Abstract
Tubular reabsorption of filtered sodium is tightly controlled to maintain body volume homeostasis. The rate of sodium transport by collecting duct (CD) cells varies widely in response to dietary sodium intake, GFR, circulating hormones, neural signals, and local regulatory factors. Reabsorption of filtered sodium by CD cells occurs via a two-step process. First, luminal sodium crosses the apical plasma membrane along its electrochemical gradient through epithelial sodium channels (ENaC). Intracellular sodium is then actively extruded into the interstitial space by the Na+,K+-ATPase located along the basolateral membrane. Mismatch between sodium entry and exit induces variations in sodium intracellular concentration and cell volume that must be maintained within narrow ranges for control of vital cell functions. Therefore, renal epithelial cells display highly coordinated apical and basolateral sodium transport rates. We review evidence from experiments conducted in vivo and in cultured cells that indicates aldosterone and vasopressin, the two major hormones regulating sodium reabsorption by CD, generate a coordinated stimulation of apical ENaC and basolateral Na+,K+-ATPase. Moreover, we discuss evidence suggesting that variations in sodium entry per se induce a coordinated change in Na+,K+-ATPase activity through the signaling of protein kinases such as protein kinase A and p38 mitogen-activated protein kinase.
Keywords: aldosterone, epithelial sodium channel, collecting ducts, ENaC, Na transport, p38 mitogen-activated protein kinase
Homeostasis of body fluids is dependent on renal function. Extracellular concentration of Na+ is the major determinant of intracellular volume while total body Na+ is, for the most part, proportional to extracellular fluid volume and thereby represents a major factor influencing BP.1 Body fluid homeostasis is maintained despite large variations of solute and water intake. This is mostly dependent on kidney tubule reabsorption and secretion processes.2 Filtered solutes are reabsorbed from the tubular lumen via a two-step process across polarized kidney tubule epithelial cells. Reabsorbed solutes first cross the apical membrane via a specific transporter and are extruded from the intracellular medium to the interstitium via a second specific transporter inserted in the basolateral membrane. Secreted solutes are taken from the interstitium across the basolateral membrane and are then extruded into the lumen after crossing the apical membrane. Both reabsorption and secretion processes are energized by the Na+ gradient generated by the Na+,K+-ATPase. It exchanges three intracellular Na+ ions against two extracellular K+ ions for each ATP hydrolyzed. This Na+,K+-ATPase activity maintains a low intracellular Na+ concentration, about ten times lower than extracellular Na+ concentration, and a high intracellular K+ concentration. The Na+-pump is electrogenic and participates in the generation and maintenance of the negative resting cell membrane potential.2 The combination of low intracellular Na+ concentration and negative potential provides the driving force for passive Na+ entry via ion channels, cotransporters, and antiporters. Vectorial transcellular transport of solutes generates a transepithelial electrochemical gradient that drives solute movement across the paracellular pathway. This pathway is especially important in electrically leaky epithelia such as the proximal tubule (PT) where about one third of NaCl reabsorption is paracellular.
Each day human kidneys filter 180 L of glomerular ultrafiltrate containing close to 140 mM Na+. The huge reabsorptive activity of kidney tubules results in the generation of 1–2 L of urine per day containing 1–5% of the filtered Na+ load. Most H2O and Na+ reabsorption is performed by the PT, loop of Henle, and distal convoluted tubule that together account for about 90% and 85% of filtered Na+ and H2O reabsorption, respectively. Connecting tubules and collecting ducts (CD) are responsible for the fine-tuning of H2O and Na+ reabsorption. The function of these terminal renal tubule segments is tightly controlled in order to achieve Na+ and H2O balance. To achieve this goal, kidney tubule epithelial cells express segment-specific apical sodium transporters while Na+,K+-ATPase is ubiquitously expressed in the basolateral membrane. All along the nephron, Na+,K+-ATPase provides the driving force for apical Na+ transport and directly accounts for the basolateral step of the Na+ reabsorption process.2 In this review we will focus on the mechanisms that coordinate apical Na+ entry and basolateral sodium exit via Na+,K+-ATPase in order to maintain intracellular Na+ concentration and thereby cell volume within narrow limits.
The Na+,K+-ATPase
Functional properties of the Na+,K+-ATPase
The Na+,K+-ATPase, also referred to as the Na+-pump, is a P-type ATPase (also called E1,E2-ATPases) characterized by the transient phosphorylation of an asparagine residue during its catalytic cycle. Na+,K+-ATPase activity is stimulated by Na+ and K+ from the cytoplasmic and extracellular side, respectively. Under conditions of low K+ concentration (5–10 mM), the apparent Na+ mean affinity constant of Na+,K+-ATPase (K0.5Na) is between 5 and 15 mM, while Vmax is attained at Na+ concentrations of between 60 and 100 mM. Because K+ and Na+ compete at the cytoplasmic site, K0.5Na is lower in intact cells where K+ concentration is high.3 Therefore, in intact cells where Na+ concentration is low (10–20 mM), Na+,K+-ATPase works largely below its Vmax.4,5 Consequently, an acute increase in intracellular Na+ stimulates Na+,K+-ATPase, leading to a rapid return of intracellular Na+ concentration to basal levels. Na+ activation of the Na+,K+-ATPase is strongly cooperative and is close to the enzyme's K0.5Na implying that small variations in intracellular Na+ concentration produce large variations in Na+,K+-ATPase activity. The apparent mean affinity constant of Na+,K+-ATPase for extracellular K+ (K0.5K) is between 0.5 and 1.5 mM. Therefore, under physiologic conditions intracellular Na+, but not extracellular K+, is rate limiting for Na+,K+-ATPase activity. In intact cells where intracellular ATP concentration is well above the K0.5 of Na+,K+-ATPase, ATP is not physiologically rate limiting for Na+,K+-ATPase activity. ATP may become rate limiting when its synthesis is decreased under ischemic or anoxic conditions.2
Structure of the Na+,K+-ATPase
The Na+,K+-ATPase is constituted of two subunits (α and β) associated in a 1:1 ratio and facultative accessory small transmembrane proteins of the FXYD family.
The α subunit contains about 1000 amino acids and its apparent molecular mass is close to 110 kDa. Four Na+,K+-ATPase α subunit isoforms (α-1 to α-4) have been identified6,7 but kidney tubule epithelial cells express only the ubiquitous α-1 isoform. The α subunit of Na+,K+-ATPase displays all the functional properties of the enzyme. It is an integral membrane protein with ten membrane-spanning domains (M1 to M10) and both NH2- and COOH-terminal domains are intracellular.8 The docking site for ATP is located in a large intracellular loop between M4 and M5. This loop also contains the aspartic acid transiently phosphorylated during the catalytic cycle.
