ABSTRACT
Purpose: Metastatic breast cancer is devastating and triple negative breast cancers (TNBC) have a higher propensity for metastasis. Improved local control upfront in this aggressive cancer could potentially decrease its propensity toward metastasis. We sought to determine if using caloric restriction (CR) as a systemic therapy, combined with radiation therapy (IR) to the primary tumor, may impact metastatic disease. Methods: An orthotopic mouse model using a highly metastatic, luciferase-tagged TNBC cell line (4T1), was used to generate palpable tumors. Mice were then treated with CR, IR, and a combination of the two. In vivo imaging was performed for metastatic evaluation. Molecular evaluation of the tumors was performed, generating a mechanistic hypothesis for CR, which was then tested with pertinent pathway inhibition in the model. Results: CR significantly increased the time to developing metastases, decreased the overall number and volume of lung metastases, and increased survival. CR decreased proliferation, increased apoptosis and globally downregulated the IGF-1R signaling pathway. Adding an IGF-1R/INSR inhibitor to local IR in vivo accomplished a decrease in metastases similar to CR plus IR, demonstrating the importance of the IGF-1R signaling pathway, and underscoring it as a possible mechanism for CR. Conclusions: CR decreased metastatic burden and therefore may complement cytotoxic therapies being used in the clinical setting for metastatic disease. Downregulation of the IGF-1R pathway, is in part responsible for this response and modulating IGF-1R directly resulted in similar improved progression-free survival. The novel use of CR has the potential to enhance clinical outcomes for patients with metastatic breast cancer.
KEYWORDS: breast cancer, Caloric restriction, metastases
Introduction
Metastatic breast cancer is a devastating diagnosis which is responsible for more than 40,000 deaths in the US annually.1 Triple negative breast cancer (TNBC), which accounts for 20% of all breast cancers, is associated with earlier time to, and high frequency of metastases.2,3 Historically, treatment of metastatic breast cancer has traditionally focused on cytotoxic systemic therapies which until recently have not shown significant improvement in overall survival. Patients with estrogen positive metastatic disease generally have the option of using anti-estrogen therapies. Recently, combination chemotherapy has been shown to improve overall survival in a subset of patients with HER2+ disease.4 Unfortunately, patients with triple-negative breast cancers (TNBC) have limited options because their tumors are defined by the lack of estrogen (ER), progesterone (PR) and HER2 receptors.5 TNBC tumors are associated with increased metastases and higher mortality rates when compared with other breast cancer subtypes and rely on signaling from other growth pathways such as the IGF-1R/Akt/PI3K pathway.6
Therefore, in the last decade, metastatic disease has been treated with novel systemic therapies via a personalized medicine approach administering treatments tailored more specifically to pathway blockade. Theoretically this idea is promising, however, clinical trials have not proven as successful as predicted. For example, Avastin which targets VEGF, has been used in combination with standard chemotherapy in the metastatic setting (RIBBON-1) and while it extended PFS slightly (8.0 months versus 9.2 months in the anthracyclin/taxane group), there was no demonstrated benefit in overall survival.7 This is likely because of redundant and multiple signaling pathways which tumors can use to survive and continue to proliferate.8,9
In this manner, a treatment which could downregulate multiple pathways without accruing toxicity from multiple single pathway inhibitors would be optimal. In several preclinical models, caloric restriction (CR), has been shown to decrease the incidence of cancer, slow tumor growth, delay the onset of metastases and downregulate multiple signaling molecules of the IGF-1R pathway including: IGF-1R, Akt and PI3K.10,11 We have previously shown the ability of CR alone and in combination with IR to decrease primary tumor growth which occurs in part through the IGF-1R pathway.12 In this study, we sought to determine whether CR alone and combined with a local therapy (ionizing radiation, IR) could have an impact on the development and progression of metastatic disease in a triple negative breast cancer mouse model.
Results
Caloric restriction coupled with radiation reduces metastatic potential and prolongs overall survival in a highly metastatic model
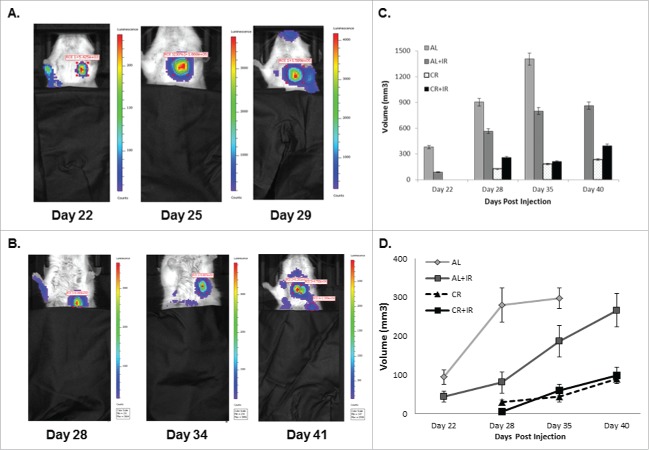
In this model, we found that the use of CR significantly decreased primary tumor growth and increased overall survival as reported in a previous study.12 We hypothesized the survival benefit seen with the use of CR was due to a decrease in metastatic burden. To test this hypothesis, cells were luciferase tagged to facilitate repetitive bioluminescent imaging of primary tumors and metastases. In addition to primary tumor growth delay seen in the current study, CR alone prolonged median overall survival of the mice by 11.3% (39.5 d vs. 35.5 d in the AL group, p = 0.0117). When radiation was added to CR (CR+IR), overall survival was increased further to 19.7% (42.5 d vs. 35.5 d in the AL group, p = 0.0047) (Table 1). Using imaging to detect metastases in the mice, we found that CR alone significantly delayed the time to visible metastases to 28 d compared to 19 d (p = 0.01) in the AL group, while CR+IR delayed the development of metastases to 29 d (Table 1).
Table 1.
