Abstract
Mitochondrial fission has been linked to the pathogenesis of diabetic nephropathy (DN). However, how mitochondrial fission affects progression of DN in vivo is unknown. Here, we report the effect of conditional podocyte–specific deletion of dynamin-related protein 1 (Drp1), an essential component of mitochondrial fission, on the pathogenesis and progression of DN. Inducible podocyte–specific deletion of Drp1 in diabetic mice decreased albuminuria and improved mesangial matrix expansion and podocyte morphology. Ultrastructure analysis revealed a significant increase in fragmented mitochondria in the podocytes of wild–type diabetic mice but a marked improvement in mitochondrial structure in Drp1-null podocytes of diabetic mice. When isolated from diabetic mice and cultured in high glucose, Drp1-null podocytes had more elongated mitochondria and better mitochondrial fitness associated with enhanced oxygen consumption and ATP production than wild-type podocytes. Furthermore, administration of a pharmacologic inhibitor of Drp1, Mdivi1, significantly blunted mitochondrial fission and rescued key pathologic features of DN in mice. Taken together, these results provide novel correlations between mitochondrial morphology and the progression of DN and point to Drp1 as a potential therapeutic target in DN.
Keywords: podocyte, mitochondria, diabetic nephropathy
Mitochondria are dynamic organelles that periodically divide (fission) and fuse (fusion).1–3 Through the fission and fusion processes, mitochondria are able to interchange their morphology between an elongated, interconnected mitochondrial network and a fragmented, denser arrangement depending on the cell type and the metabolic demands of the cell.4–6 The importance of frequent mitochondrial fission and fusion events was not clearly appreciated until fairly recently, when it was suggested that abnormal mitochondrial dynamics, an imbalance between mitochondrial fission and fusion, were linked to a number of diseases, such as neurodegenerative and cardiovascular diseases.4,7 Consistent with these observations, we have recently shown that mitochondrial fission is closely associated with key features of diabetic nephropathy (DN).8 However, how mitochondrial fission contributes to the development and progression of DN in vivo has not yet been fully elucidated.
Members of the Dynamin family are key components of mitochondrial dynamics.9,10 Importantly, dynamin-related protein 1 (Drp1) is essential for mitochondrial fission.11,12 Drp1 is an evolutionary conserved protein, which can self-assemble into large multimeric spirals, mediating mitochondrial fission through a GTP-dependent constriction of mitochondria.12,13 Drp1 is essential for embryonic development in mice, and its loss causes embryonic lethality at approximately embryonic days 11.5–12.14,15 The essential role of Drp1 in development is not surprising considering that Drp1 and mitochondrial fission play key roles in important biologic activities of the cell, such as apoptosis and mitophagy.11,16,17 However, whereas early reports indicated that enhanced Drp1 activity and consequently, mitochondrial fission were associated with increased cytochrome c release, eventually enhancing cell apoptosis by increasing reactive oxygen species (ROS) production,4,11,18 other studies have challenged the traditional pathogenic effect of high levels of Drp1 activity, suggesting that the effect of Drp1 on cell death could likely be tissue specific and context dependent.19
We have recently shown that one of the characteristic features of mitochondrial dysfunction in the diabetic kidneys is excessive mitochondrial fission.8 These findings provided the first evidence for a central role of Drp1 in mediating mitochondrial dysfunction in DN. However, the role of Drp1 in DN in vivo remained unknown. Thus, to assess the value or limitations of targeting Drp1 in progression of DN and its effect on mitochondrial function, we generated a genetically modified mouse model, in which Drp1 was selectively deleted in podocytes in an established model of type 2 diabetes. We complemented our results by using a pharmacologic inhibitor of Drp1 in diabetic mice as well as mechanistic studies in vitro using cultured podocytes. The results of our genetic approach in diabetic mice coupled with the pharmacologic findings suggest that podocytes from diabetic mice deficient for Drp1 are protected against progression of DN. Furthermore, our findings suggest that Drp1 knockdown in podocytes improves mitochondrial dysfunction in the diabetic environment. These observations strongly support the role of Drp1 and mitochondrial dynamics as new potential targets in DN.
Results
Generation and Characterization of an Inducible Podocyte–Specific Drp1–Null Mouse Model
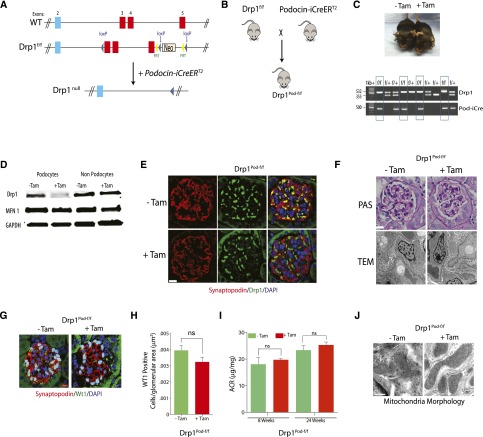
Recent studies have revealed that complete loss of function of Drp1 is embryonically lethal because of several developmental defects.14,15 Therefore, to gain insights into the relationship between the mitochondrial fission machinery and podocyte homeostasis, we generated mice with an inducible, podocyte–specific floxed allele of Drp1 (Figure 1, A and B). We first crossed Drp1flox/flox mice with podocyte–specific, tamoxifen–inducible improved Cre recombinase under the control of the human (NPHS2) podocin promoter (podocin-iCreERT2) transgenic mice previously generated in our laboratory.20 Drp1flox/flox; podocin-iCreERT2 mice (here referred to as Drp1Pod-f/f) were phenotypically normal and born at the expected Mendelian frequency and proportional male-to-female ratios (Figure 1C). At 8 weeks of age, Drp1Pod-f/f mice were induced with tamoxifen to generate podocyte–specific Drp1–null mice. Western blot analysis of Drp1 expression in podocytes isolated from tamoxifen–induced Drp1Pod-f/f mice revealed reduced Drp1 levels compared with noninduced control podocytes (Figure 1D). We also confirmed that Drp1 expression in other cell types of the kidney, comprising a mixture of endothelial, mesangial, and tubule cells of tamoxifen-induced mice, was not significantly altered (Figure 1D). Immunofluorescence analysis revealed substantially attenuated Drp1 staining in podocytes from tamoxifen-induced Drp1Pod-f/f on the basis of colocalization patterns of Drp1 and Synaptopodin, a podocyte–specific cytoplasmic protein (Figure 1E). Disruption of podocyte–specific Drp1 expression did not elicit significant histopathologic consequences as assessed by periodic acid–Schiff (PAS) staining and transmission electron microscopy (TEM) (Figure 1F). Furthermore, the total number of podocytes was similar in tamoxifen–induced Drp1Pod-f/f mice compared with noninduced mice (Figure 1, G and H). Importantly, the albumin-to-creatinine ratio was unchanged by podocyte-specific deletion of Drp1 after tamoxifen induction (Figure 1I). Finally, ultrastructure examination of mitochondria in podocytes from tamoxifen-induced Drp1Pod-f/f revealed more elongated mitochondria compared with controls (Figure 1J). Taken together, these findings suggest that conditional deletion of Drp1 in podocytes leads to elongation of mitochondria in podocytes but does not lead to significant biochemical or histologic changes.
Figure 1.