The β subunit containing about 300 amino acids exists as a single membrane-spanning domain with a large extracellular end that interacts with the extracellular loop of the α subunit located between transmembrane domains M7 and M8.9,10 The association of the β subunit with the α subunit is necessary for the adequate folding of newly synthesized subunits.11
Seven FXYD proteins have been identified and four (FXYD-1, -2, -4 and -7) can be associated with Na+,K+-ATPase.12 In the kidney, FXYD-2 decreases while FXYD-4 increases the apparent Na+ affinity of the Na+,K+-ATPase.13 FXYD-2 is mostly expressed in the PT and the thick ascending loop of Henle (TAL) where Na+,K+-ATPase displays a low apparent Na+ affinity,3,14,15 while FXYD-4 is specifically expressed in the CD where the apparent affinity of Na+,K+-ATPase is high.3,16,17
Specificity of the Na+,K+-ATPase in kidney tubule epithelial cells
Kidney tubule epithelial cells are functionally polarized and express different transporters and receptors along their apical and basolateral membrane domains delineated by tight junctions.18 Na+,K+-ATPase is located along the basolateral membrane19 where it extrudes intracellular Na+ to maintain the Na+ gradient that would be otherwise dissipated by apical Na+ entry.20 There is a close relationship between the expression levels and activity of Na+,K+-ATPase and the Na+ reabsorption capacity of kidney tubule segments.21 It is high in TAL and the distal convoluted tubule, intermediate in PT, low in CD, and extremely low in the thin segments of the loop of Henle.22 The distribution profile of Na+,K+-ATPase was similar as estimated by 3H-ouabain binding23 or by Western blotting.24
Control of Na+ reabsorption in the CD
Physiologic properties of the CD
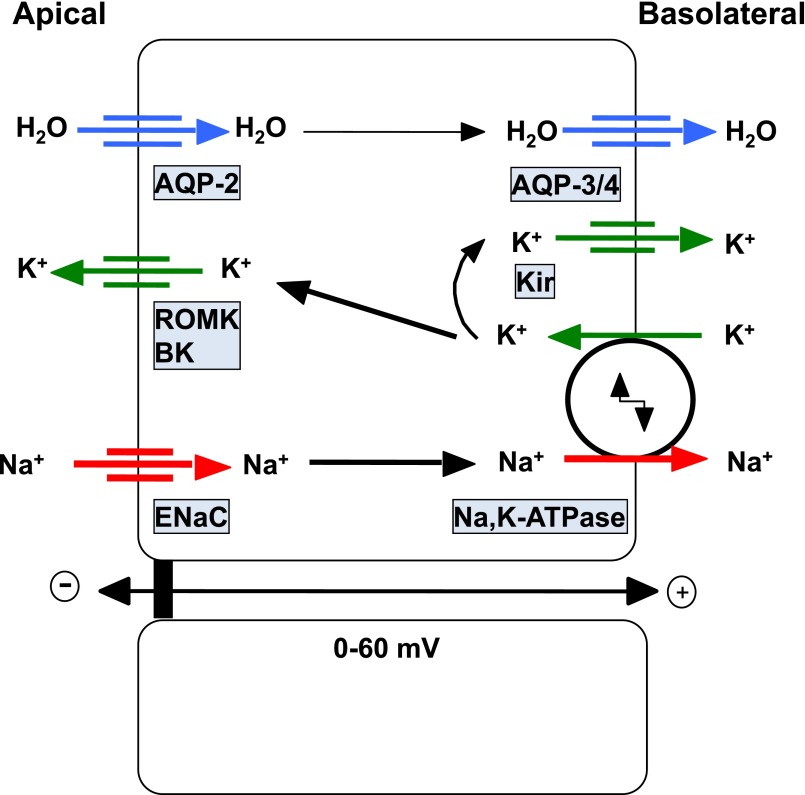
Both intercalated cells and principal cells are found in connecting tubules and CD. Intercalated cells are key players in acid/base and potassium control and will not be discussed further.25,26 Principal cells perform H2O and Na+ reabsorption as well as K+ secretion (Figure 1). Their detailed function and regulation has been recently reviewed.27–29 These cells mostly express the epithelial sodium channel (ENaC), the renal outer medullary potassium channel (ROMK), as well as calcium-activated K+ channels and the water channel aquaporin-2 (AQP2) in their apical membranes. Both apical and basolateral Cl− channels have been described in CD principal cells.30,31 Na+,K+-ATPase is located along the basolateral membrane including deep, basal invaginations surrounding the mitochondrial network. Na+,K+-ATPase along with basolateral K+ channels of the Kir family participate in the generation of the negative intracellular potential.32 Active Na+ transport generates a lumen-negative transepithelial voltage (0 to −60 mV), which is highest in the cortical collecting duct (CCD) (−10 to −60 mV) and then decreases along the medullary CD. This feature results from decreased Na+ reabsorption by principal cells combined with increased electrogenic H+ secretion by intercalated cells. Water flows through the apical membrane via AQP2 and leaves the cell through basolateral aquaporin-3 and -4 water channels. It is worth noting that Na+ reabsorption by principal cells provides the driving force for K+ secretion. Basolateral transport of K+ into the cell by Na+,K+-ATPase supplies apical ROMK for K+ secretion that is enhanced by the lumen-negative membrane potential generated by Na+ reabsorption.33
Figure 1.
Ion and water transporters in CD principal cells. Sodium reabsorption occurs sequentially through apical ENaC and basolateral Na+,K+-ATPase. This vectorial Na+ transport generates a lumen-negative transepithelial potential that drives potassium secretion via luminal K+-channels, i.e., ROMK and Big K+-channels (BK). The Na+,K+-ATPase generates the driving forces for both Na+ entry and K+ exit at the apical side of the cell. Basolateral K+ influx through Na+,K+-ATPase is also equilibrated by K+ efflux through basolateral K+-channels (Kir) to generate the negative resting transmembrane potential. Water permeability is mediated by sequential flow through apical AQP2 water channels and basolateral aquaporin-3 and -4 water channels.
In CD principal cells Na+ reabsorption is tightly controlled by hormonal, neural and local factors as well as chemical (Na+ concentration) or physical (luminal flow, membrane potential) factors. These regulatory mechanisms have been recently reviewed.28,29 In this review we will focus on the coordinated effects of aldosterone and vasopressin on apical ENaC and basolateral Na+,K+-ATPase. We will also analyze the role of apical Na+ influx and/or intracellular Na+ concentration that may play a major role in the coordination of transepithelial Na+ transport.