Overall survival and time to metastases across treatment groups.
| Treatment Group | Median Overall Survival | Median Time to Metastases |
|---|---|---|
| Ad libitum (AL) | 35.5 days | 19 days |
| AL+IR | 37.5 days | 21.3 days |
| CR | 39.5 days | 28 days |
| CR+IR | 42.5 days | 29 days |
| IGF-1R+IR | 34.5 days | 30 days |
Note. AL: Ad libitum, IR: Ionizing radiation, CR: Caloric restriction, IGF-1R: IGF-1R inhibitor
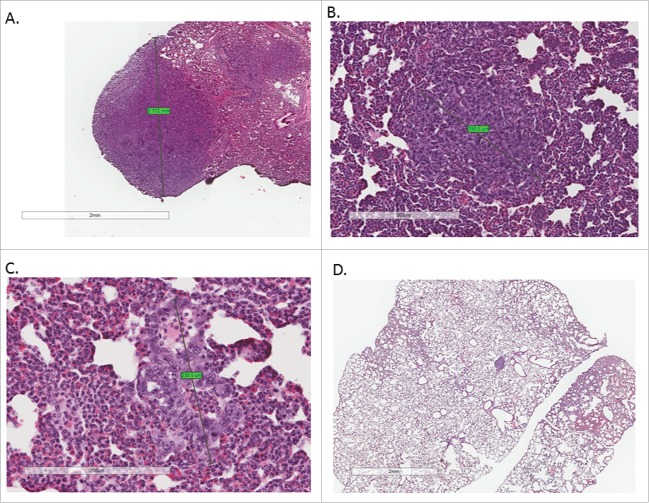
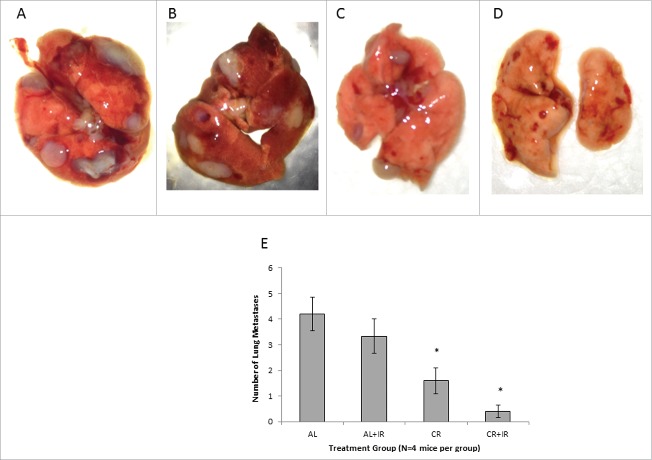
Size and number of pulmonary metastases was also significantly altered by CR and CR+IR treatments. Using imaging analysis, it was noted that mice that received CR as part of their treatment developed metastases at a later time point and the nodules that they did develop were on average smaller in size and less intense by bioluminescence when compared with AL groups (Fig. 1A and 1B). The latest imaging time point for AL mice alone was 35 d due to overwhelming tumor burden and achieving our endpoint of humane sacrifice; however, the other cohorts (AL+IR, CR and CR+IR) were imaged until day 40 post-injection. The number of visible metastases was also quantitatively evaluated histologically, which showed reduced number of nodules by radiation alone (AL+IR) from an average of 4 per tissue section in the AL group to 3.5 per tissue section in the AL+IR group, which was not statistically significant (Fig. 3A-D). CR however, reduced the total number of visible metastases to an average of 1.67 per tissue section (p = .01). Adding radiation to CR (CR+IR) further reduced the average number of visible metastases to 0.67 (p = .006) (Fig. 2E). To assess metastatic burden in a quantitative manner, the total volume of lung consumed by nodules was calculated for each mouse. At day 35 post-injection total lung volume burden was 1360 mm3 in AL fed mice, 820 mm3 in AL+IR mice, 150 mm3 in CR mice and 160 mm3 in CR+IR mice (p = 0.06 for AL+IR, p = 0.004 for CR and 0.007 for CR+IR) (Fig. 1D). Average size of metastases was measurably changed on imaging in the AL+IR, CR and CR+IR groups (Fig. 1E). The average size of lung nodules in the AL group at day 35 was 225mm3, whereas the AL+IR group was at 215 mm3 at day 40. CR alone decreased this to 99.5mm3 on day 40 and CR+IR exhibited an average size of 86.7 mm3 on day 40 (Fig. 1E). This represents a 70.5% decrease in the size of metastases in the CR+IR cohort when compared with AL control (p < 0.0001).
Figure 1.
Mice were imaged via bioluminescent imaging throughout disease progression to monitor development and spread of metastases. Mice in the ad lib (A) fed group developed visible metastases significantly faster than those in the CR fed groups (B). Imaging intensity is designed such that it is proportional to the proliferative capacity of the cells in the luminescent area. On average, metastases in the AL fed groups demonstrated increased intensity and were larger than those in the CR fed groups. The last imaging time point for AL fed mice was day 29 compared with day 41 for the CR fed group. Total volume of metastatic disease burden within the lungs was calculated for each treatment group at each respective time point (C). AL fed mice had larger total volume across time points when compared with other treatment groups, specifically at later time points as metastatic disease volume approached 1400 mm3. Average size of lung nodules on imaging was calculated for each imaging time point as well (E). AL fed mice had significantly larger lung nodules on average across imaging time points. Total volume of metastases and average nodule size were based on a representative 3 mice from each cohort, where error is defined as SEM with n = 3.
Figure 3.
Tissue sections from lungs in each of the treatment groups were H&E stained and examined for presence of visible metastases. Each tissue section was examined and the largest visible lung nodule was measured on its largest axis in each treatment group. Metastases in the AL group (A) were imaged at 2X, AL+IR and CR group at 4X (B and C) and CR+IR group at 10X (D) for data collection purposes. Nodules from the AL mice appeared larger and more frequently on histology when compared with other treatment groups. On average, mice in the CR+IR group had the smallest and least number of metastases on histology as evidenced by one micrometastasis seen on tissue section (D).
Figure 2.
Lungs from each treatment group were grossly analyzed for presence of metastases at expiration. Mice in the AL group (A) had the most metastases in number on gross inspection. Lung nodules from mice in the AL+IR (B) and CR (C) groups appeared similar on gross inspection. Lung nodules from mice in the CR+IR group appeared less frequently than in any other treatment group (D). Average number of visible metastases was calculated for each cohort of mice (E). AL mice had on average 4.25 visible metastases compared with 3.25 in the AL+IR mice, 1.67 in the CR mice and 0.67 in the CR+IR mice. * Indicates significance in comparing to AL group only and ł indicated significance in both comparison with AL and AL+IR groups (p < 0.05).
After dissection, lungs were also grossly examined for the presence of nodules. It was noted that mice in the AL fed groups (AL and AL+IR) had a greater number of metastases that appeared larger when compared with the CR fed groups (Fig. 2A-D). Lung tissue from the mice in each of the treatment groups was stained with hematoxylin and eosin for microscopic examination of metastases. The average size of metastases was also significantly decreased by on histologic examination in those mice receiving CR as part of treatment (Fig. 3A-D) with the largest of the CR nodules measuring .231 mm while the largest of the AL nodules measured 2.712 mm (p value not calculated as n = 1, this is a qualitative observation).
