % | 1.0 | 2.1a | 2.3a |
| LDL cholesterol, mg/dl (mean) | 137.7 | 138.0 | 135.3 |
| HDL, mg/dl (mean) | 50.4 | 54.9a | 55.6a |
| Triglycerides, mg/dl (mean) | 138.1 | 114.8a | 108.9a |
| On aspirin, % | 52.7 | 29.4a | 31.0a |
| On statin, % | 0.7 | 0.3a | 0.4 |
| Smoking status | |||
| Current, % | 24.7 | 29.4a | 33.6a |
| Former, % | 35.4 | 24.2a | 22.5a |
| Never, % | 39.9 | 46.2a | 43.9a |
| Annual family income <$25,000, % | 25.4 | 63.3a | 64.4a |
| Non-high school graduate, % | 17.1 | 40.2a | 42.8a |
CHD, coronary heart disease; eGFRcr, eGFR based on creatinine.
P<0.05 for comparison with the white subgroup. There were no statistically significant differences between blacks by APOL1 risk group.
Incident Events, by Race–APOL1 Risk Status
Over a median follow-up of 22.6 years, blacks in both the APOL1 low- and high-risk groups had a higher risk of total and pre-ESRD hospitalizations, AKI, ESRD, hypertension, diabetes, cardiovascular disease, and all-cause mortality compared with white participants in both crude and demographic-adjusted analyses (Table 2). For example, total hospitalization rates were 1.82 (95% confidence interval [95% CI], 1.81 to 1.84), 2.09 (95% CI, 2.05 to 2.13), and 2.17 (95% CI, 2.07 to 2.26) per 10 person-years in the white, black APOL1 low-risk, and black APOL1 high-risk groups, respectively. These differences persisted after adjustment for age and sex, but not after adjustment for multiple comorbid conditions and socioeconomic status. In the fully adjusted analyses, blacks had higher risk of incident hypertension, diabetes, and ESRD, but lower risk of total hospitalizations and incident cardiovascular disease. Among blacks, only ESRD risk was higher among the APOL1 high-risk group compared with the APOL1 low-risk group in fully adjusted analysis.
Table 2.
Risk of adverse events over 25 years of follow-up, by race/APOL1 status
| White Participants | Black APOL1 Low-Risk Participants | Black APOL1 High-Risk Participants | |
|---|---|---|---|
| Incident hypertension | |||
| Events (n) | 4540 | 891 | 125 |
| Rate per 1000 person-years | 50.98 (49.52–52.49) | 70.31 (65.85–75.09)a | 72.47 (60.82–86.36)a |
| Demographic-adjusted IRR | Reference | 1.42 (1.32–1.53)a | 1.49 (1.25–1.78)a |
| Fully adjusted IRR | Reference | 1.13 (1.04–1.22)a | 1.21 (1.00–1.45)a |
| Incident diabetes | |||
| Events (n) | 2698 | 987 | 167 |
| Rate per 1000 person-years | 14.61 (14.07–15.17) | 23.10 (21.71–24.59)a | 27.51 (23.64–32.02)ab |
| Demographic-adjusted IRR | Reference | 1.60 (1.49–1.73)a | 1.92 (1.64–2.24)ab |
| Fully adjusted IRR | Reference | 1.26 (1.16–1.38)a | 1.41 (1.20–1.67)a |
| Incident cardiovascular disease | |||
| Events (n) | 2381 | 679 | 99 |
| Rate per 1000 person-years | 11.34 (10.90–11.81) | 11.94 (11.08–12.88) | 11.86 (9.74–14.44) |
| Demographic-adjusted IRR | Reference | 1.18 (1.08–1.28)a | 1.25 (1.02–1.53)a |
| Fully adjusted IRR | Reference | 0.85 (0.77–0.93)a | 0.88 (0.72–1.09) |
| AKI during follow-up | |||
| Events (n) | 1394 | 510 | 82 |
| Rate per 1000 person-years | 5.99 (5.68–6.31) | 8.36 (7.64–9.09)a | 9.12 (7.15–11.10)a |
| Demographic-adjusted IRR | Reference | 1.54 (1.39–1.70)a | 1.71 (1.37–2.13)a |
| Fully adjusted IRR | Reference | 1.04 (0.92–1.17) | 1.11 (0.88–1.40) |
| Hospitalizations during follow-up | |||
| Events (n) | 42,470 | 12,859 | 1981 |
| Rate per 1000 person-years | 182.29 (180.56–184.03) | 208.96 (205.35–212.57)a | 216.65 (207.11–226.19)a |
