ABSTRACT
Breast cancer stem cells (BCSC) have been identified in breast carcinoma as CD44+/CD24−/low cells, which display tumorigenic activity and have the ability to self-renew, differentiate and metastasize. Previous studies showed that extracellular HSP90 (eHSP90) participates in the invasion and metastatic processes of various cancers including breast cancer. Here, we show for the first time that eHSP90 is over-expressed in mammosphere cultures that are derived from the MDA-MB-231, MDA-MB-453 and MCF-7 breast cancer cell lines. These mammospheres are highly enriched in cells of the CD44+/CD24−/low BCSC phenotype and additionally show high expression of the BCSC markers CD49f and Sox2. Thus our results indicate that eHSP90 represents a potential novel BCSC marker. Moreover, we present evidence that eHSP90 is functionally involved in BCSC activity in vitro and in vivo. Selective neutralization of eHSP90, using the monoclonal antibody mAb 4C5, has the capacity to inhibit stem cell activity in vitro because the formation of mammosphere-derived colonies is dramatically reduced in its presence. In vivo, the treatment of mice with mAb4C5 using a prophylactic protocol, significantly inhibited the primary growth of MDA-MB-231 and mammosphere-derived tumors. More importantly, administration of this antibody in a therapeutic protocol caused a statistically significant regression of established tumors derived from MDA-MB-231 originating mammospheres. Tumor regression was even greater when mAb 4C5 was administered in combination with paclitaxel. Overall, our findings implicate eHSP90 as a potential novel BCSC biomarker. Moreover they show that eHSP90 participates in BCSC-derived primary tumor growth. Finally, we provide additional support for the possible therapeutic value of mAb4C5 in the treatment of breast cancer.
KEYWORDS: Breast cancer, eHSP90, mammospheres, mAb4C5, stem cells
Abbreviations
- ALDH1
aldehyde dehydrogenase 1
- BCSC
breast cancer stem cells
- eHSP90
extracellular heat shock protein 90
- ER
estrogen receptor
- EGF
epidermal growth factor
- FGF
fibroblast growth factor
- MMP-2
matrix metalloproteinase 2
Introduction
Cancer is the outcome of a multistep process during which accumulation of somatic mutations introduce in the descendants of a single cellular progenitor a level of deregulation sufficient for the appearance of malignancy by clonal growth.1,2 In other words, cancer is a disease of malfunctioning cell signaling3 leading to deregulation of the normal cellular program for cell division and cell differentiation. A small subset of cancer cells within a tumor that have the ability to self-renew and an indefinite proliferative potential, constitute a reservoir of self-sustaining cells that are defined as cancer stem cells (CSC).4 CSCs are long-lived and display quiescent characteristics while in a dormant state, but upon activation can induce angiogenic activity, show resistance to apoptosis, differentiate into unlimited heterogeneous populations of cancer cells and metastasize.5,6 CSCs can be distinguished from other cells within the tumor by their asymmetric divisions and altered gene expression7. To avoid confusion with normal stem cells, it has been proposed that cancer stem cells be designated as “tumor initiating cells,” or “cancer stem-like cells,” thereby defining the subset of cancer cells that has the ability to drive the growth and spread of a tumor.8
Breast cancer is a significant cause of mortality in women, and has a high incidence of recurrence and treatment failure. It is a heterogeneous disease reflected both at morphological and genetic levels. Breast CSCs (BCSC) represent a small sub-population (0.1% to 1%) of breast cancer cells in primary tumors.9 The first report of CSC in breast cancer was by Al-Hajj et al., in 2003, who isolated these cells and designated them as CD44+/CD24−/low.10 One thousand of these cells were sufficient to generate tumors when xeno-transplanted into the mammary fat pad of NOD/SCID mice, whereas more than 50 thousand were needed from the unsorted population. CD44+/CD24−/low cells display features of stem cells and many studies have confirmed the poor prognosis of tumors containing cells of the CD44+/CD24−/low phenotype.11 On the other hand, Dontu and colleagues developed an in vitro culture system that allows the propagation of human mammary epithelial cells in non-adherent, non-differentiating culture conditions.12 Cells that are capable of surviving and proliferating under such conditions, formed discrete clusters of cells termed “mammospheres.” Such spheroids were enriched in progenitor cells capable of differentiating along multiple lineages, including luminal, myoepithelial and alveolar. Additionally, Ponti and colleagues13 found that 95%–96% of cells in mammospheres cultured from cell lines and primary breast tumors were of the CD44+/CD24−/low phenotype. Besides the CD44+/CD24−/low molecular phenotype, various studies have identified several other BCSC markers such as aldehyde dehydrogenase 1 (ALDH1), CD133, Sox2, CK5, α-6integrin/CD49f, β-1 integrin/CD29, and lack of estrogen receptor (ER).14 At the time of detection, most solid tumors are already genetically altered, and tend to resist therapies that target a single molecular determinant.15 Thus, a simultaneous attack on multiple nodes of a cancer cell web of overlapping signaling pathways should be more likely to affect survival than inhibition of one or even a few individual signaling nodes. Over the past years, heat shock protein 90 (HSP90) has been defined as the “cancer chaperone” since it is necessary for the stability and function of numerous oncoproteins essential for cancer processes such as blockade of apoptosis and self-renewal.16,17 Additionally, this protein interacts with a great number of molecules that are involved in the development of metastatic tumors.18-20 Considering the fact that various HSP90 clients represent nodal points of oncogenic pathways, (see also the website maintained by D. Picard, http://www.picard.ch/downloads)21, inhibition of HSP90 may prove to be a very efficient anti-cancer therapeutic strategy.22 Eustace et al. in 200423 showed that the α isoform of this chaperone is secreted and associated with matrix metalloproteinase 2 (MMP-2), an interaction that directly incriminates extracellular HSP90 (eHSP90) with cancer metastasis. More recently, we have shown that both the α and β isoforms of HSP90 are secreted by MDA-MB-453 human breast cancer cells and interact with the inactive forms of MMP-2 and MMP-9.24 In the same study we showed that mAb4C5, a previously developed and characterized cell impermeable anti-HSP90 monoclonal antibody,25 inhibits activation of these metalloproteinases by binding to eHSP90. Moreover we have reported that mAb4C5 additionally inhibits melanoma cell invasion and metastasis26, as well as MDA-MB-453 breast cancer cell invasion, due to its ability to bind selectively to the extracellular pool of HSP90. In the latter case we demonstrated that mAb4C5 disrupts the association of eHSP90 with the extracellular domain of HER2, which in turn results in inhibition of HER2-HER-3 heterodimer formation, reduced HER-2 phosphorylation and impaired downstream signaling necessary for cytoskeletal re-arrangement, which in turn is essential for cancer cell invasion.27 Finally we have reported that mAb 4C5 significantly reduces the metastatic depositions of MDA-MB-453 breast cancer cells into the lungs of NOD/SCID mice by binding to eHSP90.24 Taking into account all the above, here we investigated the presence of eHSP90 on BCSC derived from the highly metastatic MDA-MB-231, MDA-MB-453 and MCF-7 breast cancer cell lines, and compare it to that on the parental cells. Moreover we examine the effect of mAb4C5 on in vitro colony formation of the previously mentioned cancer cells. Additionally we investigate the effect of mAb 4C5 in in vivo primary growth of tumors derived from MDA-MB-231 cells and their corresponding BCSC. Finally we explore the therapeutic capacity of mAb4C5 alone and in combination with paclitaxel, an established anti-cancer agent,28,29 on the progression of established primary tumors generated by MDA-MB-231-derived BCSC.
Materials and methods
Antibodies and reagents
Mouse monoclonal mAb4C5 against HSP90 was produced in our laboratory as previously described.25 In the present study, mAb4C5 was used as concentrated serum-free supernatant and at a final concentration of 0.1 mg/mL, in all immunochemical experiments. We also used the following antibodies: rabbit anti-human HSP90α (Millipore, Catalog No. 07-2174), mouse anti-human CD44 (BD PharMingen, Catalog No. 550392), rabbit anti-human CD24 (Santa Cruz, Catalog No. sc-11406), mouse anti-human CD49f conjugated to FITC (Stem Cell Technologies, Catalog No. 60037AD), rabbit anti-human Sox2 (Abcam, Catalog No. ab59776), rabbit anti-human Ki-67 (Abcam, Catalog No. 15580), mouse anti-human CD24 conjugated to phycoerytherin (PE)(BD Biosciences, Catalog No. 555428), and mouse anti-human CD44 conjugated to fluorescein isothiocyanate (FITC)(BD Biosciences, Catalog No. 555478). TOPRO-3 (Catalog No T3605), goat anti- mouse Alexa Fluor-488 (Catalog No. A 11001), goat anti-rabbit Alexa Fluor-546 (Catalog No. A 11010), and donkey anti-rabbit Alexa Fluor-647 (Catalog No. A-31573)-conjugated antibodies were all from Life Technologies. Biotin-avidin peroxidase 2-step kit was from Vector Laboratories, DAB substrate kit from Thermo Scientific (Catalog No. 34065). Dulbecco's modified Eagle's medium (DMEM) (Catalog No. 41966-029), DMEM/F-12 (Catalog No. 11320-074), fetal bovine serum (FBS)(Catalog No. 12483-020), penicillin-streptomycin solution (Catalog No. 15140-122), B-27 supplement were all from Life Technologies (Catalog No. 12587-010). Recombinant human epidermal growth factor (EGF) (Catalog No. 236-EG) and fibroblast growth factor (FGF) (Catalog No. 233-FG-025) were from R&D Systems. Hematoxylin (Catalog No. HHS80) and eosin (Catalog No. HT1101128) were from Sigma and gelatin 0,1% (Catalog No. SF008) was from Millipore. Poly-HEMA was from Sigma-Aldrich (Catalog No. P3932-10G). Mowiol 4-88 (Catalog No. 475904) was from Millipore and DPX from VWR (Catalog No. 360294H).
