Abstract
Kidney injury implies danger signaling and a response by the immune system. The inflammasome is a central danger recognition platform that triggers local and systemic inflammation. In immune cells, inflammasome activation causes the release of mature IL-1β and of the alarmin IL-1α. Dying cells release IL-1α also, independently of the inflammasome. Both IL-1α and IL-1β ligate the same IL-1 receptor (IL-1R) that is present on nearly all cells inside and outside the kidney, further amplifying cytokine and chemokine release. Thus, the inflammasome-IL-1α/IL-β-IL-1R system is a central element of kidney inflammation and the systemic consequences. Seminal discoveries of recent years have expanded this central paradigm of inflammation. This review gives an overview of arising concepts of inflammasome and IL-1α/β regulation in renal cells and in experimental kidney disease models. There is a pipeline of compounds that can interfere with the inflammasome-IL-1α/IL-β-IL-1R system, ranging from recently described small molecule inhibitors of NLRP3, a component of the inflammasome complex, to regulatory agency–approved IL-1–neutralizing biologic drugs. Based on strong theoretic and experimental rationale, the potential therapeutic benefits of using such compounds to block the inflammasome-IL-1α/IL-β-IL-1R system in kidney disease should be further explored.
Keywords: inflammation, glomerulonephritis, acute kidney injury, chronic kidney disease, innate immunity
The immune system has a central role in maintaining homeostasis and in regaining homeostasis after injury. Infectious and noninfectious triggers of injury have an identical capacity to initiate inflammation, e.g., a gouty or bacterial arthritis both present as clinically indistinguishable acute joint inflammation. In the last 15 years the research community has unraveled the molecular mechanisms of danger signaling but this area remains a source of unexpected discoveries. Numerous data have accumulated since the Journal of the American Society of Nephrology published a first overview about inflammasomes in kidney disease in 2011.1 This brief review provides an update on inflammasome biology and extends the discussion to the alarmin IL-1α. A comprehensive view on the expression and biologic effects of the inflammasome-IL-1 axis in renal cells and its functional contribution to experimental and human kidney disease is required to appreciate the potential of inflammasome-IL-1–related drugs in this evolving area of translational nephrology.
Update on IL-1 Biology
Since its first cloning in 1984 the knowledge on the biology of the IL-1 family of cytokines has expanded to considerable complexity as reviewed elsewhere in detail (Figure 1).2–4 As the system is of enigmatic importance for the regulation of systemic and tissue inflammation, pro- and anti-inflammatory factors balance the system at all levels, i.e., the receptor ligands, the transmembrane cell surface receptors, and the signaling pathways (Figure 1).4,5
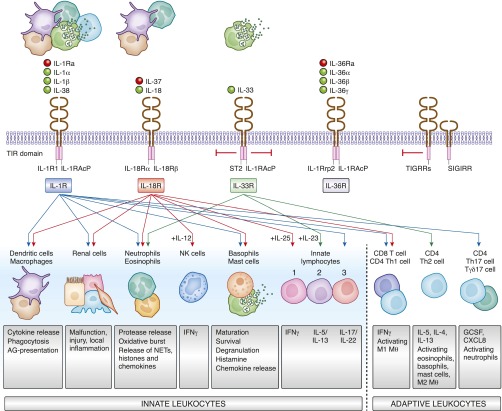
Figure 1.
The families of IL-1 cytokines and cytokine receptors activate innate and adaptive immunity. Activated or dying cells release all sorts of cytokines of the IL-1 family that specifically interact with several transmembrane surface receptors present on most cell types of the body. Some induce cell activation (IL-1R, IL-18R, IL-36R), others inhibit cell activation (IL-33R, TIGIRR, SIGIRR). This way the family elicits numerous regulatory effects on renal cells, immune cells of the innate and adaptive immune system, either activating or inhibiting their respective cell type–specific functions. AG, antigen; G-CSF, granulocyte colony-stimulating factor; MФ, macrophage; NETs, neutrophil extracellular traps; NK cell, natural killer cell; TIR domain, Toll/interleukin-1 receptor (TIR) homology domain.
IL-1α
IL-1α is constitutively present in keratinocytes and other epithelia including tubular epithelial cells, whereas macrophages, granulocytes, endothelial cells, fibroblasts, and mesangial cells express the IL-1α precursor only upon activation.5 The 31 kDa IL-1α precursor lacks a signal peptide fragment and is already biologically as active as the processed 18 kDa “mature” form.6 Therefore, cells constitutively expressing IL-1α were considered “a loaded gun” that can at any time release a proinflammatory alarmin upon cell necrosis.6–8 This way necrotic cells alert surrounding tissues and set up local tissue inflammation.
IL-1β
IL-1β is not constitutively expressed and its secretion is largely restricted to circulating monocytes that are activated for enzymatic cleavage of the inactive 266 amino acid precursor into the 153 amino acid mature form of IL-1β.5 Therefore, IL-1β mainly contributes to systemic inflammation by initiating acute phase response proteins in the liver such as C-reactive protein, by activating endothelial cells, by triggering fever, by causing neutrophil mobilization from the bone marrow (leukocytosis), and by activating all classes of leukocytes and renal cells (Figure 1).9 Within tissues, mainly resident dendritic cells and infiltrating macrophages and neutrophils can release large amounts of IL-1β, whereas parenchymal cells may release only small amounts under certain circumstances.4
Enzymatic Processing
Pro–IL-1α is a substrate for the calcium-dependent, nonlysosomal cysteine protease calpain (Figure 2),5,10,11 but little is known about how external triggers regulate calpain activity. Calcium release from intracellular stores seems sufficient to activate calpain-driven pro–IL-1α processing.10 Necrotic cells release both the 31 and 18 kDa forms of IL-1α passively from intracellular stores to alarm surrounding cells.6 In those cells that coexpress IL-1α and IL-1β, IL-1α secretion can also be inflammasome-dependent, implying that IL-1α and IL-1β are released together.12
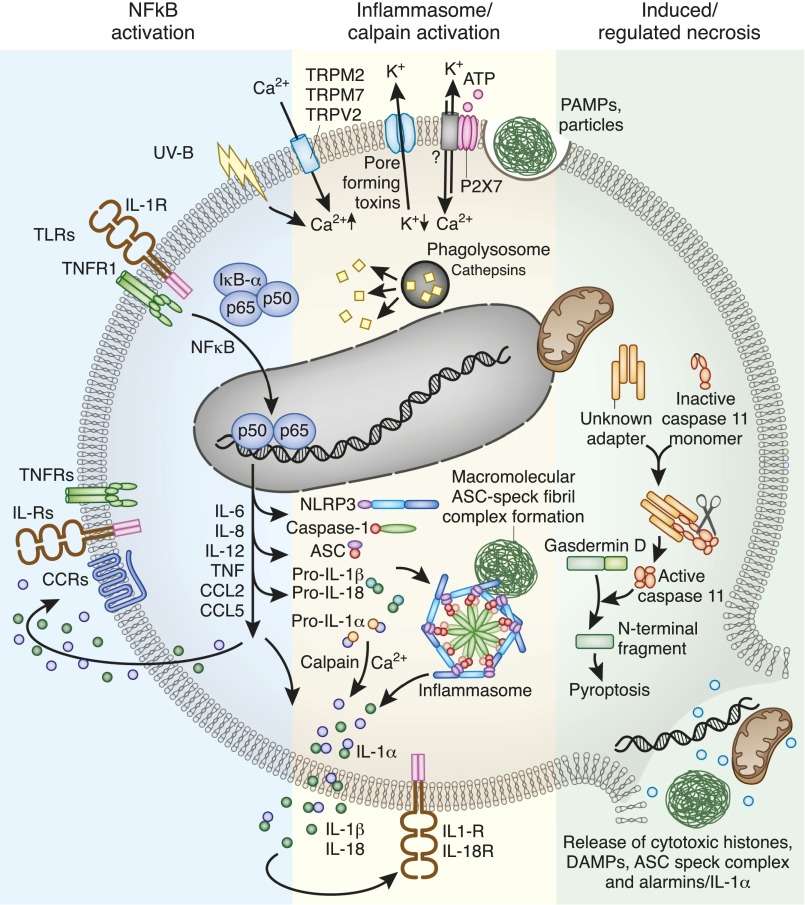
Figure 2.
Inflammasome activation in dendritic cells and macrophages involves numerous elements. Dendritic cells first need to induce the expression of the inflammasome components and of pro–IL-1α and pro–IL-1β. This can occur via cytokine receptors or Toll-like receptors (TLRs). Activation of inflammasome assembly can occur upon numerous intracellular danger signals such as mitochondrial reactive oxygen species release, lysosomal protease leakage, and potassium efflux or calcium influx. The multiprotein inflammasome complex forms a wheel-like structure to trigger caspase-1–driven IL-1β (and IL-18) enzymatic activation. Inflammasome fibrils grow in size to a single macromolecular complex called ASC speck. Calcium activates calpain, which cleaves pro–IL-1α to IL-1α, but in contrast to pro–IL-1β, IL-1α is already biologically active, and hence an alarmin. IL-1α, IL-1β, and IL-18 together with other NF-κB–dependent cytokines and chemokines activate cytokine and chemokine receptors in an autocrine, paracrine, or systemic manner. Noncanonical inflammasome signaling, e.g., triggered by cytosolic LPS, involves caspase-11 (mice) and caspase 4/5 (humans), which cleave gasdermin D. The cleaving product activates NLRP3. Noncanonical inflammasome signaling is a recognized trigger for pyroptosis, an immunogenic form of cell death that leads to the release of numerous intracellular components that have the capacity to activate a plethora of pattern recognition receptors and close the vicious cycle of necroinflammation. CCL, CC chemokine ligand; CCR, CC-chemokine receptor.