Generation of inducible podocyte–specific Drp1 knockout mice. (A) Schematic of the Drp1 conditional knockout strategy. (B) Mating strategy to generate Drp1 conditional knockout in mouse podocytes. (C, upper panel) Representative images of tamoxifen–induced and noninduced control Drp1Pod-f/f mice. (C, lower panel) PCR genotyping confirming successful transmission of Pod-iCre and Drp1f/f homozygosity. (D) Western blot analysis confirming robust reduction in Drp1 expression in isolated podocytes from tamoxifen–induced and noninduced Drp1Pod-f/f mice (n=3 mice per group). GAPDH was used as loading control. (E) Representative immunofluorescence micrographs of kidney sections stained with primary antibodies against Drp1 (green) and Synaptopodin (red) from tamoxifen–induced Drp1Pod-f/f and noninduced mice (n=5 mice per group). Sections were counterstained with DAPI. Scale bars, 50μm. (F, upper panel) Representative light micrographs of kidney sections stained with PAS and (F, lower panel) representative transmission electron micrographs from tamoxifen–induced and noninduced Drp1Pod-f/f mice (n=5 mice per group). Scale bars, 10μm in F, upper panel; 500nm in F, lower panel. (G) Representative immunofluorescence micrographs of kidney sections stained with primary antibodies against WT1 from tamoxifen–induced and noninduced Drp1Pod-f/f mice. Scale bars, 10 μm. (H) Quantification of sections stained with WT1 from G. Results are generated from 25 randomly selected kidney glomeruli per animal in each group (n=5 mice per group). (I) Albumin-to-creatinine ratio (ACR) was measured in mice 8 and 24 weeks after tamoxifen induction. (J) Representative TEM micrographs of mitochondria in podocytes from tamoxifen–induced and noninduced Drp1Pod-f/f mice (n=5 mice per group).Results are presented as means±SEMs. Scale bars, 2 μm.
Targeted Deletion of Drp1 in Podocytes Mitigates Progression of DN
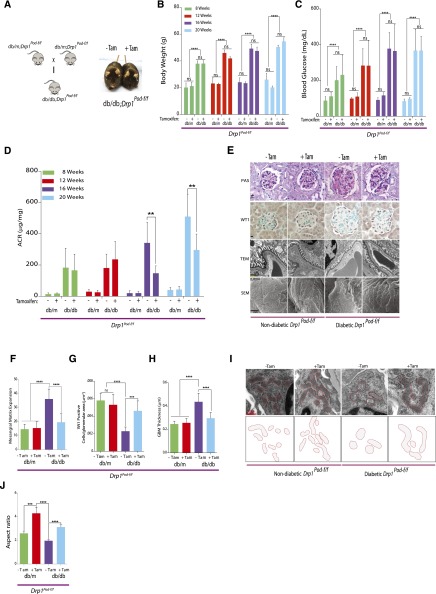
We next tested whether Drp1 deletion in podocytes exerts a renoprotective effect in DN, a condition in which podocytes are stressed under hyperglycemic conditions. To test this, we crossed Drp1Pod-f/f with Leprdb/+ mice, an established experimental model of type 2 diabetes, to generate Leprdb/db;Drp1Pod-f/f mice (hereafter referred to as db/db;Drp1Pod-f/f) (Figure 2A). To investigated the effect of Drp1 deletion in podocytes, we allocated mice into four groups: db/m;Drp1Pod-f/f (with or without tamoxifen) and db/db;Drp1Pod-f/f (with or without tamoxifen). Tamoxifen–induced diabetic Drp1Pod-f/f mice exhibited similar metabolic parameters, including body weight and blood glucose levels, compared with noninduced diabetic controls (Figure 2, B and C). However, deletion of Drp1 in podocytes significantly improved the extent of albuminuria as measured by albumin-to-creatinine ratio in tamoxifen–induced db/db;Drp1Pod-f/f mice at 16 (by approximately 60%) and 20 weeks of DN (by approximately 40%) compared with noninduced diabetic control mice (Figure 2D).
Figure 2.
Conditional deletion of Drp1 in podocytes protects against progression of DN. (A, left panel) Mating strategy to generate Drp1 conditional knockout in mouse podocytes of diabetic mice. (A, right panel) Representative image of tamoxifen–induced and noninduced diabetic db/db;Drp1Pod-f/f mice. (B) Body weight, (C)blood glucose levels, and (D) albumin-to-creatinine ratios (ACRs; micrograms per milligram) of tamoxifen-induced and noninduced controls from nondiabetic (db/m;Drp1Pod-f/f) and diabetic (db/db;Drp1Pod-f/f)mice at 4-week intervals beginning at 8 weeks of age (n=10 mice per group).(E) Representative micrographs of PAS–stained kidney sections (row 1), WT1 (row 2), transmission electron micrographs (row 3), and scanning electron micrographs (row 4) from the groups described in D. Scale bars, 10μm for rows 1 and 2; 500nm for row 3; 2 μm in row 4. (F) Quantification of mesangial matrix expansion (n=10 per group) and (G) WT1-positive glomeruli from 25 randomly selected glomeruli per animal in each indicated group (n=5 mice per group). (H) Quantification of GBM thickness (n=5–7 mice per group). (I, upper panel) Representative TEM micrographs of podocyte mitochondria from groups described in D. (I, lower panel) Tracing of mitochondria from aforementioned TEM micrographs. (J) Average aspect ratios in each group. Results are presented as means±SEMs. **P<0.01; ***P<0.01; ****P<0.001.
We then investigated the effect of Drp1 deletion in podocytes on the key histopathologic features of DN. Tamoxifen–induced db/db;Drp1Pod-f/fmice exhibited a significant reduction of mesangial expansion compared with noninduced diabetic controls as measured by PAS staining (Figure 2E, row 1). When we quantified podocyte numbers by WT1 staining, we found that tamoxifen–induced db/db;Drp1Pod-f/f mice maintain their number of podocytes compared with noninduced diabetic controls (Figure 2E, row 2). Consistent with these findings, TEM micrographs also revealed marked improvement in podocyte foot process effacement (Figure 2E, row 3) and glomerular basement membrane (GBM) in tamoxifen–induced db/db;Drp1Pod-f/f mice compared with their noninduced diabetic controls (Figure 2E). Scanning electron microscopy analysis confirmed these findings, because we observed a significant improvement in podocyte foot process flattening in tamoxifen–induced diabetic mice compared with controls (Figure 2E, row 4). Quantification of these parameters strongly confirmed that genetic deletion of Drp1 in podocytes in diabetic mice significantly prevents biochemical and histologic features of DN progression (Figure 2, F–H). Furthermore, ultrastructure examination of mitochondria in tamoxifen–induced diabetic mice revealed significant increase in elongated mitochondria (Figure 2I). Importantly, aspect ratio measurements revealed that deletion of Drp1 in podocytes blocked high glucose (HG) –mediated mitochondrial fission in vivo (Figure 2J). On the basis of these data, we concluded that targeted disruption of Drp1 expression in podocytes leads to amelioration of key features of DN.
Interruption of Mitochondrial Fission Improves Mitochondria Fitness
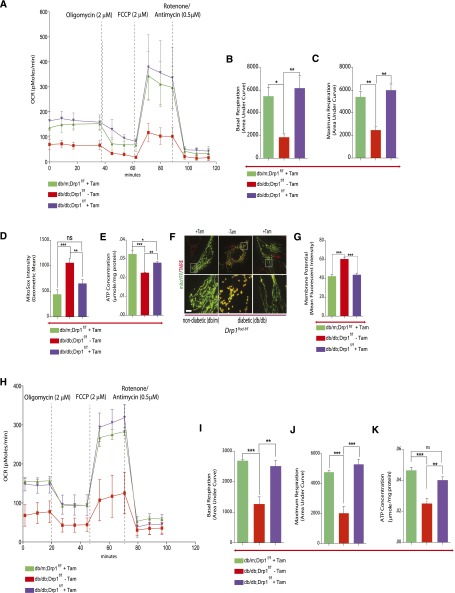
To better understand the basis for improvements in key features of DN after blocking of mitochondrial fission in podocytes, we set out to determine whether the renoprotective effects of Drp1 were attributable to changes in mitochondrial fitness. To this end, we isolated and cultured primary podocytes from tamoxifen–induced diabetic Drp1Pod-f/f mice. Cultured podocytes from noninduced diabetic and nondiabetic Drp1Pod-f/f mice were used as controls and baselines, respectively, for interpreting our findings. Early-passage podocytes were maintained in either normal glucose (NG; 5 mM) or HG (25 mM) for the duration of the study. Importantly, we found that deletion of Drp1 reverses the effect of HG on both basal and maximal oxygen consumption rate (OCR) levels (Figure 3, A–C). We found that deletion of Drp1 in podocytes from diabetic mice led to an approximately 40% reduction in ROS production but noticeably, increased total ATP content by approximately 20% compared with noninduced controls (Figure 3, D and E). Consistent with overall improvement in mitochondrial fitness, we found that deletion of Drp1 prevents HG-mediated increases in mitochondrial membrane potential by 27% (Figure 3, F and G). We also noticed that mitochondria from tamoxifen–induced nondiabetic and diabetic mice were profoundly elongated, whereas mitochondria from noninduced diabetic mice were rounded and circular, suggestive of enhanced mitochondrial fusion in induced podocytes (Figure 3F).