Coordinated control of Na+ transport by aldosterone
Aldosterone is the principal hormone that stimulates Na+ reabsorption by distal segments of the renal tubule. Aldosterone synthesized in the zona glomerulosa of the adrenal gland is secreted mainly in response to circulating angiotensin II generated in response to renin release by the afferent glomerular artery. High extracellular K+ is also an important stimulus for aldosterone secretion.34
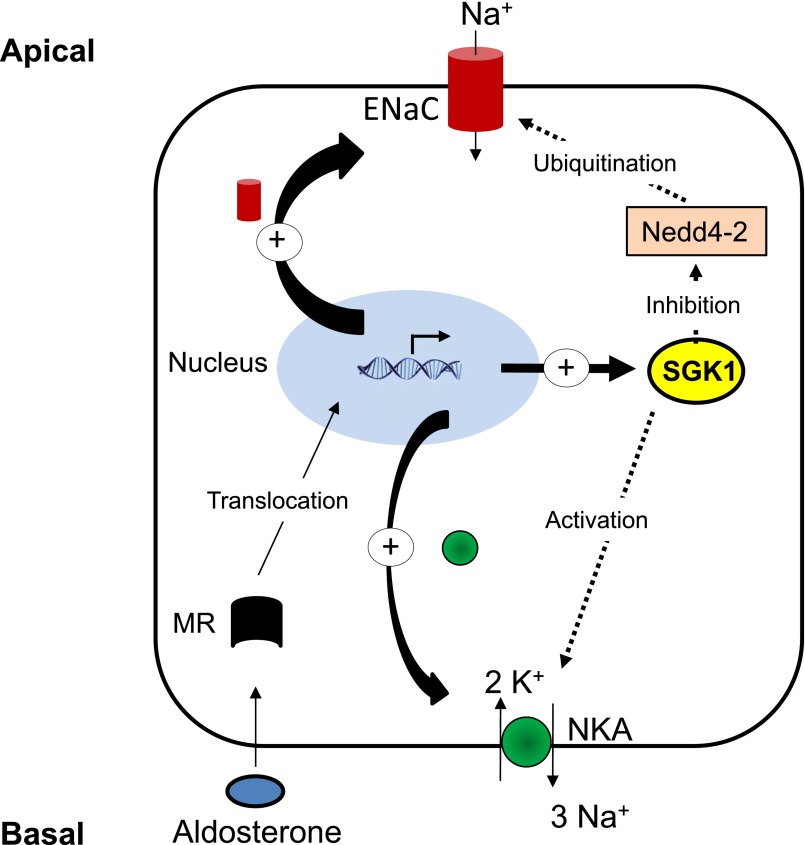
The response to aldosterone is biphasic with a short-term (after 30 minutes) and a long-term (after 1 day) effect.35–37 The short-term increase of Na+ reabsorption by the CD in response to aldosterone relies on coordinated stimulation of both ENaC and Na+,K+-ATPase activities. Stimulation of ENaC activity is in part mediated by the early induction of SGK1 that phosphorylates the ubiquitin-ligase Nedd-4.2. Nedd-4.2 phosphorylation prevents its interaction with ENaC, thereby decreasing ENaC subunits ubiquitination, endocytosis and degradation resulting in an increased number of active ENaC at the apical cell surface.38,39 Early animal studies have shown that Na+,K+-ATPase activity decreases slowly (days) after adrenalectomy but returns to basal levels 1–3 hours after administration of aldosterone.40,41 We have shown that in rat CCD as well as in cultured mpkCCDc14 principal cells aldosterone stimulates Na+,K+-ATPase activity via increased expression at the cell surface (Figure 2). This effect is independent of de novo Na+,K+-ATPase synthesis2 and may rely in part on SGK1.42 The physiologic role of SGK1 kidney Na+ handling has been confirmed in both constitutive and kidney tubule-specific inducible Sgk1-knockout mice.43,44 These mice display increased levels of urinary Na+ excretion and plasma aldosterone in response to a low Na+ diet as compared with wild-type mice. After prolonged stimulation (hours), aldosterone also increases the biosynthesis of α-ENaC as well as α-and β-subunits of Na+,K+-ATPase in a coordinated manner.45 Stimulation of α-ENaC transcription by aldosterone involves both direct binding of mineralocorticoid receptors to responsive elements and Sgk1-dependent disruption of Dot1a-Af9 and Dot1a-Sirt1 complexes and/or inhibition of their effects on chromatin.46
Figure 2.
Control of sodium transport by mineralocorticoids in CD principal cells. The steroid hormone aldosterone binds to intracellular mineralocorticoid receptor (MR) that translocates into the nucleus where it activates a transcriptional program inducing expression of modulators of sodium transport such as SGK1. SGK1 phosphorylates and inhibits Nedd-4.2, thereby increasing apical ENaC density via decreased ubiquitination, internalization and degradation of ENaC. SGK1 also increases expression of Na+,K+-ATPase and ENaC subunits. Sustained aldosterone stimulation increases the transcription of αENaC and Na,K-ATPase.
Coordinated control of Na+ transport by vasopressin
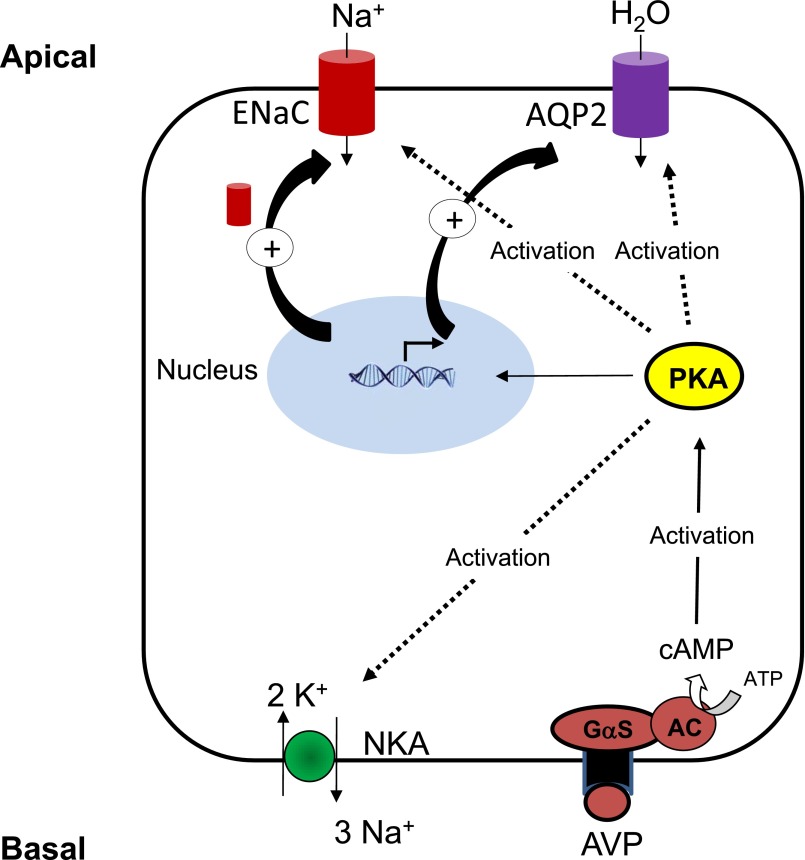
In CD principal cells, arginine vasopressin (AVP) binds to basolateral V2 receptors coupled to the cAMP/PKA pathway.21 Stimulation of water reabsorption is the major effect of AVP in the CD.47 However, AVP also stimulates Na+ reabsorption, albeit to a lesser extent. This effect has been verified by in vitro microperfusion studies in rat CCD, where vasopressin directly stimulates Na+ reabsorption,48 and by the use of V2 receptor antagonists in humans.49 An aldosterone-independent control of ENaC expression has been identified in adrenalectomized mice and in cultured CD principal cells.50,51 This AVP-dependent ENaC expression is more likely aimed at enhancing water reabsorption than maintaining Na+ balance. Both AVP and cAMP stimulate Na+ reabsorption by coordinated activation of ENaC and Na+,K+-ATPase (Figure 3). The stimulatory effect of cAMP on ENaC was demonstrated by patch-clamping experiments performed on the apical membrane of principal cells from isolated rat CDD.52 In the same preparation as well as in cultured mpkCCDc14 principal cells, cAMP rapidly stimulates Na+,K+-ATPase activity and cell surface expression53,54 independently of phosphorylation of its α−subunit.55
Figure 3.
