ABSTRACT
The targeting protein for Xenopus kinesin-like protein 2 (TPX2) is a putative oncogene in different human cancers. This study assessed TPX2 expression in gastric cancer tissue samples and then determined the effects of TPX2 knockdown on the regulation of gastric cancer cell malignant behaviors in vitro. Tissue samples from 115 gastric cancer patients were analyzed for TPX2 expression. The effects of TPX2 siRNA on gastric cancer cells were assessed in vitro, including cell viability, cell cycle distribution, apoptosis, migration, and invasion. The data showed that TPX2 was overexpressed in gastric cancer tissues compared to that in the adjacent normal epithelia. Moreover, TPX2 overexpression was associated with a poor overall survival and was an independent prognostic predictor of gastric cancer. In addition, the in vitro study further confirmed the ex vivo data, i.e., knockdown of TPX2 expression reduced gastric cancer cell viability but induced apoptosis and arrested cells at the G2/M phase of the cell cycle. Knockdown of TPX2 expression also inhibited the tumor cell migration and invasion capacity in vitro. At the gene level, knockdown of TPX2 expression upregulated the levels of cyclin B1, cdk4, p53, Bax, caspase-3, and E-cadherin, but downregulated the levels of cyclin D1, cdk2, N-cadherin, slug, matrix metalloprotease (MMP)-2, and MMP-9, suggesting that knockdown of TPX2 expression suppressed tumor cell epithelial–mesenchymal transition (EMT). This study demonstrated that detection of TPX2 overexpression could serve as a prognostic marker and therapeutic target for gastric cancer.
KEYWORDS: Apoptosis, cell cycle arrest, drug target, EMT, gastric cancer, prognostic marker, TPX2
Abbreviations
- TPX2
Targeting protein for Xklp2
- EPP86
restrictedly expressed proliferation-associated protein
- EMT
epithelial–mesenchymal transition
- AJCC
American Joint Committee on Cancer
- PBS
phosphate-buffered saline
- DMEM
Dulbecco's modified Eagle's medium
- FBS
fetal bovine serum
- CCK-8
Cell Counting Kit-8
- SDS-PAGE
dodecyl sulfate-polyacrylamide gel electrophoresis
- TBS-T
Tris-based saline-Tween
Introduction
Gastric cancer is a global health problem and reported to be the fourth most commonly occurring cancer and the second leading cause of cancer-related deaths in the world, with an estimated over 1,000,000 new cases and 738,000 deaths in 2008.1,2 Although advancements in early detection, prevention, and treatment options have led to a consistent reduction of gastric cancer incidence and mortality, the absolute number of new cases is still high, which could be due to aging population or health care inequities.1 A great portion of gastric cancer is diagnosed at the advanced stages of disease; therefore, curable surgery is impossible. Moreover, such advanced and metastatic gastric cancers are not easily treated by conventional chemotherapy. The overall survival of advanced gastric cancer patients is currently approximately 10 months without surgery.3 Thus, the identification of biomarkers for early cancer detection and the search for novel treatment targets could help us to control gastric cancer in the clinic.
Emerging data from gene expression profiling indicate that differentially expressed genes in gastric cancer could provide novel targeted therapy strategies for gastric cancer patients and serve as biomarkers for early tumor detection or prognosis prediction in the clinic.4 Toward this end, our research has focused on the targeting protein for Xenopus kinesin-like protein 2 (TPX2), also known as REPP86 (restrictedly expressed proliferation-associated protein) in various human cancers.5,6 TPX2 is a 100-kDa microtubule-associated protein whose gene is localized on chromosome 20q11.1 and plays an important role in the formation of mitotic spindles.6 Thus, TPX2 expression is strictly controlled during cell cycle progression, i.e., it appears between the G1 and S phases of the cell cycle and disappears after completion of cytokinesis of cells.6,7 Alteration of TPX2 expression has been reported to be associated with human carcinogenesis.8-13 In lung, cervical, bladder, esophageal, liver, and pancreatic cancers,8-13 TPX2 is overexpressed and associated with abnormal amplification of the centrosome, aneuploidy, and malignant transformation of cells. TPX2 overexpression also has been shown to promote cell proliferation and cell cycle progression, but reduce cell apoptosis.10 Other previous studies also have reported that TPX2 overexpression is associated with tumor metastasis and a poor prognosis in several human cancers.9-13 Gastric cancer, like most other human cancers, is involved in chromosome abnormalities, cell proliferation, and apoptosis. Therefore, in this study, we aimed to assess TPX2 expression in gastric cancer tissue specimens for association with clinicopathological data and the overall survival of patients. In addition, we explored the effects of TPX2 knockdown on the regulation of gastric cancer cell malignant behaviors in vitro. Our study will provide insightful information regarding TPX2 to serve as a prognostic marker and therapeutic target for gastric cancer.
Results
Overexpression of TPX2 protein in gastric cancer tissue samples
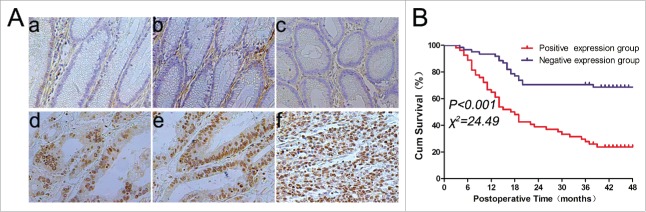
In this study, we first assessed TPX2 expression in gastric cancer and paired normal tissue samples and found that TPX2 protein was expressed in the cell nuclei. However, the expression of TPX2 protein was absent in 93/115 (81%) or at a low level in 22/115 (19%) of normal gastric mucosa samples (Fig. 1A), whereas the expression of TPX2 protein was at a high level in 54/115 (47%) of gastric cancer tissues (Fig. 1A). Therefore, TPX2 protein was overexpressed in gastric cancer tissues compared to the paired normal gastric tissues (P < 0.001).
