Abstract
IgA nephropathy (IgAN), characterized by mesangial IgA1 deposits, is a leading cause of renal failure worldwide. IgAN pathogenesis involves circulating hypogalactosylated IgA1 complexed with soluble IgA Fc receptor I (sCD89) and/or anti–hypogalactosylated-IgA1 autoantibodies, but no specific treatment is available for IgAN. The absence of IgA1 and CD89 homologs in the mouse has precluded in vivo proof-of-concept studies of specific therapies targeting IgA1. However, the α1KI‑CD89Tg mouse model of IgAN, which expresses human IgA1 and human CD89, allows in vivo testing of recombinant IgA1 protease (IgA1‑P), a bacterial protein that selectively cleaves human IgA1. Mice injected with IgA1‑P (1–10 mg/kg) had Fc fragments of IgA1 in both serum and urine, associated with a decrease in IgA1–sCD89 complexes. Levels of mesangial IgA1 deposits and the binding partners of these deposits (sCD89, transferrin receptor, and transglutaminase 2) decreased markedly 1 week after treatment, as did the levels of C3 deposition, CD11b+ infiltrating cells, and fibronectin. Antiprotease antibodies did not significantly alter IgA1‑P activity. Moreover, hematuria consistently decreased after treatment. In conclusion, IgA1‑P strongly diminishes human IgA1 mesangial deposits and reduces inflammation, fibrosis, and hematuria in a mouse IgAN model, and therefore may be a plausible treatment for patients with IgAN.
Keywords: IgA nephropathy, immune complexes, glomerulonephritis, immunology, pathology
IgA nephropathy (IgAN) is the most common primary GN worldwide. The hallmark of the disease is the mesangial deposition of IgA1-immune complexes.1,2 IgAN patients exhibit circulating galactose-deficient IgA1,3,4 which can form complexes with its soluble receptor CD895–7 and with autoantibodies that specifically recognize galactose-deficient IgA1.8 Recently, these factors have been identified as valuable biomarkers to predict disease progression and its recurrence after transplantation.9,10 Human and mouse studies have revealed pathogenic mechanisms by which IgA1 complexes get trapped in the mesangium via their interaction with an alternative IgA1 receptor, the transferrin receptor (TfR).11–13 This induces transglutaminase 2 (TG2) overexpression and activation of mesangial cells, which can be associated with the recruitment of inflammatory cells and the progressive destruction of glomerular filtration.7,14
There are no specific treatments for IgAN. Clinicians routinely use angiotensin-converting enzyme inhibitors15 or angiotensin II receptor antagonists to treat patients with proteinuric or hypertensive IgAN.16,17 In cases of severe progressive IgAN, immunosuppressive therapies are suggested. Long-term corticosteroid treatments have been shown to be effective in patients with proteinuria and preserved renal function, but their use is still controversial.18–21 Other treatments, such as tonsillectomy,22 fish oil,23 or a gluten-free diet,24,25 focus on mucosal immunity. Some of these treatments have demonstrated their efficacy in delaying disease development, but tools to specifically counter the disease are lacking.
IgA is a primary mucosal defense, and bacteria have developed various strategies to counter the host immune system, including IgA1 proteases (IgA1‑P).26 This protein, secreted by pathogenic bacteria such as Streptococcus pneumonia, Haemophilus influenza, and Neisseria meningitides, exhibits the capacity to directly cleave the hinge region of human IgA1 (hIgA1), but not human IgA2.27,28 The injection of IgA1‑P 1 hour after hIgA1 administration prevented the development of hIgA1 deposits in mice.29 However, this study did not demonstrate that IgA1‑P could treat IgAN because the model lacks continuous circulating hIgA1. We tested the capacity of IgA1‑P to treat IgAN using a humanized mouse model of IgAN,7 which spontaneously develops the disease after 6 weeks. We used α1KI‑CD89Tg mice that express both hIgA130 and human CD89.5
Recombinant IgA1‑P from H. influenzae incubated with purified IgA1 from an α1KI mouse was shown to cleave IgA1 in vitro, even at low concentrations of IgA1‑P (Supplemental Figure 1, A and B). Purified serum hIgA1 was also cleaved by IgA1‑P, whereas human IgG, myeloma IgA2, and mouse IgA were not (Supplemental Figure 1C), confirming the specificity of IgA1‑P. IgA1‑P also cleaved IgA1 in whole healthy human serum or in α1KI‑CD89Tg mouse serum in vitro as assessed by western blot analysis targeting human IgA heavy chain (Supplemental Figure 1, D and E).