Figure 3.
Interruption of mitochondrial fission improves mitochondria fitness. (A) Bioenergetics profile as measured by OCR with a Seahorse ×24 Extracellular Flux Analyzer (Seahorse Bioscience) in podocytes in each group (n=3 per group). Oligomycin (2 μM), FCCP (2 μM), and rotenone (0.5 μM)/antimycin A (0.5 μM) were added at the times indicated. (B) Basal and (C) maximal respiratory rates of podocytes were determined by calculating the area under the curve. Values are means±SEMs of two to three replicates of two separate experiments. (D) Mitochondrial ROS were described as relative mean fluorescence intensity of MitoSox Red–positive cells. (E) ATP levels were quantified in podocytes isolated from db/m;Drp1Pod-f/, db/db;Drp1Pod-f/f, and noninduced db/db;Drp1Pod-f/f control mice (n=3 per group). (F) Mito-YFP Green (mitochondria) and TMRE were used to examine mitochondrial membrane potential in podocytes. All images are merged projections of 543-nm and 488-nm Z-serial channels. (G) The fluorescent density of TMRE was used for quantitative analysis. (H) OCR analysis for podocytes freshly isolated from mice. (I) Basal and (J) maximum respiration rates as measured by calculating the area under the curve for the indicated segments. (K) ATP levels in permeabilized mouse podocytes freshly isolated from each group. Results are presented as means±SEMs. *P<0.05; **P<0.01; ***P<0.01.
To confirm the key findings from primary cultured podocytes, we isolated podocytes from 24-week-old tamoxifen–induced diabetic Drp1Pod-f/f or uninduced control mice and measured the OCR directly in freshly isolated podocytes. Consistent with our results from cultured cells, we observed that Drp1 deletion leads to a significant increase in basal and maximal OCR levels in tamoxifen–induced diabetic mice compared with noninduced control mice (Figure 3, H–J). Furthermore, we confirmed that deletion of Drp1 in podocytes leads to significantly increased ATP levels in tamoxifen–induced diabetic mice compared with controls (Figure 3K). Taken together, these results suggest that podocyte-specific deletion of Drp1 in diabetic mice leads to improved mitochondrial fitness in the diabetic milieu as evidenced by a reversal in several key bioenergetics parameters.
Pharmacologic Inhibition of Drp1 Prevents Progression of DN
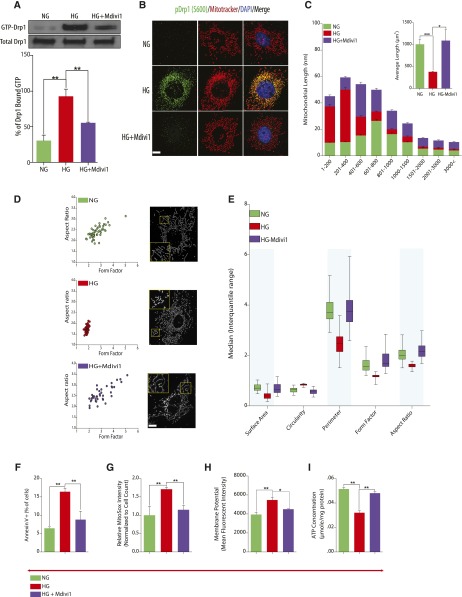
Recent observations suggest that pharmacologic inhibition and genetic ablation of Drp1 may not provide the same information.21 To address this, we deployed a well established inhibitor of Drp1 GTPase activity, Mdivi1, to investigate whether pharmacologic inhibition of Drp1 activity could lead to improvements in the DN phenotype. To this aim, we used an integrated in vitro and in vivo approach, where we initially took advantage of cultured podocytes exposed to HG conditions to assess the effect of Mdivi1 on mitochondrial morphology associated with HG conditions. Consistent with the observed effect of Mdivi1 on GTPase activity, we observed that podocytes exposed to HG exhibited a significant increase in Drp1-GTPase activity, whereas treatment of cells with Mdivi1 markedly reduced HG–induced Drp1 activity in podocytes (Figure 4A). Because we had previously shown that Ser-637 (mouse Ser-600) phosphorylation of Drp1 is required for mitochondrial fission in podocytes,8 we next examined the effect of Mdivi1 on Drp1 phosphorylation at this site in HG-treated podocytes as an indirect assessment of Drp1 activity. We observed that, although there was a marked increase in HG–induced mitochondrial fission and Ser-637 Drp1 phosphorylation after HG treatment, pretreatment with Mdivi1 resulted in a marked decrease in both mitochondrial fission and Ser-637 phosphorylation (Figure 4B). We then quantified changes in mitochondrial morphology by examining the distribution and average mitochondrial lengths in cultured podocytes treated with Mdivi1 in HG conditions. After staining with Mitotracker Red, mitochondria from podocytes cultured in NG conditions exhibited an interconnected network of tubular, elongated structures. In contrast, mitochondrial morphology was shifted toward a more fragmented, discontinuous network, with a higher proportion of smaller and rounder mitochondria in cells cultured in HG conditions (Figure 4, C–E). Importantly, we observed that treatment with Mdivi1 in podocytes exposed to HG conditions rescued the mitochondrial morphology, leading to a mitochondria morphology, which mainly resembled NG conditions (Figure 4, C–E). Indeed, mitochondrial aspect ratio and form factor from HG–induced cultured podocytes treated with Mdivi1 were significantly improved (Figure 4D). In addition to changes in aspect ratio and form factor, mitochondria from Mdivi1-treated podocytes showed increased surface area and perimeter similar to mitochondria from NG culture conditions, whereas mitochondria in podocytes in HG conditions were more fragmented as revealed by the circularity measurement, suggesting enhanced mitochondrial fission (Figure 4E). To further understand the functional consequences of preventing Drp1–mediated mitochondrial fission in podocytes, we measured cellular apoptosis. We found that Mdivi1 significantly attenuated HG–induced early apoptosis in podocytes as measured by flow cytometry for Annexin V–positive cells (Figure 4F). We also observed that, although treatment with HG significantly increased mitochondrial ROS levels, Mdivi1-treated podocytes exhibited significantly blunted HG–induced mitochondrial ROS levels (Figure 4G). In addition, we asked if pharmacologic inhibition of Drp1 had a similar effect on other parameters of mitochondrial fitness. Similar to our genetic model, we found that inhibition of Drp1 relieves HG-induced alterations in mitochondria membrane potential and ATP levels (Figure 4, H and I).
Figure 4.