Figure 1.
TPX2 overexpression in gastric cancer tissues and its association with the survival of gastric cancer patients. (A) Immunohistochemical staining of TPX2 protein in gastric tissues. TPX2 protein is localized in the tumor cell nuclei (d–f), whereas TPX2 protein is negative in the normal gastric mucosa (a–c). (B) Kaplan–Meier curves stratified by TPX2 expression levels in gastric cancer. The data showed that patients with higher TPX2-expressing tumors had a shorter overall survival, whereas patients with lower TPX2-expressing tumors had a better overall survival (P < 0.001).
Association of TPX2 overexpression with clinicopathological characteristics and overall survival `of gastric cancer patients
The data on the association of TPX2 overexpression with clinicopathological characteristics are shown in Table 1. Specifically, the expression of TPX2 protein in tumor tissues was strongly associated with the depth of tumor invasion (P = 0.022), lymph node metastasis (P = 0.036), distant metastasis (P < 0.001), and AJCC stage (P = 0.001). However, TPX2 expression was not associated with gender, age, tumor size, tumor location, or pathological type.
Table 1.
Association of TPX2 overexpression with clinicopathological characteristics from gastric cancer patients.
| TPX2 expression, n (%) |
||||
|---|---|---|---|---|
| Variable | Number of patients | + | − | P value |
| Gender | ||||
| Male | 78 | 34 (43.6) | 44 (56.4) | 0.29 |
| Female | 37 | 20 (54.1) | 17 (45.9) | |
| Age (yrs.) | ||||
| < 55 | 59 | 25 (42.4) | 34 (57.6) | 0.31 |
| ≥ 55 | 56 | 29 (51.8) | 27 (48.2) | |
| Tumor size (cm) | ||||
| ≤ 4.0 | 28 | 9 (32.1) | 19 (67.9) | 0.071 |
| > 4.0 | 87 | 45 (51.7) | 42 (48.3) | |
| Tumor location | ||||
| Gastric Cardia | 27 | 17 (63.0) | 10 (37.0) | |
| Body | 36 | 14 (38.9) | 22 (61.1) | |
| Antrum | 48 | 20 (41.7) | 28 (58.3) | |
| Diffuse | 4 | 3 (75.0) | 1 (25.0) | |
| Tumor differentiation | ||||
| Well | 31 | 15 (48.4) | 16 (51.6) | |
| Moderate | 39 | 15 (38.5) | 24 (61.5) | |
| Poor/undifferentiated | 45 | 24 (53.3) | 21 (46.7) | |
| Tumor stages | ||||
| T1+T2+T3+T4a | 84 | 34 (40.5) | 50 (59.5) | 0.022 |
| T4b | 31 | 20 (64.5) | 11 (35.5) | |
| Lymph node metastasis | ||||
| Absent (N0) | 32 | 10 (31.2) | 22 (68.8) | 0.036 |
| Present (N1–3) | 83 | 44 (53.0) | 39 (47.0) | |
| Distant metastasis | ||||
| Absent (M0) | 84 | 31 (36.9) | 53 (63.1) | <0.001 |
| Present (M1) | 31 | 23 (74.2) | 8 (25.8) | |
| AJCC stage | ||||
| I | 30 | 6 (20.0) | 24 (80.0) | |
| II | 13 | 5 (38.5) | 8 (61.5) | |
| III | 42 | 21 (50.0) | 21 (50.0) | |
| IV | 30 | 22 (73.3) | 8 (26.7) | |
TPX2, Targeting protein for Xklp2; AJCC, American Joint Committee on Cancer.
Distant metastases included the peritoneum, liver, transverse colon, pancreas, and bone.
Kaplan–Meier curves were plotted to stratify TPX2 expression for overall survival of patients with gastric cancer. Our data showed that TPX2 overexpression was associated with a poor overall survival of these patients (P < 0.05; Fig. 1B). Furthermore, the univariate analysis showed a significant association between overall survival and tumor size (HR = 2.84; 95% confidence interval (95%CI) = 1.34–5.98; P = 0.006), depth of invasion (HR = 2.48; 95%CI = 1.47–4.20; P < 0.001), lymph node metastasis (HR = 6.189; 95%CI = 2.46–15.51; P < 0.001), distant metastasis (HR = 8.51; 95%CI = 4.95–14.62; P < 0.001), AJCC stage (HR = 3.99; 95%CI = 2.75–5.79; P < 0.001), and TPX2 overexpression (HR = 3.60; 95%CI = 2.08–6.22; P < 0.001); however, there was no statistical significance between overall survival and gender, age, tumor location, or differentiation (Table 2). The multivariate analysis further showed that lymph node metastasis (HR = 3.19; 95%CI = 1.12–9.08; P < 0.029), distant metastasis (HR = 3.45; 95%CI = 1.18–10.07; P = 0.024), TPX2 overexpression (HR = 2.17; 95%CI = 1.23–3.81; P = 0.007), and AJCC stage (HR = 2.14; 95%CI = 1.07–4.28; P = 0.0071) were all independent predictors for a poor gastric cancer prognosis (Table 2).
Table 2.