Control of sodium and water transport by vasopressin in CD principal cells. The peptide hormone AVP binds to basolateral V2 receptors coupled to the activation of adenylyl cyclase via protein Gαs (Gαs). Generated cAMP binds to PKA regulatory subunits leading to release and activation of PKA catalytic subunit (PKAc) that phosphorylates a subset of substrates. PKA activation induces both post-transcriptional effects such as activation of ENaC and Na+,K+-ATPase as well as translocation of AQP2 water channels, and transcriptional effects such as increased AQP2 and ENaC subunits mRNA expression.
Control of Na+,K+-ATPase by apical Na+ entry and/or intracellular concentration
In the CD Na+,K+-ATPase displays an apparent affinity for Na+ that is two-fold higher than in the PT and TAL.14,16 Consequently, the so called “kinetic reserve” for Na+,K+-ATPase activation by increased intracellular Na+ concentration is much lower in the CD than in the more proximal nephron segments. CD luminal Na+ concentration is rather low but it is subjected to wide variations according to dietary Na+ intake and fluctuations in GFR. Therefore, CD cells must face large variations in apical Na+ entry according to requirements of body fluid homeostasis while maintaining intracellular Na+ concentration within a narrow range despite a lower kinetic reserve of the Na+,K+-ATPase. Indeed, tight control of intracellular Na+ concentration is required for many cellular functions including ENaC activity. In the absence of simultaneous stimulation of ENaC and Na+,K+-ATPase, any increase in Na+ entry would raise intracellular Na+ concentration and subsequently decrease ENaC activity by feedback inhibition.56 Coordinate control of apical and basolateral fluxes would preserve ENaC permeability over a wide range of luminal Na+ concentrations.
In the early nineties two independent research groups reported that increasing intracellular Na+ concentration in mammalian CCD rapidly and proportionally increased Na+,K+-ATPase activity and number of functional Na+,K+-ATPase units.20,57 This effect was observed after dissipation of the transmembrane Na+ gradient by either a Na+ ionophore that generates ion-permeable pores across the plasma membrane or by incubating cells in the absence of K+ which inhibits Na+,K+-ATPase activity. The Na+-induced increase in Na+,K+-ATPase activity was independent of de novo protein synthesis. We subsequently showed that increasing intracellular Na+ concentration with a Na+ ionophore increased both Na+,K+-ATPase activity and cell surface expression in isolated rat CCDs and in cultured mpkCCDc14 principal cells.58 These results suggest that intracellular Na+ concentration may control the number of active Na+,K+-ATPase units expressed at the cell surface of principal cells and thereby the rate of basolateral Na+ extrusion (Figure 4). These early findings suggested that either Na+,K+-ATPase units stored in an intracellular compartment are recruited to the cell surface or that internalization of resident plasma membrane Na+,K+-ATPase units is reduced. Activation of silent preexisting Na+,K+-ATPase units at the plasma membrane is unlikely since variations in intracellular Na+ concentration are associated with a parallel increase in Na+,K+-ATPase protein abundance at the cell surface.58
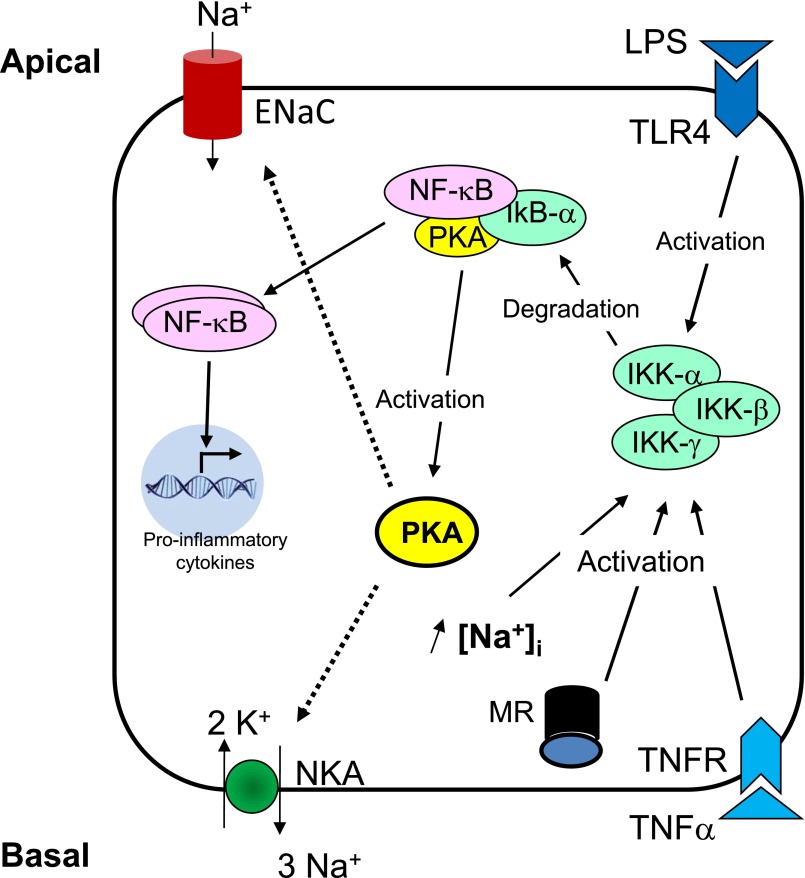
Figure 4.
Control of sodium transport by intracellular sodium in CD principal cells. The proinflammatory NF-κB pathway can be classically activated by bacterial products such as LPS via activation of Toll-like receptor 4 (TLR4) located in the apical membrane, by cytokines such as TNFα that binds to basolateral receptors, or by aldosterone via mineralocorticoid receptors. Activation of NF-κB through the classic pathway requires activation of IKKβ that phosphorylates IκBα that indices its dissociation from p65 NF-κB and its degradation by the proteasome. After dissociation from IκBα, NF-κB complexes are translocated to the nucleus where they modulate transcription of target genes including proinflammatory cytokines. Increased intracellular Na+ concentration induces dissociation of a protein complex containing IκBα, p65 NF-κB subunit and PKAc. This cascade leads to PKA activation and increased cell surface expression and activity of Na+,K+-ATPase.