Pharmacologic inhibition of Drp1 attenuates HG–induced mitochondrial dysfunction. (A, upper panel) Western blot analysis of GTP-bound Drp1 in mouse podocytes cultured in NG or HG and HG-cultured cells treated with 20μm Mdivi1. (A, lower panel) Densitometric analysis of data from A, upper panel. Values were normalized to total Drp1 levels, which remained unchanged. (B) Representative immunofluorescence micrographs of cells cultured as in A and stained with MitoTracker Red (red) and primary antibodies directed against phosphorylated Drp1 at Ser-637 (green). Nuclei were counterstained with DAPI (blue). Scale bars, 10 μm. (C) Data showing mitochondrial length measurement by ImageJ analysis. The morphology of ≥50 podocytes was determined for each condition. (D) Images of mitochondrial morphology visualized by MitoTracker Red staining of podocytes grown in NG, HG, or HG treated with Mdivi1. (E) Form factor, circularity, perimeter, surface area, and aspect ratios were quantified for each condition. Mitochondrial morphology of ≥120 mitochondria was determined for each condition and assessed by ImageJ analysis. (F) Quantitative data expressing percentage of Annexin VFITC–positive cells. (G) Mitochondrial ROS measurements described as relative mean fluorescence intensity of MitoSox Red–positive cells. (H) Quantification of mitochondrial membrane potential as measured by TMRE staining and flow cytometry analysis. (I) ATP levels were quantified in mouse podocytes cultured in NG or HG and HG-cultured cells treated with 20μm Mdivi1 (n=3 in triplicates). Results are presented as means±SEMs.*P<0.05; **P<0.01; ***P<0.01.
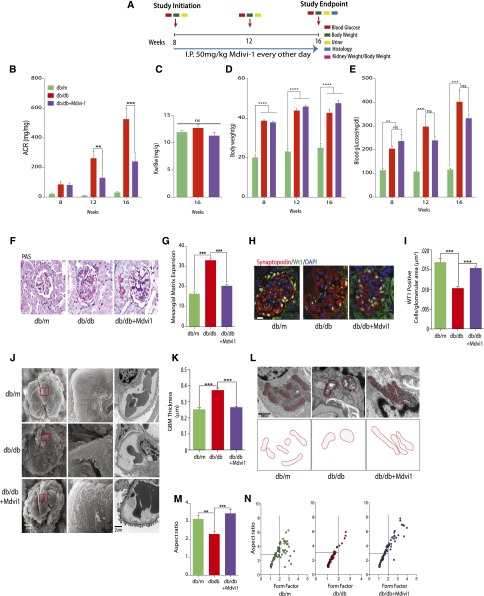
Finally, we assessed whether the administration of Mdivi1 in db/db models of DN phenocopies the results that we observed with the treatment of Mdivi1 in cultured podocytes. Figure 5A describes our schematic intervention with Mdivi1 in the db/db model of DN. Consistent with our genetic model, inhibition of Drp1 in diabetic mice led to significantly attenuated albuminuria measured at 4 and 8 weeks after initiation of Mdivi1 treatment (Figure 5B). Long-term treatment of diabetic mice with Mdivi1 did not affect body weight or kidney weight-to-body weight ratio compared with controls (Figure 5, C–E). However, blood glucose levels were decreased by approximately 25% in Mdivi1–treated diabetic mice, albeit the differences in blood glucose levels did not reach statistical significance. We also observed that mesangial expansion was significantly attenuated in diabetic mice treated with Mdivi1 as assessed by PAS staining (Figure 5, F and I), and the total number of podocytes per glomerular area was markedly improved in Mdivi1–treated diabetic mice, similar to numbers observed in nondiabetic controls (Figure 5G). Consistent with these improvements, ultrastructure analysis by TEM and scanning electron microscopy revealed significant attenuation in podocyte foot process effacement and podocyte flattening in Mdivi1–treated diabetic mice compared with controls (Figure 5H). We quantified a significant reduction in GBM thickness in Mdivi1–treated diabetic mice (Figure 5K). Analysis of mitochondrial ultrastructure in podocytes from db/db mice revealed the expected dense and fragmented mitochondria compared with db/m controls, whereas sections from Mdivi1–treated diabetic mice revealed significantly elongated mitochondria (Figure 5L). We quantified these changes as a measure of form factor and aspect ratio and found that mitochondria from Mdivi1-treated mice phenocopied those from nondiabetic controls (Figure 5M). Our interpretation of these findings is that Drp1 activation is a key step in the pathogenesis of DN and that blocking Drp1 activity could, indeed, prove beneficial in enhancing mitochondrial fitness and the progression of DN.
Figure 5.
Mdivi1 prevents progression of DN. (A) Schematic describing Mdivi1 administration protocol. (B) Albumin-to-creatinine ratios (ACRs; micrograms per milligram) of db/m (n=5), db/db (n=6), and db/db mice administered Mdivi1 (n=8) at 8, 12, and 16 weeks of age. (C) Kidney weight-to-body weight ratio, (D) body weight, and (E) blood glucose levels at each time point for the groups described in B. (F) Representative light micrographs of PAS staining of kidney sections from db/m, db/db, and db/db mice administered Mdivi1 (n=5–10 mice per group). Scale bars, 10μm. (G) Quantification of mesangial matrix expansion (n=5–7 mice per group). (H) Representative immunofluorescence micrographs of kidney sections from indicated groups. Sections were probed with primary antibodies directed against WT1 (green) and Synaptopodin (red). Nuclei were counterstained with DAPI (blue), Scale bars, 10 μm. (I) Quantification of sections stained with WT1 from H. Results are generated from 50 randomly selected kidney glomeruli per animal in each group (n=5–7 mice per group). (J) Representative TEM and scanning electron microscopy micrographs of kidneys glomeruli from the groups described in B. Scale bars, 2 μm. (K) Quantification of GBM thickness (n=5–7 mice per group). (L, upper panel) Representative TEM micrographs of podocyte mitochondria from glomeruli of each group. (L, lower panel) Tracing of mitochondria from TEM micrographs. (M) Average aspect ratio for each group from B (n=5–7 mice per group). (N) Quantification of podocyte mitochondrial aspect ratio plotted against form factor from TEM micrographs of the groups from B (n=5–7 mice per group). Results are presented as means±SEMs. **P<0.01; ***P<0.01; ****P<0.001.
Discussion
This study identifies novel correlations of mitochondrial morphology with the progression of DN and shows the importance of Drp1 in mediating mitochondrial dysfunction in the diabetic milieu and its role in the development of diabetic kidney disease. We combined Drp1 floxed allele mice with inducible podocyte–specific Cre and db/db diabetic mice to generate podocyte–specific Drp1 knockout in adult diabetic mice. Our findings indicate that conditional deletion of Drp1 in podocytes improves mitochondrial fitness in podocytes and ameliorates progression of DN. Consistent with our genetic approach, we show that pharmacologic inhibition of Drp1 activity prevents the development of DN in type 2 diabetic mice. Although short-term use of Mdivi1 has been previously shown to be safe in multiple studies, its long–term efficacy and safety profile has received less scrutiny. In this regard, our findings suggest that the long–term therapeutic use of Drp1 inhibitor, Mdivi1, is safe and efficacious in DN. Interestingly, we detected a trend between Mdivi1 administration and blood glucose levels in diabetic mice, albeit not reaching statistical significance. One interpretation of this observation is that the beneficial effects of Mdivi1 on progression of DN could be partially dependent on the mild glucose–lowering properties of Mdivi1. Our genetic approach by using Drp1 knockdown in diabetic mice in combination with our other findings presented in this study, however, provides strong evidence on a direct effect of Drp1, independent of its mild antidiabetic effect, in the development and progression of DN.