Cell line cultures
Human breast cancer cell lines MDA-MB-231, MDA-MB-453 and MCF-7 were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). All cells were maintained as monolayer cultures in DMEM, supplemented with 10% FBS, 100 units/mL penicillin and 100 μg/mL streptomycin, at 37° C in a humidified atmosphere with 5% CO2.
Mammosphere cultures
Mammosphere cultures were established according to previous reports12,30 with the following modifications. Single-cell suspensions of MDA-MB-231, MDA-MB-453 and MCF-7 breast cancer cells were seeded at density of 104 cells/ml in DMEM/F-12 supplemented with 20 ng/ml EGF, 20 ng/ml b-FGF, B-27 supplement, 100 units/mL penicillin and 100 μg/mL streptomycin at 37°C in a humidified atmosphere with 5% CO2. In order to achieve non-adherent non-differentiating culture conditions, cells were seeded in 6-well plates coated with 12 mg/ml poly-HEMA. Culture medium was renewed every week and, once mammospheres were formed, cells were passaged every 5 d in order to obtain new mammosphere generations.
Immunofluorescence experiments
For immunofluorescence studies, cells from the 3 cell lines and their corresponding mammospheres were plated on gelatin 0.1% coated coverslips, at a density of 6 × 104 cells/well in a 48-well plate and cultured as described above. After 48h, live cells were labeled by indirect immunofluorescence as previously reported.25 Briefly unfixed cells were incubated for 1 h at 37°C with rabbit anti-HSP90 (1:30), mouse anti-CD44 (1:200), rabbit anti-CD24 (1:100) primary antibodies. Next, cells were fixed with cold acetone for 3 min followed by overnight incubation at 4°C with anti-mouse Alexa Fluor-488 (1:100) or anti-rabbit Alexa Fluor-546 (1:100) conjugated antibodies. In case of rabbit anti-Sox2 (1:500) and mouse anti-CD49f (1:400) immunofluorescence, cell fixation with PFA 4%, before immunostaining with primary antibodies, was performed. Wherever necessary, a subsequent 30 min incubation with TOPRO-3 (1: 1000) was performed. For all experiments, controls were performed by omitting the primary antibodies. All preparations were viewed by a Leica TCS-SP (Solmser, Germany) confocal microscope. Confocal images were analyzed using ImagePro Plus v 5.1. and Icy v 1 software.
Immunohistochemistry experiments
Immunochemistry experiments were performed as previously described31 and modified as follows. All tissues were fixed in 10% neutral-buffered formalin for 24 h, paraffin embedded, and sectioned. 3 μm paraffin sections were de-paraffinized by incubation in xylene and rehydrated in a graded series of ethanol aqueous solutions. Antigen retrieval was performed with 7 mM citrate buffer, pH 6.0, by heating the sample in a steamer for 13 min. Endogenous peroxidase activity was blocked by incubating the slides in 3% hydrogen peroxide in TBS for 10 min. Non-specific signal blocking was achieved by incubating the slides for 1 h in antibody dilution buffer containing 1% Lys, 10% FBS. The sections were subsequently incubated with primary antibodies (anti-CD44 1:50, anti-HSP90 1:30, anti-Ki67 1:30), at 4°C overnight. The next day, sections were labeled with anti-mouse (Catalog No. BA-2001) or anti-rabbit (Catalog No.BA-1000) secondary antibodies (1:200) for 2h and subsequently for 1h with peroxidase (Catalog No.A-2004) (1:500), according to Vector Laboratories biotin-avidin 2-step protocol. Primary and secondary antibodies, as well as avidin peroxidase, were diluted in antibody dilution buffer. The immunoreaction was developed with DAB for 5 min. Sections were counterstained with hematoxylin and dehydrated in a graded series of ethanol aqueous solutions and then xylene. Finally, coverslips were mounted with one drop of DPX mounting medium. For all experiments, negative controls were performed by omitting the primary antibodies. Slide photos were taken using an Olympus BX-50 microscope, equipped with an Olympus DP71 camera. Images were analyzed with Olympus CellA software. Image processing and analysis were performed with an in-house developed algorithm in MATLAB, customized to automatically (without inserting user related biases) detect the areas of interest and export the corresponding properties, i.e., area and intensity.
Flow cytometry and FACS
MDA-MB-231, MDA-MB-453 and MCF7 and their corresponding mammospheres were labeled with combinations of fluorescent antibodies (CD44-FITC and CD24-PE) and anti–HSP90 antibody. Stained samples were analyzed on a Coulter cytometer (FC500; Cytomics) and the raw data were calculated and visualized with FlowJo software (TreeStar).
Colony formation assay
In order to perform anchorage-independent colony formation assays, cells were seeded in 48-well plates coated with 12 mg/ml poly-HEMA. In particular, single-cell suspensions of MDA-MB-231, MDA-MB-453 and MCF-7 cells were seeded in DMEM supplemented with 2% FBS, 100 units/mL penicillin and 100 μg/mL streptomycin while single-cell suspensions of mammospheres from the above mentioned cells were seeded in DMEM/F-12 supplemented with 20 ng/ml b-FGF, B-27 supplement, 100 units/mL penicillin and 100 μg/mL streptomycin; both the parental cells and the corresponding mammospheres were seeded at a density of 200 cells/well. Cells were monitored for 6 d and medium was renewed every 3 d both in the cells treated with 200 μg/ml mAb4C5 and in the untreated ones (controls). On the sixth day of incubation, photos were taken using a Leica DM IL inverted microscope, equipped with a LEICA DC300 video camera. Colony formation measurements were performed by counting the total number of colonies/well.
Cell viability assay
At the end point of the colony formation assay, cells were incubated for 4 h with Cell Counting Kit-8 (CCK8) reagent (Dojindo) and each well was subsequently measured in an ELISA reader at 450 nm. Dojindo's highly water-soluble tetrazolium salt, WST-8, is reduced by dehydrogenase activities in cells to give an orange-color formazan dye, which is soluble in the tissue culture media. The amount of the formazan dye, generated by the activities of dehydrogenases in cells, is directly proportional to the number of living cells.
Mouse lines
NOD.CB17-Prkdc SCID/NCrHsd mice were provided by Harlan Laboratories Srl, and were housed in individually ventilated cages in the authorized Laboratory Animals Facilities of the Department of Animal Models for Biomedical Research (DAMBR) of Hellenic Pasteur Institute under specific pathogen-free conditions. All animals were handled in strict accordance with the Regulatory Guide of the Department, in line with the Directive 2010/63/EU and National Decree 56/2013 requirements. All procedures were performed under the Protocol License 3075/22.10.2014 which was reviewed by the Institutional Protocol Evaluation Committee and Welfare Body and was issued by the Official Veterinary Authorities.
Orthotopic tumor growth studies
MDA-MB-231 cells and their derived mammospheres, were used for orthotopic xeno-transplantation. 7–9 week-old mice were anesthetized using a steady flow of a mixture of isofluorane gas (4%) and oxygen. During the surgical procedure, the animal's core body temperature was maintained at 36°C–37°C using a heating pad. For these orthotopic transplantations, the mice also received as analgesic a mixture of buprenorphine (Abbott Laboratories) (0.1 mg/kg) and meloxicam (Pfeizer) (2 mg/kg). With a sterile scalpel a midline incision through the skin and fascia was made, afterwards the fat-pad of both inguinal mammary glands was slightly elevated and equal numbers of MDA-MB-231 cells or mammospheres were injected into the left and right fat-pads, respectively, in order to monitor simultaneously the growth of both tumor types under identical conditions. The incision was closed using tissue adhesive (Histoacryl; B.Braun, Melsungen, Germany), and the skin was closed using Autoclips (Stoelting Europe).
Validation of the experimental model
In order to establish a xenograft model, for in vivo studies, we engrafted 2 groups of randomly chosen mice (n = 4/group), with low or high doses of MDA-MB-231 and mammosphere cells. The low dose group, which received 2,500 cells per injection site, developed non-palpable tumors from MDA-MD-231 cells and slightly palpable ones from mammospheres, 8 weeks post inoculation. In contrast, the high dose group, which received 50,000 cells per injection site, developed palpable tumors from MDA-MB-231 cells 8 weeks post injection, and from mammospheres as early as 4 weeks post injection. The experiment was repeated twice using 16 mice in total.