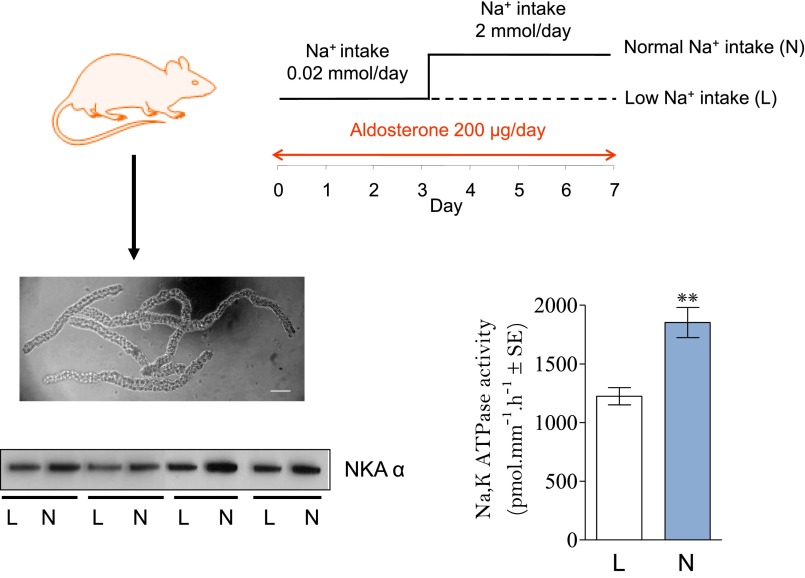
We further analyzed the coupling between apical Na+ entry through ENaC and basolateral exit via Na+,K+-ATPase in CD principal cells.59 In vivo experiments revealed that in the presence of a saturating concentration of aldosterone that is expected to maximally stimulate ENaC cell surface expression, increasing luminal Na+ availability induces an increase in Na+,K+-ATPase protein abundance and maximal hydrolytic activity in isolated rat CCD (Figure 5). Rigorous analysis of CD sections from normal and adrenalectomized rats by immuno-electron microscopy revealed an extensive gold labeling of the basolateral membrane, including basal infoldings. We did not detect any pool of intracellular vesicles displaying Na+,K+-ATPase antibody labeling although some intracellular organelles resembling multivesicular-bodies were significantly labeled with anti-Na+,K+-ATPase antibodies. This morphologic analysis indicated that the presence of an intracellular Na+,K+-ATPase storage compartment, such as exocytotic vesicles, is very unlikely. However, these labeled multivesicular bodies suggested that plasma membrane Na+,K+-ATPase is constitutively internalized and then degraded.
Figure 5.
Na+,K+-ATPase expression and activity varies in parallel with apical Na+ delivery in rat CCD. Rats were subcutaneously implanted with an osmotic minipump delivering aldosterone for 7 days. After 3 days of low sodium diet, rats were separated into two groups and given either low sodium diet (L) or normal sodium diet (N) for 4 additional days. Rats were then euthanized and CCD were isolated by microdissection from collagenase-treated kidneys. Na+,K+-ATPase α-subunit expression was assessed by Western blot on pools of 50 CCDs and Na+,K+-ATPase hydrolytic activity was determined by a radiochemical assay in isolated CCD. Results show that increased Na+ entry following reintroduction of sodium in the diet is associated with increased Na+,K+-ATPase abundance and activity in CCD.
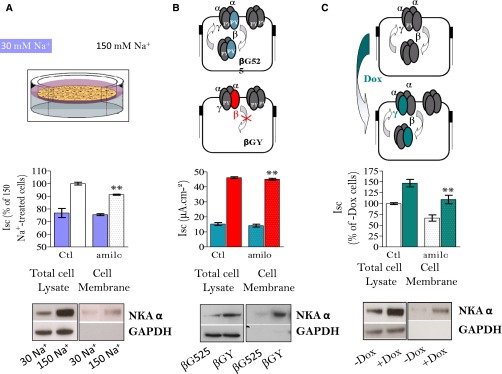
We tested the hypothesis that variations of apical Na+ entry via ENaC induce parallel changes in Na+,K+-ATPase basolateral membrane expression independent of a hormonal influence (Figure 6). In cultured mCCDcl1 principal cells overexpressing γ−ENaC in a doxycyclin-inducible manner grown on semipermeable filters, 48-hour doxycyclin treatment increased the amiloride-sensitive transepithelial current, i.e., a measurement of ENaC-dependent Na+ transport, by about 2-fold. These results confirmed that γ-ENaC is rate limiting for the activity of this channel in CD principal cells.60,61 The increase in ENaC mediated apical Na+ entry was associated with a parallel increase in total and cell-surface expression of basolateral Na+,K+-ATPase.59 These results were confirmed using mpkCCDcl4 cells, where a decrease of luminal Na+ availability at constant osmolality for 24 hours decreased both total and cell surface Na+,K+-ATPase expression levels.59 In addition, overexpression of a constitutively active mutant β-ENaC in mpkCCDcl4 cells62 increased both total and cell surface Na+,K+-ATPase expression compared with cells overexpressing wild-type β-ENaC. We analyzed whether increased Na+,K+-ATPase abundance in response to a sustained increase in ENaC activity relies on increased protein biosynthesis or decreased degradation. Analysis of Na+,K+-ATPase subunit mRNA did not reveal significant differences between cells overexpressing the γ−ENaC or not. Moreover, biosynthesis of the Na,K-ATPase α subunit, assessed by pulse labeling newly synthesized protein, remained unchanged (V. Leroy and E. Feraille, personal communication). In contrast, pulse-chase labeling of cell surface protein, used as a means to assess Na+,K+-ATPase internalization, and inhibition of lysosomal protein degradation revealed that increased apical Na+ entry via ENaC inhibits constitutive Na+,K+-ATPase endocytosis and its subsequent lysosomal degradation. This effect was specific since neither internalization rate nor degradation of E-cadherin, another basolateral transmembrane protein, was altered. These results indicate that Na+,K+-ATPase can be regulated through modulation of its degradation in ion transporting epithelia.
Figure 6.
Variation of apical Na+ entry via ENaC induces parallel changes in Na+,K+-ATPase expression in cultured CD cells. Total and amiloride-sensitive (amilo) short-circuit currents were measured on cells treated (spotted boxes) or not (plain boxes) with 10−6 M amiloride for 15 minutes, whereas Na+,K+-ATPase α-subunit (NKA-α) was detected by Western blot performed on total cellular extracts and after pull-down of biotinylated cell-surface proteins by streptavidin-agarose beads. GAPDH, a cytosolic protein, was used as a loading control for total cellular extract and its absence was taken as a control of quality for pull-down of cell-surface proteins. (A) mpkCCDcl14 cells grown to confluence on polycarbonate filters were incubated for 24 hours in presence of 150 or 30 mM apical Na+ concentration before measurement of transepithelial current and labeling of cell-surface proteins. (B) mpkCCDcl14 cells overexpressing a constitutively active mutant βENaC (βGY) or wild-type βENaC (βG525) were grown to confluence on polycarbonate filters before measurement of transepithelial current and labeling of cell-surface proteins. (C) γCCD-TetOn cells, conditionally overexpressing a wild type ENaC γ subunit in response to 5 days of doxycycline treatment, were grown to confluence on polycarbonate filters before measurement of transepithelial current and labeling of cell-surface proteins. Results show that under conditions of increased transcellular Na+ transport assessed by increased total and amiloride-sensitive currents, total and cell-surface expression of Na,K-ATPase were increased.