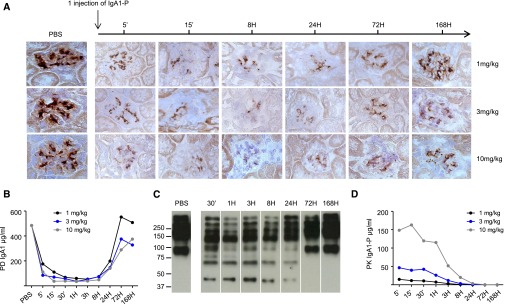
To assess the ability of IgA1‑P to clear IgA1 deposits in the kidney, an intravenous (IV) injection of IgA1‑P at one of three doses (1, 3, or 10 mg/kg) was administered to α1KI‑CD89Tg mice. A marked decrease in IgA1 deposition was observed as early as 15 minutes post injection and persisted for 3 days (Figure 1A). Thus, one injection of IgA1‑P resulted in cleavage of serum IgA1 in vivo for >24 hours and was sufficient to decrease IgA1 deposits in glomeruli for 3 days. Doses of 3 and 10 mg/kg were more efficient than 1 mg/kg. These results correlated with the concentration of total serum IgA1 (Figure 1B), which decreased soon after IgA1‑P injection and returned to its initial level 72 hours later. Fragments of IgA1 were detected by western blotting (Figure 1C). IgA1‑P was not detectable in serum beyond 24 hours after 1 or 3 mg/kg IV injection or beyond 72 hours after 10 mg/kg IV injection (Figure 1D).
Figure 1.
Decrease in serum IgA1 levels and mesangial IgA1 deposits after IgA1‑P treatment. (A) IgA1 deposit detection in the mesangium (brown) after IgA1‑P IV injection (1, 3, and 10 mg/kg) in α1KI-CD89Tg mice (n=3 mice per group) using immunohistochemistry directed against human IgA on frozen kidney sections. Representative sections. (B) Concentrations of serum IgA1 after IgA1‑P injection for each time point of euthanized mice as described in (A). (C) Detection of IgA1 fragments by western blot in serum from mice after 3 mg/kg injection. Representative samples of selected time points. (D) Pharmacokinetics (PK) of IgA1‑P in serum of α1KI-CD89Tg mice after IV injection for each time point of euthanized mice as described in (A). PD, pharmacodynamics.
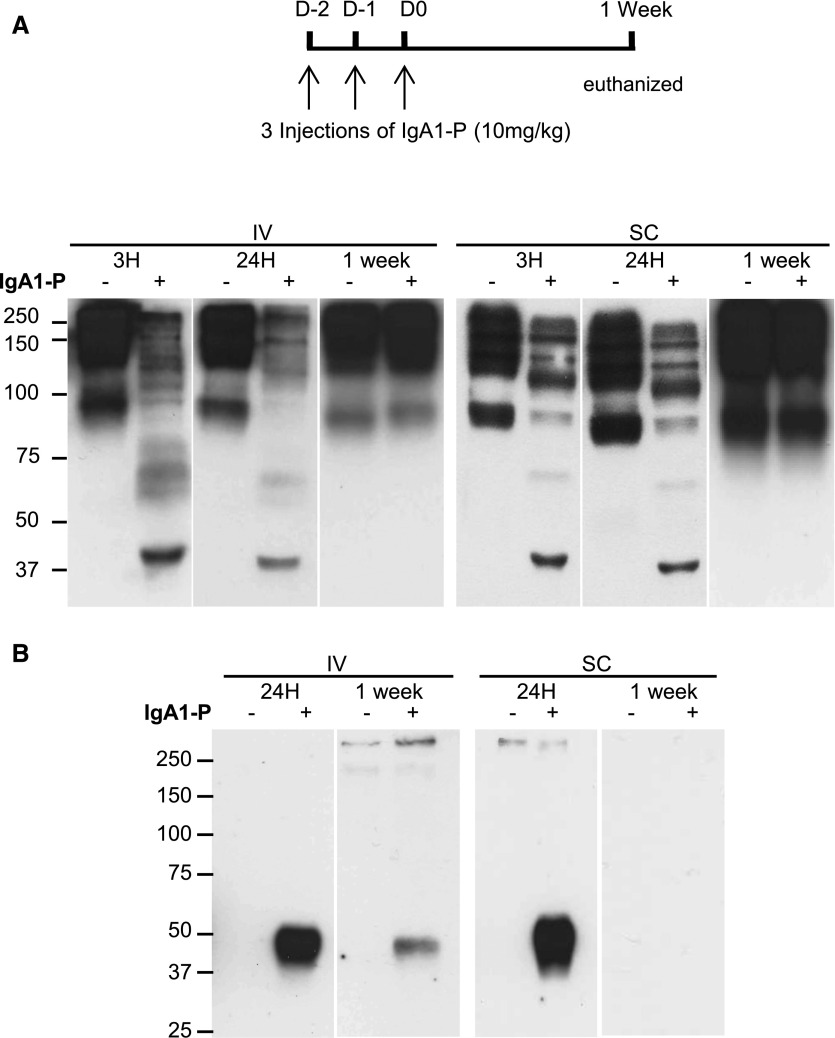
To increase the duration for which the drug was effective, mice were injected three times with IgA1‑P. Subcutaneous (SC) and IV administrations were compared with determine the superior injection route. IgA1 fragments were identified in serum 3 and 24 hours after IV or SC IgA1‑P injection at 10 mg/kg (Figure 2A), confirming the ability of IgA1‑P to cleave serum IgA1 in vivo following IV or SC injections. No fragments were detected 1 week later. Moreover, IgA1 fragments were detected in urine 24 hours after IV or SC injection and 1 week after IV injection (Figure 2B).