Prophylactic model
For the prophylactic treatment experiment we used the high dose protocol of 50,000 cells/injection site. It should be emphasized, as described above, that all mice were injected in both mammary fat pads thus yielding 2 tumors/animal and increasing the number of cases tested. After recovery from the anesthesia, the mice were divided randomly into 2 groups (n = 4/group) and treatment of the animals was initiated 2 d later. The dosing protocol was as follows. Mice were given intraperitoneal injections daily for 2 weeks, of 200 μg/ml mAb4C5 (mAb 4C5 group), and/or 200 μg/mouse of an irrelevant antibody named mAb LD33 previously used in in vivo experiments26 (control group) or PBS vehicle. Mice were then left untreated for another 6 weeks. Subsequently they were sacrificed and their tumors excised and weight measurements were made. Finally tumor samples were embedded into paraffin as described above, for further analysis. The same experiment was done twice with similar results.The total number of mice in both groups was 16.
Therapeutic model
From our initial observations using the xenograft model, we found that MDA-MB-231 cells could not form palpable tumors within 4 weeks post injection. Taking this into account, and in order to examine the therapeutic effect of mAb4C5 on tumor development under the most stringent conditions, i.e., in tumors derived from BCSCs, we chose to use only mammospheres in a therapeutic model. Additionally, in order to investigate the effect of mAb 4C5 in combination with paclitaxel (Hospira,UK), this agent alone and combined with mAb 4C5 was used in this model. The treatment protocol in this case was as follows: Mice were inoculated with 50,000 mammospheres/mammary fat pad, and divided randomly into 4 groups (n = 4/group). 4 weeks post inoculation all mice had developed palpable tumors, i.e. 100–150 mm3 in size. The therapeutic protocol was initiated with intraperitoneal injections as follows: a) mAb4C5 group: 200 μg/ml of antibody daily, b) control group: 200 μg/ml of irrelevant mAb LD33 daily or PBS vehicle, c) paclitaxel group: 20 mg/kg once a week and d) mAb4C5/paclitaxel group: 200 μg/ml daily and 20 mg/kg once a week, respectively. The administration lasted for a period of 4 weeks. Mice were sacrificed 1 day after the final injection and the tumors were excised and harvested for weight measurements and histological analysis. The same experiment was done twice with similar results.The total number of mice for all groups was 32.
Statistical analysis
Groups of data were tested for statistical significance using the Students t-test and presented as means ± SEM. Results were considered as statistically significant when p < 0.05.
Results
Production and identification of BCSC derived from mammospheres
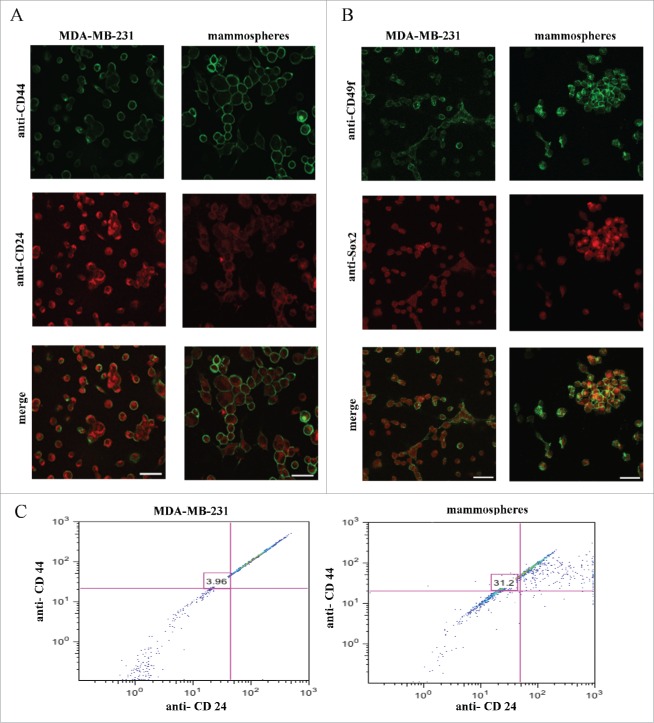
In order to investigate whether the mammosphere cultures generated were enriched in a BCSC sub-population with respect to the parental MDA-MB-231 cell line, unfixed, live mammospheres and MDA-MB-231 cells were double immunolabeled with anti-CD44/anti-CD24 and anti-CD49f/anti-Sox2 antibodies. Interestingly, most of the cells in the MDA-MB-231 cultures were CD44low/CD24+, whereas the majority of mammosphere cells switched to the CD44+/CD24−/low phenotype characteristic of BCSC (Fig. 1A). Additionally, and as expected, BCSC markers CD49f and Sox2 were over–expressed in the mammosphere cultures when compared to the parental cells (Fig. 1 B). At this point it is important to note that immunofluorescence experiments carried out in breast cancer cell lines MDA-MB-453 and MCF-7 and their corresponding mammospheres using the above mentioned BCSC markers yielded similar results (Fig. S1). Finally quantification of cells positive for CD44+/CD24−/low was performed both on the MDA-MB-231 cells and their corresponding mammospheres using FACS analysis and confirmed that indeed this phenotype is significantly increased by 8-fold (3.96% vs 31.2%) in the mammosphere cultures (Fig. 1C). Moreover FACS analysis of MDA-MB-453 and MCF-7 cells and their corresponding mammospheres showed enrichment of CD44+/CD24−/low phenotype in the mammosphere generated cultures. More specifically a 9.1-fold (4.66% vs 42.83%) and 12.03-fold (1.78% vs 21.43%) increase was observed in the MDA-MB-453 and MCF-7 derived mammospheres respectively, compared to parental cells (data not shown). Overall the above results confirm that the produced mammosphere cultures are greatly enriched in cells with the BCSC phenotype.
Figure 1.
Mammosphere cultures derived from MDA-MB-231 cells are enriched in cells expressing stem cell markers. MDA-MB-231cells and derived mammospheres were double immunolabeled using, (A) anti-CD44/anti-CD24 and, (B) anti-Sox2/anti-CD49f antibodies. (C) Flow cytometry analysis of CD44/CD24 markers revealed a 3.96% presence of cells with the CD44+/CD24−/low phenotype in the MDA-MB-231 cells, which increased 8-fold (31.2%) in the mammosphere cultures. Bars correspond to 40 μm.
eHSP90 is localized and over-expressed on BCSC
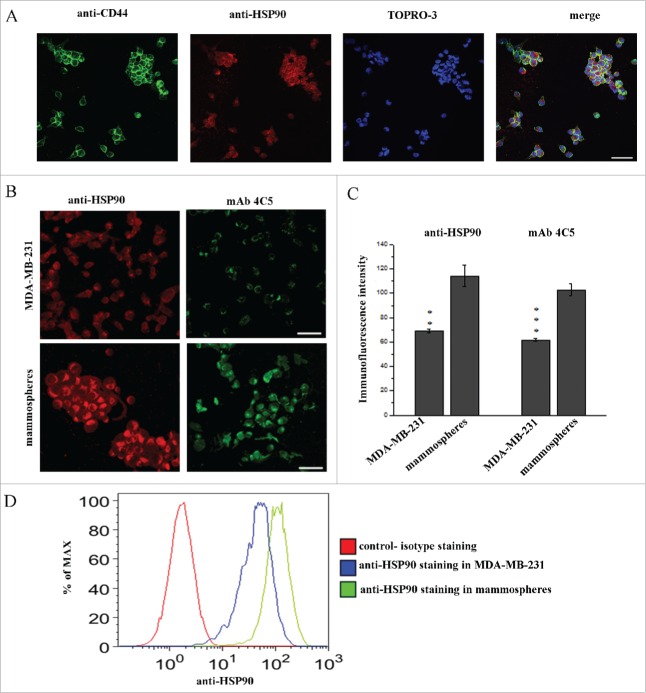
In order to further analyze the molecular phenotype of BCSC, and taking into account previous reports showing that eHSP90 is directly involved in tumor invasion and metastasis24,26,27 we investigated its expression in mammospheres. Double immunofluorescence experiments using polyclonal anti-HSP90 and anti-CD44 antibodies performed on live mammospheres, revealed that eHSP90 and CD44 were co-localized on the cell surface of most cells (Fig. 2A).
Figure 2.
eHSP90 is localized and overexpressed on BCSCs. (A) Double labeling with anti-CD44 and anti-HSP90 showed co-localization of the 2 proteins in mammospheres. Cell nuclei were stained with TOPRO-3. (B) Live MDA-MB-231 cells and mammospheres were immunostained using anti-HSP90 and mAb4C5 antibodies. (C) Immunofluorescence intensity in mammospheres as compared to MDA-MB-231 cells is 65.6% and 66.8% higher using anti-HSP90 and mAb 4C5 antibodies, respectively. (D) Flow cytometry analysis of the MDA-MB-231 cells and corresponding mammospheres showed an important increase of eHSP90 expression in the latter. Values presented are the mean ± SEM from 3 independent experiments.**p < 0.001, ***p < 0.0001. Bars correspond to 40 μm.
