Abstract
Our understanding of the process of metastatic progression has improved markedly over the past decades, yet metastasis remains the most enigmatic component of cancer pathogenesis. This lack of knowledge has serious health-related implications, since metastasis is responsible for 90% of all cancer-related mortalities. The brain is considered a sanctuary site for metastatic tumor growth, where the blood–brain barrier (BBB) and other components of the brain microenvironment, provide protection to the tumor cells from immune surveillance, chemotherapeutics and other potentially harmful substances. The interactions between tumor cells and the brain microenvironment, principally brain vascular endothelium, are the critical determinants in their progression toward metastasis, dormancy, or clearance. This review discusses current knowledge of the biology of metastatic progression, with a particular focus on the tumor cell migration and colonization in the brain.
INTRODUCTION
The term “cancer” is used to describe a heterogeneous group of more than 100 diseases, defined by dynamic changes in the genome that lead to uncontrolled cellular growth (1). Behind cardiovascular disease, cancer is the second leading cause of death in the majority of developed countries, with foreseen increased incidence in low- and middle-income countries in the upcoming decades (2). Each cancer type has its own characteristics, but several functional capabilities, acquired through alterations in normal cellular function, are considered integral components of all human cancers and are essential for their development, growth and dissemination (3). Those capabilities serve as a framework for understanding the complexity of cancer biology and include the following: self-sufficiency in proliferative signals, evasion of growth suppression, cell death resistance, replicative immortality, induction of angiogenesis, dysregulation of energy metabolism, avoidance of immune destruction and initiation of tissue invasion and metastasis (3).
While early detection of many primary tumors often allows successful treatment and cure, detection of metastatic cancers, and, in particular, brain metastases, is usually associated with poor prognosis and high mortality (4,5). The purpose of the present review was to examine the literature and summarize the current knowledge of metastatic progression, focusing on tumor cell homing in the brain and to indicate potential targets for preventive and therapeutic strategies.
METASTATIC DISSEMINATION
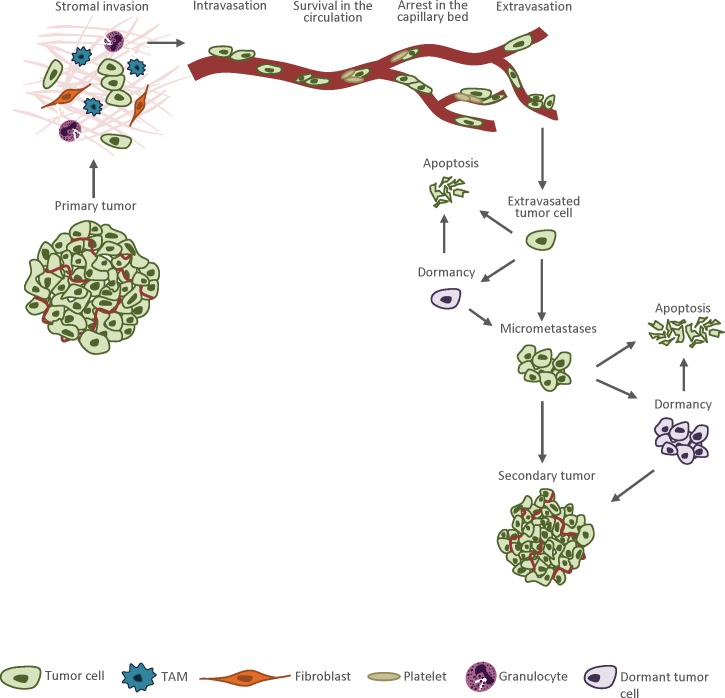
Metastatic progression is usually described as a sequence of distinct steps, termed a “metastasis cascade.” Briefly, these steps include local invasion, intravasation into the circulation (either directly into the bloodstream or via lymphatics and lymph nodes), survival in the circulation, arrest in a new organ, extravasation into the surrounding tissue, and initiation and maintenance of growth at the distant organ site (Figure 1) (6 –8). All of these steps must be completed to give rise to a secondary lesion and the success of the process depends not only on the features of tumor cell, but also on local and distant environmental factors, at both cellular and molecular levels (9). Some primary lesions shed tens of thousands of tumor cells into the circulation on daily basis, but few secondary tumors eventually develop, implying that tumor cells frequently fail to complete all of the steps of metastatic cascade (6). It has been demonstrated that the early steps of metastasis, from the time that cells enter the systemic circulation until they extravasate into secondary sites, are completed with higher efficiency compared with the final events of metastatic progression. Apoptosis of tumor cells shortly after arriving at the secondary site is considered a major source of failure in the metastatic process (4, 10,11). Moreover, neoplasms are biologically heterogeneous and contain genotypically and phenotypically diverse subpopulations of cells, indicating that the same primary tumor can shed into circulation cells of different metastatic potential (10,12,13).