How does loss of Drp1 protect against progression of DN? Recent studies have painted an interesting picture on an emerging role of mitochondrial dynamics, whereby the shape and size of mitochondria are associated with the proper function and activity of a variety of organs.7,22–24 However, despite significant progress in deciphering the role of mitochondrial dynamics in human pathologies, reports exploring the relationship between mitochondrial morphology and progression of DN are lacking. We have previously shown that activation of mitochondrial fission contributes to podocyte injury in vitro.8 Here, we translated our in vitro observations to an in vivo model by using Cre–mediated, podocyte–specific Drp1 gene ablation. We observed that changes in the mitochondrial morphology contribute to the broad spectrum of mitochondrial dysfunction in DN and identified Drp1 as an important player in mediating progression of DN. To our knowledge, this report is the first to show that mitochondrial fission has a central role in progression of DN in vivo.
Our attempts to define the molecular mechanisms for the renoprotective effects of Drp1 deletion in the diabetic environment culminated in several observations indicating that Drp1 deletion was associated with a more tubular morphology in podocytes, whereas diabetes resulted in more condensed and fragmented phenotype. Importantly, we observed increased mitochondrial ROS and decreased ATP in cells with more fragmented mitochondria. Our results are consistent with an emerging appreciation that mitochondrial morphology plays a central role in ROS production and cell apoptosis.25–29 Furthermore, we found that Drp1 knockdown rescues mitochondrial morphology in the diabetic milieu and improves mitochondrial fitness.
One important contribution of our findings is to characterize the effect of Drp1 in the diabetic environment in vivo. This is particularly relevant, because the effect of Drp1 knockdown in different organs remains the center of controversy, with seemingly opposite effects depending on specific organs/tissues and contexts.19,30,31 Here, we assessed the crosstalk between mitochondrial fitness, mitochondrial morphology, and loss of Drp1 in podocytes on progression of DN by different approaches. First, we determined the bioenergetic effect of Drp1 deletion in podocytes by conditional deletion of Drp1. Second, we followed changes of mitochondrial fitness in cultured podocytes treated with HG. Third, we characterized morphologic and bioenergetics changes in freshly isolated podocytes from an established model of type 2 diabetes. On the basis of our data, we suggest a model where the changes in mitochondrial morphology associated with Drp1 knockdown result in a significant improvement in mitochondrial fitness and function.
Recent studies have shown that Drp1 is post-translationally regulated.32 Among a variety of post-translational modifications, Drp1 phosphorylation at Ser-637/616/656(amino acid positions in human, mouse, and rat Drp1, respectively) seems to play a central regulatory role.32,33 Despite the central importance of Drp1 phosphorylation in mitochondrial fission, the functional consequences of the two conserved serine phosphorylation sites on Drp1 activation remain incompletely understood. For example, although PKA phosphorylation of Drp1 at Ser-637/656(human/rat) decreases Drp1 GTPase activity,32,33 phosphorylation of the same conserved serine residue in Drp1 isoform3 by Ca2+/calmodulin–dependent protein kinase Iα causes a significant increase in Drp1 recruitment to the mitochondria.34 We have previously shown that Rho–associated, coiled coil–containing protein kinase 1–mediated Ser-637 phosphorylation (mouse Drp1 isoform b) resulted in Drp1 translocation to the mitochondria, similar to the effect of Ca2+/calmodulin–dependent protein kinase Iα. Here, we recapitulated results that show increased Ser-637 phosphorylation in HG conditions. We have shown, furthermore, that pharmacologic inhibition of Drp1 is sufficient to block this phosphorylation, presumably because of the notion that Mdivi1 acts at the earliest stages of Drp1 oligomerization, whereas phosphorylation is required for Drp1 translocation to mitochondria after formation of Drp1 oligomers.
Notably, our results also validate the previous observations indicating that there is a significant crosstalk between podocytes and mesangial cells. Indeed, by targeting Drp1 in podocytes, we observed not only significant improvements in podocytes structure and function but also, a significant amelioration in mesangial matrix accumulation. This is of significant interest, because despite several observations describing the interactions between mesangial cells and podocytes, the effect of specific mediators generated in podocytes that could influence mesangial cells has not yet been clearly defined. The interactions of mesangial cells with podocytes and how Drp1 might influence these interactions deserve additional exploration in future studies.
In summary, the findings of this study led us to a number of conclusions: (1) mitochondrial morphology is an important determinant of the mitochondrial dysfunction in DN, (2) Drp1, a critical mediator of mitochondrial fission, serves as an important player in mediating progression of DN, (3) targeting Drp1 may provide a novel therapeutic approach in DN, and (4) our findings functionally link inhibition of mitochondrial fission with improved mitochondrial fitness and progression of DN. We suggest that the data presented herein provide a more complete framework for understanding and therapeutically exploring the role of mitochondrial fission and Drp1 in DN.
Concise Methods
Tissue Culture
Conditionally immortalized mouse podocytes were cultured as previously described.35 Briefly, cells were cultured on BD BioCoat Collagen I Plates (BD Biosciences, San Jose, CA) at 33°C in the presence of 20 U/ml mouse recombinant IFN-γ (Sigma-Aldrich, St. Louis, MO) to enhance expression of a thermosensitive T antigen. Immortalized mouse podocyte cell lines are routinely characterized in the laboratory on the basis of morphology and gene expression patterns. Immortalized cells for all experiments reported herein were between passages 4 and 12. Primary cultured podocytes were isolated as described below, and experiments done on these cells were performed on passages 2 and 3 only. Cells were confirmed to be free of mycoplasma contamination. To induce differentiation, podocytes were maintained at 37°C without IFN-γ for 10–12 days. Podocytes prepared for experiments involving HG (25mM) conditions were serum deprived for 24 hours before addition of HG. Likewise, control cells were serum deprived and cultured with NG (5mM). Cell culture experiments were repeated at least three independent times.
Animal Experiments
Animal maintenance and experimental procedures were carried out in accordance with the US National Institutes of Health guidelines for use of experimental animals and approved by the Animal Care Committee of the University of Texas MD Anderson Cancer Center. Male db/db diabetic mice in C57BLKS background and their nondiabetic littermate control db/m mice (8 weeks old) were obtained from The Jackson Laboratory (Bar Harbor, ME). Mice were housed in a room at a constant temperature of 22°C ±2°C with 12-hour light-dark cycles. Drp1 floxed mice were bred on the C57Bl6-J background. Mice were genotyped using primers according to published references.14 Mice were intercrossed with C57BLKS-Leprdb/m mice for at least six generations to make homozygous Drp1 floxed mice on the C57BLKS background. For studies involving deletion of Drp1 in podocytes, 8-week-old mice were separated into four groups as follows: db/m;Drp1Pod-f/f±20mg/kg tamoxifen or db/db;Drp1Pod-f/f±20mg/kg tamoxifen. Tamoxifen (Sigma-Aldrich) was resuspended in sesame oil and administered to mice intraperitoneally for a total of 10 injections divided over 14 days. Mice were monitored for an additional 10–14 weeks. Fasting blood glucose level, weight, and albumin levels were monitored at 8, 12, 16, and 20 weeks of age.
Mdivi1 Experimental Protocol
Mice were allocated into the following groups: nondiabetic controls (n=10), diabetic (db/db) mice administered vehicle (DMSO; n=10), and diabetic mice given Mdivi1 (n= 10). Mdivi1 was administered every other day (50 mg/kg intraperitoneally) for 8 weeks. After 8 weeks of Mdivi1 treatment, mice were euthanized for biochemical and histopathologic analyses.
Metabolic and Physiologic Parameters
Before euthanizing the mice, urine was collected over 24 hours, with each mouse individually housed in a metabolic cage and provided with water ad libitum. Urinary albumin concentration was measured by a mouse albumin ELISA kit (Exocell, Philadelphia, PA). Urinary creatinine concentration was measured using the QuantiChrom Creatinine Assay Kit (BioAssay Systems, Hayward, CA).