CR decreases proliferative potential and increases apoptosis in metastases
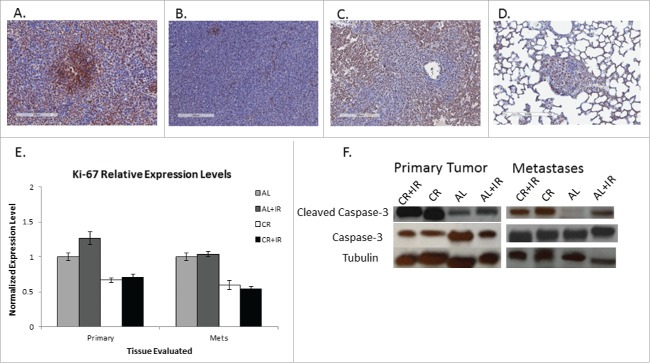
To evaluate their proliferative potential, tissue sections from both primary tumor and metastases were evaluated for Ki-67 expression. Using Aperio software to generate a Ki-67 index, data from primary tumors appeared to corroborate our previous findings. Data from the metastases demonstrated that a similar decrease in proliferation rate was noted in the lung nodules, with the nodules from CR mice demonstrated a 35.5% decrease in the cell proliferation index when compared with AL mice (CR: 34.73% +/− 3.86% vs. AL: 53.86% +/− 0.58%). Radiation (CR+IR) further decreased the cell proliferation index in metastases to 17.24% +/− 5.82% (Fig. 4A-D). Apoptosis in metastases was also evaluated by expression of cleaved caspase-3 as compared with intact caspase-3 expression. Relative expression of caspase-3 in the AL versus CR mice was 0.92 and 0.84 respectively. Relative expression of cleaved caspase-3 in the AL vs. CR metastases was 0.80 and 0.29 respectively (Fig. 4F). CR+IR also increased apoptosis in the metastases, although this increase in apoptosis was not as dramatic as CR alone.
Figure 4.
Tissue sections from both primary and metastatic tissue were examined for Ki-67 expression. Representative IHC images from metastatic tissue from each treatment group are shown from left to right: AL, AL+IR, CR, CR+IR (A-D). Each slide was imaged at 10X for presentation purposes. Tissue sections of metastases stained for Ki-67 were scored as a percentage based on staining intensity. On average AL mice exhibited a Ki-67 score of 53.8% ± 0.58, mice in the AL+IR group had Ki-67 score of 40.3% ± 0.48, mice in the CR group had Ki-67 score of 26.9% ± 3.89 and mice in the CR+IR group had Ki-67 score of 10.5% ± 0.86 (p = 0.003, p = .0.019 and p = 0.0006, respectively). Relative expression levels of Ki-67 normalized to the AL group demonstrate the decrease in proliferation across treatment groups (E). Similarly, protein gel blotting for intact caspase-3 and cleaved caspase-3 show an increase in apoptosis in CR and CR+IR groups when compared with the AL group (F). Error in panels E and F are reported as SEM. *Indicates significance in comparison with AL group.
Caloric restriction triggers a biological response in the key signaling nodes in the IGF/AKT pathway that is further enhanced with addition of ionizing radiation
Western blotting for members of the IGF-1R/Akt pathway in primary tumor tissue revealed an overall decrease in IGF-1R and PI3KCa in groups treated with CR (Fig. 5A). More notable is the decrease in expression of IGF-1R from primary tumor to metastases. IGF-1R decreased by 6-fold from primary to metastases in both groups treated with CR (Fig. 5B). Evaluation of GSK-3β expression revealed a modest elevation in the CR+IR group in primary tumor tissue. There was also a notable decrease (about 10-fold) in the phosphorylated form of Akt (pAkt) in both the CR and CR+IR groups as compared to the AL group and as compared with expression of Akt1, the primary isoform of Akt. The ratio of pAkt to Akt in the primary tumors was 0.23 in the CR cohorts versus 0.91 in the AL groups. While this same effect with regard to pAkt was not seen in the metastases (ratio 0.59 in CR cohort vs. 0.53 in AL cohort), it should be noted that the relative expression of Akt1 was overall lower in metastases of those mice treated with CR and CR+IR as compared with their respective primary tumor. A global downregulation of expression in the IGF-1R pathway was most significant in the tissue from the lung nodules (Fig. 5B).
Figure 5.
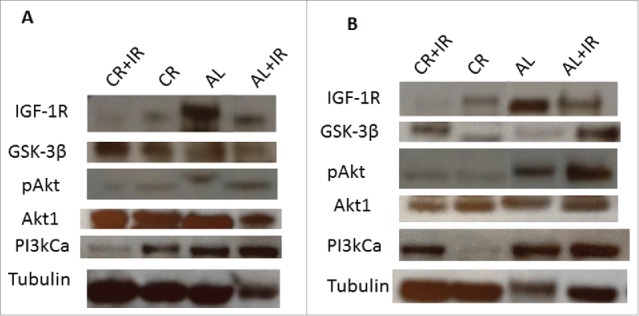
Tissue lysates were processed for analysis of expression of key signaling nodes in the IGF-1R pathway including: IGF-1R, GSK-3β, Akt1, pAkt and PI3kCa. Expression was normalized to tubulin across treatment groups as shown from left to right as: CR+IR, CR, AL and AL+IR. Expression of these molecules was significantly altered across treatment groups in both primary tumor tissue (A) and metastases (B) as quantified using ImageJ Software. The blots were cropped from a 10-well frame in which 8 samples were run and 2 wells were reserved for ladder. Each of the samples that appear juxtaposed was run simultaneously on the same gel.
Modulation of the IGF-1R pathway with radiation results in a similar decrease in metastases as caloric restriction with radiation
Since molecular analysis demonstrated the important role that IGF-1R played in the metastatic response to CR, cohorts of mice were treated with IGF-1R/INSR inhibitor therapy. The goal here was to establish that there is a similar physiologic effect of CR and IGF-1R with respect to tumor growth and metastatic disease. Consistent with the slight decrease in IGF-1R that was noted with CR in the primary tumors, IGF-1R blockade via the inhibitor did show a significant difference in primary tumor growth when added to the group receiving radiation therapy (IGF-1R+IR versus CR+IR) (Fig. 6A). The mice receiving IGF-1R/INSR inhibitor therapy did experience an increased time to metastases when compared with the AL mice and with the CR mice (Fig. 6B). The combination of an IGF-1R inhibitor with radiation prolonged development of visible lung metastases by approximately 11 d (30 d for IGF-1R+IR vs. 19 d for AL) when compared with the AL control group (Table 1) which is similar to the benefit noted when caloric restriction was added to radiation. In addition, the number of metastases was significantly decreased in mice receiving IGF-1R/INSR inhibitor with IR (Fig. 6B) which was also similar to the cohort receiving caloric restriction with IR suggesting a common mechanism since the physiologic effect on metastases was similar.