| Demographic-adjusted IRR | Reference | 1.23 (1.21–1.26)a | 1.32 (1.26–1.38)ab |
| Fully adjusted IRR | Reference | 0.92 (0.90–0.95)a | 0.93 (0.89–0.97)a |
| Pre-ESRD hospitalizations during follow-up | |||
| Events (n) | 41,748 | 11,874 | 1745 |
| Rate per 1000 person-years | 179.51 (177.79–181.23) | 194.71 (191.21–198.22)a | 194.12 (185.02–203.23)a |
| Demographic-adjusted IRR | Reference | 1.17 (1.14–1.19)a | 1.20 (1.14–1.26)a |
| Fully adjusted IRR | Reference | 0.89 (0.87–0.91)a | 0.86 (0.82–0.91)a |
| Incident ESRD | |||
| Events (n) | 153 | 145 | 34 |
| Rate per 1000 person-years | 0.65 (0.55–0.76) | 2.35 (2.00–2.77)a | 3.76 (2.69–5.26)ab |
| Demographic-adjusted IRR | Reference | 3.90 (3.10–4.91)a | 6.42 (4.42–9.33)ab |
| Fully adjusted IRR | Reference | 1.87 (1.42–2.46)a | 2.84 (1.88–4.30)ab |
| All-cause mortality | |||
| Events (n) | 3564 | 1246 | 186 |
| Rate per 1000 person-years | 15.30 (14.80–15.81) | 20.25 (19.15–21.40)a | 20.34 (17.62–23.49)a |
| Demographic-adjusted IRR | Reference | 1.52 (1.42–1.62)a | 1.63 (1.40–1.89)a |
| Fully adjusted IRR | Reference | 1.06 (0.99–1.15) | 1.03 (0.88–1.20) |
Persons with a history of hypertension were excluded from the incident hypertension analysis; persons with a history of diabetes were excluded from the incident diabetes analysis; persons with eGFR<15 ml/min per 1.73 m2 at baseline were excluded from the incident AKI and ESRD analyses. Incident AKI was censored at the development of ESRD. IRR, incident rate ratio.
P<0.05 for the comparison of the black APOL1 low-risk subgroup versus the white subgroup as well as for the comparison of the black APOL1 high-risk subgroup versus the white subgroup.
P<0.05 for the comparison of the black APOL1 high-risk subgroup versus the black APOL1 low-risk subgroup.
Annual eGFR Decline, by Race–APOL1 Risk Status
Blacks in both APOL1 risk groups had higher baseline eGFR, but faster eGFR decline than whites (Figure 1). In demographic-adjusted analysis, the average rate of eGFR decline was 1.5 ml/min per 1.73 m2 per year for whites, 2.1 ml/min per 1.73 m2 per year for blacks of APOL1 low-risk status, and 2.4 ml/min per 1.73 m2 per year for blacks of APOL1 high-risk status. In fully adjusted analysis, results were similar, with the mean annual decline in eGFR estimated as 1.3 ml/min per 1.73 m2 for whites, 1.7 ml/min per 1.73 m2 for blacks with APOL1 low-risk status, and 1.9 ml/min per 1.73 m2 for blacks with APOL1 high-risk status (P<0.001 for each comparison).
Figure 1.
Median, 10th, and 90th percentile eGFR decline from mean baseline eGFR over 25 years varied by race/APOL1 status. Slopes were estimated from a mixed model with the following covariates: age, sex, baseline coronary heart disease, body mass index, HDL cholesterol, diabetes, systolic BP, smoking, family income, and education level, and interaction terms of each covariate with time.
Variation in eGFR Decline, by Race–APOL1 Risk Status
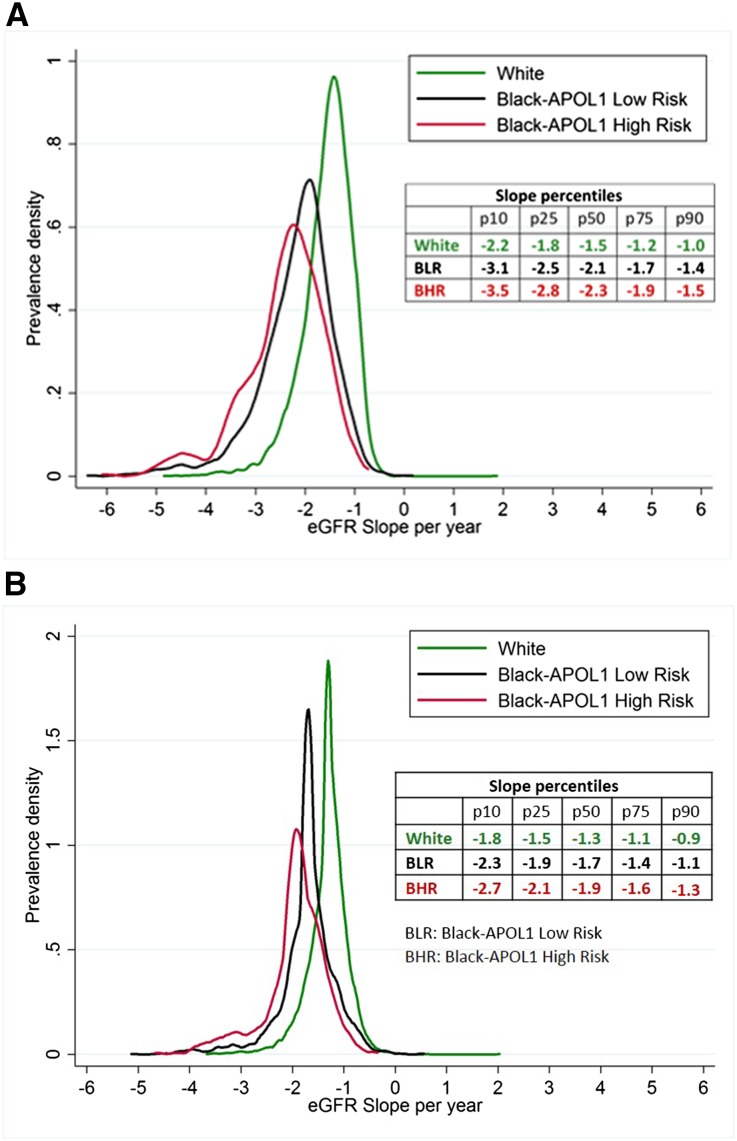
The distribution of eGFR slopes varied substantially across and within race–APOL1 risk status (Figure 2A). The median (10th–90th percentile) annual unadjusted decline was 1.5 (1.0–2.2) ml/min per 1.73 m2 for whites, 2.1 (1.4–3.1) ml/min per 1.73 m2 for blacks with APOL1 low-risk status, and 2.3 (1.5–3.5) ml/min per 1.73 m2 for blacks with APOL1 high-risk status. After taking into account differences in distributions of other risk factors such as diabetes and hypertension, differences in the adjusted annual eGFR decline remained pronounced. A greater