Kidney Histopathology
Kidneys removed from anesthetized mice were immediately cut in half, fixed in 10% formaldehyde in 0.1 mol/L PBS (pH 7.2), embedded in paraffin, and sectioned at 5 μM. Sections were stained with hematoxylin and eosin, and PAS staining was used to evaluate the general histologic changes in glomerular and tubular structures. Glomerular volume, mesangial fraction, and mesangial volume were determined from PAS and then analyzed to determine the cross-sectional area of the glomerular tuft and the mesangial area. The cross-sectional area of the glomerular tuft was determined from outlines of the tuft using the program Adobe Photoshop 7.0 (Adobe Systems, Inc., San Jose, CA). The mesangial area within the glomerular tuft was defined as the area that stained positively for PAS.36–39 Positive staining was identified using the capacity of Adobe Photoshop to select areas of matching color intensity. Mesangial fraction was calculated as the ratio of mesangial area to area of the glomerular tuft. Five mice were analyzed per group, and 50 glomeruli were measured per mouse.
Mitochondrial Morphology Assessment
Podocytes were grown until they were 70% confluent. The cells were grown in either NG or HGor treated with 20μM Mdivi1 for 30 minutes. After 48 hours, cells were incubated with 150 nM MitoTracker Red for 20 minutes, then fixed with ice-cold methanol, and mounted. Images were acquired by deconvolution microscopy. Mitochondrial shape descriptors and size measurements were obtained using ImageJ (version 1.42q; National Institutes of Health, Bethesda, MD). For aspect ratio measurements, the ratio between the major and minor axes of the ellipse equivalent to the mitochondrion was determined. The degree of branching or form factor was defined as (Pm2)/(4πAm), where Pm is the length of mitochondrial outline, and Am is the area of mitochondrion. Mitochondrial area was measured as the two-dimensional area (in micrometers2) projected to the focal plane of the image. Individual mitochondrion perimeter was measured by taking the sum of the pixels of the mitochondria edge multiplied by the length in micrometers of each pixel. Circularity was measured using mitochondrial surface area and perimeter using the equation 4π·(surface area/perimeter2). In summary, these measurements were made using ImageJ on the basis of previously published methods.40 The morphology of at least 120 mitochondria was determined for each condition.
ATP Production
Cellular ATP levels were measured using the CellTitre-Glo Luminescent Cell Viability Assay Kit (Promega, Madison, WI) according to the manufacturer’s instructions. ATP levels assays were performed on lysed podocytes as previously described.41
Mitochondrial Membrane Potential
Podocytes were plated at 40% confluency in 96-well plates. Mitochondrial membrane potential was measured using the fluorescent dye tetramethylrhodamine methyl ester (TMRE). To remove the plasma membrane contribution to the TMRE fluorescence, each assay was performed in parallel as above plus 10μM carbonyl cyanide 3-chlorophenylhydrazone, which collapses the mitochondrial membrane potential. All data are expressed as the total TMRE fluorescence minus the carbonyl cyanide 3-chlorophenylhydrazone–treated TMRE fluorescence. The results were normalized to total protein concentration in each well. For imaging, podocytes were examined in the inverted confocal microscope (Nikon, Tokyo, Japan) equipped with an incubation that allowed us to keep cells at 37°C in a controlled atmosphere with a mixed air/CO2 flow of 4 L/h and 5% CO2. The images were analyzed by ImageJ for integrated density analysis.
Seahorse Metabolic Analyzer Assays
Cultured podocytes were grown on 0.1-μg/ml collagen-coated plates in RPMI1640media (Gibco, Grand Island, NY) supplemented with 10% FBS and 1% Anti-Anti and maintained in HG (25mM) or NG (5 mM) conditions. Cells were passaged to a 24-well XF24 Plate (Seahorse Bioscience, Billerica, MA) at a cell density of 5×104 cells per well. Cartridge plates for metabolic stress injections were hydrated for at least 8 hours at 37°C before the assay with calibrant solution (Seahorse Biosciences). One hour before running the seahorse assay, the XF24 Plate’s running medium was removed and replaced with Seahorse Assay Medium (final volume of 500 μl per well). Assay conditions and setup were performed according to instructions described by Seahorse Biosciences. OCR was reported in the unit of picomoles per minute, and the results were normalized to cell number using the Cyquant Direct Cell Proliferation Assay Kit (C35011; Molecular Probes). The area under the curve was calculated for the area as previously described.42
Podocyte Isolation
Kidneys from deeply anesthetized mice were harvested, and kidney cortices were dissected and minced into small pieces with a surgical blade in ice-cold HBSS. The tissues were digested in collagenase solution containing 1 mg/ml collagenase A (Roche Diagnostics, Indianapolis, IN) and 0.2 mg/ml deoxyribonuclease I (Roche Diagnostics) in HBSS at 37°C for 60 minutes with gentle agitation. The collagenase-digested tissues were gently pressed through a 100-μm cell strainer (BD Biosciences) using a flattened pestle. The cells were finally passed through a 25-μm filter pelleted and counted. Cells were resuspended in 500μl labeling buffer with 5μg both biotinylated anti-Podocalyxin and ant-Kirrel2 (R&D Systems, Minneapolis, MN) at 4°C for 1 hour. The cells were washed three times with labeling buffer and centrifuged at 300×g. Cells were resuspended in Streptavidin M-280 Dynabeads (Life Technologies, Carlsbad, CA) and incubated for 1 hour. Podocytes bound to Dynabeads in the cell suspension were gathered by a magnetic particle concentrator and washed three times with labeling buffer. Finally, collected podocytes were suspended in a suitable amount of media and either used for analysis immediately or cultured for other downstream experiments.
Scanning Electron Microscopy
Samples were treated with a fixative containing 3% glutaraldehyde plus 2% paraformaldehyde in 0.1 M cacodylate buffer (pH 7.3) overnight at 4°C. The samples were washed with 0.1 M cacodylate buffer (pH 7.3) three times for 10 minutes each. The samples were then postfixed with 1% cacodylate–buffered osmium tetroxide for 1 hour, washed with 0.1M cacodylate buffer three times for 10 minutes each, and washed in distilled water two times for 5 minutes each. The samples were dehydrated with a graded series of increasing concentrations of ethanol for 5 minutes each. The samples were then transferred to graded series of increasing concentrations of hexamethyldisilazane for 5 minutes each and air dried overnight. Samples were mounted onto double–stick carbon tabs (Ted Pella, Inc., Redding, CA), which were mounted onto aluminum specimen mounts (Electron Microscopy Sciences, Ft. Washington, PA). The samples were then coated under vacuum using a Balzer MED 010 Evaporator (Technotrade) with platinum alloy for a thickness of 25 nm and immediately flash carbon coated under vacuum. The samples were examined in a JSM-5910 Scanning Electron Microscope (JEOL, Inc., Peabody, MA) at an accelerating voltage of 5 kV.
TEM
Fixed samples were washed in 0.1 M cacodylate buffer, postfixed with 1% buffered osmium tetroxide for 1 hour, and stained en bloc with aqueous 1% Millipore–filtered uranyl acetate (EMD Millipore, Billerica, MA). The samples were washed several times in water, dehydrated in increasing concentrations of ethanol, infiltrated, and embedded in LX-112 Medium. The samples were polymerized at 60°C. Ultrathin sections were cut in a Leica Ultracut Microtome (Leica Microsystems, Buffalo Grove, IL), stained with uranyl acetate and lead citrate in a Leica EM Stainer, and examined in a JEM 1010 Transmission Electron Microscope (JEOL, Inc.) at an accelerating voltage of 80 kV. Digital images were obtained using the AMT Imaging System (Advanced Microscopy Techniques Corp, Danvers, MA).