Experimental evidence supports a role for cell swelling in the intracellular Na+ concentration-dependent modulation of Na+,K+-ATPase cell surface expression. Early experiments performed in isolated mouse CCDs showed that the increased number of specific 3H-ouabain binding sites in response to increased intracellular Na+ concentration was dependent on cell swelling.63 In cultured CD cells (mpkCCDcl4 cells) we showed that extracellular hypotonicity increases both cell volume and Na+,K+-ATPase cell surface expression.64 In contrast, cell shrinkage in response to extracellular hypertonicity did not alter cell surface expression of Na+,K+-ATPase. The observed effect of hypotonicity was not mediated by a cell volume-dependent activation of ENaC, but relies on proteasomal-dependent PKA activation reminiscent of the effect of increased intracellular Na+.65 Along the same lines, cell swelling in response to increased apical sodium entry is likely a key initiating event in tubuloglomerular feedback signaling by macula densa cells.66
Early experiments have shown that basal levels of aldosterone are necessary to observe an increase in active Na+,K+-ATPase units in response to increased intracellular Na+ concentration in mammalian CD.20,57 This was interpreted as a requirement for aldosterone to synthesize an intracellular pool of Na+,K+-ATPase units that can be recruited to the plasma membrane. In the light of our recent results, this observation rather suggests that aldosterone and intracellular Na+ concentration both control the rate of Na+,K+-ATPase degradation. Activation of the PKA pathway may play a major role in controlling Na+,K+-ATPase expression at the cell surface. Increased Na+,K+-ATPase cell surface expression in response to AVP, cAMP and elevated intracellular Na+ concentration is dependent on PKA activity.54,58 This effect relies at least in part on cAMP-independent PKA activation following dissociation of a PKAc/IκBα/p65 complex leading on the one hand to PKA activation and on the other hand to NF-κB activation following proteasomal degradation of IκBα (Figure 4).58,65 Activation of the NF-κB pathway inhibits transepithelial Na+ transport by CD principal cells via decreased expression of α−ENaC in response to SGK1 transcriptional inhibition67; aldosterone activates NF-κB via the mineralocorticoid receptor both in vivo and in cultured CD principal cells.68 Therefore, activation of the NF-κB pathway may represent a negative feedback mechanism that prevents overstimulation of transepithelial Na+ transport by CD principal cells challenged with aldosterone and/or increased apical Na+ entry.
We have documented a key role of p38 kinase, a member of the MAP kinase family, in the inhibition of Na+,K+-ATPase endocytosis and degradation in response to an increase in Na+ entry via ENaC.59 Overexpression of γ-ENaC is associated with inhibition of p38 kinase activity, as assessed by its decreased phosphorylation. Aldosterone, which also increases Na+,K+-ATPase cell surface expression, similarly inhibits p38 kinase activity.68 The key role of p38 kinase was confirmed by its pharmacological inhibition or activation that increased or inhibited internalization of cell surface Na+,K+-ATPase.59 In contrast with results obtained in MDCK cells, that suggested that the major metabolic sensor AMPK may control Na+,K+-ATPase internalization,69 we were not able to demonstrate any effect of AMPK in cultured CD principal cells.59 In addition, salt-induced protein kinase that was shown to modulate Na+,K+-ATPase in PT cells did not alter Na+,K+-ATPase trafficking in CD principal cells (E. Féraille, personal communication).
In conclusion, increased apical Na+ influx through ENaC induces a coordinated increase in basolateral Na,K-ATPase expression and activity. Signaling through PKA and p38 kinase plays a key role in this process that may rely on the modulation of a Na+ sensor that remains to be identified.
Disclosures
None.
Acknowledgments
This work was supported by the National Center of Competence in Research Kidney control of homeostasis and a Swiss National Science Foundation grant 31003A_156736/1 to E.F.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Titze J: A different view on sodium balance. Curr Opin Nephrol Hypertens 24: 14–20, 2015 [DOI] [PubMed] [Google Scholar]
- 2.Summa V, Mordasini D, Roger F, Bens M, Martin PY, Vandewalle A, Verrey F, Féraille E: Short term effect of aldosterone on Na,K-ATPase cell surface expression in kidney collecting duct cells. J Biol Chem 276: 47087–47093, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Barlet-Bas C, Cheval L, Khadouri C, Marsy S, Doucet A: Difference in the Na affinity of Na(+)-K(+)-ATPase along the rabbit nephron: modulation by K. Am J Physiol 259: F246–F250, 1990 [DOI] [PubMed] [Google Scholar]
- 4.Cheval L, Doucet A: Measurement of Na-K-ATPase-mediated rubidium influx in single segments of rat nephron. Am J Physiol 259: F111–F121, 1990 [DOI] [PubMed] [Google Scholar]
- 5.Blot-Chabaud M, Jaisser F, Gingold M, Bonvalet JP, Farman N: Na+-K+-ATPase-dependent sodium flux in cortical collecting tubule. Am J Physiol 255: F605–F613, 1988 [DOI] [PubMed] [Google Scholar]
- 6.Shull GE, Greeb J, Lingrel JB: Molecular cloning of three distinct forms of the Na+,K+-ATPase α-subunit from rat brain. Biochemistry 25: 8125–8132, 1986 [DOI] [PubMed] [Google Scholar]
- 7.Shamraj OI, Lingrel JB: A putative fourth Na+,K(+)-ATPase α-subunit gene is expressed in testis. Proc Natl Acad Sci U S A 91: 12952–12956, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shull GE, Schwartz A, Lingrel JB: Amino-acid sequence of the catalytic subunit of the (Na+ + K+)ATPase deduced from a complementary DNA. Nature 316: 691–695, 1985 [DOI] [PubMed] [Google Scholar]
- 9.Kawakami K, Nojima H, Ohta T, Nagano K: Molecular cloning and sequence analysis of human Na,K-ATPase beta-subunit. Nucleic Acids Res 14: 2833–2844, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shull GE, Lane LK, Lingrel JB: Amino-acid sequence of the beta-subunit of the (Na+ + K+)ATPase deduced from a cDNA. Nature 321: 429–431, 1986 [DOI] [PubMed] [Google Scholar]
- 11.Hasler U, Wang X, Crambert G, Béguin P, Jaisser F, Horisberger JD, Geering K: Role of beta-subunit domains in the assembly, stable expression, intracellular routing, and functional properties of Na,K-ATPase. J Biol Chem 273: 30826–30835, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Geering K: FXYD proteins: new regulators of Na-K-ATPase. Am J Physiol Renal Physiol 290: F241–F250, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Béguin P, Crambert G, Guennoun S, Garty H, Horisberger JD, Geering K: CHIF, a member of the FXYD protein family, is a regulator of Na,K-ATPase distinct from the gamma-subunit. EMBO J 20: 3993–4002, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Féraille E, Carranza ML, Rousselot M, Favre H: Insulin enhances sodium sensitivity of Na-K-ATPase in isolated rat proximal convoluted tubule. Am J Physiol 267: F55–F62, 1994 [DOI] [PubMed] [Google Scholar]