proportion of blacks of APOL1 high-risk status had rapid eGFR decline (>3 ml/min per 1.73 m2 per year) than whites and blacks of APOL1 low-risk status (Figure 2B). Within the subgroup of patients with albuminuria >30 mg/g at study visit 4, the unadjusted median (10th–90th percentile) decline in eGFR over the subsequent 9 years showed a similar pattern: 1.8 (1.0–2.9) ml/min per 1.73 m2 per year for whites, 2.4 (1.7–3.7) ml/min per 1.73 m2 per year for blacks with APOL1 low-risk status, and 2.9 (1.7–4.1) ml/min per 1.73 m2 per year for blacks with APOL1 high-risk status.
Figure 2.
Distribution of average yearly eGFR slopes varied by race/APOL1 status. eGFR was imputed as 15 ml/min per 1.73 m2 at the time of ESRD onset. (A) reflects the unadjusted slope distribution, (B) reflects the adjusted slope distribution with slopes estimated from a mixed model with the following additional covariates: age, sex, baseline coronary heart disease, body mass index, HDL cholesterol, diabetes, systolic BP, smoking, family income, and education level, and incorporating interaction terms of each covariate with time.
Sensitivity Analyses
Associations between race–APOL1 status and eGFR decline were similar to the main results in various sensitivity analyses. When incident hypertension, diabetes, cardiovascular disease, and AKI were accounted for, the annual decline in eGFR was 1.4 ml/min per 1.73 m2 for whites, 1.9 ml/min per 1.73 m2 for blacks with APOL1 low-risk status, and 2.1 ml/min per 1.73 m2 for blacks with APOL1 high-risk status. When eGFR abstracted from cardiovascular hospitalizations was included, the annual decline in eGFR was 1.5 ml/min per 1.73 m2 for whites, 2.0 ml/min per 1.73 m2 for blacks with APOL1 low-risk status, and 2.2 ml/min per 1.73 m2 for blacks with APOL1 high-risk status. In sensitivity analyses without imputation of eGFR at ESRD onset, differences persisted with slightly attenuated results: the annual decline in eGFR was 1.4 ml/min per 1.73 m2 for whites, 1.7 ml/min per 1.73 m2 for blacks with APOL1 low-risk status, and 2.0 ml/min per 1.73 m2 for blacks with APOL1 high-risk status. On the other hand, the addition of APOL1 risk status to a multivariable model did not improve model discrimination. When APOL1 risk status was added to the fully adjusted multivariable model of ESRD risk that included race, there was no statistically significant improvement in C statistic (C statistic without APOL1 risk status, 0.862; C statistic with APOL1 risk status, 0.868; P value for difference=0.14). The slope difference for blacks compared with whites in a model without APOL1 risk status was –0.41 ml/min per 1.73 m2 per year; in a model with APOL1 status, the difference between the APOL1 low-risk group and whites was –0.39 ml/min per 1.73 m2.
Discussion
In this general population cohort of 15,140 middle-aged individuals followed for over 20 years, blacks were at higher risk for a broad range of adverse health outcomes compared with whites. These racial disparities were only minimally explained by the APOL1 high-risk genotype, which was a risk factor only for kidney function decline and incident ESRD. There was substantially heterogeneity in kidney function decline within each of the three race/APOL1 risk groups, suggesting caution in individual predictions based on genotype. Given the variability in kidney function decline among persons with the APOL1 high-risk genotype, widespread screening of the black general population seems not yet justified.