Glomerular Membrane Thickness Measurements
The TEM images were acquired by using a Hitachi H-7000 TEM System (Hitachi, Yokohama, Japan) and a JEM 1010 Transmission Electron Microscope (JEOL, Inc.) with magnification in the range of 1500–25,000. We threshold out the various regions of the glomeruli using the inbuilt threshold function in ImageJ. After separation of the various segments out, with GBM the only one remaining, we used the BoneJ, a plugin for bone image analysis in ImageJ, to measure the GBM thickness as previously described.43
Cell Apoptosis and Mitochondrial ROS Determination
For Annexin V FITC (Life Technologies) labeling, podocytes were grown in six-well plates until they were 70% confluent. The cells were then serum starved overnight. The next day, cells were either grown in NGor HG or first treated with 20μM Mdivi1 for 30 minutes. The cells were incubated at 37°C with fresh media containing 5 μM MitoSox Red Mitochondrial Superoxide Indicator (Invitrogen, Carlsbad, CA). The cells were washed once in PBS and incubated with Annexin V FITC (Life Technologies) according to the manufacturer’s instruction. The cells were incubated with DAPI (1 μg/ml) for 5 minutes on ice and then analyzed by flow cytometry (FACS Aria;Becton Dickinson, San Diego, CA). This assay discriminates between intact cells, early apoptotic cells, and late apoptotic or necrotic cells.
GTPase Activity Assay
For Drp1-GTP pulldown assay, 20μl GTP Agarose Beads (Innova Biosciences) were added to each tube and washed with IP buffer three times. Quantified protein extracts were then added on top of the GTP agarose beads at equal amounts in each tube and incubated with beads on the rotator overnight at 4°C. The next day, the beads were washed three times. The beads were boiled in sample loading buffer for 5 minutes and spun to harvest the pulldown proteins. The eluted sample proteins (sample input) were then resolved by SDS-PAGE (10% gel). Western blot analysis was then performed as described below to detect active (pulldown) and total (input) Drp1.
Western Blot Analyses
Cells were lysed in RIPA Buffer (50 mM Tris-HCl, pH 8.0 with 150 mM sodium chloride, 1.0% Igepal CA-630 [NP-40], 0.5% sodium deoxycholate, and 0.1% sodium dodecyl sulfate). Protein concentrations were determined by Bradford assay. Twenty micrograms total protein lysate was diluted in 5× Laemmli buffer, loaded on 4%–20% gradient SDS-PAGE (Bio-Rad, Hercules, CA), and transferred to nitrocellulose membranes. Membranes were probed with the indicated antibody followed by visualization by the Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE). The following antibodies were used: mouse anti–Drp1 antibody from BD Biosciences (611112; 1:500) and anti–Mitofusin 1 antibody (ab57602; 1:500) from Abcam, Inc. (Cambridge, MA). Anti-Gapdh antibody (ab9485; 1:1000) from Abcam, Inc. was used as loading control.
Immunocytochemistry
After dewaxing and rehydration, 5-μm-thick kidney sections were subjected to heat–induced epitope retrieval in Tris-EDTA Buffer (10mM Tris Base, 1mM EDTA Solution, and 0.05% Tween 20, pH 9.0). Nonspecific interactions were blocked with 10% Normal Donkey Serum in 1×Tris-Buffered Saline with Tween for 1 hour at room temperature. Sections were then incubated with anti-WT1 antibody (clone C-19; 1:100; Santa Cruz Biotechnology, Santa Cruz, CA) at 4°C overnight in blocking solution. The next day, sections were washed in Tris-Buffered Saline with Tween and treated with a 3% H2O2 solution to reduce endogenous peroxidase activity followed by incubation with anti–rabbit HRP–conjugated secondary antibody. Visualization was performed using a Vectastain ABC Kit (Vector Laboratories, Burlingame, CA). Sections were examined using a Nikon Microscope (Nikon D50i) with a 60× objective. Podocytes were identified according to cell localization and positivity to WT1 staining. Total numbers of podocytes were counted in kidney sections of randomly selected animals in 15 randomly selected kidney glomeruli per animal. The data were expressed as podocyte numbers relative to the corresponding glomerular area as assessed by a computer–aided image analysis system (Adobe Photoshop 7.0).44
Immunofluorescence
Podocytes were incubated with 25 nmol/L MitoTracker Red for 20 minutes. Cells were then washed with cold PBS, fixed in 4% formaldehyde, and permeabilized with 0.1% Triton X-100. After incubation with 2% normal horse serum (to block nonspecific staining), fixed cells were incubated overnight at 4°C with antibodies against Drp1 (1:100). Cells were washed with PBS and incubated for 60 minutes with FITC–labeled goat anti–rabbit antibody followed by incubation with DAPI (1:10,000) for 10 minutes. Drp1 mouse mAb was obtained from BD Biosciences. Drp1 phospho-637 was obtained from Biobryt. Anti-Synaptopodin was obtained from Santa Cruz Biotechnology. Mito Tracker Red CMXRos and MitoSox Red Mitochondrial Superoxide Indicator were purchased from Invitrogen. Coverslips were mounted, and slides were imaged by confocal microscopy (Nikon). Quantification was carried out using ImageJ software. For tissue sections, after antigen retrieval and blocking, 5-μm kidney paraffin sections were incubated overnight at 4°C with anti-Synaptopodin, WT1 (Santa Cruz Biotechnology) or anti-Drp1 (611112; 1:100; Becton Dickinson), anti-Drp1 phosphorylated at Ser-637 (Orb127984; 1:100; Biorbyt) primary antibodies; specific staining was detected using the FITC–conjugated donkey anti–mouse/anti–rabbit secondary antibodies. Sections were examined using a Nikon Confocal Microscope (Nikon Eclipse Ti). Podocytes were identified according to cell localization and positivity to WT1 staining. Total numbers of podocytes were counted in kidney sections of randomly selected animals in 15 randomly selected kidney glomeruli per animal. The data were expressed as podocyte numbers relative to the corresponding glomerular area as assessed by a computer–aided image analysis system (Adobe Photoshop 7.0).44
Statistical Analyses
Group data are expressed as means±SEMs. Comparisons of multiple groups were performed using one-way ANOVA followed by Tukey multiple comparisons test. Comparisons between two groups were performed using t test. All tests were two tailed, with a P<0.05 considered to be a statistically significant result. Tests were performed with GraphPad version 6.0b (GraphPad Software, La Jolla, CA).
Disclosures
None.
Acknowledgments
We thank all of the members of the laboratory of F.R.D. for their thoughtful and critical discussions and suggestions. Floxed-Drp1 mice were provided by Dr. Hiromi Sesaki (Johns Hopkins School of Medicine, Baltimore, MD).