Figure 2.
Cleavage of hIgA1 by IgA1‑P. Detection of IgA fragments by western blot in (A) serum and (B) urine before (–) or 3 hours, 24 hours, or 1 week after (+) three IV or SC injections of IgA1‑P (10 mg/kg). n=5 mice per group. Representative individual samples of each group.
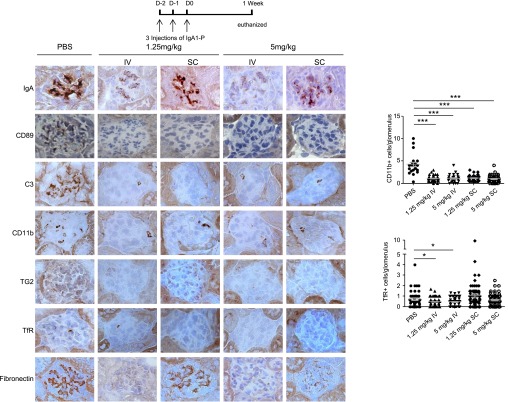
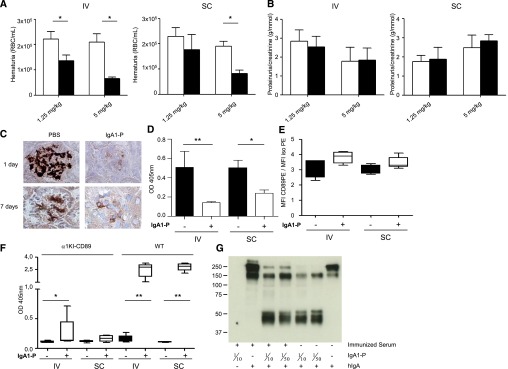
To determine whether several injections of IgA1‑P prolonged the effects, mice were euthanized 1 week after the last of the three injections (1.25 or 5 mg/kg). A readily detectable decrease in IgA1 and sCD89 deposits was observed in all animals and was more pronounced after IV injections than SC injections (Figure 3). This treatment also induced a decrease in inflammation and cell infiltration as observed by a reduction in C3 staining and the significant decrease in the number of CD11b-positive cells in treated mice compared with PBS-injected mice (Figure 3). Interestingly, TG2, which has been demonstrated to play an important role in IgAN development,7 decreased after both IV and SC treatments at 5 mg/kg. Similarly, TfR expression by mesangial cells was significantly impaired by IV treatment at either concentration. A strong decrease in fibronectin expression in the glomeruli was also observed, indicating downregulation of the extracellular matrix expansion. Taken together, these results show that a decrease in IgA1 deposits over a longer period led to an amelioration of inflammation and reduced the expression of TG2 and TfR, two molecular partners involved in the stabilization of IgA1 deposits. These results were associated with a significant decrease in hematuria in mice IV-treated at all doses and SC-treated at 5 mg/kg (Figure 4A). However, proteinuria was not modified 1 week after IgA1‑P treatment (Figure 4B).
Figure 3.
Decrease in IgA1, CD89, C3, CD11b, TG2, TfR, and fibronectin staining in mouse glomeruli after IgA1‑P treatment (three injections). Immunohistochemistry of frozen kidney sections from α1KI-CD89Tg mice, after IgA1‑P IV or SC injection, using antibodies against human IgA or mouse C3, CD11b, TG2, TfR, or fibronectin. Graphs show the corresponding number of CD11b- or TfR-positive cells per glomerulus counted in ten randomly chosen fields for each mouse at magnification ×200. n=5 mice per group. *P<0.05, unpaired t test; ***P<0.001 Mann–Whitney test.
Figure 4.