Figure 6.
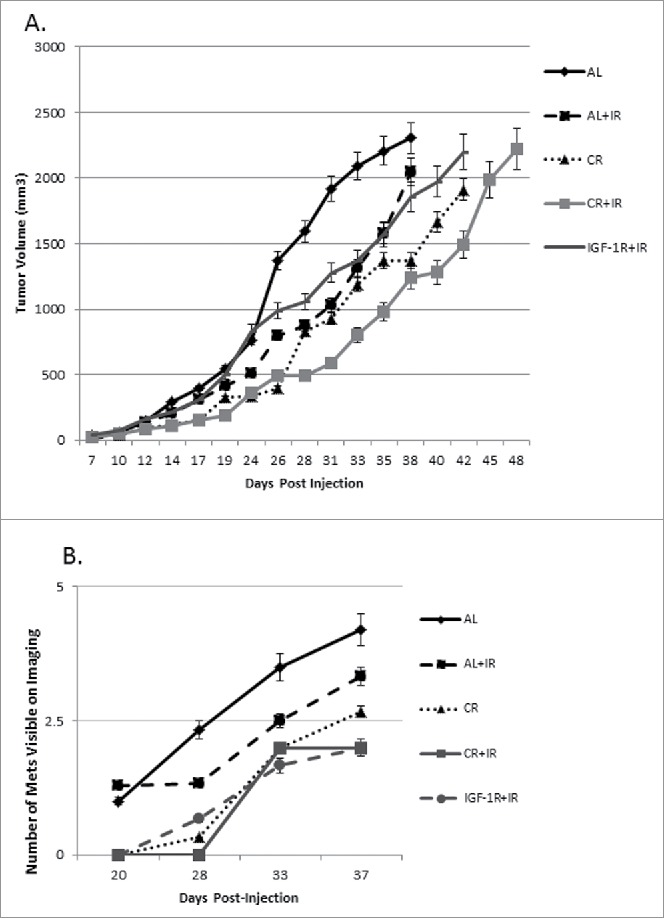
In an attempt to modify the IGF-1R pathway directly, an IGF-1R/INSR inhibitor was used in combination with CR and IR. To directly compare the effects of IGF-1R/INSR inhibitor combined with ionizing radiation, IGF-1R+IR group is plotted along with the original treatment groups. Treatment groups were followed over time similar to the 4 original cohorts and tumor volume was measured throughout disease progression (A). At a reference point of 1000 mm3, the AL+IR group demonstrated a 31% regression in tumor growth rate as compared with AL, CR alone had a 59% regression and CR+IR had a 71% regression. Similarly, IGF-1R inhibitor alone demonstrated 85% regression as compared with AL controls. Compared with IGF-1R inhibitor alone, adding IR to this induced further regression at 36%, inhibitor with CR regression was 42% and CR with IR and the inhibitor was 52%. Visible metastases were measured and were smaller on average across treatment groups receiving either CR, CR+IR or IGF-1R+IR inhibitor as compared with the original treatment groups (B).
Discussion
With few treatment options available for patients with metastatic triple negative breast cancer, it is crucial to explore new therapeutic options. Our findings demonstrate that caloric restriction can complement local cytotoxic therapy to decrease metastases and therefore, dietary alterations may be used in the clinical setting for metastatic disease. In an aggressive orthotopic model of triple negative breast cancer treated with caloric restriction and local radiation, metastases took longer to develop, were fewer in number and smaller in size. The advantages of CR for metastases were also noted molecularly with decreased proliferation, increased apoptosis and a global downregulation of the IGF-1R signaling pathway. Together, these findings demonstrate that dietary interventions can alter the systemic milieu to potentially prevent metastases and increase the response to cytotoxic treatment.
Approximately 33.9% of triple negative breast cancer patients ultimately develop metastases and median overall survival is significantly shorter for those patients with triple negative disease (4.2 y versus 6 years).13 In this study we have demonstrated that administering a dietary alteration at the time of initial primary cancer treatment, significantly lengthens the time to metastases in triple negative breast cancer. This led to a significant (p = 0.005) survival benefit, which can be attributed to the change in time to metastases. Molecularly we show that modulation of multiple nodes of the IGF-1R signaling pathway decreases the metastatic propensity. The importance of IGF-1R in decreasing the time to metastases has previously been shown by others in both lung cancer and gastric cancer models.14-16 In this manner, CR may be changing the subpopulation of cells that escape the primary tumor to make a less favorable environment for metastases.
In addition to the prolonged time to metastases that CR offers, the general molecular alterations suggest that CR could be used as a novel treatment for metastatic disease since treatment options for metastatic TNBC are limited and are fraught with toxicity. We show that the metastases from the mice treated with CR, have decreased proliferation as noted by Ki-67 and increased cell death as noted by caspase expression. These favorable molecular changes induced by CR could be harnessed by using dietary alterations to complement cytotoxic therapy of metastases to induce more cell kill. We also demonstrate that CR significantly reduced the number and size of lung metastases, particularly when used concurrently with radiation. It has been previously shown that CR alone has a substantial impact on tumor growth and metastases in vivo,10,17-19. This has been found by De Lorenzo et al., who have previously shown that CR affects the growth of metastases, particularly for spontaneous metastases. To build on this, Phoenix et al. examined the effects of metformin, a potential CR mimetic, but also included some data on CR and reported a reduction in the average number of metastatic nodules per lung. We have demonstrated that targeting the primary tumor locally with radiation and systemically with caloric restriction can change the molecular profile of metastases. Using the “seed to soil” hypothesis, metastases require not only a source, but an appropriate environment in which to grow. CR in combination with IR may be affecting not only the seed, but the soil as well, both of which result in not only decreased size and number of metastases, but an alteration in the makeup of this metastases that do develop. Taken together, these observations suggest metastases treated with CR would be rendered more susceptible to cytotoxic treatments.