Univariate and multivariate analyses of individual parameters for association with overall survival of gastric cancer patients.
| Univariate |
Multivariate |
|||||
|---|---|---|---|---|---|---|
| Variable | HR | CI (95%) | P value | HR | CI (95%) | P value |
| Gender | 1.16 | 0.68–1.97 | 0.58 | |||
| Age | 1.38 | 0.83–2.29 | 0.21 | |||
| Tumor location | 0.99 | 0.72–1.35 | 0.96 | |||
| Pathological type | 0.95 | 0.69–1.31 | 0.72 | |||
| Tumor size | 2.84 | 1.34–5.98 | 0.006 | 1.32 | 0.60–2.93 | 0.48 |
| Depth of invasion | 2.48 | 1.47–4.20 | 0.001 | 1.70 | 0.97–2.97 | 0.06 |
| Lymph node metastasis | 6.18 | 2.46–15.51 | <0.001 | 3.19 | 1.12–9.08 | 0.029 |
| Distant metastasis | 8.51 | 4.95–14.62 | <0.001 | 3.45 | 1.18–10.07 | 0.024 |
| AJCC stage | 3.99 | 2.75–5.79 | <0.001 | 2.14 | 1.07–4.28 | 0.031 |
| TPX2 | 3.60 | 2.08–6.22 | <0.001 | 2.17 | 1.23–3.81 | 0.007 |
HR, hazard ratio; CI, confidence interval; AJCC, American Joint Committee on Cancer; TPX2, Targeting protein for Xklp2.
Effects of TPX2 knockdown on gastric cancer cell viability, cell cycle arrest, and apoptosis
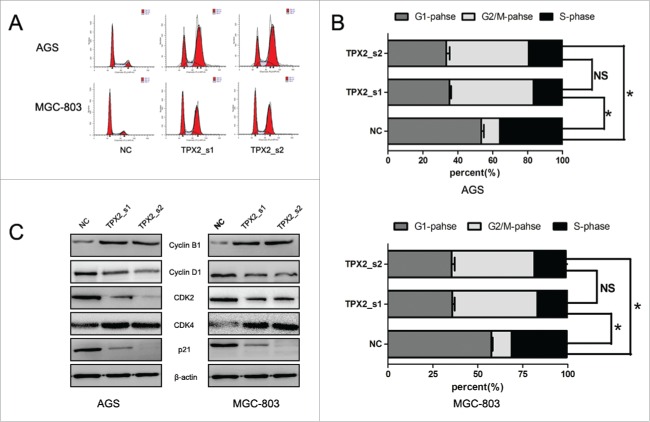
To further confirm the role of TPX2 in gastric cancer development and progression, we performed in vitro experiments by first assessing the level of TPX2 protein in 5 human gastric cancer cell lines: AGS, BGC-823, MKN-28, MGC-803, and SGC-7901. We found that TPX2 protein was highly expressed in AGS and MGC-803 cells compared to that of BGC-823, MKN-28, and SGC-7901 cells (Fig. 2A). Next, we knocked down TPX2 expression in AGS and MGC-803 cell lines using 2 TPX2 siRNA constructs and found that TPX2 expression was significantly knocked down after transient siRNA transfection for 48 h (Fig. 2B). We then assessed the effects of TPX2 knockdown on gastric cancer cell viability, cell cycle arrest, and apoptosis. Specifically, knockdown of TPX2 expression caused a marked reduction in the viability of AGS and MGC-803 cells compared to the negative siRNA control cells (P < 0.01; Fig. 2C). The cell cycle distribution data showed that knockdown of TPX2 expression had a dramatic increase in the G2-M fraction, from less than 20% in the control samples to more than 40% for TPX2_s1 siRNA and TPX2_s2 siRNA in both cell lines (P < 0.01; Fig. 3A-B), but a significant decrease in the G1 and S fraction, from more than 55% and 30% in the control samples to < 36% and 18% for TPX2_s1 and TPX2_s2 siRNA in both cell lines, respectively (P < 0.01; Fig. 3A-B). In addition, we found that TPX2 siRNA-transfected AGS and MGC-803 cells had a marked increase in apoptosis (P < 0.01; Fig. 4A-B).
Figure 2.
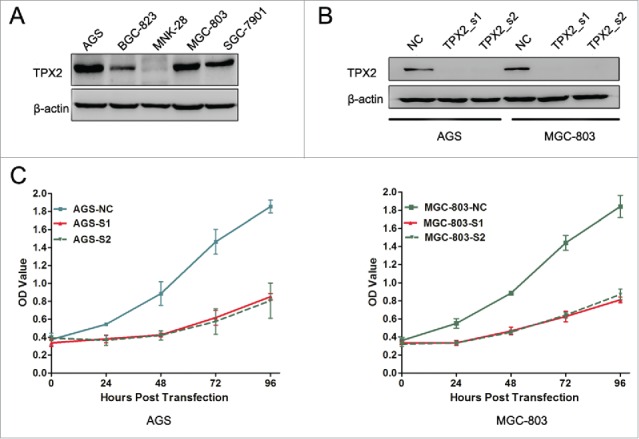
Effects of reduced TPX2 expression on the inhibition of gastric cancer cell viability. (A) Western blot analysis of TPX2 protein in gastric cancer cell lines. (B) Western blot. Two different siRNA constructs were transiently transfected into gastric cancer cells to knock down TPX2 expression. (C) CCK-8 assay. Two TPX2 siRNAs were transiently transfected into gastric cancer cells and then subjected to the CCK-8 cell viability assay.
Figure 3.
Knockdown of TPX2 expression in induction of gastric cancer cell cycle arrest. (A-B) Flow cytometric cell cycle analysis. Two TPX2 siRNAs were transiently transfected into gastric cancer cells and then subjected to flow cytometric cell cycle analysis. (C) Western blot. Two TPX2 siRNA constructs were transiently transfected into gastric cancer AGS and MGC-803 cell lines and then subjected to western blot analysis of cell cycle-related proteins. Columns, mean of 3 independent experiments; bars, SD. *P < 0.05, NS = not significant.
Figure 4.