Decrease of hematuria and IgA1 deposits after a longer treatment with IgA1‑P despite the production of antibodies recognizing IgA1‑P. (A) Hematuria counts in α1KI-CD89Tg mice before and 1 week after three IgA1‑P injections (red blood cells [RBC]/ml). (B) Proteinuria in α1KI-CD89Tg mice before and 1 week after three IgA1‑P injections. (C) Immunohistochemistry of frozen kidney sections from four treated α1KI-CD89Tg mice using antibodies against human IgA. Mice were injected four times during 3 weeks and euthanized the day after the last injection (1 day) or 1 week later (7 days). (D) Levels of IgA1–sCD89 complexes in vehicle (–) or IgA1‑P (+) treated mouse serum assessed by ELISA. (E) CD89 expression on vehicle (–) or IgA1‑P (+) treated mouse splenic CD11b-positive cell surface analyzed by flow cytometry. The results are displayed as the ratio of CD89 mean fluorescent intensity (MFI) to control isotype MFI. (F) Anti–IgA1‑P IgG in vehicle (–) or IgA1‑P (+) treated mouse serum 3 weeks after the first of four injections. (G) The capacity of IgA1‑P to cleave IgA1 was tested by incubating the serum of an IgA1‑P-immunized wild-type mouse with IgA1‑P and then adding hIgA1. Cleavage was detected by a western blot targeting IgA. n=5 mice per group. Empty bars represent the PBS-treated animals, and filled bars show mice treated with IgA1‑P. *P<0.05; **P<0.01 Wilcoxon or Mann–Whitney test.
To test whether longer treatment is effective, mice were injected once a week with IgA1‑P (IV or SC) and euthanized the day after the fourth injection (3 weeks after the first one). Mesangial IgA1 deposits were nearly absent in α1KI‑CD89Tg mice after this treatment and slowly reappeared 1 week later (Figure 4C). In serum from the treated mice, a significant decrease in IgA1–sCD89 complexes was observed (IV or SC treatment) when compared with mice injected with vehicle, measured by an IgA1–sCD89 ELISA (Figure 4D). Moreover, CD89 expression on the surface of splenic monocytes was assessed by flow cytometry to determine the origin of the reduction in circulating sCD89. CD89 expression tended to increase after treatment, indicating a possible decrease in sCD89 shedding (Figure 4E). The putative upregulation of transmembrane CD89 expression could be the result of a decrease in its cleavage at lower IgA1–complex concentrations.
Because 3 weeks is sufficient to develop a strong adaptive immune response against pathogens, the production of IgG antibodies targeting IgA1‑P was tested by ELISA. A specific IgG response was present in α1KI‑CD89Tg mice (Figure 4F), although it was at lower magnitude when compared with wild-type mice. In fact, knocking in of the human Cα1 Ig gene in place of the mouse S mu region altered IgG production in these mice.30 The capacity of IgG to inhibit IgA1‑P efficacy was then tested in vitro by incubating serum from immunized wild-type mice with IgA1‑P followed 2 hours later by the addition of hIgA1. Serum IgG anti–IgA1‑P antibodies from immunized mice were not sufficient to fully inhibit the activity of IgA1‑P (Figure 4G). Finally, IgA1‑P treatment was well tolerated with no death, no body or organ weight loss, and no unrelated histologic changes (brain, heart, thymus, liver, spleen, and lymph nodes, data not shown).
Taken together, these results demonstrate the ability of IgA1‑P to markedly reduce hIgA1 in serum and IgA1 deposits in the mesangium of α1KI‑CD89Tg mice after only a single injection. The effect was observed after only 5 minutes, suggesting that the IgA1‑P can cleave IgA1 deposits as soon as it reaches the mesangium. However, one injection was not sufficient to prevent the recurrence of IgA1 deposits. Three daily injections of IgA1‑P at 5 mg/kg strongly reduced IgA1 deposits in the mesangium, an effect that was maintained for at least 1 week even after IgA1‑P disappeared 24 hours post injection. The lack of recurrence of IgA1 deposits during serum IgA1 recovery (see Figure 1B) could be due to the secondary effect of IgA1‑P on mesangial receptors and partners. Indeed, sCD89, TfR, and TG2 expression were markedly impaired after IgA1‑P treatment. These proteins bind, stabilize, and amplify IgA1 deposits,7 and reaching levels of these proteins sufficient to avoid IgA1 deposits may require a longer time. As a result, there was a strong decrease in C3 deposition and CD11b-positive cell infiltration, confirming that a decrease in mesangial IgA1 deposits has the capacity to decrease inflammatory responses and ameliorate symptoms in α1KI‑CD89Tg mice with a decrease in hematuria. However, we did not observe changes in proteinuria, which might indicate a need for either longer IgA1‑P treatment or combined complementary reparative drugs to recover normal glomerular filtration. The low level of mesangial IgA1 deposits after 3 weeks of treatment in α1KI‑CD89Tg mice suggests that IgA1‑P might be efficient as a long-term treatment. Moreover, this treatment significantly reduced IgA1–sCD89 complexes and tended to restore CD89 expression on the surface of splenic macrophages.