To further investigate whether eHSP90 is overexpressed on BCSC compared to their respective parental cell lines, immunostainings were performed on both MDA-MB-231 cells and mammospheres using either anti-HSP90 or mAb4C5. A typical punctuate immunolabeling was observed in all cases, confirming the cell surface localization of eHSP90. Interestingly, increased immunostaining was observed in the mammosphere cultures when compared to MDA-MB-231 cells, with both antibodies (Fig. 2B). More specifically, anti-HSP90 and mAb4C5 labeling revealed a 65.6% and 66.8% increase on mammospheres compared to MDA-MB-231 cells, respectively (Fig. 2C). With the aim of further evaluating the increased expression of eHSP90 on mammospheres, FACS analysis was performed in MDA-MB-231 cells and the corresponding mammospheres, revealing that indeed presence of eHSP90 was more important in the mammosphere cultures as compared to the parental cells (Fig. 2D). The higher expression of eHSP90 in mammospheres compared to parental cells indicates a potentially crucial role of this molecule in BCSCs. At this point it should be mentioned that double immunofluorescence using anti-HSP90 and anti-CD44 performed on live mammosheres derived from the MDA-MB-453 and MCF-7 cell lines, showed co-expression of these proteins on the cell surface (Fig. S2). Finally, immunolabeling of MDA-MB-453 and MCF-7cells and their corresponding mammospheres with anti-HSP90 and mAb4C5 confirmed the increased expression of eHSP90 in the mammosphere cultures (Fig. S2).
MAb4C5 inhibits colony formation of mammospheres
In order to examine the effect of mAb 4C5 on tumor cell growth in vitro we performed anchorage independent clonogenic assays, taking into consideration that mammospheres are grown and established under conditions that inhibit adherence to the substrate12. Assays were performed in the absence or presence of mAb4C5, using MDA-MB-231 cells and the corresponding mammospheres. Our results showed that, after a 6-day incubation in non-adherent conditions, in control cultures, not including mAb4C5, MDA-MB-231 cells did not form colonies and notable necrosis was observed. Interestingly, in the presence of mAb4C5, these phenomena were significantly intensified. When the same experiment was performed using mammospheres, an important number of colonies was observed after a 6-day incubation in the control cultures. However, in the presence of mAb4C5, colony formation was dramatically inhibited (Fig. 3A). More precisely, mAb4C5-treated mammospheres showed a 73.3% reduction of their clonogenic potential as compared to control cultures (Fig. 3B). It should be noted that using the CCK8 assay we observed that the metabolic activity of mAb 4C5 treated MDA-MB-231 parental cells was reduced by 79.5 % in comparison to the untreated cells, mostly due to cell necrosis. Moreover the metabolic activity of mammospheres treated with mAb 4C5 was reduced by 65.1% withrespect to the control cultures as a result of a decreased number of colony formation (Fig. 3C). Finally, it is important to note that clonogenic assays were performed in the absence or presence of mAb4C5 using MDA-MB-453 and MCF-7 cells and their corresponding mammospheres and yielded similar results to those obtained with the MDA-MB-231 cells and their mammospheres. In fact, the addition of mAb 4C5 in MDA-MB-453- and MCF-7- derived mammospheres resulted in a 58% and 66% decrease respectively of the number of newly-formed colonies as compared to the controls (Fig. S3). Reduced metabolic activity of the mab4C5 treated cultures for both cell lines was verified as mentioned above using the CCK8 assay (data not shown). Overall, these results further support the importance of eHSP90 in BCSC function.
Figure 3.
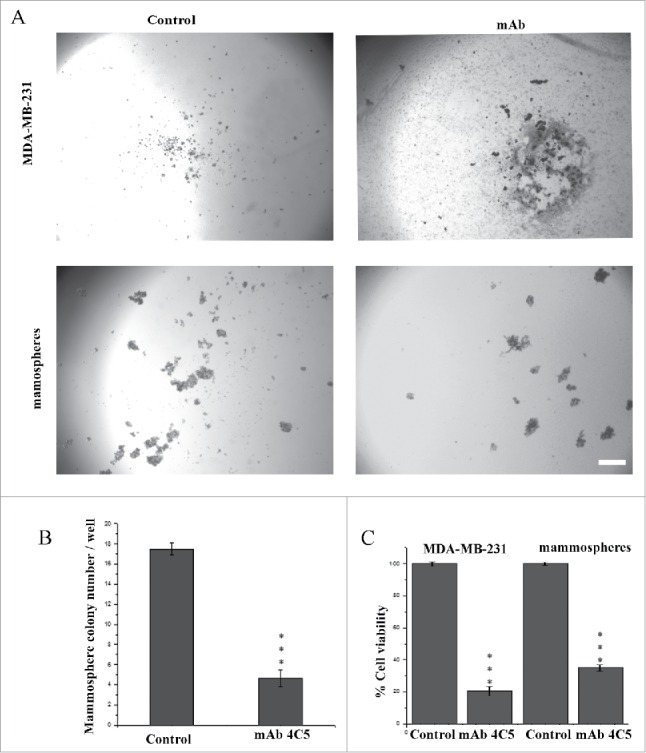
MAb4C5 inhibits colony formation of BCSCs. (A) Anchorage-independent assay using MDA-MB-231cells and mammospheres, in the absence (left panel) or presence (right panel) of mAb4C5. (B) Addition of mAb4C5 resulted in a 73.3% decrease in the number of newly-formed colonies with respect to the controls. (C) quantification of cell viability of all cases studied. Values presented are the mean ± SEM from 3 independent experiments.***p value < 0.0001. Bars correspond to 200 μm.
Enrichment of CD44 positive cells in tumors derived from mammospheres
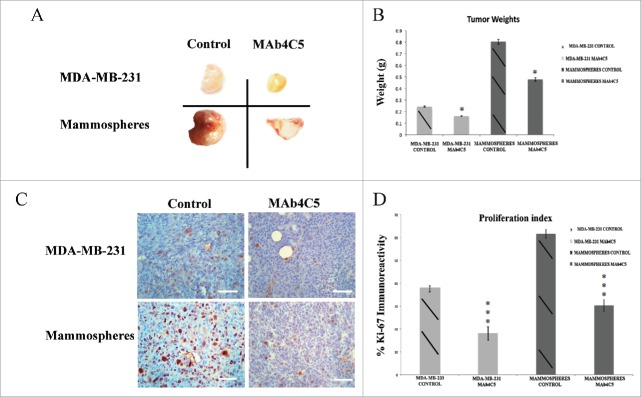
The above results prompted us to further explore the effect of mAb4C5 on primary tumor growth in vivo. For this reason we initially sought to characterize and establish an in vivo model using MDA-MB-231 cells and BCSC enriched mammospheres. We inoculated left and right inguinal mammary fat-pads of each mouse with MDA-MB-231 cells and mammospheres, respectively (Fig. 4A). This was done in order to simultaneously monitor the growth of both tumor types under identical conditions. Two different cell concentrations were used, and mice were sacrificed 8 weeks after inoculation. At the low concentration of 2,500 cells per injection site (Fig. 4B and C), for the MDA-MB-231 xenografts, very small, non-palpable tumors were generated which were detectable only at the microscopic level (arrow in Fig. 4C), while for the mammosphere grafts, larger palpable tumors were generated (arrowhead in Fig. 4B). At the high concentration of 50,000 cells, palpable tumors were obtained in both cases, however as anticipated, the mammosphere derived tumors were larger when compared to the ones derived from the parental cells both at the macroscopic (Fig. 4B) and microscopic (Fig. 4C) levels. Furthermore, anti-CD44 immunostaining of tumors derived from mice injected with the high cell concentration revealed a 53.63% higher number of CD44 positive cells in the mammosphere derived tumors, as compared to the MDA-MB-231 derived ones (Fig. 4D and E). This result shows that mammosphere derived tumors are greatly enriched with cells possessing the BCSC phenotype. Interestingly, anti-HSP90 immunostaining of these tumors, which was abundant in both cases, showed an increase of 36.2% of HSP90 immunoreactivity in the mammosphere derived tumors, which was not statistically significant (Fig. 4D and E).
Figure 4.