The results of this general population study extend earlier work examining eGFR trajectories in CKD cohorts.6,14 Similar to results in the Chronic Renal Insufficiency Cohort (CRIC) and African American Study of Kidney Disease and Hypertension, we found that blacks with two high-risk variants of the APOL1 gene had higher risk of ESRD and a faster decline in eGFR compared with whites (adjusted slope difference in CRIC: –0.81 ml/min per 1.73 m2 per year in blacks with APOL1 high-risk compared with whites; slope difference in ARIC: –0.77 ml/min per 1.73 m2 per year). Like our study, variability in eGFR decline was high in CRIC, where the SD of unadjusted eGFR decline ranged from 3.1 to 5.6 ml/min per 1.73 m2 per year in subgroups of race/APOL1 risk and diabetes status. Although CRIC results demonstrated faster declines in eGFR among blacks without the APOL1 high-risk genotype than whites in unadjusted analyses, this difference did not persist in analyses adjusted for potential confounders. In contrast, we found that blacks of APOL1 low-risk status had faster declines in eGFR than whites in all analyses. These differences may be due to differences in length of follow-up: CRIC participants were followed for a mean of 4.4 years, whereas ARIC participants had up to 25 years of follow-up. Alternatively, ethnic differences in disease progression may be less pronounced among populations with prevalent CKD.
Our results also complement a recent publication from the Coronary Artery Risk Development in Young Adults (CARDIA) study.15 In a sample of 3030 adults with mean baseline age of 35 years and 9 years of follow-up, both APOL1 high- and low-risk blacks were at higher risk than whites for the development of incident albuminuria and eGFR decline in demographic-adjusted analyses. In fully adjusted analyses, however, only the APOL1 high-risk group remained at statistically significant higher risk. Differences in our results may be due to the different follow-up time between studies. Other explanations include differences in estimating equations, since CARDIA used eGFR based on cystatin C levels rather than creatinine, differences in adjustment variables (including albuminuria, which was not available at the baseline visit of the ARIC study), or differences in age of the cohort, since CARDIA participants were on average 20 years younger at baseline. Given that most incident CKD occurs after the age of 50 years,1 there were likely fewer CKD events in the CARDIA cohort, thereby limiting power. On the other hand, the ARIC cohort may inadvertently select for more stable disease by not capturing persons who developed ESRD prior to middle age. This type of selection bias would be expected to result in underestimation of the differences between blacks and whites, since blacks tend to develop kidney disease at younger age than whites.16
Regardless of APOL1 genotype, blacks were at higher risk for each adverse health event evaluated in this study. These results are consistent with previous publications demonstrating pervasive health disparities among the United States population.17–19 Risks of adverse outcomes associated with black race were attenuated in fully adjusted analyses, suggesting that the poor outcomes are explained in part by differences at baseline, including lower income and education levels, which may be proxies for access to and quality of care. Blacks with APOL1 high-risk status were at higher risk of ESRD; surprisingly, they were also at higher risk of incident diabetes and had a higher rate of hospitalizations than blacks without the APOL1 high-risk genotype. The latter two risks have not previously been described and may be a byproduct of the higher kidney disease risk. Indeed, there was no difference in hospitalization rates by APOL1 genotype when only pre-ESRD hospitalizations were included. Similarly, a diagnosis of incident diabetes requires contact with the medical system, which is frequent among persons with ESRD or advanced CKD. Notably, the association between APOL1 risk status, incident diabetes, and hospitalizations was not statistically significant in fully adjusted analyses, suggesting that the distribution of risk factors varied by APOL1 risk status, likely by chance.
The mechanism by which APOL1 high-risk variants might affect kidney disease progression remains uncertain. APOL1 is upregulated in response to proinflammatory cytokines and encodes the circulating protein apolipoprotein 1.20 Apolipoprotein 1 is involved in the innate immune response, most notably to Trypansoma brucei infection, where it causes osmotic lysis of the parasite.5,21 The G1 and G2 variants are mutations that appear to prevent neutralization of apolipoprotein 1, a defense mechanism which has evolved in Trypansoma brucei rhodesiense.22 Whether G1-/G2-encoded apolipoprotein 1 has additional nephrotoxic action is not known. There is some evidence that active infection or inflammation may modify APOL1-related risk: in one study of HIV-infected individuals, eGFR trajectory differed by APOL1 high-risk status only among those with unsuppressed viremia.23 In another, associations between hemostatic factors and ESRD were strongest in those with the APOL1 high-risk genotype compared with whites or blacks with the APOL1 low-risk genotype.24
A novel aspect of this study is the examination of the distribution of eGFR trajectories. Similar to studies in populations with CKD and HIV,6,23 we observed that, on average, blacks with the APOL1 high-risk genotype experienced faster decline in eGFR. However, much of the difference was driven by the tail of the distribution, or the few individuals with extremely rapid decline in eGFR. In contrast, the majority of individuals with APOL1 high-risk status had eGFR declines similar to other race–APOL1 groups. This variability suggests that using the APOL1 genotype alone or with the currently known risk factors to forecast future kidney disease would be difficult. It also suggests that APOL1-associated susceptibility may be in part modifiable. Identifying factors associated with rapid eGFR decline among APOL1 high-risk individuals may help to uncover genetic or environmental second hits, which can compound APOL1-related renal risk.