This therapeutic test suggests that the decrease in IgA1 deposits in the α1KI‑CD89Tg mouse model was sufficient to block IgA1 deposition, fibrosis, and hematuria. Our study confirms that IgA1‑P may be a potential treatment for IgAN patients. The immunogenicity against IgA1‑P is high, as shown previously in humans,31 where, in contrast to the mouse model, it diminishes its action in vitro. One could propose that a combined immunosuppressive therapy will avoid immune responses against IgA1‑P, but toxicities of immunosuppressive therapy should be also considered. Compared with the other currently used treatments, IgA1‑P has the specificity to target the main protein responsible for the disease and take secondary action on the receptor responsible for IgA1 deposits in the mesangium. This treatment could represent a potential option for a future specific therapy for IgAN.
Concise Methods
Mouse Procedures
All strains were raised and maintained at the mouse facilities of the Center of Research on Inflammation. All experiments were performed in accordance with the national ethical guidelines and with the approval of local authorities of the Comité d’Éthique Expérimentation Animale Bichat-Debré. Twelve-week-old α1KI‑CD89Tg mice7 were injected IV or SC, one, three, or four times, with 1, 3, 5, or 10 mg/kg of IgA1-P or with the vehicle (PBS). Following urine and serum collection, mice were euthanized, and the kidney, spleen, heart, liver, thymus, brain, inguinal, and cervical lymph node were collected. Organs were conserved in either medium optimal cutting temperature (CML, Nemours, France) or formalin 10% solution (Sigma-Aldrich, St. Louis, MO).
IgA1‑P
IgA1‑P from H. influenzae was expressed intracellularly as inclusion bodies in Escherichia coli fed-batch fermentation. The fermentation culture was centrifuged and the recovered cells were stored frozen at –20°C. Cells were thawed and resuspended in cell suspension buffer, followed by high-pressure homogenization and clarification by centrifugation. The obtained inclusion bodies were stored frozen at –80°C. The inclusion bodies were subsequently thawed and washed three times and stored frozen at –80°C as slurry in purified water.
The washed inclusion bodies were thawed and solubilized using urea, followed by product refolding. The refolded IgA1‑P was captured and purified through a series of chromatography steps, including ion-exchange and hydrophobic interaction chromatography, followed by an ultrafiltration and diafiltration step to concentrate IgA1‑P to 2–3 mg/ml in PBS (pH 7.1). The final purified IgA1‑P was 0.22-μm filtered and stored frozen at –80°C until use.
Histology and Immunohistochemistry
Paraffin-embedded kidney sections 4-µm thick were stained with periodic acid-Schiff or hematoxylin-eosin for morphologic analysis. For immunohistochemistry, 4-μm thick cryostate frozen kidney sections were first incubated for 1 hour with BSA (Euromedex, Souffelweyersheim, France), followed by 1 hour 30 minutes at room temperature with biotinylated mouse anti-human IgA (BD Biosciences, San Jose, CA), rat anti-mouse CD11b (BD Biosciences), goat anti-mouse fibronectin (Santa Cruz Biotechnology, Santa Cruz, CA) or rat anti-mouse TfR (BD Biosciences). For unconjugated antibodies: purified rabbit anti-mouse TG2 (Thermo Fisher Scientific, Vernon Hills, IL) or rabbit anti-mouse C3 (Abcam, Inc., Cambridge, MA), a supplementary step followed by incubation with a biotinylated goat anti-rabbit antibody (Southern Biotech, Birmingham, AL) for 30 minutes. Detection was performed with vectastain elite ABCkit (Vector Laboratories, Burlingame, CA). Slides were mounted with the Immu-mount (Thermo Fisher Scientific) mounting medium and read with an upright microscope, DM2000 (Leica Microsystems, Buffalo Grove, IL) at 400× magnification using the IM50 software (Leica Microsystems).