One of the posited mechanisms behind the effect that CR has on growth of primary tumor and metastases is that perhaps CR is affecting the IGF-1R pathway.20-22 Downregulation of the IGF-1R signaling cascade leads to widespread apoptosis of cancer cells through the PI3K/AKT pathway which makes CR an intriguing therapeutic option since 46% of TNBC show upregulation of this pathway and this upregulation has been shown to be correlated with poor outcomes.23-26 Breast tumors often express high IGF-1 levels and thus, IGF-1R pathway members have been implicated as possible therapeutic targets of this disease. The downstream effects of IGF-1R activation include signaling cascades via IRS-1 (insulin receptor substrate) to the Akt/PI3K and RAF/MAPK pathways. It has been shown that IRS-1 is upregulated significantly in breast cancers.27 Additionally, it has also been demonstrated the triple-negative cells have shown a notable growth response to IGF-1 via the AKT pathway.28 Based on these principals, clinical trials using IGF-1R inhibitors have been designed and performed but have had limited success.29,30 This may be due in part to dose limiting side effects noted in the use of IGF-1R inhibitors including hyperglycemia combined with thrombocytopenia resulting in stroke or other cardiovascular complications.31,32 Our published research implicates the IGF-1R/Akt pathway as important in the cooperative cytotoxicity between RT and diet modification in the primary tumor. In this study, we found that CR alone appears to downregulate key signaling nodes in the IGF/Akt/PI3K pathway in both primary tumor and metastases, however the expression of IGF-1R was decreased by 6-fold in the metastases suggesting the importance of this pathway in metastases. Our initial findings related to IGF-1R downregulation as well as the significant downregulation of the IGF-1R molecule in metastatic tissue, prompted us to investigate the effects of an IGF-1R inhibitor in addition to radiation.6,28. We attempted to modulate this pathway directly by adding a concurrent IGF-1R/INSR inhibitor with radiation. We found that mice receiving IGF-1R inhibitor with IR took a significantly longer amount of time to develop lung metastases when compared with AL controls (30 d on average for IGF-1R+IR compared with 19 d for AL). This delay in metastatic initiation was similar to the delay note in mice treated with CR+IR suggesting CR acts in part via harnessing IGF-1R to affect its metastatic change when combined with radiation.
Clinically, the use of CR in the metastatic setting may not be ideal in all patients due to their potentially altered metabolic state, more specifically those patients who experience cachexia.33 Therefore, we suggest that alternatives to the administration of a dietary intervention like CR that have less potential for significant weight loss, warrant further study to determine if a similar benefit can be achieved using a ketogenic diet or time restricted/intermittent diet.34 Since we did note a similar benefit of CR+IR to IGF-1R inhibitor+IR, further use of IGF-1R inhibitors should be investigated in combination with cytotoxic therapy such as radiation therapy. However, for some patients, such as those with brain metastases, it becomes difficult to deliver effective therapy across the blood-brain barrier.35-37 A dietary intervention may prove the most efficient way to achieve an added benefit to IR since most IGF-1R inhibitors do not cross the blood-brain barrier.
In summary, the combination of CR and IR showed promising results in the decreased growth and invasion of metastases. Therapeutic options for TNBC are limited and future research should explore dietary alternatives suitable to augment cytotoxic therapy in metastatic disease. Investigations should be done to determine the population of patients who may benefit the most from this combination in the clinic.
Materials and methods
Mice and diets
To determine the effect of caloric restriction, radiation, or a combination of both treatments on metastases, 40 female 12 week-old BALB/c mice were acquired from Charles River Laboratories under an Institutional Animal Care and Use Committee approved protocol at Thomas Jefferson University. Every effort was made to decrease suffering and minimize pain for the animals utilized in this study. Mice were randomly assigned to one of 4 treatment groups in cohorts of 10: they were fed ad libitum (AL), received local radiation of 8 Gy to their primary tumor (IR), were placed on a 30% reduction of their total caloric intake (after being singly housed and measuring their baseline intake for 2 weeks - calorie restriction (CR)), or were treated with both CR and IR. The treatments have been previously described in detail12 but briefly, at 13 weeks, all mice received orthotopic injections of 50,000 4T1 luciferase-tagged cells (a gift from Patricia Steeg) into the #4 mammary fat pad. Cells were verified prior to use with isoenzymology using the Authentikit system (ATCC). Once tumors reached a palpable size, roughly 5 mm × 5 mm, radiation was delivered to the primary tumor with shielding of the remainder of the body (Pantak H-320 (320 kV) Precision X-Ray, N. Bradford, CT). Mice treated with CR were stepped down to 90% of their baseline chow intake for 5 days, followed by 2 successive 10% decreases every 5 d until a 30% total reduction was achieved (LabDiet 5010).
Monitoring and tissue collection
Mice were monitored by weighing them and measuring their primary tumor growth 3 times weekly. Tumors were measured using calipers in the longest dimension (L) and at a right angle to that axis (W). Tumor volume was calculated using the formula L × W × W × π/6. Mice were euthanized when the primary tumor reached 2500 mm3 in size or earlier if required by humane endpoints. Mice were euthanized according to protocol, were grossly examined for metastases and primary tumors, and their corresponding lung metastases were isolated and divided such that equal pieces were fixed in formalin for histologic evaluation, snap frozen for protein evaluation and preserved in RNAlater (Ambion, Life Technologies, Grand Island, NY) to assess RNA.
Live bioluminescent imaging
Since the tumors were generated from luciferase tagged cells, the primary tumors and metastases were able to be visualized in real time, 3 times a week throughout the experiment. Images were obtained using the IVIS Lumina XR System and Live Imaging Software (PerkinElmer, Waltham, MA) and were acquired 15 minutes after mice were injected with 100 µl of D-luciferin at a concentration of 5 ng/µl. Mice were imaged in supine and prone positions. Focal point was set to 12.5 cm and filter parameters were set to auto-detect appropriate exposure times depending on achieving a minimum photon flux threshold. In order to correlate images with proliferative activity with the tumors, fluorescent intensities for both primary tumor and metastases were measured. Primary tumor intensity was measured as average photon counts within the tumor volume that reached at least a 30% fluorescence threshold. Metastatic intensity within the lungs was measured as average photon counts within the volume reaching at least a 15% fluorescence threshold. This threshold level was chosen because lung metastases demonstrated on average less intense signal than primary tumors. This is presumably due to the superficial nature of the primary tumors in comparison with the lung nodules which appear deeper and more embedded within normal surrounding tissue. Each lung lesion was singly measured in pixels and converted to millimeters using a conversion factor provided by the image analysis software. These measurements were used in the previously described formula (L × W × W × π/6) to calculate the volume of each nodule. Additionally, the total volume of all visible metastases was calculated.