Pro–IL-1β is a true precursor that requires enzymatic processing for activation. Extrinsic or intrinsic danger signals that initiate the canonical inflammasome–dependent activation of caspase-1 have been extensively studied in mononuclear phagocytes.13 In other immune cells other proteases may activate IL-1β, e.g., proteinase-3 in neutrophils, granzyme A in NK cells, chymase in mast cells, and metalloendopeptidase meprin A in epithelial cells.5,14 Inflammasomes are cytosolic wheel-like complexes composed by the assembly of NACHT, LRR and PYD domains-containing proteins (NLRP), the linker molecule apoptosis-associated specklike protein (ASC), and caspase-1 (Figure 2).15 Different inflammasomes are defined by the different NLRs that serve as sensors for intracellular danger signals.15 Whereas the NLRC4, NLRP6, -7, -12, or AIM2 inflammasomes recognize preferably pathogen-associated molecular patterns (PAMPs), NLRP1 and NLRP3 are more promiscuous and have the capacity to translate a large variety of different cytosolic danger signals into the caspase-1–dependent secretion of IL-1β.15 Many of these may also occur during kidney injury, such as mitochondrial release of reactive oxygen species and pore forming toxins, ATP, or osmotic pressure that affect intracellular potassium and calcium concentrations.15–17 Other danger-associated molecular patterns (DAMPs) that contribute to NLRP3-driven renal inflammation include uromodulin particles,18 biglycan,19 extracellular histones,20 oxalate, or urate crystals that destabilize lysosomes for cathepsin leakage into the cytosol like other phagocytosed microparticles.21–23 Cytosolic LPS is a known trigger for noncanonical inflammasome signaling involving caspase-11 in mice and caspases-4 and -5 in humans.24–27 Caspase-11/4/5 activation leads to cleavage of the cytosolic protein gasdermin,24,28 and gasdermin’s N–terminally cleaved product p70 subsequently activates the NLRP3 inflammasome for caspase-1–dependent pro–IL-1β processing.24
Unlike macrophages, dendritic cells do not constitutively express pro–IL-1β and the inflammasome components. Dendritic cells first require a priming signal via Toll-like receptors or cytokine receptors that induce NF-ĸB–dependent transcription of NLRP3, ASC, caspase-1, pro–IL-1α, and IL-1β.15 It is of note that also other enzymes have been found to cleave pro–IL-1β, such as serine proteases in neutrophil granules.29
The activation of the NLRP3 inflammasome is tightly regulated.30 A20-driven ubiquitination of NLRP3, PYRIN domain-only protein POP1-dependent inhibition of inflammasome assembly, or reactive oxygen species–driven suppression of caspase-1 are just three such regulatory mechanisms.31–33
IL-1 Receptor
IL-1R1 is expressed on nearly all cells and hardly regulated.4 Its activation induces the release of the soluble IL-1 receptor antagonist that can neutralize the biologic effects of IL-1α and IL-1β.9,34 IL-1R2 has the same inhibitory effect.4,35 IL-1R1 ligation signals via the adaptor protein MyD88 to the kinases IRAK-2 and -4 and uses TRAF6 to involve NF-ĸB, p38, Janus kinase, and ERK for initiating the transcription of inflammatory cytokines including pro–IL-1α and pro–IL-1β.4
Consequences of Inflammasome Activation or NLRP3/ASC Induction Other Than IL-1 Release
Inflammasome activation also induces the secretion of mature IL-18 (Figure 1).36 IL-18 is clearly expressed by tubular epithelial cells in addition to infiltrating immune cells,37 but if the tubular expression of IL-18 is inflammasome-dependent or not and its contribution to kidney injury remains under debate.38,39 The strong induction of IL-18 inside the kidney upon injury has raised much attention as IL-18 could be a useful urinary biomarker of kidney injury as discussed in detail elsewhere.40 Inflammasome activation by intracellular bacteria or LPS can induce necrotic cell death, referred to as pyroptosis.41,42 In murine macrophages this process is largely caspase-11–dependent (caspase-4/5 in humans).24,26 Pyroptosis re-exposes intracellular bacteria to extracellular host defense elements and increases local inflammation by DAMP and alarmin release including IL-1α (Figure 2).41 Whether NLRP3 agonists other than cytosolic LPS induce pyroptosis in cells other than macrophages is still under debate but human immunodeficiency virus infection depletes CD4 T cells in a caspase-1–dependent manner.43 Recently, it was shown that Syk- and Jnk-dependent Tyr144 phosphorylation of ASC induces ASC speck aggregate formation.44 ASC specks grow to fibril-like macromolecular structures inside the cytosol that are eventually released into the extracellular space, e.g., upon pyroptosis.45,46 Such extracellular ASC specks elicit DAMP-like proinflammatory effects conceptually similar to crystals and other microparticles once they get phagocytosed and induce lysosomal leakage and activate the NLRP3 inflammasome of the phagocyte.46,47 Finally, several studies suggest that NLRP3 and ASC have other, inflammasome-independent biologic functions, such as facilitating the anti-inflammatory and profibrotic effects of TGF-βR signaling.48,49
Data in Renal Cells: Expression, Release
Kidney injury exposes renal cells to numerous inflammasome activators, such as ATP, uric acid, histones, uromodulin, oxalate or cystine crystals, and matrix degradation products.17,18,20,50–53 But are the inflammasome components expressed at all in the kidney and, if so, do they contribute to canonical inflammasome signaling54,55? IL-1α and IL-1β are both induced by NF-ĸB signaling but still display heterogeneous expression patterns in solid organs of healthy mice.56 Especially IL-1β can be induced in tubular epithelial cells in kidneys of young mice and the expression of both IL-1α and IL-1β increases with age.56 Essential elements of the NLRP3 inflammasome have meanwhile been found to be expressed in most renal parenchymal cell types (Table 1) but their functional roles inside the kidney and species-specific differences remain under debate.57–59 For example, albumin overload induces NLRP3 expression in proximal tubular cells of rats and in cultured HK-2 cells, and IL-1β release could be demonstrated but only in cell lines in vitro.59 Indeed, the roles of NLRP3, ASC, and caspase-1 for canonical inflammasome signaling in macrophages and dendritic cells are consistent in literature.21 The published data become conflicting when reporting pro–IL-1β processing and IL-1β release from renal parenchymal cells.60,61 Only a few studies demonstrate the secretion of mature IL-1β from immune cell–free tissue specimens or from nonimmune renal cell cultures by ELISA and western blot. Those that do, obtain inconclusive results.60–64 Convincing evidence was demonstrated for renal fibroblasts.65 Others conclude on inflammasome activation from immunostaining or transcript analysis,66–68 which is inadequate given the post-transcriptional nature of inflammasome activity. Eventually, confocal laser microscopy might be a feasible way to prove intrarenal inflammasome activation by documenting ASC speck formation,46,69 but ASC speck complexes have not yet been reported inside the kidney. In fact, recent reports on renal cell or kidney tissue NLRP3/ASC immunostaining depict diffuse cytoplasmic positivity rather than ASC specks. In addition, Nlrp3/Asc−/− control sections were not used to prove specificity of immunolabeling.66–68 However, renal parenchymal cells may process pro–IL-1β into mature IL-1β via the metalloendopeptidase meprin A.5,14 Data on caspase-11 and renal cell pyroptosis are still sparse. Yang, et al. proposed tubule cell pyroptosis to occur upon ischemia-reperfusion injury based on an association with caspase-11 activity,70 but a functional role of caspase-11 was not proven and others did not find any effect on postischemic tubular necrosis with pan-caspase inhibitor treatment.71,72 IL-1α has potent immunostimulatory effects on renal cells73 but little is known about the capacity of renal parenchymal cells to release IL-1α from intracellular stores and elicit alarmin-like effects on surrounding cells.6
Table 1.
Expression and function of inflammasome components in renal parenchymal cells
| Component | NLRP3, ASC Expression | Speck Formation | IL-1β Release | Pyroptosis | IL-1α Release |
|---|---|---|---|---|---|
| Endothelial cells | Yes61,112 | ? | 61 | ? | ? |
| No63 | |||||
| Mesangial cells | No63 | ? | — | ? | 113 |
| Yes113,114 | |||||
| Podocytes | Yes61,66,95 | ? | Yes61,115 | ? | ? |
| No63 | No63 | ||||
| Tubular epithelial cells | Yes49,59,62,76,78,116–119 | ? | Yes70 | ||
| No21,62 | No71 | ||||
| Fibroblasts | ? | ? | 65 | ? | 65 |
?, unknown; —, absent.