The interplay of apolipoprotein 1 with the inflammatory and innate immune system has led some to hypothesize that APOL1 high-risk status might promote both CKD and cardiovascular disease. In this study, we found no difference in cardiovascular disease risk by APOL1 genotype. The lack of association is consistent with findings from the Systolic Blood Pressure Intervention trial, where APOL1 high-risk status was not associated with clinical cardiovascular disease.25 In contrast, a combined study of the Jackson Heart Study and the Women’s Health Initiative found an association between APOL1 high-risk status and cardiovascular disease incidence, defined as myocardial infarction, stroke, or therapeutic vascular interventions.26 Interestingly, APOL1 high-risk status was associated with lower coronary calcium scores, when higher scores are a strong predictor of cardiovascular risk.27,28 Differences in these results may be due to differences in the selection criteria for each of the studies, outcomes assessed, or the length of follow-up, but suggest caution in genetic counseling given the likely variability in APOL1 high-risk genotype expression and/or penetrance.
There are notable strengths and limitations of this study. The ARIC cohort is a general population-based study with long-term follow-up of >15,000 individuals. Study visits were conducted with careful attention to research protocol. Creatinine measurements were standardized and calibrated over time to reduce the possibility of bias from laboratory drift, a major concern in longitudinal studies evaluating change in biomarkers.29 Blacks were directly genotyped for APOL1. Although whites were not genotyped, previous population-based studies have demonstrated a very low rate of homozygous status among populations of European descent.7 Participants were followed not only at study visits, but also by active surveillance of hospitalizations and linkage to the US Renal Data System ESRD registry, a process that helps alleviate concerns of differential loss to follow-up. Sensitivity analyses were performed using eGFR abstracted from hospitalizations and without imputation of ESRD at ESRD onset, and results were similar. A key measure of kidney disease, albuminuria, was not available at the baseline visit, and adjustment for this variable may have attenuated differences by race and APOL1 risk status. On the other hand, albuminuria may mediate the kidney function decline seen among blacks with APOL1 high-risk status, and adjustment for factors on the causal pathway between exposure and outcome is not always desirable.30 The effect of APOL1 high-risk status in persons known to have elevated albuminuria requires further study.
In conclusion, we report on the prognosis of the APOL1 high-risk genotype in the general population, demonstrating a higher risk of ESRD and faster eGFR decline over 22.6 years of follow-up. However, we also demonstrate large variability in eGFR trajectory, such that the majority of blacks with the high-risk genotype experience eGFR decline similar to blacks without the high-risk genotype. More striking are the pervasive racial disparities in adverse health outcomes, which are not explained by APOL1 risk alleles. Our findings suggest that general population screening for the APOL1 high-risk genotype is not yet justified, and that interventions to improve outcomes in blacks with and without the APOL1 high-risk genotype are desperately needed.
Concise Methods
Study Population
The ARIC study is a prospective cohort of 15,792 persons drawn from four communities (Washington County, Maryland; Jackson, Mississippi; Forsyth County, North Carolina; suburban Minneapolis, Minnesota) and has been described previously.31 Participants were aged 45–64 years at baseline, visit 1 (1987–1989). Additional clinic examinations took place from 1990 to 1992 (visit 2), 1993–1995 (visit 3), 1996–1998 (visit 4), and 2011–2013 (visit 5). For this study, persons with self-reported race other than black or white (n=34), missing serum creatinine at baseline (n=150), or missing genotyping data (the latter requirement for blacks only, n=468) were excluded, for a final sample size of 15,140 participants. The institutional review board at all study sites approved the study prior to data collection.
Assessment of APOL1 Risk Status
Genotyping was performed on 3757 blacks who gave informed consent for genetic studies. The APOL1 risk alleles were directly genotyped using Taqman assays. As described previously,32 the G1 risk allele was defined by missense mutations at rs73885319 and rs60919145, and the G2 allele was the 6-bp deletion at rs71785313. APOL1 high-risk status was characterized as the presence of ≥2 risk alleles (G1/G1, G1/G2, or G2/G2). APOL1 low-risk status was defined as <2 risk alleles. All whites were imputed as APOL1 low-risk due to the low prevalence of APOL1 alleles in whites, as reported elsewhere.7
Covariate Assessment
Key demographic factors included age, sex, and self-reported race. Body mass index was calculated using height and weight as measured in light clothing at study visit 1. Diabetes at baseline was defined as self-reported physician diagnosis, fasting glucose ≥126 mg/dl, random glucose ≥200 mg/dl, or the use of antidiabetes medications. Blood pressure was assessed after 5 minutes of rest in a series of three measurements; we used the average of the second and third measurement. Antihypertension medications were identified based on inspection of medication bottles supplied by the participant. Coronary heart disease was defined on the basis of self-reported history of myocardial infarction, coronary artery bypass surgery, angioplasty of a coronary artery, or history of myocardial infarction detected on electrocardiogram performed at visit 1. HDL cholesterol was measured using the Monotest Cholesterol enzymatic assay in the ARIC Central Lipid Laboratory at Baylor College of Medicine (Houston, Texas). Smoking was assessed by self-report and defined as current, former, or never. Family income was assessed as an annual income ≥$25,000, <$25,000, or not reported. Education was dichotomized as high school graduate (yes/no).