Western blotting
To determine if increased apoptosis was induced in the tissues after the treatments, western blots were performed for caspase-3. Previously, CR has been shown to augment radiation by increasing apoptosis in vivo.12 Since prior studies revealed that the members of the IGF-1R pathway were affected in primary tumors treated with CR and IR, we wanted to evaluate the role of this pathway in metastases. Briefly, tissue lysates were prepared from the primary tumor and lung metastases using RIPA buffer (50 mm Tris-Cl, 150 mm NaCl, 1% NP40, 0.25% Sodium deoxycholate, 1 mm PMSF) with proteinase inhibitor cocktail (Genentech, San Francisco, CA) until homogenized. Protein concentration was quantified using a BSA assay, diluted in 6x SDS buffer, and run on a NuPAGE 4–12% Bis-Tris gels (Life Technologies, Philadelphia, PA). Westerns were performed to assess for the concentration of primary antibodies including anti-β-tubulin (Sigma Aldrich, St. Louis, MO), anti-IGF-1R, anti-Akt1, anti-GSK3β, anti-pAkt, anti-PI3KCa (Upstate Biotechnology, Lake Placid, NY), anti-caspase-3 and anti-cleaved caspase-3 ( all antibodies from Cell Signaling Technology, Danvers, MA, unless otherwise noted) according to the manufacturer's instructions and quantified using ImageJ software (NIH, Bethesda, MD).38
Tissue evaluation and immunohistochemistry
Tissue samples from both the primary and lung metastases were fixed in formalin, paraffin-embedded and cut into 5 µm sections. To evaluate both the primary tumor and metastases for histologic changes, tissues were stained with hematoxylin and eosin (Sigma Aldrich, St. Louis, MO) as previously described. Sections were studied microscopically with an Olympus BX 41 microscope and representative images were acquired with an Olympus DP25 camera. The microscopic exam focused on the search for metastases using the Aperio ImageScope software (Aperio, Vista, CA)39; a pathologist (JP) also evaluated them separately.
To determine the effect of treatments on proliferation, immunohistochemistry was performed for Ki-67. Antigen retrieval was performed by heating the sections in 10 mmol/L citrate buffer pH 6.0 for 50 minutes with the use of a pressure cooker. The primary antibody for Ki-67 was used at a 1:600 dilution (Cell Signaling Technology, Cat#9027, Danvers, MA) for 60 minutes. The immune complexes were visualized with Mouse ABC (Vector Laboratories, Inc., Burlingame, CA) and the chromogenic substrate Dako Liquid DAB_Substrate-Chromogen Solution (Dako North America, Inc., Carpinteria, CA; catalog no. K3468; diaminobenzidine tetrahydrochloride) for 3 minutes. Ki-67 labeling index was determined by counting ≥500 nuclei in areas of the section with the highest labeling rates and was considered high when ≥10 % of tumor cells were stained. The scoring was performed by an experienced staff pathologist (JP) and was also counted on the automated system (Aperio). IGF-1R/INSR Inhibitor Intervention
As the results will indicate, the IGF-1R pathway was globally downregulated by CR. To determine the actual effect of IGF-1R on the interaction of CR with IR on the metastases, we sought to modulate the response with an IGF-1R inhibitor in vivo. In this manner, 10 mice per cohort were again randomized to either control, the IGF-1R/INSR inhibitor (BMS-754807, Active Biochem, Maplewood, NJ), or IR+BMS-754807 to determine if the inhibitor + IR would mimic the results seen with CR + IR. Mice were treated as per prior description for the control and IR conditions, and the BMS-754807 was first dissolved in PEG 1:400 at concentration of 100 µg/µl and injected subcutaneously twice daily for 14 d.40 All of the endpoints previously described including tumor measurements, bioluminescent imaging and molecular evaluation were reevaluated for this cohort.
Statistics
Statistical significance between tumor growth curves was calculated as previously described.41 Standard deviations were derived and used in a Student's t-test to obtain p values for comparison of experimental cohorts to the AL group. Results for tumor growth curves, size and number of metastases and relative protein expression are expressed as mean plus or minus standard error of the mean (SEM) unless otherwise stated. Significance for data regarding size and number of metastases and relative protein expression data was determined by comparisons using a 2-tailed Student's T-test with p ≤ .05 considered significant.
The study was designed to have 80% power for the log-rank test to detect an improvement in overall survival with a HR of 0.80 for CR as a treatment. The planned sample size was 10 mice per cohort for a total of 60 mice, with 36 deaths required for final analysis. Overall survival data was determined using the Mantel-Cox test to generate a Kaplan-Meier curve and censoring those mice that were euthanized for serum analyses as opposed to those mice that died of disease. The median survival for each of the 4 treatment groups was compared and significance was determined using a pre-specified α=0.025 (.05/2). Time to metastases was determined in a similar manner using time of first visible metastasis as an event. Statistical analyses were done using the commercially available software GraphPad Prism (GraphPad Software, La Jolla, CA) and SPSS Statistics (IBM Software, Armonk, NY).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
Research support was in part through Grant # IRG - 08-060-04 from the American Cancer Society, in part by the ASTRO Junior Faculty Award, and in part by the Kimmel Cancer Center's NCI Cancer Center Support Grant P30 CA56036.