Role in Animal Models of Kidney Disease
AKI Models
Necroinflammation occurs mainly in AKI, e.g., in infective pyelonephritis, thrombotic microangiopathy, necrotizing GN, and tubular necrosis (Figure 3).74 Up to now only the latter two have been studied using mice deficient for Nlrp3, Asc, Casp1/11, Il1α, or Il1β (Table 2). Whenever tested, mutant mice were consistently protected from renal necroinflammation.21,75 However, postischemic tubular necrosis depends on NLRP376–79 but not on ASC.76 This finding could imply an additional, inflammasome-independent, biologic effect of ASC in postischemic AKI. Studies performed on the model of acute oxalosis suggest inflammasome signaling to be restricted to intrarenal dendritic cells.21 The independent contribution of IL-1α to AKI has so far been addressed only by Lee et al.80 Il1α-deficient mice were protected from cisplatin-induced AKI but, unexpectedly, markers of inflammation were not different from wild-type control mice.80 However, not all studies document a consistent blockade of intrarenal inflammation and tubular necrosis in postischemic or toxic AKI in models upon blockade of the IL-1 axis.81–83 These findings may imply that IL-1 is not a universal mediator of AKI and its role is context-dependent.
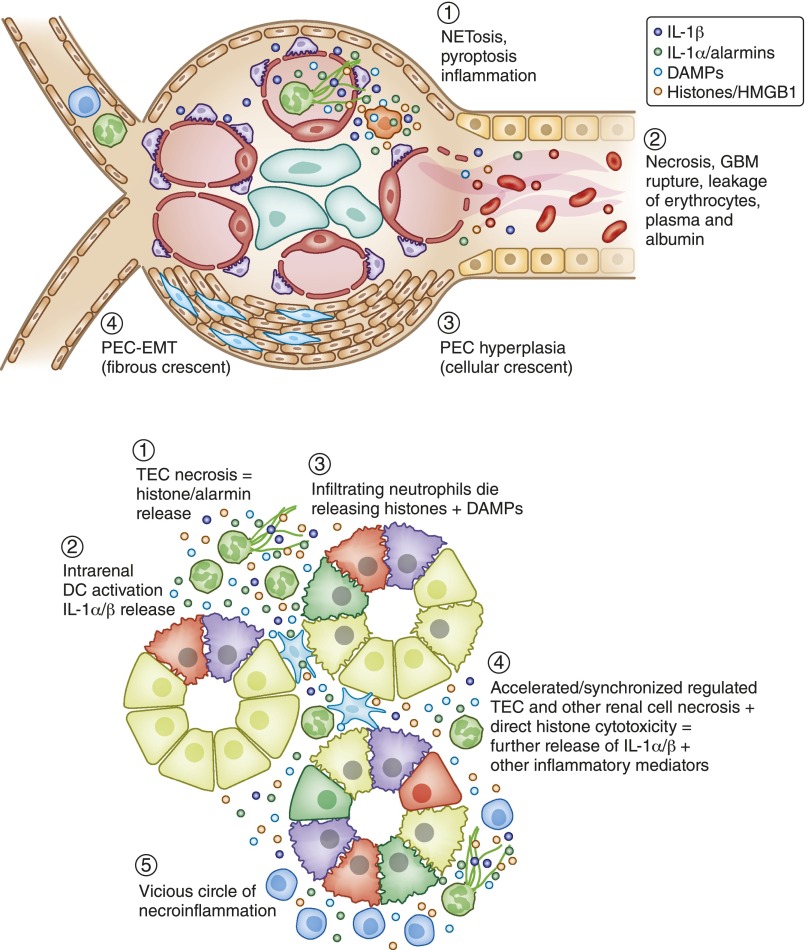
Figure 3.
The inflammasome/IL-1 system contributes to renal necroinflammation. Necroinflammation can occur in the glomerulus (A) or the tubulointerstitial compartment (B). The primary event can be intravascular NETosis, as in ANCA vasculitis, or renal cell necrosis, such as in ischemic tubular injury. Histone, DAMP, and alarmin (IL-1α) released from dying cells activate the NLRP3 inflammasome (IL-1α/β release) and induce IL-1R signaling, which implies local inflammation. Especially histones also kill other cells, resulting in a crescendo of tissue inflammation and necrosis, i.e., necroinflammation. The consequences in the glomerulus and the tubules are illustrated. Both lead to a drop out of nephrons and the clinical syndrome of AKI.
Table 2.
AKI models in Nlrp3-, Asc-, Casp1/11-, Il-1α–, and Il-β–deficient mice
| Disease Model | Gene | Inflammation | Renal Function | Reference |
|---|---|---|---|---|
| Severe GN | ||||
| Heterologous anti-GBM GN | Nlrp3−/− | — | — | 63 |
| Asc−/− | — | — | 63 | |
| Casp1/11−/− | — | — | 63 | |
| Autologous anti-GBM GN | Nlrp3−/− | ↓ | Better | 86 |
| Asc−/− | ↓ | Better | 86 | |
| IL1β−/− | ↓ | Better | 84,85 | |
| Sepsis | ||||
| LPS-induced | Casp1/11−/− | ↓ | Better | 120 |
| Tubular necrosis | ||||
| Ischemia- reperfusion | Nlrp3−/− | — | Better | 76,77,79 |
| Asc−/− | — | — | 76 | |
| Casp1/11−/− | ↓ | Better | 121 | |
| IL1α−/− | ↓ | ? | 122 | |
| IL1β−/− | ↓ | ? | 122 | |
| Cisplatin | Nlrp3−/− | — | — | 79 |
| Asc−/− | ? | ? | 123 | |
| Casp1/11−/− | ↓ | Better | 118 | |
| IL1α−/−- | — | Better | 80 | |
| Rhabdomyolysis | Nlrp3−/− | ↓ | Better | 75 |
| Asc−/− | ↓ | Better | 75 | |
| Casp1/11−/− | ↓ | Better | 75 | |
| IL1β−/− | ↓ | Better | 75 | |
| Acute oxalosis | Nlrp3−/− | ↓ | Better | 21 |
| Asc−/− | ↓ | Better | 21 | |
| Casp1/11−/− | ↓ | Better | 21 | |
Anti-GBM GN, anti-glomerular basement membrane GN; —, absent; ↓, suppressed; ?, unknown.
Models of acute glomerular injury pose new questions. Autologous anti-GBM disease is a model of GN triggered by in situ immune complex formation. Ten years after Timoshanko et al. had demonstrated that autologous anti-GBM GN is attenuated in Il1β-deficient mice,84,85 Andersen et al. identified the NLRP3/ASC inflammasome as the mechanism of glomerular IL-1β production in this model.86 However, Lichtnekert et al. could not find any phenotype of mice deficient for Nlrp3, Asc, and Casp1/11 with heterologous anti-GBM GN, a model with little leukocyte infiltration.63 In fact, in 2004 Timoshanko et al. already identified infiltrating leukocytes as the only source of glomerular IL-1β release.85 Altogether, AKI involves canonical inflammasome–mediated IL-1β secretion mostly from the tubulointerstitial network of dendritic cells and infiltrating leukocytes as part of the autoamplification loop of necroinflammation (Figure 3). The role of leukocyte pyroptosis, NETosis, and IL-1α in this context remains poorly studied. In ANCA vasculitis neutrophil serine proteases such as cathepsin G, elastase, and proteinase 3 mediate intrarenal IL-1β processing.29
CKD Models
Glomerular pathology drives CKD progression in most cases, diabetic nephropathy being the clinically most prevalent disease category (Figure 4). Shahzad et al. provided a comprehensive data set documenting a role of the NLRP3/ASC inflammasome for the progression of STZ-induced diabetic nephropathy (Table 3).61 Their analysis also included bone marrow transplant studies documenting a significant contribution of NLRP3-mediated caspase-1 activation inside nonimmune cells to podocyte loss, albuminuria, and glomerulosclerosis.60,61 As their experiments involved conventional knockout mice it remains unclear if this relates to NLRP3 in nonimmune cells in- or outside the kidney. Reports on other CKD models such as hypertensive nephrosclerosis, Western diet-, toxin-, or oxalate crystal–induced nephropathy or ureter obstruction were only conducted using Nlrp3-deficient mice, but consistently displayed a protected phenotype (Table 3). Bone marrow–derived vehicle cells overexpressing IL-1Ra suppress interstitial inflammation in obstructive nephropathy.87 However, in lupus nephritis of C57BL/6-(Fas)lpr mice lack of NLRP3 and ASC consistently presented an aggravated phenotype.48 As the same was not found for lack of IL-1R or IL-18 an inflammasome-independent effect was postulated.48 For example, NLPR3 and ASC mediate TGFR-dependent SMAD phosphorylation,48 a signaling pathway known to suppress lupus nephritis via the known immunoregulatory effects of TGF-β signaling on autoimmunity.88
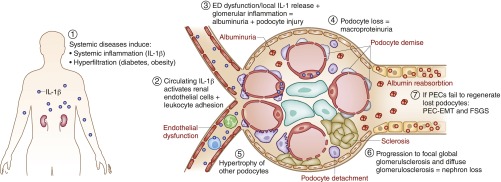
Figure 4.