Outcome Assessment
Kidney function was assessed through measurement of serum or plasma creatinine at all visits except visit 3. Creatinine was assayed by the modified kinetic Jaffe method, standardized to the National Institute of Standards and Technology, and calibrated across visits by a 200-sample calibration substudy with repeated assays from each visit.29 eGFR was calculated using these values and the Chronic Kidney Disease Epidemiology Collaboration equation.33 Cases of ESRD were determined through linkage to the US Renal Data System registry34; eGFR at the time of ESRD onset was imputed as 15 ml/min per 1.73 m2. In sensitivity analysis, we also included eGFR abstracted from intervening cardiovascular hospitalizations. These data were collected starting in 2005 for heart failure hospitalizations and 2004 for cardiovascular hospitalizations.
Additional outcomes were determined through ARIC study surveillance using both annual telephone interviews and abstraction of hospitalization records, with follow-up until December 31, 2011.35–37 Incident hypertension was assessed at each annual telephone interview as self-reported hypertension diagnosis or antihypertension medication use, or systolic BP ≥140 mmHg, diastolic BP ≥90 mmHg, or the use of antihypertension medications at a subsequent study visit. Incident diabetes was defined as self-reported diabetes diagnosis or use of diabetes medications during study visits or annual telephone interviews. Incident cardiovascular disease was defined as adjudicated incident coronary heart disease (definite or probable myocardial infarction, fatal coronary heart disease, or cardiac procedure) or adjudicated incident stroke. AKI was defined as an International Classification of Diseases, Ninth Revision, Clinical Modification code of 584.* in any position in the hospitalization discharge diagnostic codes.38 Mortality was determined by linkage to the National Death Index as well as the aforementioned active surveillance techniques.
Statistical Analyses
Baseline characteristics were compared by race and APOL1 risk status using t tests and chi-squared tests for continuous and categorical variables, respectively. Incident events were calculated for ESRD, hypertension, diabetes, cardiovascular disease, and mortality using person-time since baseline visit as the denominator and excluding those with prevalent disease from the risk set. Age- and sex- adjusted incidence rate ratios were determined for incident ESRD, hypertension, diabetes, and mortality as well as overall rates of hospitalizations and AKI using Poisson regression.
To estimate eGFR slopes, we fit mixed models relating eGFR measured at study visits to race–APOL1 risk status. Because persons with ESRD are much more likely to miss subsequent follow-up visits, we imputed eGFR as 15 ml/min per 1.73 m2 at the time of ESRD onset in the primary analysis. Random intercept and random slopes were used to incorporate individual variation in trajectories. Best linear unbiased prediction estimates of eGFR slope over time were obtained for each race–APOL1 high-risk group in unadjusted and adjusted analyses.39 Risk factors used in adjustment were age, sex, baseline coronary heart disease, body mass index, HDL cholesterol, systolic BP, diabetes, smoking status, family income, and education as covariates, incorporating interaction terms of each risk factor with time. We compared a model including these covariates and race with a model including these covariates, race, and APOL1 risk status for the outcome of eGFR trajectory as well as for the discrimination of incident ESRD with the use of C statistics. We also evaluated eGFR trajectory among the 343 people with urine albumin-to-creatinine ratios >30 mg/g at visit 4 (the first study visit in which urine albuminuria was quantified) and available subsequent trajectory data (9 years of follow-up).
Several sensitivity analyses were performed. First, models were additionally adjusted for time-varying hypertension, diabetes, cardiovascular disease, and AKI. Second, eGFR measured during hospitalizations was incorporated in the mixed models. Finally, persons developing ESRD were censored at their last study visit rather than imputing eGFR at the time of ESRD onset. All analyses were performed using StataMP version 13.
Disclosures
None.
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions.
M.G. receives support from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDKD; K08DK092287). C.M.R. receives support from a National Heart, Lung, and Blood Institute (NHLBI) training grant in Cardiovascular Epidemiology (T32 HL007024). A.T. receives support from a Renal Disease Epidemiology training grant from the NIDDKD (T32 DK007732). The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by NHLBI contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C).