Figure 1.
The metastatic cascade. Tumor cells that acquired an invasive phenotype detach from the primary lesion, invade the surrounding tissues and move toward neighboring blood vessels. Then tumor cells intravasate into the blood circulation are carried by the flow, usually until they arrest by size restriction in small capillaries at the distant site. In the following step, tumor cells exit the circulation and invade the foreign microenvironment. In a secondary site, tumor cells can exist as small pre-angiogenic micrometastases, solitary dormant tumor cells or dormant micrometastatic lesions. To develop into an active macrometastatic lesion, the tumor cells must evade destruction by host defense mechanisms, adapt to the new microenvironment, initiate proliferation and create vascular network. Very few extravasated tumor cells are able to accomplish these tasks, and the majority of them undergo apoptosis.
For years, lack of appropriate technological resources restrained the advancement in the field of metastasis. Progress in microscopy techniques, better tumor models and the development of reliable tools that allow to track tumor cells in vivo brought our understanding of the process to a new level, yet metastasis remains the most enigmatic component of cancer pathogenesis (4,10).
LOCAL INVASION
Local invasion involves detachment of cancer cells from the primary tumor, entry and migration through the surrounding stroma and subsequent invasion into the neighboring normal tissue (5,13).
The acquisition of invasive phenotype is the first step for tumor cells to initiate the metastatic process. To migrate, the tumor cell body must adapt its shape and stiffness (14,15). First, the tumor cell elongates, pseudopodia are formed and then the entire cell body contracts, generating traction force that leads to amoeboid (for example, lymphoma, small-cell lung carcinoma) or mesenchymal-type movement (for example, fibrosarcoma, glioblastoma) (7,14,15). Cell motility is driven by cycles of actin polymerization, cell adhesion and acto–myosin contraction. Recent studies demonstrated that a low number of cells within the primary tumor are motile, but the tumor cells that acquired this ability move at high speeds (up to 15 μm a minute) and can rapidly change shape and direction of the movement (10). Some types of cancers instead of individual tumor cell dissemination use collective migration strategy (15). Disseminating clusters or cohorts of tumor cells are common in epithelial cancers that maintain high or intermediate levels of differentiation, including breast and colon carcinoma, prostate cancer, as well as melanoma (14).
To invade the stroma, tumor cells must overcome the basement membrane. The degradation of the basement membrane barrier is achieved though active proteolysis by matrix metalloproteinases (MMPs) (5,16). Whereas the function of MMPs is complex and they affect multiple signaling pathways, in cancer, their principal tasks are to downregulate cellular adhesion and degrade extracellular matrix (ECM), paving the way through the peripheral tissue to the blood or lymphatic vessels (16). It has been suggested that the expression of MMPs might be particularly important in the process of migration of tumor cells through the blood–brain barrier (BBB). Indeed, MMP-9 was found to be overexpressed in brain metastatic lung adenocarcinoma cells, whereas it has been demonstrated that MMP-2 plays a crucial role in brain metastasis formation of breast cancer and melanoma cells (17 –19). MMPs secreted by metastasizing tumor cells disrupt tight junctions, which are proteins that seal brain microvascular endothelium and represent the core structure of the BBB (20,21). Additionally, it has been reported that metastasizing melanoma cells produce high levels of serine proteases, which degrade components of the basement membrane surrounding brain microvessels (22).
When metastasizing tumor cells manage to overcome the basement membrane, they enter the stroma and encounter a variety of stromal cells, including fibroblasts, adipocytes, macrophages, granulocytes and other immune cells (5,23,24). The stromal cells can further enhance the metastatic potential of tumor cells through various types of heterotypic signaling (5,24). Invasion through the stroma is often stimulated by tumor-associated macrophages (TAMs) that attract tumor cells toward blood vessels by secreting epidermal growth factor (EGF) and contribute to ECM remodeling by secretion of matrix-degrading enzymes (7,10,25).