Cleavage Experiments
Purified IgA1 (produced in the laboratory from α1KI mice), human IgG1 (purified from human sera), hIgA1 (MP Biomedicals, Illkirch, France), IgA2 (Abcam, Inc.), mouse IgA (BD Biosciences), mouse sera, or human sera were incubated for 2 hours at 37°C with or without IgA1‑P. This was followed by SDS-10% PAGE analysis and Coomassie blue staining except for the sera, which were western blotted. For cleavage inhibition, immunized wild-type mouse serum was incubated for 2 hours with IgA1‑P at a concentration of 1/10 or 1/50 that of IgG in mouse serum. hIgA1 (MP Biomedicals) was added for 2 hours followed by SDS-10% PAGE analysis and a western blot.
Western Blot
Serum or urine was solubilized in the SDS sample buffer under reducing or nonreducing conditions and subjected to SDS-10% PAGE. Proteins were electroblotted on nitrocellulose membranes (EMD Millipore, Billerica, MA) and subjected to western blot analysis using a biotinylated goat anti-human IgA (Southern Biotech) followed by streptavidin coupled to horseradish peroxidase (R&D Systems, Minneapolis, MN) or donkey anti-goat horseradish peroxidase. Membranes were developed by enhanced chemical luminescence treatment (GE Healthcare, Waukesha, WI).
ELISA
Plates were coated with IgA1‑P (5 µg/ml) or A3 anti-CD89 mAb (10 µg/well) (produced in the laboratory). Sera were incubated in the wells in PBS, containing 0.05% Tween, 0.1% sodium azide, and 1% BSA for 2 hours. After washing, the goat anti-mouse IgG or mouse anti-human IgA coupled with the alkaline phosphatase (BD Biosciences) was added at 1:2000 dilution for 1 hour. The reaction was developed by adding the alkaline phosphatase substrate (SIGMAFAST p‑nitrophenyl phosphate tablets; Sigma-Aldrich).
Cells and Flow Cytometry
Splenocytes were stained with anti-mouse CD11b–PE-Cy7 and anti–CD89-PE antibodies (BD Biosciences).
Biochemical Analyses
Measurements of protein and creatinine were performed in the urine of mice using the Olympus AU400 chemistry analyzer by Bichat biochemistry platform. For hematuria, 10 µl of fresh urine were mounted on a Malassez hemocytometer, and red cells were counted.
Statistical Analyses
All data are expressed as the mean±SEM. Data from different groups were compared using the Wilcoxon test to compare mice before and after IgA1‑P treatment. The Mann–Whitney test was used for comparison between mice treated with IgA1‑P and mice treated with only the vehicle. P<0.05 was considered statistically significant.
Disclosures
This study was supported in part by Shire. Christelle Moal was financed by a Shire grant. Y.W., A.L., and P.G.V.M. are members of Shire, Bioprocess Development and Discovery Biology and Translational Research, Lexington, Massachussetts.
Supplementary Material
Acknowledgments
We thank Olivier Thibaudeau and Sofiane Ameur of the Bichat histology platform and Nicolas Sorhindo of the Center of Research on Inflammation Biochemistry platform.
This study was supported by Equipe French Foundation for Medical Research, French Francilian Cariovascular-Obesity-Kidney-Diabetes Network, Laboratory of Excellency, Laboratory of Inflamex Excellency, (ANR-11-IDEX-0005-02), and National French Agency for Research, Young Researcher, Transglutaminase Role in IgA Nephropathy project.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015080856/-/DCSupplemental.
References
- 1.Berger J, Yaneva H, Nabarra B, Barbanel C: Recurrence of mesangial deposition of IgA after renal transplantation. Kidney Int 7: 232–241, 1975 [DOI] [PubMed] [Google Scholar]
- 2.Wyatt RJ, Julian BA: IgA nephropathy. N Engl J Med 368: 2402–2414, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Mestecky J, Tomana M, Moldoveanu Z, Julian BA, Suzuki H, Matousovic K, Renfrow MB, Novak L, Wyatt RJ, Novak J: Role of aberrant glycosylation of IgA1 molecules in the pathogenesis of IgA nephropathy. Kidney Blood Press Res 31: 29–37, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanaka M, Seki G, Someya T, Nagata M, Fujita T: Aberrantly glycosylated IgA1 as a factor in the pathogenesis of IgA nephropathy. Clin Dev Immunol 2011: 470803, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Launay P, Grossetête B, Arcos-Fajardo M, Gaudin E, Torres SP, Beaudoin L, Patey-Mariaud