- 15.Wetzel RK, Sweadner KJ: Immunocytochemical localization of Na-K-ATPase alpha- and gamma-subunits in rat kidney. Am J Physiol Renal Physiol 281: F531–F545, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Féraille E, Rousselot M, Rajerison R, Favre H: Effect of insulin on Na+,K(+)-ATPase in rat collecting duct. J Physiol 488: 171–180, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi H, Levy-Holzman R, Cluzeaud F, Farman N, Garty H: Membrane topology and immunolocalization of CHIF in kidney and intestine. Am J Physiol Renal Physiol 280: F505–F512, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Balda MS, Matter K: Tight junctions at a glance. J Cell Sci 121: 3677–3682, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Takada T, Yamamoto A, Omori K, Tashiro Y: Quantitative immunogold localization of Na, K-ATPase along rat nephron. Histochemistry 98: 183–197, 1992 [DOI] [PubMed] [Google Scholar]
- 20.Blot-Chabaud M, Wanstok F, Bonvalet JP, Farman N: Cell sodium-induced recruitment of Na(+)-K(+)-ATPase pumps in rabbit cortical collecting tubules is aldosterone-dependent. J Biol Chem 265: 11676–11681, 1990 [PubMed] [Google Scholar]
- 21.Féraille E, Doucet A: Sodium-potassium-adenosinetriphosphatase-dependent sodium transport in the kidney: hormonal control. Physiol Rev 81: 345–418, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Katz AI, Doucet A, Morel F: Na-K-ATPase activity along the rabbit, rat, and mouse nephron. Am J Physiol 237: F114–F120, 1979 [DOI] [PubMed] [Google Scholar]
- 23.El Mernissi G, Doucet A: Quantitation of [3H]ouabain binding and turnover of Na-K-ATPase along the rabbit nephron. Am J Physiol 247: F158–F167, 1984 [DOI] [PubMed] [Google Scholar]
- 24.McDonough AA, Magyar CE, Komatsu Y: Expression of Na(+)-K(+)-ATPase α- and β-subunits along rat nephron: isoform specificity and response to hypokalemia. Am J Physiol 267: C901–C908, 1994 [DOI] [PubMed] [Google Scholar]
- 25.Alper SL, Natale J, Gluck S, Lodish HF, Brown D: Subtypes of intercalated cells in rat kidney collecting duct defined by antibodies against erythroid band 3 and renal vacuolar H+-ATPase. Proc Natl Acad Sci U S A 86: 5429–5433, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roy A, Al-bataineh MM, Pastor-Soler NM: Collecting duct intercalated cell function and regulation. Clin J Am Soc Nephrol 10: 305–324, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eladari D, Hübner CA: Novel mechanisms for NaCl reabsorption in the collecting duct. Curr Opin Nephrol Hypertens 20: 506–511, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Pearce D, Soundararajan R, Trimpert C, Kashlan OB, Deen PM, Kohan DE: Collecting duct principal cell transport processes and their regulation. Clin J Am Soc Nephrol 10: 135–146, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Staruschenko A: Regulation of transport in the connecting tubule and cortical collecting duct. Compr Physiol 2: 1541–1584, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nissant A, Paulais M, Lachheb S, Lourdel S, Teulon J: Similar chloride channels in the connecting tubule and cortical collecting duct of the mouse kidney. Am J Physiol Renal Physiol 290: F1421–F1429, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Lu M, Dong K, Egan ME, Giebisch GH, Boulpaep EL, Hebert SC: Mouse cystic fibrosis transmembrane conductance regulator forms cAMP-PKA-regulated apical chloride channels in cortical collecting duct. Proc Natl Acad Sci U S A 107: 6082–6087, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lachheb S, Cluzeaud F, Bens M, Genete M, Hibino H, Lourdel S, Kurachi Y, Vandewalle A, Teulon J, Paulais M: Kir4.1/Kir5.1 channel forms the major K+ channel in the basolateral membrane of mouse renal collecting duct principal cells. Am J Physiol Renal Physiol 294: F1398–F1407, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Vinciguerra M, Mordasini D, Vandewalle A, Feraille E: Hormonal and nonhormonal mechanisms of regulation of the NA,K-pump in collecting duct principal cells. Semin Nephrol 25: 312–321, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Bollag WB: Regulation of aldosterone synthesis and secretion. Compr Physiol 4: 1017–1055, 2014 [DOI] [PubMed] [Google Scholar]
- 35.Horisberger JD, Diezi J: Effects of mineralocorticoids on Na+ and K+ excretion in the adrenalectomized rat. Am J Physiol 245: F89–F99, 1983 [DOI] [PubMed] [Google Scholar]
- 36.El Mernissi G, Doucet A: Short-term effect of aldosterone on renal sodium transport and tubular Na-K-ATPase in the rat. Pflugers Arch 399: 139–146, 1983 [DOI] [PubMed] [Google Scholar]
- 37.El Mernissi G, Chabardès D, Doucet A, Hus-Citharel A, Imbert-Teboul M, Le Bouffant F, Montégut M, Siaume S, Morel F: Changes in tubular basolateral membrane markers after chronic DOCA treatment. Am J Physiol 245: F100–F109, 1983 [DOI] [PubMed] [Google Scholar]
- 38.Debonneville C, Flores SY, Kamynina E, Plant PJ, Tauxe C, Thomas MA, Münster C, Chraïbi A, Pratt JH, Horisberger JD, Pearce D, Loffing J, Staub O: Phosphorylation of Nedd4-2 by Sgk1 regulates epithelial Na(+) channel cell surface expression. EMBO J 20: 7052–7059, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Snyder PM, Olson DR, Kabra R, Zhou R, Steines JC: cAMP and serum and glucocorticoid-inducible kinase (SGK) regulate the epithelial Na(+) channel through convergent phosphorylation of Nedd4-2. J Biol Chem 279: 45753–45758, 2004 [DOI] [PubMed] [Google Scholar]
- 40.El Mernissi G, Doucet A: Specific activity of Na-K-ATPase after adrenalectomy and hormone replacement along the rabbit nephron. Pflugers Arch 402: 258–263, 1984 [DOI] [PubMed] [Google Scholar]
- 41.Horster M, Schmid H, Schmidt U: Aldosterone in vitro restores nephron Na-K-ATPase of distal segments from adrenalectomized rabbits. Pflugers Arch 384: 203–206, 1980 [DOI] [PubMed] [Google Scholar]
- 42.Zecevic M, Heitzmann D, Camargo SM, Verrey F: SGK1 increases Na,K-ATP cell-surface expression and function in Xenopus laevis oocytes. Pflugers Arch 448: 29–35, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Wulff P, Vallon V, Huang DY, Völkl H, Yu F, Richter K, Jansen M, Schlünz M, Klingel K, Loffing J, Kauselmann G, Bösl MR, Lang F, Kuhl D: Impaired renal Na(+) retention in the sgk1-knockout mouse. J Clin Invest 110: 1263–1268, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Faresse N, Lagnaz D, Debonneville A, Ismailji A, Maillard M, Fejes-Toth G, Náray-Fejes-Tóth A, Staub O: Inducible kidney-specific Sgk1 knockout mice show a salt-losing phenotype. Am J Physiol Renal Physiol 302: F977–F985, 2012 [DOI] [PubMed] [Google Scholar]
