The demand for building blocks in cancer cells differs greatly from the ones of a normal cell. In order for a cell to divide, it must duplicate its protein content, a process that requires large energy and amino acid resources. Cancer cells achieve this by rewiring their metabolic networks to cope with higher demands of energy and building blocks. The metabolic changes a tumor undergoes to adapt to deregulated growth might represent vulnerabilities that can be exploited for therapy. For example, certain types of tumors are auxotrophic for a particular amino acid, suggesting tumor starvation as a possible interesting approach for therapy. This was successfully demonstrated for the amino acid asparagine, resulting in a very effective combined treatment of chemotherapy and L-asparaginase in acute lymphoblastic leukemia (ALL).1 In addition, arginine starvation by using ADI-PEG20 (pegylated arginine deimidase) has shown promising results in tumors negative for ASS1 and currently it's been tested in clinical trials.2
To exploit amino acid vulnerabilities for cancer therapy, one must first identify which amino acid is the most restrictive to the tumor. Amino acid demand and availability depend on many genetic and environmental factors such as extracellular free amino acid supply, amino acid uptake, synthesis and degradation, protein synthesis rate, the divergent use of amino acid for energy, and tRNA levels. Some metabolomics-based approaches generate profiles of plasma free amino acids (PFAAs), providing specific patterns in cancer patients; however, the profiles represent a systemic response that may not reflect the metabolic adaptations of the tumor.3
Recently, we developed a novel approach to detect restrictive amino acids in cells and tumors. The rationale of our approach is based on differential ribosome codon reading (diricore); we make use of ribosome profiling4 to detect ribosomes stalled at specific codons. The accumulation of ribosomes at a particular codon indicates that the corresponding aminoacylated tRNA might be limiting and suggests a deficiency of the amino acid (Fig. 1).5
Figure 1.
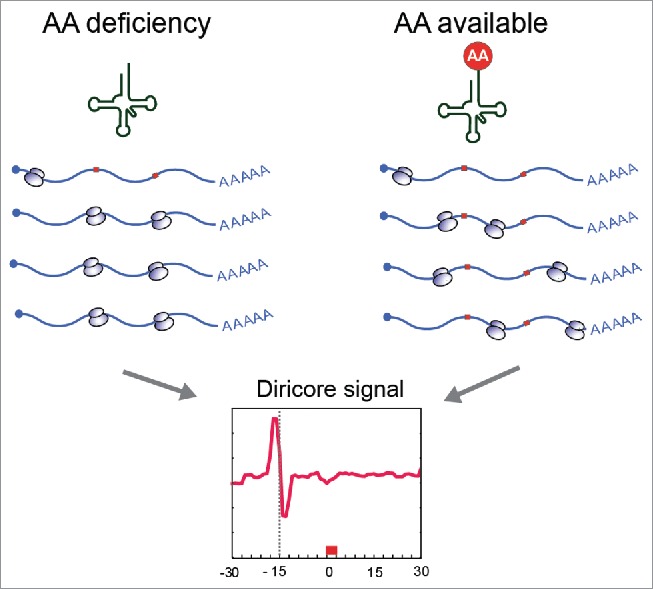
Scheme of differential ribosome codon reading (diricore). The deficiency of an amino acid result in tRNA deaminoacylation and stalling of ribosome in the corresponding codon.
We validated our approach in yeast cells treated with an inhibitor of histidine synthesis, we detected a strong signal at the 2 histidine-codons at the site A of the ribosome. Furthermore, cells treated with L-asparaginase showed specific signals at asparagine codons. Interestingly, the expression of ASNS (asparagine synthetase) markedly increased following treatment, providing evidence for a feedback loop, similar to the observation seen in patients that developed resistance to L-asparaginase.
To test whether diricore could be used to detect amino acid deficiencies in cancer, we compared samples of clear cell renal cell carcinoma (ccRCC) and normal tissue from the same patient. Some tumors showed increased signals at methionine and proline codons, and others only at methionine. For methionine, the accumulation of ribosomes occurred only at the initiating AUG codons, indicating increased translation initiation rates in the tumors as the underling mechanism. Importantly, accumulation of ribosomes in the position P of the first AUG does not necessarily mean a higher rate of initiation; a low rate of elongation might also lead to accumulation of ribosomes at this position, additional evidence like phosphorylation levels of key regulators must always be taken in account.
In contrast, the proline signal was associated with a limiting availability of proline for protein synthesis. Proline is synthesized in the cell by the mitochondrial protein PYCR (Pyrroline-5-Carboxylate Reductase). PYCR produces proline from glutamine via P5C (pyrroline-5-carboxylate), while proline degradation occurs through proline dehydrogenases/oxidases (PRODH). Of note, PYCR1 is one of the metabolic genes most commonly overexpressed in cancer. The metabolism of proline serves as a source of energy during stress, provides signaling reactive oxygen species for epigenetic reprogramming and regulates redox homeostasis. Interesting links of proline pathway to cancer are the rapid transcriptional activation of PRODH by the tumor suppressor P53, and loss-of PRODH2 in cancer.6
Tumors that showed a proline signal significantly up-regulated PYCR1 suggesting a compensatory attempt of feedback loop similar to the one observed following L-asparaginase treatment. Cells in culture that were starved of glutamine (the main precursor of proline) also upregulated PYCR1. Tumor xenografts of high-PYCR1 breast cancer cells showed proline signals when compared with their expansion ex vivo. Using CRISPR-Cas9 we knocked out PYCR1 and interrogated tumor development. PYCR1 knockout cells proliferated normally ex vivo, however their capacity to expand in vivo was severely compromised.
Like with the proline pathway, different types of tumors can rewire other amino acid pathways to generate energy and building blocks. Recently, it was shown that aspartate is essential for nucleotide synthesis in ASS1-defficient cancer cells.7 It is well established that many types of tumors are highly dependent of the amino acid glutamine. Furthermore, the expression of many essential amino acid transporters is altered in cancer. We anticipate that diricore can be used as a platform to sense these amino acid deficiencies in cells and tumors and to expose the weaknesses of tumor's metabolic remodeling.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Richards NG, et al.. Annu Rev Biochem 2006; 75:629-54; PMID:16756505; http://dx.doi.org/ 10.1146/annurev.biochem.75.103004.142520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Synakiewicz A, et al.. Expert Opin Investig Drugs 2014; 23:1517-29; PMID:24965808; http://dx.doi.org/ 10.1517/13543784.2014.934808 [DOI] [PubMed] [Google Scholar]
- [3].Mayers JR, et al.. Nat Med 2014; 20:1193-8; PMID:25261994; http://dx.doi.org/ 10.1038/nm.3686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ingolia NT, et al.. Science 2009; 324:218-23; PMID:19213877; http://dx.doi.org/ 10.1126/science.1168978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Loayza-Puch F, et al.. Nature 2016; 530:490-4; PMID:26878238; http://dx.doi.org/ 10.1038/nature16982 [DOI] [PubMed] [Google Scholar]
- [6].Phang JM, et al.. Front Oncol 2012; 2:60; PMID:22737668; http://dx.doi.org/ 10.3389/fonc.2012.00060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Rabinovich S, et al.. Nature 2015; 527:379-83; PMID:26560030; http://dx.doi.org/ 10.1038/nature15529 [DOI] [PMC free article] [PubMed] [Google Scholar]