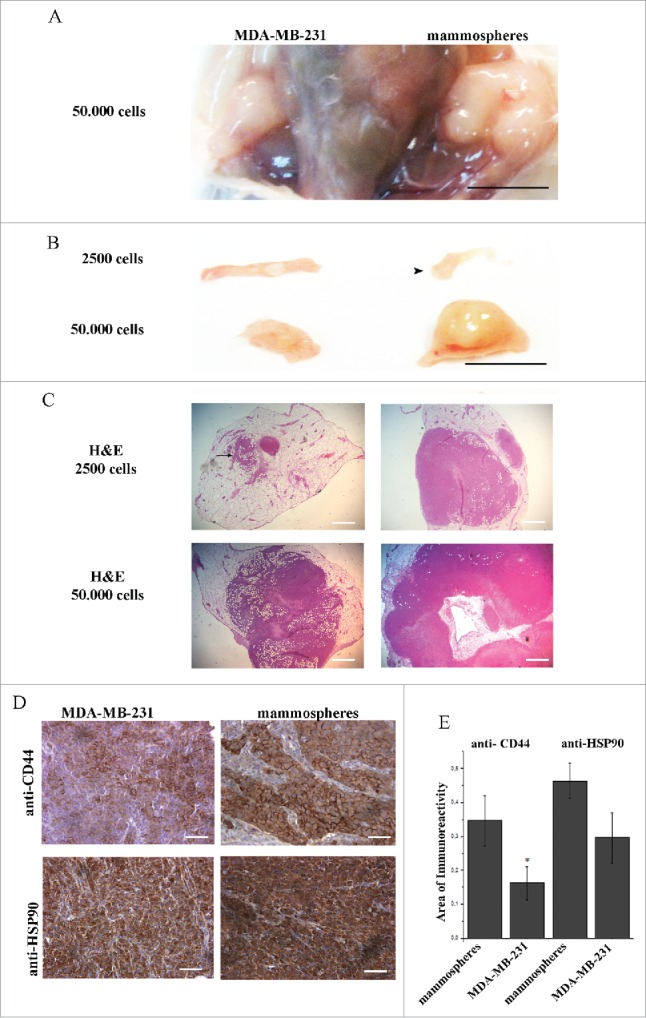
Orthotopic inoculation of MDA-MB-231 cells and mammospheres, revealed enrichment of CD44 positive cells in mammosphere-derived tumors. Eight weeks post inoculation, left and right panels show MDA-MB-231- and mammosphere-derived tumors, respectively. (A) The anatomical site demonstrating tumors formed in both mammary fat pads after inoculation of 50,000 cells/site. (B) Macroscopic pictures of orthotopic xenografts. Arrowhead shows a palpable mammosphere-derived tumor after injection of 2,500 cells. (C) Corresponding hematoxylin/eosin (H&E) stained sections of the xenografts shown in (B). Administration of 2,500 MDA-MB231 cells results in a very small niche of cancer cells (arrow). (D) Immunostaining of tumors derived from mice injected with 50,000 cells/site, using anti-CD44 and anti-HSP90 antibodies. (E) Quantification of CD44 expression showed a significant enhancement (*p < 0.05) in the mammosphere-derived tumors as compared to the MDA-MB-231-derived ones, whereas increased HSP90 expression in the mammosphere-derived tumors as compared to the MDA-MB-231 derived ones was not statistically significant. *p < 0.05. In (A) and (B) bars correspond to 10 mm, in (C) bars correspond to 1100 μm and in (D) bar corresponds to 100 μm.
MAb4C5 has a prophylactic effect on the development of primary tumors derived from MDA-MB-231 cells and mammospheres
We next investigated the possible prophylactic effect of mAb4C5 on primary tumor growth using the high dose protocol, ie 50,000 cells/injection site. The treatment scheme followed is described in detail in Materials and Methods. Our results showed that 8 weeks after inoculation, both the MDA-MB-231 and the mammosphere derived tumors were smaller in the mAb4C5 treated mice as compared to the controls (Fig. 5A). Weight measurements of tumors (Fig. 5B) revealed a 33.3% and 40.4% reduction in the mAb4C5-treated mice regarding the MDA-MB-231 and mammosphere derived tumors, respectively, when compared to the controls. When tumors from the above cases were sectioned and immunolabeled with anti-Ki-67, to monitor proliferating cells,32 an important reduction of Ki-67 positive cells was observed in mAb4C5 treated animals with respect to controls both in the MDA-MB-231- and the mammosphere-derived tumors (Fig. 5C). More specifically, MDA-MB-231-derived tumors of the mAb4C5 treated group exhibited a 47.34% reduction in proliferation compared to controls, whereas mammosphere-derived tumors of the mAb4C5-treated group showed a 49.1% reduction compared to the untreated tumors (Fig. 5D).
Figure 5.
MAb4C5 inhibits primary tumor growth in an in vivo prophylactic model. Eight weeks after inoculation: (A) Gross pictures of tumors derived from MDA-MB-231 and mammosphere injected mice. Treatment of animals with mAb 4C5 significantly inhibited tumor growth as compared to the control mice. (B) Mean weight measurements of the MDA-MB-231- and mammosphere-derived tumors were 0.25g and 0.80g respectively in the control group, 0.16g and 0.47g respectively in the mAb4C5 treated group. These values mirror a 33.3% and 40.4% weight reduction of MDA-MB-231- and mammosphere-derived tumors respectively, by mAb4C5 treatment, compared to non-treated controls. (C) Immunolabeling of tumors corresponding to the above cases using the anti-Ki-67 antibody. (D) Treatment with mAb4C5 resulted in a 40.8% and 47.9% reduction of Ki-67 positive cells in the MDA-MB-231- and mammosphere-derived tumors respectively, when compared to controls.*p < 0.05, ***p value < 0.0001. Bars correspond to 200 μm.
MAb4C5 alone and combined with paclitaxel has a therapeutic effect on the progression of primary tumors derived from mammospheres
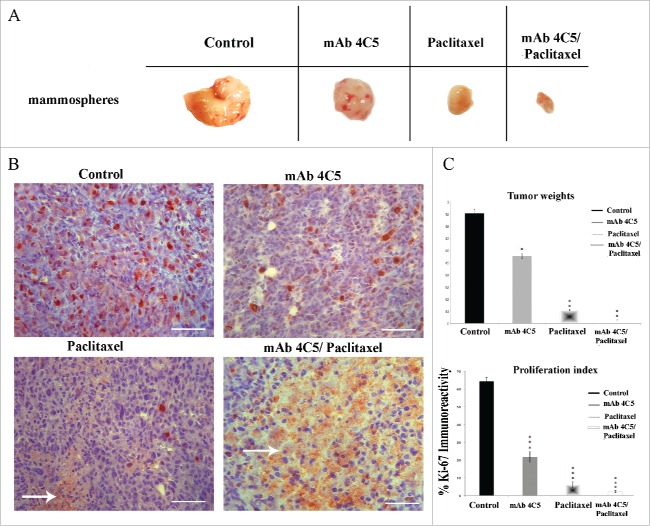
Considering accumulating evidence incriminating BCSC as the sub-population of cancer cells responsible for tumor initiation and metastasis,33 we went on to investigate the potential therapeutic effect of mAb4C5 on established primary tumors. Additionally we examined the possible therapeutic capacity of mAb4C5 when combined with paclitaxel, an established anti-cancer agent used in the clinic.34 The protocol followed was as described comprehensively in Materials and Methods. Our results showed that treatment of mice having palpable tumors with mAb4C5, paclitaxel or a combination of the two, resulted in statistically significant tumor regression in all cases compared to controls (Fig. 6A). More precisely, in the mAb4C5, paclitaxel and mAb4C5/paclitaxel groups a 44.2%, 88.4% and 97.3% reduction of tumor weight was obtained, respectively, in comparison to the control group (Fig. 6C). Ki-67 immunohistochemical analysis and quantification of these tumors revealed reduced immunolabeling in the mAb 4C5 treated mice (67.3%), which became even less in the paclitaxel group (90.1%), and was almost absent when the animals received a combination of the 2 agents (96.7%), compared to controls (Fig. 6B and C). Moreover, areas of cell necrosis were observed in paclitaxel-treated mice, and were markedly enhanced in the mAb4C5/paclitaxel group (arrows in Fig. 6B). Importantly, using either tumor weight or the Ki-67 proliferation index as criteria, the combinational treatment of mice with mAb4C5 and paclitaxel resulted in a far more significant reduction as compared to the mono-therapeutic protocols.
Figure 6.
MAb4C5 alone and combined with paclitaxel, causes tumor regression in an in vivo therapeutic tumor model. Eight weeks after inoculation: (A) Gross pictures of mammosphere-derived tumors, treated or not with mab4C5, paclitaxel and a combination of both. (B) Immunolabeling of tumors corresponding to the above cases with the anti-Ki-67 antibody. Arrows show areas of cell necrosis. (C) Mean tumor weight measurements were 0.90g, 0.55g, 0.11g and 0.02g for the control, mAb4C5, paclitaxel and mAb4C5/paclitaxel-treated groups, respectively. These values corresponded to a 44.2%, 88.4%and 97.3% reduction in tumor weight of the mAb4C5, paclitaxel and mAb4C5/paclitaxel groups, respectively, as compared to controls. Quantification of Ki-67 immunolabeling of the mAb4C5-, paclitaxel- and mAb4C5/paclitaxel-treated mice, showed a 67.3%, 90.1%and 96.7% decrease in proliferation, respectively, when compared to controls.*p < 0.05,**p < 0.001, ***p value < 0.0001. Bars correspond to 200 μm.
Discussion
BCSC are shown to exhibit unique characteristics including tumorigenicity, self-renewal, differentiation, metastatic potential and most importantly, therapeutic resistance to most anti-cancer agents.8 Consequently there is an urgent need, not only to further define the nature of heterogeneity in breast tumors, but also to develop new therapeutic protocols for targeting this sub-population of “hard core” cancer cells. HSP90 has been considered as an attractive molecular target for cancer therapy for more than a decade, because it can simultaneously affect numerous oncogenic proteins and pathways.35 We have previously reported that the selective targeting of eHSP90 with mAb4C5 results in inhibition of breast cancer cell invasion in vitro and metastatic deposition of breast cancer cells in vivo.