The inflammasome/IL-1 system promotes CKD. CKD is devoid of tissue necrosis and currently there is little evidence for renal cell necroptosis or other forms of regulated cell necrosis being involved in CKD progression. There are data supporting a role of the NLRP3 inflammasome and IL-1 in CKD but the mechanisms are unclear. Most likely systemic release of IL-1, e.g., in diabetes or systemic lupus, contributes to systemic endothelial dysfunction (ED), a process also promoting leukocyte adhesion and vascular leakage (microalbuminuria) in the kidney. Furthermore, intrarenal inflammasome activation in immune cells, and eventually also in renal parenchymal cells, may contribute to local inflammation, cell stress, and cell loss. For example, podocyte detachment promotes albuminuria, hypertrophy of the remaining podocytes, FSGS, and subsequently first focal-global and later diffuse glomerulosclerosis-related nephron loss. In this process the inflammasome components NLRP3 and ASC may contribute to SMAD phosphorylation downstream of TGFR signaling during epithelial-mesenchymal transition (EMT) of parietal epithelial cells (PEC), and induce extracellular matrix production by tubular epithelial cells (not depicted). Whether the same also occurs in interstitial fibroblasts is currently unknown (not depicted). A role of NLRP3 and ASC has also been reported on systemic autoimmunity where that assures the immunosuppressive effect of TGFR signaling on immune cells (not depicted).
Table 3.
CKD models in Nlrp3-, Asc-, caspase-1/11–, Il-1α–, and Il-β–deficient mice
| Disease Model | Gene | Inflammation | Renal Function | Fibrosis | Reference |
|---|---|---|---|---|---|
| Glomerulopathy | |||||
| Lupus nephritis | Nlrp3−/− | ↑ | Worse | ? | 48 |
| Asc−/− | ↑ | Worse | ? | 48 | |
| Casp1/11−/− | ↑ | Worse | ? | 48 | |
| Diabetic nephropathy | Nlrp3−/− | ↓ | Better | ↓ | 61 |
| Asc−/− | ↓ | Better | ↓ | 61 | |
| Casp1/11−/− | ↓ | Better | ↓ | 61 | |
| Toxic glomerulopathy | Nlrp3−/− | ↓ | Better | ↓ | 124 |
| Hypertensive nephrosclerosis | Asc−/− | ↓ | ? | ↓ | 125 |
| Western diet nephropathy | Nlrp3−/− | ↓ | ? | ↓ | 106 |
| Tubulopathy | |||||
| Obstructive nephropathy | Nlrp3−/− | ↓/— | N/A | ↓/— | 62,126 |
| Chronic oxalosis | Nlrp3−/− | ↓ | Better | ? | 107 |
↑, increased; ?, unknown; ↓, suppressed; —, absent; N/A, not applicable.
IL-1–Related Drugs and Clinical Data
The relevance of the NLRP3 inflammasome for human disease becomes obvious from the cryopyrin-associated periodic syndrome (CAPS), a group of genetic disorders of periodic fever and multifocal tissue inflammation caused by gain-of-function mutations in the NLRP3 gene.89 Genetic variants in IL-1–related genes associate with the risk for ESRD.90,91 The pathogenic concept of CAPS was verified by dramatic clinical responses to IL-1 inhibitors such as the IL-1β neutralizing antibody canakinumab (Ilaris), the soluble decoy receptor rilonacept (Arcalyst), and the recombinant human IL-1Ra (Kineret).89 Important for nephrologists is renal amyloidosis secondary to CAPS or any other hereditary systemic (auto-)inflammatory disorder that responds well to therapeutic IL-1 inhibition (Table 4).92–96 Numerous other IL-1β–, IL-1α–, or IL-1R1–specific biologic drug candidates and one oral caspase-1 inhibitor are currently in clinical trials.7 In 2013, canakinumab was approved in Europe for preventing remittent gouty arthritis based on its capacity to suppress painful inflammation in acute gout attacks faster than intramuscular steroid injection.97 Unlike most other drugs used in gout, canakinumab can also be used in patients with CKD.98 Interestingly, renal function seems to improve when gout is treated with IL-1 inhibition.99 Also, safety and pharmacokinetics of rilonacept are not affected by CKD or even ESRD.100 Now, this finally allows for the testing of the old theory that IL-1 induction seen in hemodialysis patients contributes to dialysis-related systemic inflammation and complications.101 A first interventional study showed that anakinra can significantly improve C-reactive protein, IL-6, and serum albumin levels in patients on hemodialysis.102 These data create hopes that blocking IL-1–driven systemic inflammation could improve nutritional status and body wasting, and eventually dampen accelerated cardiovascular disease in ESRD.103 A trial with monoclonal anti–IL-1β IgG gevokizumab in diabetic kidney disease is ongoing.104 Clinical trials testing whether IL-1 blockade can also improve outcomes in diseases such as GN, vasculitis, or AKI are still awaited.
Table 4.
Clinical reports and ongoing trials of IL-1 blockade in patients with presumed or manifest kidney disease
| Drug | Disease | Trial Phase | NCT Identifier | Reference |
|---|---|---|---|---|
| Canakinumab | CAPS-related (renal) amyloidosis | Case reports | 93 | |
| Gout in CKD | 3 | NCT01029652 | 97 | |
| NCT01080131 | ||||
| Proliferative diabetic retinopathy | 1 | NCT01589029 | ||
| Anakinra | CAPS-related (renal) amyloidosis | Case reports | 93 | |
| Gout in CKD | 2–3 | NCT02578394 | ||
| Inflammation in CKD | 2 | NCT00420290 | 102 | |
| Nutrition, insulin resistance, and inflammation in CKD | 2 | NCT02278562 | ||
| Resistance and inflammation in CKD | 2 | NCT02278562 | ||
| Henoch–Schönlein purpura | Case report | 127 | ||
| Rilonacept | Vascular dysfunction in CKD | 2a | NCT01663103 | |
| Inflammation in CKD | 2a | NCT00897715 | ||
| Gevokizumab | Type 2 diabetic kidney disease | 2a | EudraCT2013–003610–41 | 104 |
NCT, ClinicalTrials.gov identifier; EudraCT, European Union Clinical Trials register.
Perspectives for Targeting the Inflammasome-IL-1 Axis in Kidney Disease
Which types of human kidney disease would be eligible for IL-1 blockade? In analogy to its clinical effectiveness in gout and the role of the NLRP3-IL-1 axis in crystal- and microparticle-related tissue inflammation, IL-1 blockade may also be instrumental in crystalline nephropathies. Primary hyperoxaluria, cystinosis, light chain–related Fanconi syndrome, fibrillary GN, or crystalloglobulinemia are all as rare as CAPS but secondary oxalosis-related AKI is more common.105 Timing is probably an issue, because the diagnosis of AKI, based on its current definition, is not recognized before the kidney is largely destroyed. Therefore, IL-1 blockade might be more feasible in chronic disorders for which some experimental evidence exists such as primary hyperoxaluria or diabetes.61,106,107 The ongoing Canakinumab Anti-Inflammatory Thrombosis Outcomes Study aims to evaluate whether IL-1β inhibition can reduce rates of recurrent myocardial infarction, stroke, and cardiovascular death among high risk patients with persistent elevations of CRP.108
Several small molecule–based NLRP3 antagonists have been validated in preclinical studies. Arglabin, a sesquiterpene lactone from the Chinese herb Artemisia myriantha, can specifically inhibit cholesterol crystal–induced IL-1β, but not IL-6 and IL-12, production in vitro and markedly reduce vascular wall inflammation and atherosclerosis in ApoE2.Ki mice.109 Coll et al. described MCC950 as a selective small molecule inhibitor of the NLRP3 (but not the AIM2, NLRC4, or NLRP1) inflammasome, which can attenuate experimental multiple sclerosis and a mouse model of CAPS.110 The ketone body and short-chain fatty acid β-hydroxybutyrate is another compound that can block NLRP3-IL-1–mediated inflammatory disease.111 β-hydroxybutyrate can be administered in a low carbohydrate ketogenic diet and can block murine CAPS or uric acid crystal–induced peritoneal inflammation. Now it is necessary also to test these compounds in animal models of kidney disease.
Summary and Perspective
Inflammasomes turn a broad variety of danger signals into the release of inflammatory cytokines that rapidly set off inflammation for host defense. Upon activation, the inflammasome assembles to a single ASC speck macromolecular complex per cell that can induce canonical caspase-1 or noncanonical caspase-11/5 activation. The subsequent release of cytokines and immunogenic cell death promotes local and systemic inflammation. Inside the kidney mainly myeloid cells express the NLRP3 inflammasome and secrete IL-1 upon activation but renal parenchymal cells may contribute in certain settings. In many cases, IL-1α release is probably more relevant inside the kidney, whereas IL-1β elicits systemic inflammation. In addition, NLRP3 and ASC have biologic effects unrelated to IL-1 release, e.g., they promote STAT phosphorylation downstream of the TGF-βR, which implies anti-inflammatory and profibrotic effects. As IL-1–dependent inflammation is involved in the pathogenesis of numerous acute and chronic kidney diseases, the inflammasome-IL-1 axis is an evolving therapeutic target. Nephrologists can already use IL-1 antagonists for patients with CAPS-related amyloidosis and relapsing gout. More clinical trials are ongoing that hold the perspective for broader clinical applications, e.g., in diabetes, cardiovascular disease, or CKD-related systemic inflammation. Whether the inflammasome-IL-1 axis holds further promises to better control intrarenal inflammation in immune-mediated kidney diseases remains to be explored.