Some of the data reported here have been supplied by the US Renal Data System. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US Government.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Grams ME, Chow EK, Segev DL, Coresh J: Lifetime incidence of CKD stages 3-5 in the United States. Am J Kidney Dis 62: 245–252, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.United States Renal Data System : USRDS 2013 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2013 [Google Scholar]
- 3.Hsu CY, Lin F, Vittinghoff E, Shlipak MG: Racial differences in the progression from chronic renal insufficiency to end-stage renal disease in the United States. J Am Soc Nephrol 14: 2902–2907, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Freedman BI, Kopp JB, Langefeld CD, Genovese G, Friedman DJ, Nelson GW, Winkler CA, Bowden DW, Pollak MR: The apolipoprotein L1 (APOL1) gene and nondiabetic nephropathy in African Americans. J Am Soc Nephrol 21: 1422–1426, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, Bowden DW, Langefeld CD, Oleksyk TK, Uscinski Knob AL, Bernhardy AJ, Hicks PJ, Nelson GW, Vanhollebeke B, Winkler CA, Kopp JB, Pays E, Pollak MR: Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 329: 841–845, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parsa A, Kao WH, Xie D, Astor BC, Li M, Hsu CY, Feldman HI, Parekh RS, Kusek JW, Greene TH, Fink JC, Anderson AH, Choi MJ, Wright JT Jr, Lash JP, Freedman BI, Ojo A, Winkler CA, Raj DS, Kopp JB, He J, Jensvold NG, Tao K, Lipkowitz MS, Appel LJ AASK Study Investigators CRIC Study Investigators : APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med 369: 2183–2196, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Seaghdha CM, Parekh RS, Hwang SJ, Li M, Köttgen A, Coresh J, Yang Q, Fox CS, Kao WH: The MYH9/APOL1 region and chronic kidney disease in European-Americans. Hum Mol Genet 20: 2450–2456, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freedman BI, Skorecki K: Gene-gene and gene-environment interactions in apolipoprotein L1 gene-associated nephropathy. Clin J Am Soc Nephrol 9: 2006–2013, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen DM, Mittalhenkle A, Scott DL, Young CJ, Norman DJ: African American living-kidney donors should be screened for APOL1 risk alleles. Transplantation 92: 722–725, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kopp JB, Nelson GW, Sampath K, Johnson RC, Genovese G, An P, Friedman D, Briggs W, Dart R, Korbet S, Mokrzycki MH, Kimmel PL, Limou S, Ahuja TS, Berns JS, Fryc J, Simon EE, Smith MC, Trachtman H, Michel DM, Schelling JR, Vlahov D, Pollak M, Winkler CA: APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol 22: 2129–2137, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palmer ND, Freedman BI: APOL1 and progression of nondiabetic nephropathy. J Am Soc Nephrol 24: 1344–1346, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bostrom MA, Kao WH, Li M, Abboud HE, Adler SG, Iyengar SK, Kimmel PL, Hanson RL, Nicholas SB, Rasooly RS, Sedor JR, Coresh J, Kohn OF, Leehey DJ, Thornley-Brown D, Bottinger EP, Lipkowitz MS, Meoni LA, Klag MJ, Lu L, Hicks PJ, Langefeld CD, Parekh RS, Bowden DW, Freedman BI Family Investigation of Nephropathy and Diabetes (FIND) Research Group : Genetic association and gene-gene interaction analyses in African American dialysis patients with nondiabetic nephropathy. Am J Kidney Dis 59: 210–221, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Divers J, Palmer ND, Lu L, Langefeld CD, Rocco MV, Hicks PJ, Murea M, Ma L, Bowden DW, Freedman BI: Gene-gene interactions in APOL1-associated nephropathy. Nephrol Dial Transplant 29: 587–594, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lipkowitz MS, Freedman BI, Langefeld CD, Comeau ME, Bowden DW, Kao WH, Astor BC, Bottinger EP, Iyengar SK, Klotman PE, Freedman RG, Zhang W, Parekh RS, Choi MJ, Nelson GW, Winkler CA, Kopp JB SK Investigators : Apolipoprotein L1 gene variants associate with hypertension-attributed nephropathy and the rate of kidney function decline in African Americans. Kidney Int 83: 114–120, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peralta CA, Bibbins-Domingo K, Vittinghoff E, Lin F, Fornage M, Kopp JB, Winkler CA: APOL1 genotype and race differences in incident albuminuria and renal function decline. J Am Soc Nephrol 27: 887–893, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.United States Renal Data System : USRDS 2012 Annual Data Report: Atlas of End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2012 [Google Scholar]
- 17.Safford MM, Brown TM, Muntner PM, Durant RW, Glasser S, Halanych JH, Shikany JM, Prineas RJ, Samdarshi T, Bittner VA, Lewis CE, Gamboa C, Cushman M, Howard V, Howard G REGARDS Investigators : Association of race and sex with risk of incident acute coronary heart disease events. JAMA 308: 1768–1774, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grams ME, Matsushita K, Sang Y, Estrella MM, Foster MC, Tin A, Kao WH, Coresh J: Explaining the racial difference in AKI incidence. J Am Soc Nephrol 25: 1834–1841, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ayanian JZ, Landon BE, Newhouse JP, Zaslavsky AM: Racial and ethnic disparities among enrollees in Medicare Advantage plans. N Engl J Med 371: 2288–2297, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nichols B, Jog P, Lee JH, Blackler D, Wilmot M, D’Agati V, Markowitz G, Kopp JB, Alper SL, Pollak MR, Friedman DJ: Innate immunity pathways regulate the nephropathy gene Apolipoprotein L1. Kidney Int 87: 332–342, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomson R, Genovese G, Canon C, Kovacsics D, Higgins MK, Carrington M, Winkler CA, Kopp J, Rotimi C, Adeyemo A, Doumatey A, Ayodo G, Alper SL, Pollak MR, Friedman DJ, Raper J: Evolution of the primate trypanolytic factor APOL1. Proc Natl Acad Sci U S A 111: E2130–E2139, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kruzel-Davila E, Wasser WG, Aviram S, Skorecki K: APOL1 nephropathy: from gene to mechanisms of kidney injury [published online ahead of print January 5, 2015]. Nephrol Dial Transplant doi: 10.1093/ndt/gfu391 [DOI] [PubMed] [Google Scholar]