Our present findings demonstrate that eHSP90 is over-expressed on the surface of BCSC derived from MDA-MB-231, MDA-MB-453 and MCF-7 breast cancer cell lines. Moreover we show that mAb 4C5 inhibits colony formation of these cells, and in vivo primary tumor growth of MDA-MB-231 cells and their corresponding mammospheres. Finally and most importantly, we demonstrate that this antibody has a therapeutic effect on established tumors derived from BCSC originating from MDA-MB-231 cells, alone and in combination with the approved anti-cancer agent paclitaxel.
In order to study the expression of eHSP90 on BCSC, we initially generated a BCSC sub-population by creating mammospheres from the breast cancer cell lines studied. Double immunofluorescence labeling against CD44 and CD24 molecules revealed that most of the cells in the parental cell cultures showed a CD44low/CD24+ phenotype, but this was reversed in the mammosphere cultures, which were greatly enriched in cells of the CD44+/CD24−/low phenotype, an established molecular profile for BCSC.12 Moreover double immunofluorescence experiments using the BCSC markers CD49f and Sox2 confirmed that these proteins were overexpressed in the mammosphere cultures, compared to the parental cells.
We have previously demonstrated the presence of eHSP90 in several cancer cell lines, including the MDA-MB-231 breast cancer cell line where it interacts with the ErbB receptor EGFR.36 In the present study we examined the expression of eHSP90 on mammospheres derived from MDA-MB-231, MDA-MB-453 and MCF-7 breast cancer cells, and compared it to that in the parental cells. Double immunolabeling experiments on mammospheres using anti-CD44 and anti-HSP90 antibodies, revealed the co-localization of these molecules on the cell surface of the great majority of cells, thus confirming presence of eHSP90 on BCSC. We next compared the expression of eHSP90 between mammospheres and their parental cells, using polyclonal anti-HSP90 and mAb4C5. Interestingly, a higher expression of eHSP90 was observed in mammospheres when compared to the parental cells using both antibodies. Taking into account the documented metastatic potential of BCSC37 this result is in line with previously reported data relating eHSP90 with invasion and metastasis.24,26,27 These observations suggest that eHSP90 could be considered as a potential CSC marker in the case of breast cancer.
In previous studies, we show that mAb4C5 inhibits cancer cell invasion and metastasis by selectively targeting eHSP90. In this study we sought to investigate the effect of mAb 4C5 on tumor growth in vitro and in vivo. Anchorage independent colony formation assays were performed using MDA-MB-231, MDA-MB-453 and MCF-7cells and their corresponding mammospheres in the absence and presence of mAb4C5. Our results revealed that the parental cells showed poor survival under these conditions in control cultures. This is not surprising since substrate adherence is necessary for these cells to grow.30,38,39 The presence of mAb4C5 in the culture medium resulted in an even greater cell necrosis, suggesting that eHSP90 may be involved either in cell-cell and/or cell-substrate interactions or in cell proliferation processes. In line with our suggestion regarding the participation of eHSP90 in cell-substrate interactions, there are previously reported findings, which show that eHSP90 in MDA-MB-231 cells interacts with fibronectin and is involved in fibronectin matrix assembly and/or stability.40 Regarding mammospheres grown in the absence of mAb4C5, an important number of colonies was formed which is suggestive of the stemness of these cells. However, when mAb 4C5 was included in the culture medium, colony formation was dramatically reduced. It is interesting to note that in the mammosphere cultures, no cell necrosis was observed either in the absence or the presence of mAb 4C5. These results further suggest that eHSP90 may be essential either for the cell-cell interactions occurring during colony formation or for cell proliferation events that are necessary for sphere formation. Further experimentation is necessary, however, to elucidate the precise molecular mechanisms underlying the above observations. Similar results to ours were reported for the adhesion molecule P-cadherin. More precisely, Vieira et al.41 showed that P-cadherin is not only a BCSC biomarker, but might also be directly related to stem cell activity, since a sub-population of BT549 breast cancer cells with high levels of P-cadherin expression had an increased colony formation capacity when compared to cells derived from the same parental line with low-level P-cadherin expression.
Prompted by the in vitro data we further studied the role of mAb4C5 in primary tumor growth of MDA-MB-231 cells in vivo. To establish an experimental model, orthotopic inoculations into the mammary fat pads of NOD/SCID mice were performed using MDA-MB-231 cells and mammospheres. The injection of mammospheres resulted in larger tumors than the injection of parental cells. This result was consistent both for the low and high dose protocols used, ie 2,500 and 50,000 cells respectively. As anticipated, immunohistochemical analysis revealed that mammosphere-derived tumors were enriched in CD44 positive cells while tumors originating from both MDA-MB-231 cells and mammospheres were enriched in HSP90 expression. A minor increase in the levels of HSP90 immunoreactivity was observed in the mammosphere tumors, thus confirming our in vitro immunochemical results. Notably, abundant HSP90 immunolabeling in both tumor types is not surprising, since tissue section immunostaining reveals not only the membrane but also the cytoplasmic pool of HSP90 which, as is well documented is high in breast cancer cells.42 These results further confirm the stemness of the mammospheres generated in this study and are in line with previously reported data demonstrating that orthotopic inoculation of mammospheres into the mammary fat-pad of NOD/SCID mice give rise to much larger tumors with respect to the ones formed from the parental MDA-MB-231 cell line.12 In this study, and in a first attempt to assess the effect of mAb4C5 on primary tumor growth we initially applied a prophylactic model, orthotopically engrafting 50,000 of MDA-MB-231 cells or mammospheres, and commencing treatment or not with mAb4C5, 2 d after cancer cell injection. Our results showed that for both MDA-MB-231 and mammosphere-derived tumors, mAb4C5 had a statistically significant inhibitory effect when compared to controls, as judged by tumor weight measurements and cell proliferation monitoring, using the Ki-67 index. These data are in accordance with our in vitro observations, suggesting that eHSP90 may be involved either in cell-matrix and/or cell-cell interactions, or cell proliferation processes, necessary for tumor growth. Surface molecules participating in cell/cell and cell/matrix interactions during breast tumor growth have been reported previously. Such examples are the Notch receptors,43 the insulin-like growth factor I receptor44 and the CD44 receptor, which is also an established marker for BCSC.45 Interestingly, and although EGFR over-expression is associated with breast cancer cell proliferation and invasion,46 Kari et al.47 reported that malignant tumor cells faced with inadequate cell-matrix contacts, critically depend on EGFR activation for survival. Taking into account that mAb4C5 significantly inhibits the growth of MDA-MB-231 and mammosphere tumors, we next examined the possible therapeutic effect of this antibody on palpable tumors derived from mammospheres, taking into consideration that this sub-population is greatly enriched in cells with the CD44+/CD24−/low BCSC phenotype and additionally over-expresses eHSP90. Moreover, using this protocol, we also investigated the combined effect of mAb4C5 and paclitaxel, an anticancer used in the clinic.48 Our results showed that a 4 week daily treatment with mAb4C5, of palpable tumors that were formed 1 month after the initial inoculations, resulted in a reduction of tumor development as judged by tumor weight measurements and Ki-67 immunolabeling. As expected, treatment with paclitaxel resulted in a much more profound decrease of tumor growth. In this case areas of cell necrosis could be observed by microscopic analysis of tumors. This however was not surprising since paclitaxel, targets cancer cells by driving them to apoptosis.29 When administration of mAb 4C5 was combined with paclitaxel, regression of the tumors was statistically very significant, as estimated by weight measurements and Ki-67 immunolabeling which was almost absent. Additionally, in these tumors vast areas of cell necrosis were observed. At this point it should be noted that HSP90 expression in all cases studied and in the non-necrotic areas of the tumors, is approximately the same and independent of the treatment applied (data not shown). The above results indicate that a combinational treatment of breast cancer tumors using mAb4C5 and paclitaxel could be considered as a promising new therapeutic protocol. However further studies need to be made in order to claim a synergistic relation between these 2 molecules. Similar results to ours were reported by Sawai et al.49 who showed that co-administration of the HSP90 inhibitor 17-AAG and paclitaxel to mutant EGFR lung adenocarcinoma xenografts, was significantly more effective than administration of either drug alone. Previous works have reported that targeting HSP90 in MDA-MB-231 cells in vitro and in vivo with small molecule inhibitors, induced down regulation and inactivation of numerous client oncoproteins involved in cell proliferation, and invasive potential, including the tyrosine kinase receptor EGFR.46,50 Moreover Huang et al.,51 have recently shown that FW-04- 806, a novel HSP90 inhibitor, displayed anti-tumor activity against breast cancer cells by inhibiting the HSP90/Cdc37 interaction. We have previously demonstrated the interaction of eHSP90 with EGFR and eCdc37 on the cell surface of MDA-MB-231 cells.36 Moreover in the same work we have reported that mAb4C5 disrupts the formation and/or stabilization of the eHSP90/eCdc37/EGFR complex thus leading to inhibition of the invasive capacity of the MDA-MB-231 cells. Taking into account all the above, it is tempting to speculate that eHSP90, by acting as an extracellular chaperone, is crucial not only for the invasive potential of breast cancer cells24,27 but also for the maintenance of stem cell properties such as self-renewal capacity and tumorigenic ability that are necessary for primary tumor growth. Finally our results further support and re-inforce the anti-cancer potential of mAb 4C5. More precisely they show that by targeting eHSP90, this antibody can significantly inhibit primary growth of MDA-MB-231 breast tumors but can also induce, either alone or in combination with paclitaxel, important regression of BCSC derived tumors.