References
- [1].Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013; 63:11-30; PMID:23335087 [DOI] [PubMed] [Google Scholar]
- [2].Smid M, Wang Y, Zhang Y, Sieuwerts AM, Yu J, Klijn JG, Foekens JA, Martens JW. Subtypes of breast cancer show preferential site of relapse. Cancer Res 2008; 68:3108-14; PMID:18451135; http://dx.doi.org/ 10.1158/0008-5472.CAN-07-5644 [DOI] [PubMed] [Google Scholar]
- [3].Lin NU, Claus E, Sohl J, Razzak AR, Arnaout A, Winer EP. Sites of distant recurrence and clinical outcomes in patients with metastatic triple-negative breast cancer: high incidence of central nervous system metastases. Cancer 2008; 113:2638-45; PMID:18833576; http://dx.doi.org/ 10.1002/cncr.23930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Swain SM, Baselga J, Kim SB, Ro J, Semiglazov V, Campone M, Ciruelos E, Ferrero JM, Schneeweiss A, Heeson S, et al.. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med 2015; 372:724-34; PMID:25693012; http://dx.doi.org/ 10.1056/NEJMoa1413513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hudis CA, Gianni L. Triple-negative breast cancer: an unmet medical need. Oncologist 2011; 16 Suppl 1:1-11; PMID:21278435; http://dx.doi.org/ 10.1634/theoncologist.2011-S1-01 [DOI] [PubMed] [Google Scholar]
- [6].Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, Pietenpol JA. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest 2011; 121:2750-67; PMID:21633166; http://dx.doi.org/ 10.1172/JCI45014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Robert NJ, Dieras V, Glaspy J, Brufsky AM, Bondarenko I, Lipatov ON, Perez EA, Yardley DA, Chan SY, Zhou X, et al.. RIBBON-1: randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J Clin Oncol 2011; 29:1252-60; PMID:21383283; http://dx.doi.org/ 10.1200/JCO.2010.28.0982 [DOI] [PubMed] [Google Scholar]
- [8].Wang J, Sinnberg T, Niessner H, Dolker R, Sauer B, Kempf WE, Meier F, Leslie N, Schittek B. PTEN regulates IGF-1R-mediated therapy resistance in melanoma. Pigment Cell Melanoma Res 2015; 28:572-89; PMID:26112748; http://dx.doi.org/ 10.1111/pcmr.12390 [DOI] [PubMed] [Google Scholar]
- [9].Dey N, Williams C, Leyland-Jones B, De P. A critical role for HER3 in HER2-amplified and non-amplified breast cancers: function of a kinase-dead RTK. Am J Transl Res 2015; 7:733-50; PMID:26064441 [PMC free article] [PubMed] [Google Scholar]
- [10].De Lorenzo MS, Baljinnyam E, Vatner DE, Abarzua P, Vatner SF, Rabson AB. Caloric restriction reduces growth of mammary tumors and metastases. Carcinogenesis 2011; 32:1381-7; PMID:21665891; http://dx.doi.org/ 10.1093/carcin/bgr107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Buschemeyer WC 3rd, Klink JC, Mavropoulos JC, Poulton SH, Demark-Wahnefried W, Hursting SD, Cohen P, Hwang D, Johnson TL, Freedland SJ. Effect of intermittent fasting with or without caloric restriction on prostate cancer growth and survival in SCID mice. Prostate 2010; 70:1037-43; PMID:20166128; http://dx.doi.org/ 10.1002/pros.21136 [DOI] [PubMed] [Google Scholar]
- [12].Saleh AD, Simone BA, Palazzo J, Savage JE, Sano Y, Dan T, Jin L, Champ C, Zhao S, Lim M, et al.. Caloric restriction augments radiation efficacy in breast cancer. Cell Cycle 2013; 12:1955-63; PMID:23708519; http://dx.doi.org/ 10.4161/cc.25016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P, Narod SA. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 2007; 13:4429-34; PMID:17671126; http://dx.doi.org/ 10.1158/1078-0432.CCR-06-3045 [DOI] [PubMed] [Google Scholar]
- [14].Bahr C, Groner B. The IGF-1 receptor and its contributions to metastatic tumor growth-novel approaches to the inhibition of IGF-1R function. Growth Factors 2005; 23:1-14; PMID:16019422; http://dx.doi.org/ 10.1080/08977190400020229 [DOI] [PubMed] [Google Scholar]
- [15].Qian J, Dong A, Kong M, Ma Z, Fan J, Jiang G. Suppression of type 1 Insulin-like growth factor receptor expression by small interfering RNA inhibits A549 human lung cancer cell invasion in vitro and metastasis in xenograft nude mice. Acta Biochim Biophys Sin (Shanghai) 2007; 39:137-47; PMID:17277889; http://dx.doi.org/ 10.1111/j.1745-7270.2007.00257.x [DOI] [PubMed] [Google Scholar]
- [16].Gryko M, Kisluk J, Cepowicz D, Zinczuk J, Kamocki Z, Guzinska-Ustymowicz K, Pryczynicz A, Czyzewska J, Kemona A, Kedra B. Expression of insulin-like growth factor receptor type 1 correlate with lymphatic metastases in human gastric cancer. Pol J Pathol 2014; 65:135-40; PMID:25119174; http://dx.doi.org/ 10.5114/pjp.2014.42678 [DOI] [PubMed] [Google Scholar]
- [17].Bonorden MJ, Rogozina OP, Kluczny CM, Grossmann ME, Grande JP, Lokshin A, Cleary MP. Cross-sectional analysis of intermittent vs. chronic caloric restriction in the TRAMP mouse. Prostate 2009; 69:317-26; PMID:19016490; http://dx.doi.org/ 10.1002/pros.20878 [DOI] [PubMed] [Google Scholar]
- [18].Cleary MP, Jacobson MK, Phillips FC, Getzin SC, Grande JP, Maihle NJ. Weight-cycling decreases incidence and increases latency of mammary tumors to a greater extent than does chronic caloric restriction in mouse mammary tumor virus-transforming growth factor-α female mice. Cancer Epidemiol Biomarkers Prev 2002; 11:836-43. [PubMed] [Google Scholar]
- [19].Ershler WB, Berman E, Moore AL. Slower B16 melanoma growth but greater pulmonary colonization in calorie-restricted mice. J Natl Cancer Inst 1986; 76:81-5; PMID:3455745 [PubMed] [Google Scholar]
- [20].Lee C, Longo VD. Fasting vs dietary restriction in cellular protection and cancer treatment: from model organisms to patients. Oncogene 2011; 30:3305-16; PMID:21516129; http://dx.doi.org/ 10.1038/onc.2011.91 [DOI] [PubMed] [Google Scholar]