Disclosures
None.
Acknowledgments
Support from the Deutsche Forschungs-gemeinschaft (AN372/9-2, 14-3, 16-1, 17-1, 20-1, 23-1) is gratefully acknowledged.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Anders HJ, Muruve DA: The inflammasomes in kidney disease. J Am Soc Nephrol 22: 1007–1018, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Auron PE, Webb AC, Rosenwasser LJ, Mucci SF, Rich A, Wolff SM, Dinarello CA: Nucleotide sequence of human monocyte interleukin 1 precursor cDNA. Proc Natl Acad Sci U S A 81: 7907–7911, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lomedico PT, Gubler U, Hellmann CP, Dukovich M, Giri JG, Pan YC, Collier K, Semionow R, Chua AO, Mizel SB: Cloning and expression of murine interleukin-1 cDNA in Escherichia coli. Nature 312: 458–462, 1984 [DOI] [PubMed] [Google Scholar]
- 4.Garlanda C, Dinarello CA, Mantovani A: The interleukin-1 family: back to the future. Immunity 39: 1003–1018, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Netea MG, van de Veerdonk FL, van der Meer JW, Dinarello CA, Joosten LA: Inflammasome-independent regulation of IL-1-family cytokines. Annu Rev Immunol 33: 49–77, 2015 [DOI] [PubMed] [Google Scholar]
- 6.Kim B, Lee Y, Kim E, Kwak A, Ryoo S, Bae SH, Azam T, Kim S, Dinarello CA: The Interleukin-1α Precursor is Biologically Active and is Likely a Key Alarmin in the IL-1 Family of Cytokines. Front Immunol 4: 391, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dinarello CA, Simon A, van der Meer JW: Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov 11: 633–652, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan JK, Roth J, Oppenheim JJ, Tracey KJ, Vogl T, Feldmann M, Horwood N, Nanchahal J: Alarmins: awaiting a clinical response. J Clin Invest 122: 2711–2719, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dinarello CA: The role of the interleukin-1-receptor antagonist in blocking inflammation mediated by interleukin-1. N Engl J Med 343: 732–734, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Gross O, Yazdi AS, Thomas CJ, Masin M, Heinz LX, Guarda G, Quadroni M, Drexler SK, Tschopp J: Inflammasome activators induce interleukin-1α secretion via distinct pathways with differential requirement for the protease function of caspase-1. Immunity 36: 388–400, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Carruth LM, Demczuk S, Mizel SB: Involvement of a calpain-like protease in the processing of the murine interleukin 1 alpha precursor. J Biol Chem 266: 12162–12167, 1991 [PubMed] [Google Scholar]
- 12.Yazdi AS, Drexler SK: Regulation of interleukin 1α secretion by inflammasomes. Ann Rheum Dis 72[Suppl 2]: ii96–ii99, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Man SM, Kanneganti TD: Converging roles of caspases in inflammasome activation, cell death and innate immunity. Nat Rev Immunol 16: 7–21, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herzog C, Haun RS, Kaushal V, Mayeux PR, Shah SV, Kaushal GP: Meprin A and meprin alpha generate biologically functional IL-1beta from pro-IL-1beta. Biochem Biophys Res Commun 379: 904–908, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strowig T, Henao-Mejia J, Elinav E, Flavell R: Inflammasomes in health and disease. Nature 481: 278–286, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J: Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol 11: 136–140, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM: Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440: 228–232, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Darisipudi MN, Thomasova D, Mulay SR, Brech D, Noessner E, Liapis H, Anders HJ: Uromodulin triggers IL-1β-dependent innate immunity via the NLRP3 inflammasome. J Am Soc Nephrol 23: 1783–1789, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Babelova A, Moreth K, Tsalastra-Greul W, Zeng-Brouwers J, Eickelberg O, Young MF, Bruckner P, Pfeilschifter J, Schaefer RM, Gröne HJ, Schaefer L: Biglycan, a danger signal that activates the NLRP3 inflammasome via toll-like and P2X receptors. J Biol Chem 284: 24035–24048, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allam R, Darisipudi MN, Tschopp J, Anders HJ: Histones trigger sterile inflammation by activating the NLRP3 inflammasome. Eur J Immunol 43: 3336–3342, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Mulay SR, Kulkarni OP, Rupanagudi KV, Migliorini A, Darisipudi MN, Vilaysane A, Muruve D, Shi Y, Munro F, Liapis H, Anders HJ: Calcium oxalate crystals induce renal inflammation by NLRP3-mediated IL-1β secretion. J Clin Invest 123: 236–246, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J: Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440: 237–241, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E: Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol 9: 847–856, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kayagaki N, Stowe IB, Lee BL, O’Rourke K, Anderson K, Warming S, Cuellar T, Haley B, Roose-Girma M, Phung QT, Liu PS, Lill JR, Li H, Wu J, Kummerfeld S, Zhang J, Lee WP, Snipas SJ, Salvesen GS, Morris LX, Fitzgerald L, Zhang Y, Bertram EM, Goodnow CC, Dixit VM: Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526: 666–671, 2015 [DOI] [PubMed] [Google Scholar]
- 25.Kayagaki N, Wong MT, Stowe IB, Ramani SR, Gonzalez LC, Akashi-Takamura S, Miyake K, Zhang J, Lee WP, Muszyński A, Forsberg LS, Carlson RW, Dixit VM: Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science 341: 1246–1249, 2013 [DOI] [PubMed] [Google Scholar]
- 26.Shi J, Zhao Y, Wang Y, Gao W, Ding J, Li P, Hu L, Shao F: Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 514: 187–192, 2014 [DOI] [PubMed] [Google Scholar]
- 27.Viganò E, Diamond CE, Spreafico R, Balachander A, Sobota RM, Mortellaro A: Human caspase-4 and caspase-5 regulate the one-step non-canonical inflammasome activation in monocytes. Nat Commun 6: 8761, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, Shao F: Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526: 660–665, 2015 [DOI] [PubMed] [Google Scholar]
- 29.Schreiber A, Pham CT, Hu Y, Schneider W, Luft FC, Kettritz R: Neutrophil serine proteases promote IL-1β generation and injury in necrotizing crescentic glomerulonephritis. J Am Soc Nephrol 23: 470–482, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Man SM, Kanneganti TD: Regulation of inflammasome activation. Immunol Rev 265: 6–21, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schreiber A, Luft FC, Kettritz R: Phagocyte NADPH oxidase restrains the inflammasome in ANCA-induced GN. J Am Soc Nephrol 26: 411–424, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vande Walle L, Van Opdenbosch N, Jacques P, Fossoul A, Verheugen E, Vogel P, Beyaert R, Elewaut D, Kanneganti TD, van Loo G, Lamkanfi M: Negative regulation of the NLRP3 inflammasome by A20 protects against arthritis. Nature 512: 69–73, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Almeida L, Khare S, Misharin AV, Patel R, Ratsimandresy RA, Wallin MC, Perlman H, Greaves DR, Hoffman HM, Dorfleutner A, Stehlik C: The PYRIN Domain-only Protein POP1 Inhibits Inflammasome Assembly and Ameliorates Inflammatory Disease. Immunity 43: 264–276, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liao Z, Grimshaw RS, Rosenstreich DL: Identification of a specific interleukin 1 inhibitor in the urine of febrile patients. J Exp Med 159: 126–136, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rauschmayr T, Groves RW, Kupper TS: Keratinocyte expression of the type 2 interleukin 1 receptor mediates local and specific inhibition of interleukin 1-mediated inflammation. Proc Natl Acad Sci U S A 94: 5814–5819, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Novick D, Kim S, Kaplanski G, Dinarello CA: Interleukin-18, more than a Th1 cytokine. Semin Immunol 25: 439–448, 2013 [DOI] [PubMed] [Google Scholar]