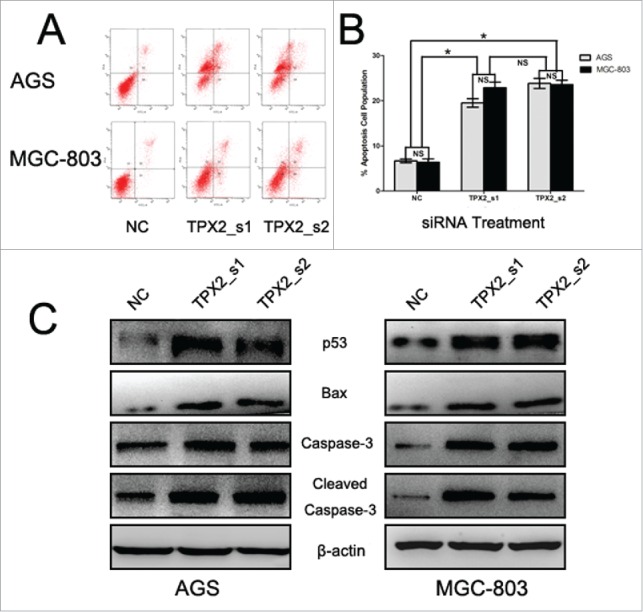
Knockdown of TPX2 expression in inhibition of gastric cancer cell apoptosis. (A-B) Flow cytometric apoptosis assay. Two TPX2 siRNAs were transiently transfected into gastric cancer cells and then subjected to a flow cytometric apoptosis assay. (C) Western blot. Two TPX2 siRNA constructs were transiently transfected into gastric cancer AGS and MGC-803 cell lines and then subjected to protein gel blot analysis of apoptosis-related proteins. Columns, mean of 3 independent experiments; bars, SD. *P < 0.05, NS = not significant.
Effects of TPX2 knockdown on inhibition of gastric cancer cell migration and invasion
Since our ex vivo data showed that TPX2 overexpression was associated with gastric cancer metastasis, we assessed whether TPX2 knockdown could inhibit gastric cancer cell migration and invasion. Our data showed that TPX2 siRNAs significantly reduced the migration (Fig. 5A) and invasion capacity (Fig. 5B) of AGS and MGC-803 cells.
Figure 5.

Knockdown of TPX2 expression in inhibition of gastric cancer cell migration and invasion. (A) Tumor cell Transwell migration assay. Two TPX2 siRNAs were transiently transfected into gastric cancer AGS and MGC-803 cell lines and subjected to the Transwell migration assay. (B) Tumor cell Transwell invasion assay. Two TPX2 siRNAs were transiently transfected into gastric cancer AGS and MGC-803 cell lines and then subjected to the Transwell invasion assay. (C) Western blot. Two TPX2 siRNAs were transiently transfected into gastric cancer AGS and MGC-803 cell lines and then subjected to western blot analysis of EMT-related proteins. Columns, mean of 3 independent experiments; bars, SD. *P < 0.05, NS = not significant.
Effects of TPX2 knockdown on the expression of cell growth, apoptosis, and epithelial–mesenchymal transition (EMT)-related genes
Next, we further explored the underlying molecular events after knockdown of TPX2 expression in gastric cancer cells. Our data showed that knockdown of TPX2 expression significantly upregulated the levels of cyclinB1 and cdk4 proteins, but downregulated the levels of cyclinD1 and cdk2 proteins (Fig. 3C). These data indicate that TPX2 regulated cell cycle progression in gastric cancer cells. Furthermore, we found that knockdown of TPX2 expression increased the expression of p53, Bax, caspase-3, and cleaved caspase-3 in AGS and MGC-803 cells (Fig. 4C). After that, we assessed tumor cell EMT-related gene expression; for example, during cell EMT, the expression of epithelial markers, such as E-cadherin, will be downregulated, whereas the expression of mesenchymal markers, such as N-cadherin and slug, and cell mobility markers, such as MMP-2 and MMP-9, will be upregulated. Our data showed that knockdown of TPX2 expression significantly modulated the expression of these proteins (Fig. 5C).
Discussion
Gastric cancer at an early stage often shows no symptoms or only causes nonspecific symptoms, such as heartburn, nausea, or loss of appetite; but by the time symptoms occur, the tumor may have reached an advanced stage.1,2 Thus, without cancer screening, like endoscopy, many patients are diagnosed with an advanced incurable disease at the time of presentation. For those with potentially resectable gastric cancer in conjunction with adjuvant chemotherapy or chemoradiotherapy, the overall 5-year survival rate can reach up to 35%;14,15 whereas the majority of patients with metastatic disease can be treated with a combination of triple chemotherapy regimens, and their overall survival rate is approximately 9 to 11 months.16,17 Therefore, research and identification of molecular markers could help clinicians to diagnose gastric cancer early and to improve the survival rate. In the current study, we detected TPX2 expression in gastric tissue specimens for association with gastric cancer behaviors and the overall survival of patients. Then, we assessed the effects of TPX2 knockdown on the regulation of gastric cancer cell malignant behaviors in vitro to confirm our ex vivo data. We found that TPX2 protein was overexpressed in tumor tissues compared to that in the adjacent normal epithelia, the expression of which was associated with the depth of tumor invasion, tumor lymph node and distant metastases, and advanced AJCC stages. Furthermore, our data showed that TPX2 overexpression was associated with a poor overall survival and was an independent prognostic predictor of gastric cancer. Our in vitro data demonstrated that knockdown of TPX2 expression reduced gastric cancer cell viability but induced apoptosis and arrested tumor cells at the G2/M phase of the cell cycle. Knockdown of TPX2 expression also inhibited the tumor cell migration and invasion capacity in vitro. At the gene level, knockdown of TPX2 expression upregulated the expression of cyclinB1, cdk4, p53, Bax, caspase-3, and E-cadherin, but downregulated the levels of cyclinD1, cdk2, N-cadherin, slug, MMP-2, and MMP-9. Our current study demonstrated that TPX2 protein could serve as a prognostic marker and therapeutic target for gastric cancer.