- 23.Estrella MM, Li M, Tin A, Abraham AG, Shlipak MG, Penugonda S, Hussain SK, Palella FJ Jr, Wolinsky SM, Martinson JJ, Parekh RS, Kao WH: The association between APOL1 risk alleles and longitudinal kidney function differs by HIV viral suppression status. Clin Infect Dis 60: 646–652, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tin A, Grams ME, Maruthur NM, Astor BC, Couper D, Mosley TH, Fornage M, Parekh RS, Coresh J, Kao WH: Hemostatic Factors, APOL1 Risk Variants, and the Risk of ESRD in the Atherosclerosis Risk in Communities Study. Clin J Am Soc Nephrol 10: 784–790, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Langefeld CD, Divers J, Pajewski NM, Hawfield AT, Reboussin DM, Bild DE, Kaysen GA, Kimmel PL, Raj DS, Ricardo AC, Wright JT Jr, Sedor JR, Rocco MV, Freedman BI Systolic Blood Pressure Intervention Trial (SPRINT) : Apolipoprotein L1 gene variants associate with prevalent kidney but not prevalent cardiovascular disease in the Systolic Blood Pressure Intervention Trial. Kidney Int 87: 169–175, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ito K, Bick AG, Flannick J, Friedman DJ, Genovese G, Parfenov MG, Depalma SR, Gupta N, Gabriel SB, Taylor HA Jr, Fox ER, Newton-Cheh C, Kathiresan S, Hirschhorn JN, Altshuler DM, Pollak MR, Wilson JG, Seidman JG, Seidman C: Increased burden of cardiovascular disease in carriers of APOL1 genetic variants. Circ Res 114: 845–850, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O’Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA: Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med 358: 1336–1345, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Folsom AR, Kronmal RA, Detrano RC, O’Leary DH, Bild DE, Bluemke DA, Budoff MJ, Liu K, Shea S, Szklo M, Tracy RP, Watson KE, Burke GL: Coronary artery calcification compared with carotid intima-media thickness in the prediction of cardiovascular disease incidence: the Multi-Ethnic Study of Atherosclerosis (MESA). Arch Intern Med 168: 1333–1339, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parrinello CM, Grams ME, Couper D, Ballantyne CM, Hoogeveen RC, Eckfeldt JH, Selvin E, Coresh J: Recalibration of blood analytes over 25 years in the atherosclerosis risk in communities study: impact of recalibration on chronic kidney disease prevalence and incidence. Clin Chem 61: 938–947, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schisterman EF, Cole SR, Platt RW: Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology 20: 488–495, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.The ARIC Investigators : The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol 129: 687–702, 1989 [PubMed] [Google Scholar]
- 32.Foster MC, Coresh J, Fornage M, Astor BC, Grams M, Franceschini N, Boerwinkle E, Parekh RS, Kao WH: APOL1 variants associate with increased risk of CKD among African Americans. J Am Soc Nephrol 24: 1484–1491, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, Ckd EPI CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rebholz CM, Coresh J, Ballew SH, McMahon B, Whelton SP, Selvin E, Grams ME: Kidney failure and ESRD in the Atherosclerosis Risk in Communities (ARIC) Study: comparing ascertainment of treated and untreated kidney failure in a cohort study. Am J Kidney Dis 66: 231–239, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang M, Matsushita K, Sang Y, Ballew SH, Astor BC, Coresh J: Association of kidney function and albuminuria with prevalent and incident hypertension: the Atherosclerosis Risk in Communities (ARIC) study. Am J Kidney Dis 65: 58–66, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Selvin E, Rawlings AM, Grams M, Klein R, Steffes M, Coresh J: Association of 1,5-anhydroglucitol with diabetes and microvascular conditions. Clin Chem 60: 1409–1418, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Selvin E, Rawlings AM, Grams M, Klein R, Sharrett AR, Steffes M, Coresh J: Fructosamine and glycated albumin for risk stratification and prediction of incident diabetes and microvascular complications: a prospective cohort analysis of the Atherosclerosis Risk in Communities (ARIC) study. Lancet Diabetes Endocrinol 2: 279–288, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grams ME, Waikar SS, MacMahon B, Whelton S, Ballew SH, Coresh J: Performance and limitations of administrative data in the identification of AKI. Clin J Am Soc Nephrol 9: 682–689, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robinson GK: That BLUP is a good thing: the estimation of random effects. Stat Sci 6: 15–32, 1991 [Google Scholar]