- 37.Stokman G, Kers J, Yapici Ü, Hoelbeek JJ, Claessen N, de Boer OJ, Netea MG, Hilbrands L, Bemelman FJ, Ten Berge IJ, Florquin S: Predominant Tubular Interleukin-18 Expression in Polyomavirus-Associated Nephropathy [published online ahead of print February 9, 2016]. Transplantation doi:10.1097/TP.0000000000001086 [DOI] [PubMed] [Google Scholar]
- 38.Wyburn K, Wu H, Chen G, Yin J, Eris J, Chadban S: Interleukin-18 affects local cytokine expression but does not impact on the development of kidney allograft rejection. Am J Transplant 6: 2612–2621, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Nozaki Y, Kinoshita K, Yano T, Asato K, Shiga T, Hino S, Niki K, Nagare Y, Kishimoto K, Shimazu H, Funauchi M, Matsumura I: Signaling through the interleukin-18 receptor α attenuates inflammation in cisplatin-induced acute kidney injury. Kidney Int 82: 892–902, 2012 [DOI] [PubMed] [Google Scholar]
- 40.Lin X, Yuan J, Zhao Y, Zha Y: Urine interleukin-18 in prediction of acute kidney injury: a systemic review and meta-analysis. J Nephrol 28: 7–16, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chow SH, Deo P, Naderer T: Macrophage cell death in microbial infections. Cell Microbiol 18: 466–474, 2016 [DOI] [PubMed] [Google Scholar]
- 42.Fernandes-Alnemri T, Wu J, Yu JW, Datta P, Miller B, Jankowski W, Rosenberg S, Zhang J, Alnemri ES: The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ 14: 1590–1604, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Doitsh G, Galloway NL, Geng X, Yang Z, Monroe KM, Zepeda O, Hunt PW, Hatano H, Sowinski S, Muñoz-Arias I, Greene WC: Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature 505: 509–514, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hara H, Tsuchiya K, Kawamura I, Fang R, Hernandez-Cuellar E, Shen Y, Mizuguchi J, Schweighoffer E, Tybulewicz V, Mitsuyama M: Phosphorylation of the adaptor ASC acts as a molecular switch that controls the formation of speck-like aggregates and inflammasome activity. Nat Immunol 14: 1247–1255, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu A, Magupalli VG, Ruan J, Yin Q, Atianand MK, Vos MR, Schröder GF, Fitzgerald KA, Wu H, Egelman EH: Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell 156: 1193–1206, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Franklin BS, Bossaller L, De Nardo D, Ratter JM, Stutz A, Engels G, Brenker C, Nordhoff M, Mirandola SR, Al-Amoudi A, Mangan MS, Zimmer S, Monks BG, Fricke M, Schmidt RE, Espevik T, Jones B, Jarnicki AG, Hansbro PM, Busto P, Marshak-Rothstein A, Hornemann S, Aguzzi A, Kastenmüller W, Latz E: The adaptor ASC has extracellular and ‘prionoid’ activities that propagate inflammation. Nat Immunol 15: 727–737, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baroja-Mazo A, Martín-Sánchez F, Gomez AI, Martínez CM, Amores-Iniesta J, Compan V, Barberà-Cremades M, Yagüe J, Ruiz-Ortiz E, Antón J, Buján S, Couillin I, Brough D, Arostegui JI, Pelegrín P: The NLRP3 inflammasome is released as a particulate danger signal that amplifies the inflammatory response. Nat Immunol 15: 738–748, 2014 [DOI] [PubMed] [Google Scholar]
- 48.Lech M, Lorenz G, Kulkarni OP, Grosser MO, Stigrot N, Darisipudi MN, Günthner R, Wintergerst MW, Anz D, Susanti HE, Anders HJ: NLRP3 and ASC suppress lupus-like autoimmunity by driving the immunosuppressive effects of TGF-β receptor signalling. Ann Rheum Dis 74: 2224–2235, 2015 [DOI] [PubMed] [Google Scholar]
- 49.Wang W, Wang X, Chun J, Vilaysane A, Clark S, French G, Bracey NA, Trpkov K, Bonni S, Duff HJ, Beck PL, Muruve DA: Inflammasome-independent NLRP3 augments TGF-β signaling in kidney epithelium. J Immunol 190: 1239–1249, 2013 [DOI] [PubMed] [Google Scholar]
- 50.Kim SM, Lee SH, Kim YG, Kim SY, Seo JW, Choi YW, Kim DJ, Jeong KH, Lee TW, Ihm CG, Won KY, Moon JY: Hyperuricemia-induced NLRP3 activation of macrophages contributes to the progression of diabetic nephropathy. Am J Physiol Renal Physiol 308: F993–F1003, 2015 [DOI] [PubMed] [Google Scholar]
- 51.Mulay SR, Evan A, Anders HJ: Molecular mechanisms of crystal-related kidney inflammation and injury. Implications for cholesterol embolism, crystalline nephropathies and kidney stone disease. Nephrol Dial Transplant 29: 507–514, 2014 [DOI] [PubMed] [Google Scholar]
- 52.Anders HJ, Schaefer L: Beyond tissue injury-damage-associated molecular patterns, toll-like receptors, and inflammasomes also drive regeneration and fibrosis. J Am Soc Nephrol 25: 1387–1400, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Prencipe G, Caiello I, Cherqui S, Whisenant T, Petrini S, Emma F, De Benedetti F: Inflammasome activation by cystine crystals: implications for the pathogenesis of cystinosis. J Am Soc Nephrol 25: 1163–1169, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Anders HJ, Lech M: NOD-like and Toll-like receptors or inflammasomes contribute to kidney disease in a canonical and a non-canonical manner. Kidney Int 84: 225–228, 2013 [DOI] [PubMed] [Google Scholar]
- 55.Lorenz G, Darisipudi MN, Anders HJ: Canonical and non-canonical effects of the NLRP3 inflammasome in kidney inflammation and fibrosis. Nephrol Dial Transplant 29: 41–48, 2014 [DOI] [PubMed] [Google Scholar]
- 56.Hacham M, Argov S, White RM, Segal S, Apte RN: Different patterns of interleukin-1alpha and interleukin-1beta expression in organs of normal young and old mice. Eur Cytokine Netw 13: 55–65, 2002 [PubMed] [Google Scholar]
- 57.Lech M, Avila-Ferrufino A, Skuginna V, Susanti HE, Anders HJ: Quantitative expression of RIG-like helicase, NOD-like receptor and inflammasome-related mRNAs in humans and mice. Int Immunol 22: 717–728, 2010 [DOI] [PubMed] [Google Scholar]
- 58.Leemans JC, Kors L, Anders HJ, Florquin S: Pattern recognition receptors and the inflammasome in kidney disease. Nat Rev Nephrol 10: 398–414, 2014 [DOI] [PubMed] [Google Scholar]
- 59.Liu D, Wen Y, Tang TT, Lv LL, Tang RN, Liu H, Ma KL, Crowley SD, Liu BC: Megalin/Cubulin-Lysosome-mediated Albumin Reabsorption Is Involved in the Tubular Cell Activation of NLRP3 Inflammasome and Tubulointerstitial Inflammation. J Biol Chem 290: 18018–18028, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shahzad K, Bock F, Al-Dabet MM, Gadi I, Kohli S, Nazir S, Ghosh S, Ranjan S, Wang H, Madhusudhan T, Nawroth PP, Isermann B: Caspase-1, but Not Caspase-3, Promotes Diabetic Nephropathy [published online ahead of print February 1, 2016]. J Am Soc Nephrol doi:10.1681/ASN.2015060676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shahzad K, Bock F, Dong W, Wang H, Kopf S, Kohli S, Al-Dabet MM, Ranjan S, Wolter J, Wacker C, Biemann R, Stoyanov S, Reymann K, Söderkvist P, Groß O, Schwenger V, Pahernik S, Nawroth PP, Gröne HJ, Madhusudhan T, Isermann B: Nlrp3-inflammasome activation in non-myeloid-derived cells aggravates diabetic nephropathy. Kidney Int 87: 74–84, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vilaysane A, Chun J, Seamone ME, Wang W, Chin R, Hirota S, Li Y, Clark SA, Tschopp J, Trpkov K, Hemmelgarn BR, Beck PL, Muruve DA: The NLRP3 inflammasome promotes renal inflammation and contributes to CKD. J Am Soc Nephrol 21: 1732–1744, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lichtnekert J, Kulkarni OP, Mulay SR, Rupanagudi KV, Ryu M, Allam R, Vielhauer V, Muruve D, Lindenmeyer MT, Cohen CD, Anders HJ: Anti-GBM glomerulonephritis involves IL-1 but is independent of NLRP3/ASC inflammasome-mediated activation of caspase-1. PLoS One 6: e26778, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ma CH, Kang LL, Ren HM, Zhang DM, Kong LD: Simiao pill ameliorates renal glomerular injury via increasing Sirt1 expression and suppressing NF-κB/NLRP3 inflammasome activation in high fructose-fed rats. J Ethnopharmacol 172: 108–117, 2015 [DOI] [PubMed] [Google Scholar]
- 65.Lonnemann G, Engler-Blum G, Müller GA, Koch KM, Dinarello CA: Cytokines in human renal interstitial fibrosis. II. Intrinsic interleukin (IL)-1 synthesis and IL-1-dependent production of IL-6 and IL-8 by cultured kidney fibroblasts. Kidney Int 47: 845–854, 1995 [DOI] [PubMed] [Google Scholar]