Indeed, cancer development is characterized by uncontrolled cell proliferation and transformation. Moreover, at the molecular level, a great number of genes are altered, including proteins that regulate cell proliferation and death as well as genomic stability.18,19 In this context, the human TPX2 gene is localized at chromosome 20q11, a region that has been shown to be amplified frequently in human cancers.20,21 Other studies have demonstrated that the DNA copy number of chromosome 20q is increased and associated with TPX2 overexpression in various cancers,22-25 whereas additional studies have shown that TPX2 overexpression in many cancers is not related to DNA amplification.26,27 Although our current study did not assess DNA copy number changes, our data, for the first time, show TPX2 overexpression in gastric cancer tissues. TPX2 overexpression was associated with gastric cancer progression and a poor overall survival of patients, which is consistent with previous studies in other cancer types.8,9,13,28
Gastric cancer is a heterogeneous and complex disease as gastric cancer development involves multiple gene alterations.3 Our current study only focused on TPX2 protein overexpression and the knockdown of TPX2 expression, which inhibited gastric cancer malignant behaviors in vitro, consistent with a previously published study in another cancer type.10 For example, after knockdown of TPX2 expression, gastric cell proliferation was remarkably reduced. As a mitotic regulator, the TPX2 protein regulates formation of the mitotic spindle and cell mitosis. Upon knockdown of TPX2 expression, tumor cells are unable to form a normal level of the mitotic spindle and are arrested in the G2/M phase of the cell cycle, which is consistent with previous studies in other tumor types.6,8,10,13 However, Yan et al. found that the cell cycle is arrested in the G0/G1 phase in bladder carcinoma after siRNA treatment.11 At the molecular level, knockdown of TPX2 expression modulated the expression of multiple genes that regulate cell proliferation, apoptosis, and EMT.
Tumor cell EMT plays an important role in tumor cell invasion and metastasis. During EMT, tumor cells lose even more polarity and gain the ability to migrate and invade by degradation of the extracellular matrix (ECM).29 MMPs are a family of zinc-dependent endopeptidases and function to degrade ECM components in tissues.30,31 Our current data revealed that knockdown of TPX2 expression suppressed gastric cancer cell migration and invasion by downregulation of MMP-2 and MMP-9 expression. We also found that knockdown of TPX2 expression upregulated the levels of E-cadherin and downregulated N-cadherin and slug. Previous studies have shown that knockdown of TPX2 expression also suppresses the activity of the PI3K/Akt, Ras/Raf/MEK/ERK, and Wnt/β-catenin pathways in hepatocellular carcinoma and colon cancer cells.13,28,32 Moreover, TPX2 is recognized as an oncogene in many tumors.8-13,28 Targeting TPX2 expression or activity could be further explored as a therapeutic strategy in the future control of gastric cancer. However, our current study did not assess or explore how TPX2 expression was altered or how TPX2 regulates the cell cycle, apoptosis, or EMT.
Materials and methods
Patients and tissue samples
Paired gastric cancer and normal gastric tissues from 115 gastric cancer patients were obtained from The Second Affiliated Hospital to Nanchang University. These patients were histologically diagnosed with gastric cancer by the Department of Pathology, according to the World Health Organization criteria,33 and underwent surgery between 2009 and 2013. In this study, fresh tissue samples were cut into 4-mm cubic blocks, snap-frozen in liquid nitrogen, and stored at −80°C. Clinicopathological data, such as gender, age, tumor size, tumor location, differentiation, depth of invasion, lymph node metastasis, distant metastasis, and American Joint Committee on Cancer (AJCC) stage, were obtained from their medical record. This study was approved by the Ethics Committee of The Second Affiliated Hospital to Nanchang University, and a written informed consent was obtained from all the participants. This study was performed in accordance with the ethical standards of the Declaration of Helsinki.
Immunohistochemistry
Paraffin-embedded tissue blocks were cut into 4-µm thick sections, and these sections were first stained by hematoxylin and eosin for confirmation of diagnosis. For immunohistochemistry, we utilized a 2-step immunohistochemical method. Specifically, tissue sections were first deparaffinized twice in xylene for 10 min each, rehydrated in a graded alcohol series (100–50%) for 2 min each, and then subjected to antigen repair in a pressure cooker at 130°C for 10 min. The sections were incubated with hydrogen peroxide (0.3%) to block potential endogenous peroxidase activity, washed 3 times for 3 min each with phosphate-buffered saline (PBS), and then washed with 20% normal goat serum for 30 min at room temperature. After that, the sections were subsequently incubated with a mouse monoclonal anti-TPX2 antibody (BioLegend, San Diego, CA, USA) at a dilution of 1:200 at 4°C overnight. On the next day, the sections were washed 3 times with PBS briefly and then incubated with a biotinylated goat anti-mouse IgG (#PV-6000, ZSGB-Bio, Beijing, China) for 30 min at 37°C. After washing 3 times with PBS, the sections were subsequently subjected to a color reaction with 3,3′-diaminobenzidine solution, counterstained with hematoxylin, and mounted with coverslips. The immunostained tissue sections were then reviewed and scored under a microscope (Nikon, Tokyo, Japan) independently by 2 pathologists in a blinded fashion. TPX2 protein was localized in the nuclei of the positive cells. Our scoring system was based on staining intensity and the percentage of staining, i.e., the staining intensity was graded as 0, no staining; 1+, mild staining; 2+, moderate staining; or 3+, strong staining. The percentage of staining was scored as 0, no staining; 1, <10% staining; 2, 10–40% staining; or 3, >40% staining. The overall staining index was then computed by multiplying these 2 scores to reach 0 to 9 for each immunostained section and summarized and designated as follows: 0–1, negative TPX2 expression or 2–9, positive expression, according to a previous study.8
Cell lines and culture
The gastric cancer cell lines AGS, BGC-823, MKN-28, MGC-803, and SGC-7901 were obtained from the Cell Bank of Type Culture Collection of Chinese Academy of Sciences (Shanghai, China) and cultured in high-glucose Dulbecco's modified Eagle's medium (DMEM; Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS, Hyclone, Logan, UT, USA) at 37°C in a humidified incubator with 5% CO2 and 95% air. Cells were grown in the exponential phase and subcultured at approximately 80% confluency.