- 45.Verrey F, Beron J, Spindler B: Corticosteroid regulation of renal Na,K-ATPase. Miner Electrolyte Metab 22: 279–292, 1996 [PubMed] [Google Scholar]
- 46.Kone BC: Epigenetics and the control of the collecting duct epithelial sodium channel. Semin Nephrol 33: 383–391, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hasler U, Leroy V, Martin P-Y, Féraille E: Aquaporin-2 abundance in the renal collecting duct: new insights from cultured cell models. Am J Physiol Renal Physiol 297: F10–F18, 2009 [DOI] [PubMed] [Google Scholar]
- 48.Hawk CT, Li L, Schafer JA: AVP and aldosterone at physiological concentrations have synergistic effects on Na+ transport in rat CCD. Kidney Int Suppl 57: S35–S41, 1996 [PubMed] [Google Scholar]
- 49.Perucca J, Bichet DG, Bardoux P, Bouby N, Bankir L: Sodium excretion in response to vasopressin and selective vasopressin receptor antagonists. J Am Soc Nephrol 19: 1721–1731, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mironova E, Bugaj V, Roos KP, Kohan DE, Stockand JD: Aldosterone-independent regulation of the epithelial Na+ channel (ENaC) by vasopressin in adrenalectomized mice. Proc Natl Acad Sci U S A 109: 10095–10100, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gaeggeler HP, Guillod Y, Loffing-Cueni D, Loffing J, Rossier BC: Vasopressin-dependent coupling between sodium transport and water flow in a mouse cortical collecting duct cell line. Kidney Int 79: 843–852, 2011 [DOI] [PubMed] [Google Scholar]
- 52.Frindt G, Silver RB, Windhager EE, Palmer LG: Feedback regulation of Na channels in rat CCT. III. Response to cAMP. Am J Physiol 268: F480–F489, 1995 [DOI] [PubMed] [Google Scholar]
- 53.Deschênes G, Gonin S, Zolty E, Cheval L, Rousselot M, Martin PY, Verbavatz JM, Féraille E, Doucet A: Increased synthesis and avp unresponsiveness of Na,K-ATPase in collecting duct from nephrotic rats. J Am Soc Nephrol 12: 2241–2252, 2001 [DOI] [PubMed] [Google Scholar]
- 54.Gonin S, Deschênes G, Roger F, Bens M, Martin PY, Carpentier JL, Vandewalle A, Doucet A, Féraille E: Cyclic AMP increases cell surface expression of functional Na,K-ATPase units in mammalian cortical collecting duct principal cells. Mol Biol Cell 12: 255–264, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mordasini D, Bustamante M, Rousselot M, Martin PY, Hasler U, Féraille E: Stimulation of Na+ transport by AVP is independent of PKA phosphorylation of the Na-K-ATPase in collecting duct principal cells. Am J Physiol Renal Physiol 289: F1031–F1039, 2005 [DOI] [PubMed] [Google Scholar]
- 56.Kellenberger S, Gautschi I, Rossier BC, Schild L: Mutations causing Liddle syndrome reduce sodium-dependent downregulation of the epithelial sodium channel in the Xenopus oocyte expression system. J Clin Invest 101: 2741–2750, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barlet-Bas C, Khadouri C, Marsy S, Doucet A: Enhanced intracellular sodium concentration in kidney cells recruits a latent pool of Na-K-ATPase whose size is modulated by corticosteroids. J Biol Chem 265: 7799–7803, 1990 [PubMed] [Google Scholar]
- 58.Vinciguerra M, Deschênes G, Hasler U, Mordasini D, Rousselot M, Doucet A, Vandewalle A, Martin PY, Féraille E: Intracellular Na+ controls cell surface expression of Na,K-ATPase via a cAMP-independent PKA pathway in mammalian kidney collecting duct cells. Mol Biol Cell 14: 2677–2688, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang YB, Leroy V, Maunsbach AB, Doucet A, Hasler U, Dizin E, Ernandez T, de Seigneux S, Martin PY, Féraille E: Sodium transport is modulated by p38 kinase-dependent cross-talk between ENaC and Na,K-ATPase in collecting duct principal cells. J Am Soc Nephrol 25: 250–259, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Husted RF, Volk KA, Sigmund RD, Stokes JB: Discordant effects of corticosteroids and expression of subunits on ENaC activity. Am J Physiol Renal Physiol 293: F813–F820, 2007 [DOI] [PubMed] [Google Scholar]
- 61.Volk KA, Husted RF, Sigmund RD, Stokes JB: Overexpression of the epithelial Na+ channel gamma subunit in collecting duct cells: interactions of Liddle’s mutations and steroids on expression and function. J Biol Chem 280: 18348–18354, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Coutry N, Farman N, Bonvalet JP, Blot-Chabaud M: Role of cell volume variations in Na(+)-K(+)-ATPase recruitment and/or activation in cortical collecting duct. Am J Physiol 266: C1342–C1349, 1994 [DOI] [PubMed] [Google Scholar]
- 63.Auberson M, Hoffmann-Pochon N, Vandewalle A, Kellenberger S, Schild L: Epithelial Na+ channel mutants causing Liddle’s syndrome retain ability to respond to aldosterone and vasopressin. Am J Physiol Renal Physiol 285: F459–F471, 2003 [DOI] [PubMed] [Google Scholar]
- 64.Vinciguerra M, Arnaudeau S, Mordasini D, Rousselot M, Bens M, Vandewalle A, Martin PY, Hasler U, Feraille E: Extracellular hypotonicity increases Na,K-ATPase cell surface expression via enhanced Na+ influx in cultured renal collecting duct cells. J Am Soc Nephrol 15: 2537–2547, 2004 [DOI] [PubMed] [Google Scholar]
- 65.Vinciguerra M, Hasler U, Mordasini D, Roussel M, Capovilla M, Ogier-Denis E, Vandewalle A, Martin PY, Feraille E: Cytokines and sodium induce protein kinase A-dependent cell-surface Na,K-ATPase recruitment via dissociation of NF-kappaB/IkappaB/protein kinase A catalytic subunit complex in collecting duct principal cells. J Am Soc Nephrol 16: 2576–2585, 2005 [DOI] [PubMed] [Google Scholar]
- 66.Peti-Peterdi J: Confocal imaging and function of the juxtaglomerular apparatus. Curr Opin Nephrol Hypertens 14: 53–57, 2005 [DOI] [PubMed] [Google Scholar]
- 67.de Seigneux S, Leroy V, Ghzili H, Rousselot M, Nielsen S, Rossier BC, Martin PY, Féraille E: NF-kappaB inhibits sodium transport via down-regulation of SGK1 in renal collecting duct principal cells. J Biol Chem 283: 25671–25681, 2008 [DOI] [PubMed] [Google Scholar]
- 68.Leroy V, De Seigneux S, Agassiz V, Hasler U, Rafestin-Oblin M-E, Vinciguerra M, Martin P-Y, Féraille E: Aldosterone activates NF-kappaB in the collecting duct. J Am Soc Nephrol 20: 131–144, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alves DS, Farr GA, Seo-Mayer P, Caplan MJ: AS160 associates with the Na+,K+-ATPase and mediates the adenosine monophosphate-stimulated protein kinase-dependent regulation of sodium pump surface expression. Mol Biol Cell 21: 4400–4408, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]