- 66.Abais JM, Xia M, Li G, Chen Y, Conley SM, Gehr TW, Boini KM, Li PL: Nod-like receptor protein 3 (NLRP3) inflammasome activation and podocyte injury via thioredoxin-interacting protein (TXNIP) during hyperhomocysteinemia. J Biol Chem 289: 27159–27168, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Abais JM, Xia M, Li G, Gehr TW, Boini KM, Li PL: Contribution of endogenously produced reactive oxygen species to the activation of podocyte NLRP3 inflammasomes in hyperhomocysteinemia. Free Radic Biol Med 67: 211–220, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Komada T, Usui F, Shirasuna K, Kawashima A, Kimura H, Karasawa T, Nishimura S, Sagara J, Noda T, Taniguchi S, Muto S, Nagata D, Kusano E, Takahashi M: ASC in renal collecting duct epithelial cells contributes to inflammation and injury after unilateral ureteral obstruction. Am J Pathol 184: 1287–1298, 2014 [DOI] [PubMed] [Google Scholar]
- 69.Stutz A, Horvath GL, Monks BG, Latz E: ASC speck formation as a readout for inflammasome activation. Methods Mol Biol 1040: 91–101, 2013 [DOI] [PubMed] [Google Scholar]
- 70.Yang JR, Yao FH, Zhang JG, Ji ZY, Li KL, Zhan J, Tong YN, Lin LR, He YN: Ischemia-reperfusion induces renal tubule pyroptosis via the CHOP-caspase-11 pathway. Am J Physiol Renal Physiol 306: F75–F84, 2014 [DOI] [PubMed] [Google Scholar]
- 71.Linkermann A, Bräsen JH, Himmerkus N, Liu S, Huber TB, Kunzendorf U, Krautwald S: Rip1 (receptor-interacting protein kinase 1) mediates necroptosis and contributes to renal ischemia/reperfusion injury. Kidney Int 81: 751–761, 2012 [DOI] [PubMed] [Google Scholar]
- 72.Krautwald S, Linkermann A: The fire within: pyroptosis in the kidney. Am J Physiol Renal Physiol 306: F168–F169, 2014 [DOI] [PubMed] [Google Scholar]
- 73.Deckers JG, Van Der Woude FJ, Van Der Kooij SW, Daha MR: Synergistic effect of IL-1alpha, IFN-gamma, and TNF-alpha on RANTES production by human renal tubular epithelial cells in vitro. J Am Soc Nephrol 9: 194–202, 1998 [DOI] [PubMed] [Google Scholar]
- 74.Mulay SR, Linkermann A, Anders HJ: Necroinflammation in Kidney Disease. J Am Soc Nephrol 27: 27–39, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Komada T, Usui F, Kawashima A, Kimura H, Karasawa T, Inoue Y, Kobayashi M, Mizushina Y, Kasahara T, Taniguchi S, Muto S, Nagata D, Takahashi M: Role of NLRP3 Inflammasomes for Rhabdomyolysis-induced Acute Kidney Injury. Sci Rep 5: 10901, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shigeoka AA, Mueller JL, Kambo A, Mathison JC, King AJ, Hall WF, Correia JS, Ulevitch RJ, Hoffman HM, McKay DB: An inflammasome-independent role for epithelial-expressed Nlrp3 in renal ischemia-reperfusion injury. J Immunol 185: 6277–6285, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Iyer SS, Pulskens WP, Sadler JJ, Butter LM, Teske GJ, Ulland TK, Eisenbarth SC, Florquin S, Flavell RA, Leemans JC, Sutterwala FS: Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc Natl Acad Sci U S A 106: 20388–20393, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bakker PJ, Butter LM, Claessen N, Teske GJ, Sutterwala FS, Florquin S, Leemans JC: A tissue-specific role for Nlrp3 in tubular epithelial repair after renal ischemia/reperfusion. Am J Pathol 184: 2013–2022, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim HJ, Lee DW, Ravichandran K, O Keys D, Akcay A, Nguyen Q, He Z, Jani A, Ljubanovic D, Edelstein CL: NLRP3 inflammasome knockout mice are protected against ischemic but not cisplatin-induced acute kidney injury. J Pharmacol Exp Ther 346: 465–472, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee JW, Nam WJ, Han MJ, Shin JH, Kim JG, Kim SH, Kim HR, Oh DJ: Role of IL-1α in cisplatin-induced acute renal failure in mice. Korean J Intern Med 26: 187–194, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rusai K, Huang H, Sayed N, Strobl M, Roos M, Schmaderer C, Heemann U, Lutz J: Administration of interleukin-1 receptor antagonist ameliorates renal ischemia-reperfusion injury. Transpl Int 21: 572–580, 2008 [DOI] [PubMed] [Google Scholar]
- 82.Faubel S, Lewis EC, Reznikov L, Ljubanovic D, Hoke TS, Somerset H, Oh DJ, Lu L, Klein CL, Dinarello CA, Edelstein CL: Cisplatin-induced acute renal failure is associated with an increase in the cytokines interleukin (IL)-1beta, IL-18, IL-6, and neutrophil infiltration in the kidney. J Pharmacol Exp Ther 322: 8–15, 2007 [DOI] [PubMed] [Google Scholar]
- 83.Haq M, Norman J, Saba SR, Ramirez G, Rabb H: Role of IL-1 in renal ischemic reperfusion injury. J Am Soc Nephrol 9: 614–619, 1998 [DOI] [PubMed] [Google Scholar]
- 84.Timoshanko JR, Kitching AR, Iwakura Y, Holdsworth SR, Tipping PG: Contributions of IL-1beta and IL-1alpha to crescentic glomerulonephritis in mice. J Am Soc Nephrol 15: 910–918, 2004 [DOI] [PubMed] [Google Scholar]
- 85.Timoshanko JR, Kitching AR, Iwakura Y, Holdsworth SR, Tipping PG: Leukocyte-derived interleukin-1beta interacts with renal interleukin-1 receptor I to promote renal tumor necrosis factor and glomerular injury in murine crescentic glomerulonephritis. Am J Pathol 164: 1967–1977, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Andersen K, Eltrich N, Lichtnekert J, Anders HJ, Vielhauer V: The NLRP3/ASC inflammasome promotes T-cell-dependent immune complex glomerulonephritis by canonical and noncanonical mechanisms. Kidney Int 86: 965–978, 2014 [DOI] [PubMed] [Google Scholar]
- 87.Yamagishi H, Yokoo T, Imasawa T, Mitarai T, Kawamura T, Utsunomiya Y: Genetically modified bone marrow-derived vehicle cells site specifically deliver an anti-inflammatory cytokine to inflamed interstitium of obstructive nephropathy. J Immunol 166: 609–616, 2001 [DOI] [PubMed] [Google Scholar]
- 88.Kumar SV, Anders HJ: Glomerular disease: limiting autoimmune tissue injury: ROS and the inflammasome. Nat Rev Nephrol 10: 545–546, 2014 [DOI] [PubMed] [Google Scholar]
- 89.de Jesus AA, Canna SW, Liu Y, Goldbach-Mansky R: Molecular mechanisms in genetically defined autoinflammatory diseases: disorders of amplified danger signaling. Annu Rev Immunol 33: 823–874, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Manchanda PK, Kumar A, Bid HK, Mittal RD: Interleukin-1beta and receptor antagonist (IL-1Ra) gene polymorphisms and the prediction of the risk of end-stage renal disease. Biomarkers 11: 164–173, 2006 [DOI] [PubMed] [Google Scholar]
- 91.Wetmore JB, Hung AM, Lovett DH, Sen S, Quershy O, Johansen KL: Interleukin-1 gene cluster polymorphisms predict risk of ESRD. Kidney Int 68: 278–284, 2005 [DOI] [PubMed] [Google Scholar]
- 92.Moser C, Pohl G, Haslinger I, Knapp S, Rowczenio D, Russel T, Lachmann HJ, Lang U, Kovarik J: Successful treatment of familial Mediterranean fever with Anakinra and outcome after renal transplantation. Nephrol Dial Transplant 24: 676–678, 2009 [DOI] [PubMed] [Google Scholar]
- 93.Ozcakar ZB, Ozdel S, Yilmaz S, Kurt-Sukur ED, Ekim M, Yalcinkaya F: Anti-IL-1 treatment in familial Mediterranean fever and related amyloidosis. Clin Rheumatol 35: 441–446, 2016 [DOI] [PubMed] [Google Scholar]
- 94.Pagel G: [Do children need fairytales?]. Krankenpfl J 26: 35–38, 1988 [PubMed] [Google Scholar]
- 95.Stankovic Stojanovic K, Delmas Y, Torres PU, Peltier J, Pelle G, Jéru I, Colombat M, Grateau G: Dramatic beneficial effect of interleukin-1 inhibitor treatment in patients with familial Mediterranean fever complicated with amyloidosis and renal failure. Nephrol Dial Transplant 27: 1898–1901, 2012 [DOI] [PubMed] [Google Scholar]
- 96.Thornton BD, Hoffman HM, Bhat A, Don BR: Successful treatment of renal amyloidosis due to familial cold autoinflammatory syndrome using an interleukin 1 receptor antagonist. Am J Kidney Dis 49: 477–481, 2007 [DOI] [PubMed] [Google Scholar]
- 97.Schlesinger N, Alten RE, Bardin T, Schumacher HR, Bloch M, Gimona A, Krammer G, Murphy V, Richard D, So AK: Canakinumab for acute gouty arthritis in patients with limited treatment options: results from two randomised, multicentre, active-controlled, double-blind trials and their initial extensions. Ann Rheum Dis 71: 1839–1848, 2012 [DOI] [PubMed] [Google Scholar]