Protein extraction and western blot
The total cellular protein was extracted using RIPA buffer (KeyGEN, Nanjing, China) containing 1% phenylmethanesulfonyl fluoride and quantified using the Bradford method. The protein samples were then separated using sodium dodecyl sulfate-PAGE (SDS-PAGE) in an SDS-PAGE minigel and transferred onto polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA). For protein gel blotting, the membranes were first incubated in 5% bovine serum albumin in Tris-based saline-Tween 20 (TBS-T) and then subsequently incubated with the respective primary antibodies overnight at 4°C. On the next day, the membranes were washed with TBS-T 3 times for 10 min each and further incubated with a horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature and then subjected to color development with Enhanced Chemiluminescence reagent (ECL; Millipore). The antibodies against cyclin B1, cyclin D1, CDK2, and CDK4 were from Bioworld (Dublin, OH, USA); while anti-caspase-3, cleaved caspase-3, p53, Bax, E-cadherin, N-cadherin, slug, matrix metalloprotease (MMP)-2, and MMP-9 antibodies were from Cell Signaling Technology (Danvers, MA, USA) and used according to the manufacturers' instructions. An anti-β-actin antibody (ZSGB-Bio, Beijing, China) was used as an endogenous loading control.
siRNA and gene transfection
Human TPX2 siRNA reagents were obtained from GenePharma RNAi Company (Shanghai, China), and their sequences were TPX2_s1, 5′-GAAUGGAACUGGAGGGCUUTT-3′ and TPX2_s2, 5′-AUGAAAGUUUCUAACAACAAATT-3′. GAPDH siRNA was used as a positive control (5′-GUAUGACAACAGCCUCAAGTT-3′), and the negative control sequence was 5′-UUCUCCGAACGUGUCACGUTT-3′. Gastric cancer AGS and MGC-803 cell lines were grown in 6-well plates and transiently transfected with these siRNA reagents for 48 h using Lipofectamine 2000 (Invitrogen), according to the manufacturer's protocol. Transfected cells were used in further assays or for protein extraction.
Cell proliferation assay
The CCK-8 assay kit (KeyGEN, Nanjing, China) was utilized to measure cell proliferation after TPX2 knockdown. Briefly, cells were seeded in 96-well plates at a density of 5000 cells per well, incubated for 12 h, and then transiently transfected with these indicated siRNA reagents. The cells were grown for 24 h, 48 h, 72 h, and 96 h, respectively, with regular growth medium replacement. At the end of each experiment, cell culture medium was added with 10 μL of CCK-8 reagent to each well, the mixture was incubated for an additional hour, and then the optical density was measured by using a microplate reader (PerkinElmer, Pomona, CA, USA) at a wavelength of 450 nm. The data were summarized as the mean ± standard error and calculated as a percentage of the control. The experiments were performed in triplicate and repeated at least 3 times.
Flow cytometric cell cycle and apoptosis assay
To analyze the cell cycle distribution, cells were grown and transiently transfected with the siRNA reagents for 48 h. Then, 1 × 106 cells were harvested using trypsinization, washed with PBS, and fixed with 70% ethanol at 4°C overnight. On the next day, the cells were resuspended in 500 μL of propidium iodide (PI)/RNase staining solution (Sungene, Tianjin, China) and incubated at 37°C for 30 min. The samples were then analyzed by a FACScan flow cytometer (BD Immunocytometry Systems, San Jose, CA, USA).
To detect cell apoptosis, duplicate cells from the cell cycle analysis were subjected to measurement with the Annexin V-FITC/PtdIns Apoptosis Detection Kit (KeyGEN, Nanjing, China), according to the manufacturer's instructions. Briefly, the cells were washed in ice-cold PBS and incubated with Annexin V-FITC and PI solution in the dark for 15 min and then analyzed for apoptosis rates using a FACScan flow cytometer (BD Immunocytometry Systems). For each sample, at least 10,000 cells were analyzed, and the data were summarized as the mean ± SE and calculated as the percentage of the control.
Transwell tumor cell migration and invasion assay
To assess the tumor cell migration and invasion capacity, we utilized modified 24-well Boyden chambers with the filter either uncoated or precoated with Matrigel (BD Biosciences). Gastric cancer cells were grown and transiently transfected using siRNA reagents in serum-free DMEM at a density of 1 × 106/mL. Next, we seeded 300 µL of the cell solution into the upper chamber with a filter containing 8-µm pores, and DMEM containing 20% FBS was added to the bottom chambers. After incubation for 48 h, the cells in the upper chambers were removed using a cotton swap, and the cells that had migrated or invaded into the lower chamber of the filter were fixed in 4% paraformaldehyde and stained with 0.5% crystal violet. The number of migrated or invaded cells was counted under an inverted microscope (Nikon, Tokyo, Japan) in 5 randomly selected fields at 10× magnification.