In conclusion the present study shows that eHSP90 is over-expressed in mammospheres derived from the MDA-MB-231 MDA-MB-453 and MCF-7 breast cancer cell lines, that are enriched in cells of CD44+/CD24−/low BCSC phenotype and additionally over-express BCSC markers CD49f and Sox2. Moreover it demonstrates that targeting of eHSP90 with mAb4C5, inhibits cancer stem cell activity in vitro, as judged by the reduced capacity of mammospheres to form colonies. Finally it reveals that eHSP90 participates in BCSC-derived primary tumor growth. More specifically, we demonstrate the in vivo anti-cancer activity of mAb4C5 on MDA-MB-231 primary tumor growth, and more importantly its effect either alone or in combination with paclitaxel on the regression of established tumors derived from BCSC.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors would like to thank Dr. K.Soteriadou for kindly providing the LD33 antibody and Dr. Lesley Probert for reviewing and editing the manuscript.
Funding
This research was co–financed by the European Union (European Social Fund—ESF) and Greek national funds through the Operational Program “Education and Lifelong Learning” of the National Strategic Refer- ence Framework (NSRF)—Research Funding Program: ARCHIMEDES III 770 Investing in knowledge society through the European Social Fund.
Authors' contributions
EP and DT designed and conceived the study. TS, DS, and DT developed the experimental methodologies. TS, DS, GV, and DT carried out all experiments. TS, DS and EP wrote the manuscript. EP and DT supervised the study. All authors read and approved the final manuscript.
References
- 1.Vogelstein B, Kinzler KW. The multistep nature of cancer. Trends Genet: TIG 1993; 9:138-41; PMID:8516849; http://dx.doi.org/ 10.1016/0168-9525(93)90209-Z [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000; 100:57-70; PMID:10647931; http://dx.doi.org/ 10.1016/S0092-8674(00)81683-9 [DOI] [PubMed] [Google Scholar]
- 3.Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature 2001; 411:342-8; PMID:11357141; http://dx.doi.org/ 10.1038/35077213 [DOI] [PubMed] [Google Scholar]
- 4.Dalerba P, Cho RW, Clarke MF. Cancer stem cells: models and concepts. Ann Rev Med 2007; 58:267-84; PMID:17002552; http://dx.doi.org/ 10.1146/annurev.med.58.062105.204854 [DOI] [PubMed] [Google Scholar]
- 5.Hill RP, Perris R. “Destemming” cancer stem cells. J Natl Cancer Inst 2007; 99:1435-40; PMID:17895479; http://dx.doi.org/ 10.1093/jnci/djm136 [DOI] [PubMed] [Google Scholar]
- 6.Fabian A, Barok M, Vereb G, Szollosi J. Die hard: are cancer stem cells the Bruce Willises of tumor biology? Cytometry Part A: J Int Soc Analytical Cytol 2009; 75:67-74; PMID:19051297; http://dx.doi.org/ 10.1002/cyto.a.20690 [DOI] [PubMed] [Google Scholar]
- 7.Rosen JM, Jordan CT. The increasing complexity of the cancer stem cell paradigm. Science (New York, NY) 2009; 324:1670-3; PMID:19556499; http://dx.doi.org/ 10.1126/science.1171837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geng SQ, Alexandrou AT, Li JJ. Breast cancer stem cells: multiple capacities in tumor metastasis. Cancer Lett 2014; 349:1-7; PMID:24727284; http://dx.doi.org/ 10.1016/j.canlet.2014.03.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu H, Clauser KR, Tam WL, Frose J, Ye X, Eaton EN, Reinhardt F, Donnenberg VS, Bhargava R, Carr SA, et al.. A breast cancer stem cell niche supported by juxtacrine signalling from monocytes and macrophages. Nat Cell Biol 2014; 16:1105-17; PMID:25266422; http://dx.doi.org/ 10.1038/ncb3041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A 2003; 100:3983-8; PMID:12629218; http://dx.doi.org/ 10.1073/pnas.0530291100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmed MA, Aleskandarany MA, Rakha EA, Moustafa RZ, Benhasouna A, Nolan C, Green AR, Ilyas M, Ellis IO. A CD44(−)/CD24(+) phenotype is a poor prognostic marker in early invasive breast cancer. Breast Cancer Res Treat 2012; 133:979-95; PMID:22119938; http://dx.doi.org/ 10.1007/s10549-011-1865-8 [DOI] [PubMed] [Google Scholar]
- 12.Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, Wicha MS. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev 2003; 17:1253-70; PMID:12756227; http://dx.doi.org/ 10.1101/gad.1061803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ponti D, Costa A, Zaffaroni N, Pratesi G, Petrangolini G, Coradini D, Pilotti S, Pierotti MA, Daidone MG. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res 2005; 65:5506-11; PMID:15994920; http://dx.doi.org/ 10.1158/0008-5472.CAN-05-0626 [DOI] [PubMed] [Google Scholar]
- 14.Liu Y, Nenutil R, Appleyard MV, Murray K, Boylan M, Thompson AM, Coates PJ. Lack of correlation of stem cell markers in breast cancer stem cells. Br J Cancer 2014; 110:2063-71; PMID:24577057; http://dx.doi.org/ 10.1038/bjc.2014.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kitano H. Cancer robustness: tumour tactics. Nature 2003; 426:125; PMID:14614483; http://dx.doi.org/ 10.1038/426125a [DOI] [PubMed] [Google Scholar]
- 16.Neckers L. Hsp90 inhibitors as novel cancer chemotherapeutic agents. Trends Mol Med 2002; 8:S55-61; PMID:11927289; http://dx.doi.org/ 10.1016/S1471-4914(02)02316-X [DOI] [PubMed] [Google Scholar]
- 17.Zhang H, Burrows F. Targeting multiple signal transduction pathways through inhibition of Hsp90. J Mol Med (Berlin, Germany) 2004; 82:488-99; PMID:15168026 [DOI] [PubMed] [Google Scholar]
- 18.Calderwood SK, Khaleque MA, Sawyer DB, Ciocca DR. Heat shock proteins in cancer: chaperones of tumorigenesis. Trends Biochem Sci 2006; 31:164-72; PMID:16483782; http://dx.doi.org/ 10.1016/j.tibs.2006.01.006 [DOI] [PubMed] [Google Scholar]
- 19.Isaacs JS, Xu W, Neckers L. Heat shock protein 90 as a molecular target for cancer therapeutics. Cancer Cell 2003; 3:213-7; PMID:12676580; http://dx.doi.org/ 10.1016/S1535-6108(03)00029-1 [DOI] [PubMed] [Google Scholar]
- 20.Maloney A, Workman P. HSP90 as a new therapeutic target for cancer therapy: the story unfolds. Exp Opin Biol Ther 2002; 2:3-24; PMID:11772336; http://dx.doi.org/ 10.1517/14712598.2.1.3 [DOI] [PubMed] [Google Scholar]
- 21.Picard Lb. Hsp90 molecular chaperone complex; Department of Cell Biology University of Geneva March 2016 Switzerland; http://www.picard.ch/downloads [Google Scholar]
- 22.Trepel J, Mollapour M, Giaccone G, Neckers L. Targeting the dynamic HSP90 complex in cancer. Nat Rev Cancer 2010; 10:537-49; PMID:20651736; http://dx.doi.org/ 10.1038/nrc2887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eustace BK, Jay DG. Extracellular roles for the molecular chaperone, hsp90. Cell Cycle (Georgetown, Tex) 2004; 3:1098-100; PMID:15326368; http://dx.doi.org/ 10.4161/cc.3.9.1088 [DOI] [PubMed] [Google Scholar]
- 24.Stellas D, El Hamidieh A, Patsavoudi E. Monoclonal antibody 4C5 prevents activation of MMP2 and MMP9 by disrupting their interaction with extracellular HSP90 and inhibits formation of metastatic breast cancer cell deposits. BMC Cell Biol 2010; 11:51; PMID:20602761; http://dx.doi.org/ 10.1186/1471-2121-11-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomaidou D, Patsavoudi E. Identification of a novel neuron-specific surface antigen in the developing nervous system, by monoclonal antibody 4C5. Neuroscience 1993; 53:813-27; PMID:8487957; http://dx.doi.org/ 10.1016/0306-4522(93)90626-Q [DOI] [PubMed] [Google Scholar]
- 26.Stellas D, Karameris A, Patsavoudi E. Monoclonal antibody 4C5 immunostains human melanomas and inhibits melanoma cell invasion and metastasis. Clin Cancer Res: Off J Am Assoc Cancer Res 2007; 13:1831-8; PMID:17363539; http://dx.doi.org/ 10.1158/1078-0432.CCR-06-1585 [DOI] [PubMed] [Google Scholar]