- 98.Singh JA: Emerging therapies for gout. Expert Opin Emerg Drugs 17: 511–518, 2012 [DOI] [PubMed] [Google Scholar]
- 99.Balasubramaniam G, Almond M, Dasgupta B: Improved renal function in diabetic patients with acute gout treated with anakinra. Kidney Int 88: 195–196, 2015 [DOI] [PubMed] [Google Scholar]
- 100.Radin A, Marbury T, Osgood G, Belomestnov P: Safety and pharmacokinetics of subcutaneously administered rilonacept in patients with well-controlled end-stage renal disease (ESRD). J Clin Pharmacol 50: 835–841, 2010 [DOI] [PubMed] [Google Scholar]
- 101.Dinarello CA, Koch KM, Shaldon S: Interleukin-1 and its relevance in patients treated with hemodialysis. Kidney Int Suppl 24: S21–S26, 1988 [PubMed] [Google Scholar]
- 102.Hung AM, Ellis CD, Shintani A, Booker C, Ikizler TA: IL-1β receptor antagonist reduces inflammation in hemodialysis patients. J Am Soc Nephrol 22: 437–442, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Machowska A, Carrero JJ, Lindholm B, Stenvinkel P: Therapeutics targeting persistent inflammation in chronic kidney disease. Transl Res 167: 204–213, 2016 [DOI] [PubMed] [Google Scholar]
- 104.Perez-Gomez MV, Sanchez-Niño MD, Sanz AB, Martín-Cleary C, Ruiz-Ortega M, Egido J, Navarro-González JF, Ortiz A, Fernandez-Fernandez B: Horizon 2020 in Diabetic Kidney Disease: The Clinical Trial Pipeline for Add-On Therapies on Top of Renin Angiotensin System Blockade. J Clin Med 4: 1325–1347, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bhasin B, Ürekli HM, Atta MG: Primary and secondary hyperoxaluria: Understanding the enigma. World J Nephrol 4: 235–244, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bakker PJ, Butter LM, Kors L, Teske GJ, Aten J, Sutterwala FS, Florquin S, Leemans JC: Nlrp3 is a key modulator of diet-induced nephropathy and renal cholesterol accumulation. Kidney Int 85: 1112–1122, 2014 [DOI] [PubMed] [Google Scholar]
- 107.Scarpioni R, Rigante D, Cantarini L, Ricardi M, Albertazzi V, Melfa L, Lazzaro A. Renal involvement in secondary amyloidosis of Muckle-Wells syndrome: marked improvement of renal function and reduction of proteinuria after therapy with human anti-interleukin-1β monoclonal antibody canakinumab. Clin Rheumatol 34: 1311–1316, 2015 [DOI] [PubMed]
- 108.Ridker PM, Thuren T, Zalewski A, Libby P: Interleukin-1β inhibition and the prevention of recurrent cardiovascular events: rationale and design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS). Am Heart J 162: 597–605, 2011 [DOI] [PubMed] [Google Scholar]
- 109.Abderrazak A, Couchie D, Mahmood DF, Elhage R, Vindis C, Laffargue M, Matéo V, Büchele B, Ayala MR, El Gaafary M, Syrovets T, Slimane MN, Friguet B, Fulop T, Simmet T, El Hadri K, Rouis M: Anti-inflammatory and antiatherogenic effects of the NLRP3 inflammasome inhibitor arglabin in ApoE2.Ki mice fed a high-fat diet. Circulation 131: 1061–1070, 2015 [DOI] [PubMed] [Google Scholar]
- 110.Coll RC, Robertson AA, Chae JJ, Higgins SC, Muñoz-Planillo R, Inserra MC, Vetter I, Dungan LS, Monks BG, Stutz A, Croker DE, Butler MS, Haneklaus M, Sutton CE, Núñez G, Latz E, Kastner DL, Mills KH, Masters SL, Schroder K, Cooper MA, O’Neill LA: A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med 21: 248–255, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Youm YH, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D, D’Agostino D, Planavsky N, Lupfer C, Kanneganti TD, Kang S, Horvath TL, Fahmy TM, Crawford PA, Biragyn A, Alnemri E, Dixit VD: The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med 21: 263–269, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Martin-Rodriguez S, Caballo C, Gutierrez G, Vera M, Cruzado JM, Cases A, Escolar G, Diaz-Ricart M: TLR4 and NALP3 inflammasome in the development of endothelial dysfunction in uraemia. Eur J Clin Invest 45: 160–169, 2015 [DOI] [PubMed] [Google Scholar]
- 113.Tomita N, Morishita R, Tomita S, Kaneda Y, Higaki J, Ogihara T, Horiuchi M: Inhibition of TNF-alpha, induced cytokine and adhesion molecule. Expression in glomerular cells in vitro and in vivo by transcription factor decoy for NFkappaB. Exp Nephrol 9: 181–190, 2001 [DOI] [PubMed] [Google Scholar]
- 114.Xiao J, Fu C, Zhang X, Zhu D, Chen W, Lu Y, Ye Z: Soluble monosodium urate, but not its crystal, induces toll like receptor 4-dependent immune activation in renal mesangial cells. Mol Immunol 66: 310–318, 2015 [DOI] [PubMed] [Google Scholar]
- 115.Solini A, Menini S, Rossi C, Ricci C, Santini E, Blasetti Fantauzzi C, Iacobini C, Pugliese G: The purinergic 2X7 receptor participates in renal inflammation and injury induced by high-fat diet: possible role of NLRP3 inflammasome activation. J Pathol 231: 342–353, 2013 [DOI] [PubMed] [Google Scholar]
- 116.Chung SD, Lai TY, Chien CT, Yu HJ: Activating Nrf-2 signaling depresses unilateral ureteral obstruction-evoked mitochondrial stress-related autophagy, apoptosis and pyroptosis in kidney. PLoS One 7: e47299, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kasimsetty SG, DeWolf SE, Shigeoka AA, McKay DB: Regulation of TLR2 and NLRP3 in primary murine renal tubular epithelial cells. Nephron Clin Pract 127: 119–123, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lee DW, Faubel S, Edelstein CL: A pan caspase inhibitor decreases caspase-1, IL-1α and IL-1β, and protects against necrosis of cisplatin-treated freshly isolated proximal tubules. Ren Fail 37: 144–150, 2015 [DOI] [PubMed] [Google Scholar]
- 119.Liu D, Xu M, Ding LH, Lv LL, Liu H, Ma KL, Zhang AH, Crowley SD, Liu BC: Activation of the Nlrp3 inflammasome by mitochondrial reactive oxygen species: a novel mechanism of albumin-induced tubulointerstitial inflammation. Int J Biochem Cell Biol 57: 7–19, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang W, Faubel S, Ljubanovic D, Mitra A, Falk SA, Kim J, Tao Y, Soloviev A, Reznikov LL, Dinarello CA, Schrier RW, Edelstein CL: Endotoxemic acute renal failure is attenuated in caspase-1-deficient mice. Am J Physiol Renal Physiol 288: F997–F1004, 2005 [DOI] [PubMed] [Google Scholar]
- 121.Melnikov VY, Faubel S, Siegmund B, Lucia MS, Ljubanovic D, Edelstein CL: Neutrophil-independent mechanisms of caspase-1- and IL-18-mediated ischemic acute tubular necrosis in mice. J Clin Invest 110: 1083–1091, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Furuichi K, Wada T, Iwata Y, Kokubo S, Hara A, Yamahana J, Sugaya T, Iwakura Y, Matsushima K, Asano M, Yokoyama H, Kaneko S: Interleukin-1-dependent sequential chemokine expression and inflammatory cell infiltration in ischemia-reperfusion injury. Crit Care Med 34: 2447–2455, 2006 [DOI] [PubMed] [Google Scholar]
- 123.Faubel S, Ljubanovic D, Reznikov L, Somerset H, Dinarello CA, Edelstein CL: Caspase-1-deficient mice are protected against cisplatin-induced apoptosis and acute tubular necrosis. Kidney Int 66: 2202–2213, 2004 [DOI] [PubMed] [Google Scholar]
- 124.Xia M, Conley SM, Li G, Li PL, Boini KM: Inhibition of hyperhomocysteinemia-induced inflammasome activation and glomerular sclerosis by NLRP3 gene deletion. Cell Physiol Biochem 34: 829–841, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Krishnan SM, Dowling JK, Ling YH, Diep H, Chan CT, Ferens D, Kett MM, Pinar A, Samuel CS, Vinh A, Arumugam TV, Hewitson TD, Kemp-Harper BK, Robertson AA, Cooper MA, Latz E, Mansell A, Sobey CG, Drummond GR: Inflammasome activity is essential for one kidney/deoxycorticosterone acetate/salt-induced hypertension in mice. Br J Pharmacol 173: 752–765, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pulskens WP, Butter LM, Teske GJ, Claessen N, Dessing MC, Flavell RA, Sutterwala FS, Florquin S, Leemans JC: Nlrp3 prevents early renal interstitial edema and vascular permeability in unilateral ureteral obstruction. PLoS One 9: e85775, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Boyer EM, Turman M, O’Neil KM: Partial response to anakinra in life-threatening Henoch-Schönlein purpura: case report. Pediatr Rheumatol Online J 9: 21, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]