Statistical analysis
The Mann-Whitney U test was used to compare TPX2 expression between gastric cancer and paired normal tissues, while the Chi-squared test was performed to detect statistical significance between TPX2 expression and clinicopathological factors. Overall survival was assessed using Kaplan–Meier curves, and the difference in overall survival was stratified by TPX2 expression and evaluated using the log-rank test. The Cox proportional hazards regression model was used to assess the hazard ratio (HR) and to identify factors that independently predicted overall survival. The in vitro data were expressed as the mean ± standard error and analyzed using one-way analysis of variance using factorial design to compare the growth curves of the different siRNA treatment groups. All P values were based on a 2-sided statistical analysis, and a P value ≤ 0.05 was considered statistically significant.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This study was supported in part by grants from the Second Affiliated Hospital of Nanchang University (#2016YNQN12004) and Natural Science Foundation (#20114BAB205087) of Jiangxi Province, China.rgg.
References
- 1.Grabsch HI, Tan P. Gastric cancer pathology and underlying molecular mechanisms. Dig Surg 2013; 30:150-8; PMID:23867592; http://dx.doi.org/ 10.1159/000350876 [DOI] [PubMed] [Google Scholar]
- 2.Duraes C, Almeida GM, Seruca R, Oliveira C, Carneiro F. Biomarkers for gastric cancer: prognostic, predictive or targets of therapy? Virchows Arch 2014; 464:367-78; PMID:24487788; http://dx.doi.org/ 10.1007/s00428-013-1533-y [DOI] [PubMed] [Google Scholar]
- 3.Cho JY. Molecular diagnosis for personalized target therapy in gastric cancer. J Gastric Cancer 2013; 13:129-35; PMID:24156032; http://dx.doi.org/ 10.5230/jgc.2013.13.3.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smyth EC, Cunningham D. Targeted therapy for gastric cancer. Curr Treat Options Oncol 2012; 13:377-89; PMID:22552927; http://dx.doi.org/ 10.1007/s11864-012-0192-6 [DOI] [PubMed] [Google Scholar]
- 5.Perez de Castro I, Malumbres M. Mitotic stress and chromosomal instability in cancer: the case for TPX2. Genes Cancer 2012; 3:721-30; PMID:23634259; http://dx.doi.org/ 10.1177/1947601912473306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neumayer G, Belzil C, Gruss OJ, Nguyen MD. TPX2: of spindle assembly, DNA damage response, and cancer. Cell Mol Life Sci 2014; 71:3027-47; PMID:24556998; http://dx.doi.org/ 10.1007/s00018-014-1582-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kufer TA, Sillje HH, Korner R, Gruss OJ, Meraldi P, Nigg EA. Human TPX2 is required for targeting Aurora-A kinase to the spindle. J Cell Biol 2002; 158:617-23; PMID:12177045; http://dx.doi.org/ 10.1083/jcb.200204155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Warner SL, Stephens BJ, Nwokenkwo S, Hostetter G, Sugeng A, Hidalgo M, Trent JM, Han H, Von Hoff DD. Validation of TPX2 as a potential therapeutic target in pancreatic cancer cells. Clin Cancer Res 2009; 15:6519-28; PMID:19861455; http://dx.doi.org/ 10.1158/1078-0432.CCR-09-0077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma Y, Lin D, Sun W, Xiao T, Yuan J, Han N, Guo S, Feng X, Su K, Mao Y, et al.. Expression of targeting protein for xklp2 associated with both malignant transformation of respiratory epithelium and progression of squamous cell lung cancer. Clin Cancer Res 2006; 12:1121-7; PMID:16489064; http://dx.doi.org/ 10.1158/1078-0432.CCR-05-1766 [DOI] [PubMed] [Google Scholar]
- 10.Chang H, Wang J, Tian Y, Xu J, Gou X, Cheng J. The TPX2 gene is a promising diagnostic and therapeutic target for cervical cancer. Oncol Rep 2012; 27:1353-9; PMID:22307108; http://dx.doi.org/ 10.3892/or.2012.1668 [DOI] [PubMed] [Google Scholar]
- 11.Yan L, Li S, Xu C, Zhao X, Hao B, Li H, Qiao B. Target protein for Xklp2 (TPX2), a microtubule-related protein, contributes to malignant phenotype in bladder carcinoma. Tumour Biol 2013; 34:4089-100; PMID:23873098; http://dx.doi.org/ 10.1007/s13277-013-1000-z [DOI] [PubMed] [Google Scholar]
- 12.Hsu PK, Chen HY, Yeh YC, Yen CC, Wu YC, Hsu CP, Hsu WH, Chou TY. TPX2 expression is associated with cell proliferation and patient outcome in esophageal squamous cell carcinoma. J Gastroenterol 2014; 49:1231-40; PMID:23963785; http://dx.doi.org/ 10.1007/s00535-013-0870-6 [DOI] [PubMed] [Google Scholar]
- 13.Liang B, Jia C, Huang Y, He H, Li J, Liao H, Liu X, Bai X, Yang D. TPX2 level correlates with hepatocellular carcinoma cell proliferation, apoptosis, and EMT. Dig Dis Sci 2015; 60:2360-72; PMID:26025609; http://dx.doi.org/ 10.1007/s10620-015-3730-9 [DOI] [PubMed] [Google Scholar]
- 14.Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, et al.. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006; 355:11-20; PMID:16822992; http://dx.doi.org/ 10.1056/NEJMoa055531 [DOI] [PubMed] [Google Scholar]