This work was supported by National Institutes of Health grants R01DK091310 (to F.R.D.) and R01DK078900 (to F.R.D.). Work performed at the University of Texas MD Anderson Cancer Center High–Resolution Microscopy Facility was supported by institutional funds through Core Grant CA16672.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Youle RJ, van der Bliek AM: Mitochondrial fission, fusion, and stress. Science 337: 1062–1065, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Westermann B: Mitochondrial fusion and fission in cell life and death. Nat Rev Mol Cell Biol 11: 872–884, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Osman C, Noriega TR, Okreglak V, Fung JC, Walter P: Integrity of the yeast mitochondrial genome, but not its distribution and inheritance, relies on mitochondrial fission and fusion. Proc Natl Acad Sci U S A 112: E947–E956, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nunnari J, Suomalainen A: Mitochondria: In sickness and in health. Cell 148: 1145–1159, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoppins S, Nunnari J: Cell biology. Mitochondrial dynamics and apoptosis--the ER connection. Science 337: 1052–1054, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Rafelski SM, Viana MP, Zhang Y, Chan YH, Thorn KS, Yam P, Fung JC, Li H, Costa LF, Marshall WF: Mitochondrial network size scaling in budding yeast. Science 338: 822–824, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen H, Chan DC: Mitochondrial dynamics--fusion, fission, movement, and mitophagy--in neurodegenerative diseases. Hum Mol Genet 18[R2]: R169–R176, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang W, Wang Y, Long J, Wang J, Haudek SB, Overbeek P, Chang BH, Schumacker PT, Danesh FR: Mitochondrial fission triggered by hyperglycemia is mediated by ROCK1 activation in podocytes and endothelial cells. Cell Metab 15: 186–200, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Low HH, Löwe J: A bacterial dynamin-like protein. Nature 444: 766–769, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Praefcke GJ, McMahon HT: The dynamin superfamily: Universal membrane tubulation and fission molecules? Nat Rev Mol Cell Biol 5: 133–147, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Frank S, Gaume B, Bergmann-Leitner ES, Leitner WW, Robert EG, Catez F, Smith CL, Youle RJ: The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell 1: 515–525, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Smirnova E, Griparic L, Shurland DL, van der Bliek AM: Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell 12: 2245–2256, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Bliek AM, Payne GS: Dynamin subunit interactions revealed. Dev Cell 18: 687–688, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wakabayashi J, Zhang Z, Wakabayashi N, Tamura Y, Fukaya M, Kensler TW, Iijima M, Sesaki H: The dynamin-related GTPase Drp1 is required for embryonic and brain development in mice. J Cell Biol 186: 805–816, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishihara N, Nomura M, Jofuku A, Kato H, Suzuki SO, Masuda K, Otera H, Nakanishi Y, Nonaka I, Goto Y, Taguchi N, Morinaga H, Maeda M, Takayanagi R, Yokota S, Mihara K: Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nat Cell Biol 11: 958–966, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Cassidy-Stone A, Chipuk JE, Ingerman E, Song C, Yoo C, Kuwana T, Kurth MJ, Shaw JT, Hinshaw JE, Green DR, Nunnari J: Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer membrane permeabilization. Dev Cell 14: 193–204, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Youle RJ, Karbowski M: Mitochondrial fission in apoptosis. Nat Rev Mol Cell Biol 6: 657–663, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Chan DC: Mitochondria: Dynamic organelles in disease, aging, and development. Cell 125: 1241–1252, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Song M, Mihara K, Chen Y, Scorrano L, Dorn GW 2nd: Mitochondrial fission and fusion factors reciprocally orchestrate mitophagic culling in mouse hearts and cultured fibroblasts. Cell Metab 21: 273–285, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang J, Wang Y, Long J, Chang BH, Wilson MH, Overbeek P, Danesh FR: Tamoxifen-inducible podocyte-specific iCre recombinase transgenic mouse provides a simple approach for modulation of podocytes in vivo. Genesis 48: 446–451, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song M, Dorn GW 2nd: Mitoconfusion: Noncanonical functioning of dynamism factors in static mitochondria of the heart. Cell Metab 21: 195–205, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brand MD, Nicholls DG: Assessing mitochondrial dysfunction in cells. Biochem J 435: 297–312, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brooks C, Wei Q, Cho SG, Dong Z: Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J Clin Invest 119: 1275–1285, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smirnova E, Shurland DL, Ryazantsev SN, van der Bliek AM: A human dynamin-related protein controls the distribution of mitochondria. J Cell Biol 143: 351–358, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Autret A, Martin SJ: Emerging role for members of the Bcl-2 family in mitochondrial morphogenesis. Mol Cell 36: 355–363, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Brownlee M: Biochemistry and molecular cell biology of diabetic complications. Nature 414: 813–820, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Sheridan C, Martin SJ: Mitochondrial fission/fusion dynamics and apoptosis. Mitochondrion 10: 640–648, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Yu T, Robotham JL, Yoon Y: Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc Natl Acad Sci U S A 103: 2653–2658, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhan M, Usman IM, Sun L, Kanwar YS: Disruption of renal tubular mitochondrial quality control by Myo-inositol oxygenase in diabetic kidney disease. J Am Soc Nephrol 26: 1304–1321, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cerveny KL, McCaffery JM, Jensen RE: Division of mitochondria requires a novel DMN1-interacting protein, Net2p. Mol Biol Cell 12: 309–321, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ikeda Y, Shirakabe A, Maejima Y, Zhai P, Sciarretta S, Toli J, Nomura M, Mihara K, Egashira K, Ohishi M, Abdellatif M, Sadoshima J: Endogenous Drp1 mediates mitochondrial autophagy and protects the heart against energy stress. Circ Res 116: 264–278, 2015 [DOI] [PubMed] [Google Scholar]
- 32.Cribbs JT, Strack S: Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO Rep 8: 939–944, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang CR, Blackstone C: Cyclic AMP-dependent protein kinase phosphorylation of Drp1 regulates its GTPase activity and mitochondrial morphology. J Biol Chem 282: 21583–21587, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Han XJ, Lu YF, Li SA, Kaitsuka T, Sato Y, Tomizawa K, Nairn AC, Takei K, Matsui H, Matsushita M: CaM kinase I alpha-induced phosphorylation of Drp1 regulates mitochondrial morphology. J Cell Biol 182: 573–585, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mundel P, Reiser J, Zúñiga Mejía Borja A, Pavenstädt H, Davidson GR, Kriz W, Zeller R: Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res 236: 248–258, 1997 [DOI] [PubMed] [Google Scholar]
- 36.Chen S, Iglesias-de la Cruz MC, Jim B, Hong SW, Isono M, Ziyadeh FN: Reversibility of established diabetic glomerulopathy by anti-TGF-beta antibodies in db/db mice. Biochem Biophys Res Commun 300: 16–22, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Cohen MP, Sharma K, Jin Y, Hud E, Wu VY, Tomaszewski J, Ziyadeh FN: Prevention of diabetic nephropathy in db/db mice with glycated albumin antagonists. A novel treatment strategy. J Clin Invest 95: 2338–2345, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Craven PA, Melhem MF, Phillips SL, DeRubertis FR: Overexpression of Cu2+/Zn2+ superoxide dismutase protects against early diabetic glomerular injury in transgenic mice. Diabetes 50: 2114–2125, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Ziyadeh FN, Hoffman BB, Han DC, Iglesias-De La Cruz MC, Hong SW, Isono M, Chen S, McGowan TA, Sharma K: Long-term prevention of renal insufficiency, excess matrix gene expression, and glomerular mesangial matrix expansion by treatment with monoclonal antitransforming growth factor-beta antibody in db/db diabetic mice. Proc Natl Acad Sci U S A 97: 8015–8020, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Picard M, White K, Turnbull DM: Mitochondrial morphology, topology, and membrane interactions in skeletal muscle: A quantitative three-dimensional electron microscopy study. J Appl Physiol (1985) 114: 161–171, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Manfredi G, Yang L, Gajewski CD, Mattiazzi M: Measurements of ATP in mammalian cells. Methods 26: 317–326, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Lee KY, Gesta S, Boucher J, Wang XL, Kahn CR: The differential role of Hif1β/Arnt and the hypoxic response in adipose function, fibrosis, and inflammation. Cell Metab 14: 491–503, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Doube M, Kłosowski MM, Arganda-Carreras I, Cordelières FP, Dougherty RP, Jackson JS, Schmid B, Hutchinson JR, Shefelbine SJ: BoneJ: Free and extensible bone image analysis in ImageJ. Bone 47: 1076–1079, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Riedl E, Pfister F, Braunagel M, Brinkkötter P, Sternik P, Deinzer M, Bakker SJ, Henning RH, van den Born J, Krämer BK, Navis G, Hammes HP, Yard B, Koeppel H: Carnosine prevents apoptosis of glomerular cells and podocyte loss in STZ diabetic rats. Cell Physiol Biochem 28: 279–288, 2011 [DOI] [PubMed] [Google Scholar]