- 27.Sidera K, Gaitanou M, Stellas D, Matsas R, Patsavoudi E. A critical role for HSP90 in cancer cell invasion involves interaction with the extracellular domain of HER-2. J Biol Chem 2008; 283:2031-41; PMID:18056992; http://dx.doi.org/; http://dx.doi.org/ 10.1074/jbc.M701803200 [DOI] [PubMed] [Google Scholar]
- 28.Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly in vitro by taxol. Nature 1979; 277:665-7; PMID:423966; http://dx.doi.org/ 10.1038/277665a0 [DOI] [PubMed] [Google Scholar]
- 29.Ferlini C, Cicchillitti L, Raspaglio G, Bartollino S, Cimitan S, Bertucci C, Mozzetti S, Gallo D, Persico M, Fattorusso C, et al.. Paclitaxel directly binds to Bcl-2 and functionally mimics activity of Nur77. Cancer Res 2009; 69:6906-14; PMID:19671798; http://dx.doi.org/ 10.1158/0008-5472.CAN-09-0540 [DOI] [PubMed] [Google Scholar]
- 30.Wang R, Lv Q, Meng W, Tan Q, Zhang S, Mo X, Yang X. Comparison of mammosphere formation from breast cancer cell lines and primary breast tumors. J Thoracic Dis 2014; 6:829-37; PMID:24977009; http://dx.doi.org/ 10.3978/j.issn.2072-1439.2014.03.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duan C, Yang X, Zhang X, Feng J, Liu Z, Que H, Johnson H, Zhao Y, Fan Y, Lu Y, et al.. Generation of monoclonal antibodies against MGA and comparison of their application in breast cancer detection by immunohistochemistry. Sci Rep 2015; 5:13073; PMID:26272389; http://dx.doi.org/ 10.1038/srep13073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jonat W, Arnold N. Is the Ki-67 labelling index ready for clinical use? Ann Oncol 2011; 22:500-2; PMID:21343384; http://dx.doi.org/ 10.1093/annonc/mdq732 [DOI] [PubMed] [Google Scholar]
- 33.Wong SY, Kumar S. Matrix regulation of tumor-initiating cells. Prog Mol Biol Trans Sci 2014; 126:243-56; PMID:25081621; http://dx.doi.org/ 10.1016/B978-0-12-394624-9.00010-5 [DOI] [PubMed] [Google Scholar]
- 34.Liu P, Kumar IS, Brown S, Kannappan V, Tawari PE, Tang JZ, Jiang W, Armesilla AL, Darling JL, Wang W. Disulfiram targets cancer stem-like cells and reverses resistance and cross-resistance in acquired paclitaxel-resistant triple-negative breast cancer cells. Br J Cancer 2013; 109:1876-85; PMID:24008666; http://dx.doi.org/ 10.1038/bjc.2013.534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sidera K, Patsavoudi E. HSP90 inhibitors: current development and potential in cancer therapy. Recent Patents Anti-Cancer Drug Disc 2014; 9:1-20; PMID:23312026; http://dx.doi.org/ 10.2174/15748928113089990031 [DOI] [PubMed] [Google Scholar]
- 36.El Hamidieh A, Grammatikakis N, Patsavoudi E. Cell surface Cdc37 participates in extracellular HSP90 mediated cancer cell invasion. Plos One 2012; 7:e42722; PMID:22912728; http://dx.doi.org/ 10.1371/journal.pone.0042722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Allan AL, Vantyghem SA, Tuck AB, Chambers AF. Tumor dormancy and cancer stem cells: implications for the biology and treatment of breast cancer metastasis. Breast Dis 2006; 26:87-98; PMID:17473368 [DOI] [PubMed] [Google Scholar]
- 38.Chiotaki R, Polioudaki H, Theodoropoulos PA. Differential nuclear shape dynamics of invasive andnon-invasive breast cancer cells are associated with actin cytoskeleton organization and stability. Biochem Cell Biol = Biochimie et Biologie Cellulaire 2014; 92:287-95; PMID:25053513; http://dx.doi.org/ 10.1139/bcb-2013-0120 [DOI] [PubMed] [Google Scholar]
- 39.Kim SY, Kang JW, Song X, Kim BK, Yoo YD, Kwon YT, Lee YJ. Role of the IL-6-JAK1-STAT3-Oct-4 pathway in the conversion of non-stem cancer cells into cancer stem-like cells. Cell Signalling 2013; 25:961-9; PMID:23333246; http://dx.doi.org/ 10.1016/j.cellsig.2013.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hunter MC, O'Hagan KL, Kenyon A, Dhanani KC, Prinsloo E, Edkins AL. Hsp90 binds directly to fibronectin (FN) and inhibition reduces the extracellular fibronectin matrix in breast cancer cells. Plos One 2014; 9:e86842; PMID:24466266; http://dx.doi.org/ 10.1371/journal.pone.0086842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vieira AF, Ricardo S, Ablett MP, Dionisio MR, Mendes N, Albergaria A, Farnie G, Gerhard R, Cameselle-Teijeiro JF, Seruca R, et al.. P-cadherin is coexpressed with CD44 and CD49f and mediates stem cell properties in basal-like breast cancer. Stem Cells (Dayton, Ohio) 2012; 30:854-64; PMID:22389315; http://dx.doi.org/ 10.1002/stem.1075 [DOI] [PubMed] [Google Scholar]
- 42.Pick E, Kluger Y, Giltnane JM, Moeder C, Camp RL, Rimm DL, Kluger HM. High HSP90 expression is associated with decreased survival in breast cancer. Cancer Res 2007; 67:2932-7; PMID:17409397; http://dx.doi.org/ 10.1158/0008-5472.CAN-06-4511 [DOI] [PubMed] [Google Scholar]
- 43.Baker AT, Zlobin A, Osipo C. Notch-EGFR/HER2 bidirectional crosstalk in breast cancer. Front Oncol 2014; 4:360; PMID:25566499; http://dx.doi.org/ 10.3389/fonc.2014.00360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mauro L, Salerno M, Morelli C, Boterberg T, Bracke ME, Surmacz E. Role of the IGF-I receptor in the regulation of cell-cell adhesion: implications in cancer development and progression. J Cell Physiol 2003; 194:108-16; PMID:12494449; http://dx.doi.org/ 10.1002/jcp.10207 [DOI] [PubMed] [Google Scholar]
- 45.Li L, Qi L, Liang Z, Song W, Liu Y, Wang Y, Sun B, Zhang B, Cao W. Transforming growth factor-beta1 induces EMT by the transactivation of epidermal growth factor signaling through HA/CD44 in lung and breast cancer cells. Int J Mol Med 2015; 36:113-22; PMID:26005723; http://dx.doi.org/ 10.1007/s00894-015-2654-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caldas-Lopes E, Cerchietti L, Ahn JH, Clement CC, Robles AI, Rodina A, Moulick K, Taldone T, Gozman A, Guo Y, et al.. Hsp90 inhibitor PU-H71, a multimodal inhibitor of malignancy, induces complete responses in triple-negative breast cancer models. Proc Natl Acad Sci U S A 2009; 106:8368-73; PMID:19416831; http://dx.doi.org/ 10.1073/pnas.0903392106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kari C, Chan TO, Rocha de Quadros M, Rodeck U. Targeting the epidermal growth factor receptor in cancer: apoptosis takes center stage. Cancer Res 2003; 63:1-5; PMID:12517767 [PubMed] [Google Scholar]
- 48.Cleator S, Heller W, Coombes RC. Triple-negative breast cancer: therapeutic options. Lancet Oncol 2007; 8:235-44; PMID:17329194; http://dx.doi.org/ 10.1016/S1470-2045(07)70074-8 [DOI] [PubMed] [Google Scholar]
- 49.Sawai A, Chandarlapaty S, Greulich H, Gonen M, Ye Q, Arteaga CL, Sellers W, Rosen N, Solit DB. Inhibition of Hsp90 down-regulates mutant epidermal growth factor receptor (EGFR) expression and sensitizes EGFR mutant tumors to paclitaxel. Cancer Res 2008; 68:589-96; PMID:18199556; http://dx.doi.org/ 10.1158/0008-5472.CAN-07-1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Friedland JC, Smith DL, Sang J, Acquaviva J, He S, Zhang C, Proia DA. Targeted inhibition of Hsp90 by ganetespib is effective across a broad spectrum of breast cancer subtypes. Investigat New Drugs 2014; 32:14-24; PMID:23686707; http://dx.doi.org/ 10.1007/s10637-013-9971-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang W, Ye M, Zhang LR, Wu QD, Zhang M, Xu JH, Zheng W. FW-04-806 inhibits proliferation and induces apoptosis in human breast cancer cells by binding to N-terminus of Hsp90 and disrupting Hsp90-Cdc37 complex formation. Mol Cancer 2014; 13:150; PMID:24927996; http://dx.doi.org/ 10.1186/1476-4598-13-150 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.