- 15.Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, Haller DG, Ajani JA, Gunderson LL, Jessup JM, et al.. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med 2001; 345:725-30; PMID:11547741; http://dx.doi.org/ 10.1056/NEJMoa010187 [DOI] [PubMed] [Google Scholar]
- 16.Cunningham D, Okines AF, Ashley S. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 2010; 362:858-9; PMID:20200397; http://dx.doi.org/ 10.1056/NEJMc0911925 [DOI] [PubMed] [Google Scholar]
- 17.Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, Rodrigues A, Fodor M, Chao Y, Voznyi E, et al.. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol 2006; 24:4991-7; PMID:17075117; http://dx.doi.org/ 10.1200/JCO.2006.06.8429 [DOI] [PubMed] [Google Scholar]
- 18.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer 2002; 2:563-72; PMID:12154349; http://dx.doi.org/ 10.1038/nrc865 [DOI] [PubMed] [Google Scholar]
- 19.Sieber O, Heinimann K, Tomlinson I. Genomic stability and tumorigenesis. Semin Cancer Biol 2005; 15:61-6; PMID:15613289; http://dx.doi.org/ 10.1016/j.semcancer.2004.09.005 [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Heidebrecht H, Rott A, Schlegelberger B, Parwaresch R. Assignment of human proliferation associated p100 gene (C20orf1) to human chromosome band 20q11.2 by in situ hybridization. Cytogenet Cell Genet 1999; 84:182-3; PMID:10393424; http://dx.doi.org/ 10.1159/000015251 [DOI] [PubMed] [Google Scholar]
- 21.Beroukhim R, Mermel CH, Porter D, Wei G, Raychaudhuri S, Donovan J, Barretina J, Boehm JS, Dobson J, Urashima M, et al.. The landscape of somatic copy-number alteration across human cancers. Nature 2010; 463:899-905; PMID:20164920; http://dx.doi.org/ 10.1038/nature08822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scotto L, Narayan G, Nandula SV, Arias-Pulido H, Subramaniyam S, Schneider A, Kaufmann AM, Wright JD, Pothuri B, Mansukhani M, et al.. Identification of copy number gain and overexpressed genes on chromosome arm 20q by an integrative genomic approach in cervical cancer: potential role in progression. Genes Chromosomes Cancer 2008; 47:755-65; PMID:18506748; http://dx.doi.org/ 10.1002/gcc.20577 [DOI] [PubMed] [Google Scholar]
- 23.Tonon G, Wong KK, Maulik G, Brennan C, Feng B, Zhang Y, Khatry DB, Protopopov A, You MJ, Aguirre AJ, et al.. High-resolution genomic profiles of human lung cancer. Proc Natl Acad Sci U S A 2005; 102:9625-30; PMID:15983384; http://dx.doi.org/ 10.1073/pnas.0504126102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith LT, Mayerson J, Nowak NJ, Suster D, Mohammed N, Long S, Auer H, Jones S, McKeegan C, Young G, et al.. 20q11.1 amplification in giant-cell tumor of bone: Array CGH, FISH, and association with outcome. Genes Chromosomes Cancer 2006; 45:957-66; PMID:16847944; http://dx.doi.org/ 10.1002/gcc.20354 [DOI] [PubMed] [Google Scholar]
- 25.Ramakrishna M, Williams LH, Boyle SE, Bearfoot JL, Sridhar A, Speed TP, Gorringe KL, Campbell IG. Identification of candidate growth promoting genes in ovarian cancer through integrated copy number and expression analysis. PLoS One 2010; 5:e9983; PMID:20386695; http://dx.doi.org/ 10.1371/journal.pone.0009983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perez de Castro I, de Carcer G, Malumbres M. A census of mitotic cancer genes: new insights into tumor cell biology and cancer therapy. Carcinogenesis 2007; 28:899-912; PMID:17259655; http://dx.doi.org/ 10.1093/carcin/bgm019 [DOI] [PubMed] [Google Scholar]
- 27.Asteriti IA, Rensen WM, Lindon C, Lavia P, Guarguaglini G. The Aurora-A/TPX2 complex: a novel oncogenic holoenzyme? Biochim Biophys Acta 2010; 1806:230-9; PMID:20708655; http://dx.doi.org/ 10.1016/j.bbcan.2010.08.001 [DOI] [PubMed] [Google Scholar]
- 28.Wei P, Zhang N, Xu Y, Li X, Shi D, Wang Y, Li D, Cai S. TPX2 is a novel prognostic marker for the growth and metastasis of colon cancer. J Transl Med 2013; 11:313; PMID:24341487; http://dx.doi.org/ 10.1186/1479-5876-11-313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bogachek MV, De Andrade JP, Weigel RJ. Regulation of epithelial-mesenchymal transition through SUMOylation of transcription factors. Cancer Res 2015; 75:11-5; PMID:25524900; http://dx.doi.org/ 10.1158/0008-5472.CAN-14-2824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev 2006; 25:9-34; PMID:16680569; http://dx.doi.org/ 10.1007/s10555-006-7886-9 [DOI] [PubMed] [Google Scholar]
- 31.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell 2010; 141:52-67; PMID:20371345; http://dx.doi.org/ 10.1016/j.cell.2010.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andl CD. Targeting HCC Therapy: On or Off ToPiX? Dig Dis Sci 2015; 60:2219-21; PMID:26062821; http://dx.doi.org/ 10.1007/s10620-015-3749-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flucke U, Monig SP, Baldus SE, Zirbes TK, Bollschweiler E, Thiele J, Dienes HP, Holscher AH. Differences between biopsy- or specimen-related Lauren and World Health Organization classification in gastric cancer. World J Surg 2002; 26:137-40; PMID:11865338; http://dx.doi.org/ 10.1007/s00268-001-0195-0 [DOI] [PubMed] [Google Scholar]