de Serre N, Lehuen A, Monteiro RC: Fcalpha receptor (CD89) mediates the development of immunoglobulin A (IgA) nephropathy (Berger’s disease). Evidence for pathogenic soluble receptor-Iga complexes in patients and CD89 transgenic mice. J Exp Med 191: 1999–2009, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vuong MT, Hahn-Zoric M, Lundberg S, Gunnarsson I, van Kooten C, Wramner L, Seddighzadeh M, Fernström A, Hanson LÅ, Do LT, Jacobson SH, Padyukov L: Association of soluble CD89 levels with disease progression but not susceptibility in IgA nephropathy. Kidney Int 78: 1281–1287, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Berthelot L, Papista C, Maciel TT, Biarnes-Pelicot M, Tissandie E, Wang PHM, Tamouza H, Jamin A, Bex-Coudrat J, Gestin A, Boumediene A, Arcos-Fajardo M, England P, Pillebout E, Walker F, Daugas E, Vrtosvnik F, Flamant M, Benhamou M, Cogné M, Moura IC, Monteiro RC: Transglutaminase is essential for IgA nephropathy development acting through IgA receptors. J Exp Med 209: 793–806, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suzuki H, Fan R, Zhang Z, Brown R, Hall S, Julian BA, Chatham WW, Suzuki Y, Wyatt RJ, Moldoveanu Z, Lee JY, Robinson J, Tomana M, Tomino Y, Mestecky J, Novak J: Aberrantly glycosylated IgA1 in IgA nephropathy patients is recognized by IgG antibodies with restricted heterogeneity. J Clin Invest 119: 1668–1677, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berthoux F, Suzuki H, Thibaudin L, Yanagawa H, Maillard N, Mariat C, Tomino Y, Julian BA, Novak J: Autoantibodies targeting galactose-deficient IgA1 associate with progression of IgA nephropathy. J Am Soc Nephrol 23: 1579–1587, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berthelot L, Robert T, Vuiblet V, Tabary T, Braconnier A, Dramé M, Toupance O, Rieu P, Monteiro RC, Touré F: Recurrent IgA nephropathy is predicted by altered glycosylated IgA, autoantibodies and soluble CD89 complexes. Kidney Int 88: 815–822, 2015 [DOI] [PubMed] [Google Scholar]
- 11.Moura IC, Centelles MN, Arcos-Fajardo M, Malheiros DM, Collawn JF, Cooper MD, Monteiro RC: Identification of the transferrin receptor as a novel immunoglobulin (Ig)A1 receptor and its enhanced expression on mesangial cells in IgA nephropathy. J Exp Med 194: 417–425, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moura IC, Arcos-Fajardo M, Sadaka C, Leroy V, Benhamou M, Novak J, Vrtovsnik F, Haddad E, Chintalacharuvu KR, Monteiro RC: Glycosylation and size of IgA1 are essential for interaction with mesangial transferrin receptor in IgA nephropathy. J Am Soc Nephrol 15: 622–634, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Moura IC, Arcos-Fajardo M, Gdoura A, Leroy V, Sadaka C, Mahlaoui N, Lepelletier Y, Vrtovsnik F, Haddad E, Benhamou M, Monteiro RC: Engagement of transferrin receptor by polymeric IgA1: evidence for a positive feedback loop involving increased receptor expression and mesangial cell proliferation in IgA nephropathy. J Am Soc Nephrol 16: 2667–2676, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Kanamaru Y, Arcos-Fajardo M, Moura IC, Tsuge T, Cohen H, Essig M, Vrtovsnik F, Loirat C, Peuchmaur M, Beaudoin L, Launay P, Lehuen A, Blank U, Monteiro RC: Fc α receptor I activation induces leukocyte recruitment and promotes aggravation of glomerulonephritis through the FcR γ adaptor. Eur J Immunol 37: 1116–1128, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Coppo R, Troyanov S, Bellur S, Cattran D, Cook HT, Feehally J, Roberts ISD, Morando L, Camilla R, Tesar V, Lunberg S, Gesualdo L, Emma F, Rollino C, Amore A, Praga M, Feriozzi S, Segoloni G, Pani A, Cancarini G, Durlik M, Moggia E, Mazzucco G, Giannakakis C, Honsova E, Sundelin BB, Di Palma AM, Ferrario F, Gutierrez E, Asunis AM, Barratt J, Tardanico R, Perkowska-Ptasinska A VALIGA study of the ERA-EDTA Immunonephrology Working Group : Validation of the Oxford classification of IgA nephropathy in cohorts with different presentations and treatments. Kidney Int 86: 828–836, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohtake T, Oka M, Maesato K, Mano T, Ikee R, Moriya H, Kobayashi S: Pathological regression by angiotensin II type 1 receptor blockade in patients with mesangial proliferative glomerulonephritis. Hypertens