- [21].Lee C, Safdie FM, Raffaghello L, Wei M, Madia F, Parrella E, Hwang D, Cohen P, Bianchi G, Longo VD. Reduced levels of IGF-I mediate differential protection of normal and cancer cells in response to fasting and improve chemotherapeutic index. Cancer Res 2010; 70:1564-72; PMID:20145127; http://dx.doi.org/ 10.1158/0008-5472.CAN-09-3228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Longo VD, Fontana L. Calorie restriction and cancer prevention: metabolic and molecular mechanisms. Trends Pharmacol Sci 2010; 31:89-98; PMID:20097433; http://dx.doi.org/ 10.1016/j.tips.2009.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Baserga R, Hongo A, Rubini M, Prisco M, Valentinis B. The IGF-I receptor in cell growth, transformation and apoptosis. Biochim Biophys Acta 1997; 1332:F105-26; PMID:9196021 [DOI] [PubMed] [Google Scholar]
- [24].Metalli D, Lovat F, Tripodi F, Genua M, Xu SQ, Spinelli M, Alberghina L, Vanoni M, Baffa R, Gomella LG, et al.. The insulin-like growth factor receptor I promotes motility and invasion of bladder cancer cells through Akt- and mitogen-activated protein kinase-dependent activation of paxillin. Am J Pathol 2010; 176:2997-3006; PMID:20395438; http://dx.doi.org/ 10.2353/ajpath.2010.090904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wang Y, Ji QS, Mulvihill M, Pachter JA. Inhibition of the IGF-I receptor for treatment of cancer. Kinase inhibitors and monoclonal antibodies as alternative approaches. Recent Results Cancer Res 2007; 172:59-76; PMID:17607936; http://dx.doi.org/ 10.1007/978-3-540-31209-3_5 [DOI] [PubMed] [Google Scholar]
- [26].Shin SJ, Gong G, Lee HJ, Kang J, Bae YK, Lee A, Cho EY, Lee JS, Suh KS, Lee DW, et al.. Positive expression of insulin-like growth factor-1 receptor is associated with a positive hormone receptor status and a favorable prognosis in breast cancer. J Breast Cancer 2014; 17:113-20; PMID:25013431; http://dx.doi.org/ 10.4048/jbc.2014.17.2.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lee AV, Jackson JG, Gooch JL, Hilsenbeck SG, Coronado-Heinsohn E, Osborne CK, Yee D. Enhancement of insulin-like growth factor signaling in human breast cancer: estrogen regulation of insulin receptor substrate-1 expression in vitro and in vivo. Mol Endocrinol 1999; 13:787-96; PMID:10319328; http://dx.doi.org/ 10.1210/mend.13.5.0274 [DOI] [PubMed] [Google Scholar]
- [28].Davison Z, de Blacquiere GE, Westley BR, May FE. Insulin-like growth factor-dependent proliferation and survival of triple-negative breast cancer cells: implications for therapy. Neoplasia 2011; 13:504-15; PMID:21677874; http://dx.doi.org/ 10.1593/neo.101590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Haluska P, Shaw HM, Batzel GN, Yin D, Molina JR, Molife LR, Yap TA, Roberts ML, Sharma A, Gualberto A, et al.. Phase I dose escalation study of the anti insulin-like growth factor-I receptor monoclonal antibody CP-751,871 in patients with refractory solid tumors. Clin Cancer Res 2007; 13:5834-40; PMID:17908976; http://dx.doi.org/ 10.1158/1078-0432.CCR-07-1118 [DOI] [PubMed] [Google Scholar]
- [30].Soria JC, Massard C, Lazar V, Ozoux ML, Mery-Mignard D, Deslandes A, Tolcher AW. A dose finding, safety and pharmacokinetic study of AVE1642, an anti-insulin-like growth factor-1 receptor (IGF-1R/CD221) monoclonal antibody, administered as a single agent and in combination with docetaxel in patients with advanced solid tumours. Eur J Cancer 2013; 49:1799-807; PMID:23485230; http://dx.doi.org/ 10.1016/j.ejca.2013.01.003 [DOI] [PubMed] [Google Scholar]
- [31].Chen HX, Sharon E. IGF-1R as an anti-cancer target–trials and tribulations. Chinese J Cancer 2013; 32:242-52; PMID:23601239; http://dx.doi.org/ 10.5732/cjc.012.10263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hixon ML, Paccagnella L, Millham R, Perez-Olle R, Gualberto A. Development of inhibitors of the IGF-IR/PI3K/Akt/mTOR pathway. Rev Recent Clin Trials 2010; 5:189-208; PMID:20533896; http://dx.doi.org/ 10.2174/157488710792007329 [DOI] [PubMed] [Google Scholar]
- [33].Meriggi F. Cancer Cachexia: One Step Ahead. Rev Recent Clin Trials 2015; 10:246-50; PMID:26435290; http://dx.doi.org/ 10.2174/1574887110666150916141351 [DOI] [PubMed] [Google Scholar]
- [34].Simone BA, Champ CE, Rosenberg AL, Berger AC, Monti DA, Dicker AP, Simone NL. Selectively starving cancer cells through dietary manipulation: methods and clinical implications. Future Oncol 2013; 9:959-76; PMID:23837760; http://dx.doi.org/ 10.2217/fon.13.31 [DOI] [PubMed] [Google Scholar]
- [35].Mittapalli RK, Vaidhyanathan S, Dudek AZ, Elmquist WF. Mechanisms limiting distribution of the threonine-protein kinase B-RaF(V600E) inhibitor dabrafenib to the brain: implications for the treatment of melanoma brain metastases. J Pharmacol Exp Ther 2013; 344:655-64; PMID:23249624; http://dx.doi.org/ 10.1124/jpet.112.201475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Alonso A. Ultrasound-induced blood-brain barrier opening for drug delivery. Front Neurol Neurosci 2015; 36:106-15; PMID:25531667; http://dx.doi.org/ 10.1159/000366242 [DOI] [PubMed] [Google Scholar]
- [37].Barani IJ, Larson DA, Berger MS. Future directions in treatment of brain metastases. Surg Neurol Int 2013; 4:S220-30; PMID:23717793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 2012; 9:671-5; PMID:22930834; http://dx.doi.org/ 10.1038/nmeth.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Martina JD, Simmons C, Jukic DM. High-definition hematoxylin and eosin staining in a transition to digital pathology. J Pathol Inform 2011; 2:45; PMID:22059146; http://dx.doi.org/ 10.4103/2153-3539.86284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Carboni JM, Wittman M, Yang Z, Lee F, Greer A, Hurlburt W, Hillerman S, Cao C, Cantor GH, Dell-John J, et al.. BMS-754807, a small molecule inhibitor of insulin-like growth factor-1R/IR. Mol Cancer Ther 2009; 8:3341-9; PMID:19996272; http://dx.doi.org/ 10.1158/1535-7163.MCT-09-0499 [DOI] [PubMed] [Google Scholar]
- [41].Mitchell JB, Choudhuri R, Fabre K, Sowers AL, Citrin D, Zabludoff SD, Cook JA. In vitro and in vivo radiation sensitization of human tumor cells by a novel checkpoint kinase inhibitor, AZD7762. Clin Cancer Res 2010; 16:2076-84; PMID:20233881; http://dx.doi.org/ 10.1158/1078-0432.CCR-09-3277 [DOI] [PMC free article] [PubMed] [Google Scholar]