Res 31: 387–394, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Moriyama T, Amamiya N, Ochi A, Tsuruta Y, Shimizu A, Kojima C, Itabashi M, Takei T, Uchida K, Nitta K: Long-term beneficial effects of angiotensin-converting enzyme inhibitor and angiotensin receptor blocker therapy for patients with advanced immunoglobulin A nephropathy and impaired renal function. Clin Exp Nephrol 15: 700–707, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Pozzi C, Andrulli S, Del Vecchio L, Melis P, Fogazzi GB, Altieri P, Ponticelli C, Locatelli F: Corticosteroid effectiveness in IgA nephropathy: long-term results of a randomized, controlled trial. J Am Soc Nephrol 15: 157–163, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Radhakrishnan J, Cattran DC: The KDIGO practice guideline on glomerulonephritis: reading between the (guide)lines--application to the individual patient. Kidney Int 82: 840–856, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Rauen T, Eitner F, Fitzner C, Sommerer C, Zeier M, Otte B, Panzer U, Peters H, Benck U, Mertens PR, Kuhlmann U, Witzke O, Gross O, Vielhauer V, Mann JF, Hilgers RD, Floege J: STOP-IgAN Investigators. N Engl J Med 373: 2225–2236, 2015 [DOI] [PubMed] [Google Scholar]
- 21.Floege J: Glomerular disease: Efficacy of corticosteroids in high-risk IgA nephropathy. Nat Rev Nephrol 11: 319–320, 2015 [DOI] [PubMed] [Google Scholar]
- 22.Kawamura T, Yoshimura M, Miyazaki Y, Okamoto H, Kimura K, Hirano K, Matsushima M, Utsunomiya Y, Ogura M, Yokoo T, Okonogi H, Ishii T, Hamaguchi A, Ueda H, Furusu A, Horikoshi S, Suzuki Y, Shibata T, Yasuda T, Shirai S, Imasawa T, Kanozawa K, Wada A, Yamaji I, Miura N, Imai H, Kasai K, Soma J, Fujimoto S, Matsuo S, Tomino Y Special IgA Nephropathy Study Group : A multicenter randomized controlled trial of tonsillectomy combined with steroid pulse therapy in patients with immunoglobulin A nephropathy. Nephrol Dial Transplant 29: 1546–1553, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donadio JV Jr, Bergstralh EJ, Offord KP, Spencer DC, Holley KE Mayo Nephrology Collaborative Group : A controlled trial of fish oil in IgA nephropathy. N Engl J Med 331: 1194–1199, 1994 [DOI] [PubMed] [Google Scholar]
- 24.Coppo R, Roccatello D, Amore A, Quattrocchio G, Molino A, Gianoglio B, Amoroso A, Bajardi P, Piccoli G: Effects of a gluten-free diet in primary IgA nephropathy. Clin Nephrol 33: 72–86, 1990 [PubMed] [Google Scholar]
- 25.Papista C, Lechner S, Ben Mkaddem S, LeStang M-B, Abbad L, Bex-Coudrat J, Pillebout E, Chemouny JM, Jablonski M, Flamant M, Daugas E, Vrtovsnik F, Yiangou M, Berthelot L, Monteiro RC: Gluten exacerbates IgA nephropathy in humanized mice through gliadin-CD89 interaction. Kidney Int 88: 276–285, 2015 [DOI] [PubMed] [Google Scholar]
- 26.Plaut AG: The IgA1 proteases of pathogenic bacteria. Annu Rev Microbiol 37: 603–622, 1983 [DOI] [PubMed] [Google Scholar]
- 27.Senior BW, Woof JM: The influences of hinge length and composition on the susceptibility of human IgA to cleavage by diverse bacterial IgA1 proteases. J Immunol 174: 7792–7799, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Long S, Phan E, Vellard MC: The expression of soluble and active recombinant Haemophilus influenzae IgA1 protease in E. coli. [published online ahead of print November 30, 2010]. J Biomed Biotechnol doi: 10.1155/2010/253983 [DOI] [PMC free article] [PubMed]
- 29.Lamm ME, Emancipator SN, Robinson JK, Yamashita M, Fujioka H, Qiu J, Plaut AG: Microbial IgA protease removes IgA immune complexes from mouse glomeruli in vivo: potential therapy for IgA nephropathy. Am J Pathol 172: 31–36, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duchez S, Amin R, Cogné N, Delpy L, Sirac C, Pascal V, Corthésy B, Cogné M: Premature replacement of μ with α immunoglobulin chains impairs lymphopoiesis and mucosal homing but promotes plasma cell maturation. Proc Natl Acad Sci U S A 107: 3064–3069, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Palois F, Beghetto E, Spadoni A, Montagnani F, Felici F, Oggioni MR, Gargano N: Identification of a human immunodominant B-cell epitope within the immunoglobulin A1 protease of Streptococcus pneumoniae. BMC Microbiol 7: 113, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.