INTRAVASATION
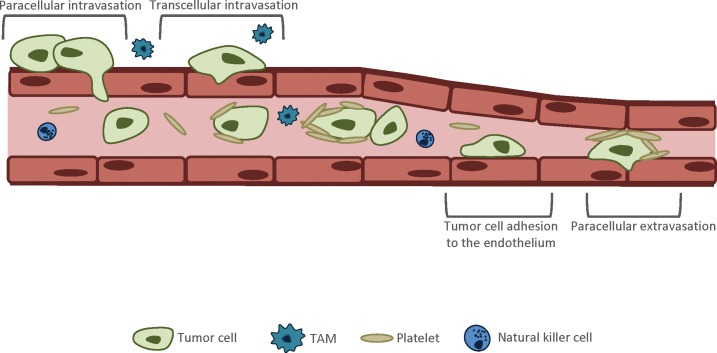
Crossing the endothelial barrier is commonly considered a rate-limiting step against tumor dissemination during metastatic progression (26). Two routes are used by tumor cells to cross the endothelial barrier: paracellular and transcellular transendothelial migration (TEM) (Figure 2) (25,26). Paracellular TEM involves disruption of endothelial cell junctions between neighboring endothelial cells, which allows tumor cells to squeeze between them. Tumor cells induce endothelial cell junction opening by secreting a variety of factors (25). For example, transforming growth factor (TGF)-β and vascular endothelial growth factor (VEGF) were reported to locally reduce endothelial barrier function by disrupting the VE-cadherin–β-catenin complex and therefore inducing endothelial cell junction opening (25,27,28). Some tumor cells during TEM secrete pro-apoptotic factors, which results in permanent damage to the endothelium (25). In addition, TAMs can promote TEM of tumor cells by secreting tumor necrosis factor-α, which induces opening of endothelial junctions (7,25). Transcellular TEM refers to the migration of tumor cells directly through the endothelial cell body, and this route of TEM seems to be used by tumor cells less often than the paracellular migratory mechanism (29). It is hypothesized that the mechanism of intravasation depends on the structural features of the vessels and on the cancer type (25).
Figure 2.
Crossing the endothelial barrier. There are two routes used by tumor cells to cross the endothelial cell barrier: paracellular and transcellular TEM. Paracellular TEM involves disruption of endothelial cell junctions between neighboring endothelial cells, which allows tumor cells to squeeze between them. Tumor cells induce endothelial cell junction opening by secreting a variety of factors, for example, TGF-β and VEGF. Additionally, the opening of endothelial junctions can be promoted by tumor-associated macrophages (TAMs). Transcellular TEM refers to the migration of tumor cells through the endothelial cell body, and this route of TEM seems to be used by tumor cells less often than the paracellular migratory mechanism. As tumor cells enter into the bloodstream, they encounter various challenges that dramatically decrease their chances to survive and arrive to the distant site. Aggregation with platelets provides tumor cells protection from immune cells, reduces shear stress and facilitates tumor cell adhesion and extravasation to the secondary site. Extravasation involves the specific interaction of tumor cells with vascular endothelium via cell adhesion-and chemokine-related processes. Similarly to the process of intravasation, extravasating tumor cells can cross the endothelial barrier via a para-or transcellular mechanism.
SURVIVAL IN THE CIRCULATION
As tumor cells intravasate into blood vessels, they encounter an entirely different microenvironment. It is estimated that as few as only 0.01% of all tumor cells entering the bloodstream survive to create secondary lesions (9,23,25). Various challenges, including mechanical destruction caused by the shear stress of the blood circulation and surveillance by immune cells, make the blood a particularly severe environment for a metastasizing cell (23). Tumor cells developed several strategies designed to enhance their chances of survival. One of them is using platelets as a shield. P-selectin, expressed on the surface of platelets, binds a wide variety of tumor cell lines (27,30). The aggregation with platelets provides tumor cell protection from NK cell–mediated lysis and reduces shear stress that can destroy individually circulating cells (23,27). Moreover, platelets can escort tumor cells through the steps of metastatic progression, facilitating tumor cell adhesion, migration and extravasation to the secondary site (27).
Although tumor cells can also enter the circulation indirectly (through the lymphatic vessels), the central nervous system (CNS) lacks a classic lymphatic drainage system, and the only entry route for tumor cells into the brain is via the bloodstream (31). Because of the existence of the BBB, to migrate into the brain tissue, tumor cells need markedly more time compared with other organs (31,32). For instance, it has been reported that ~48 h is required for lung cancer cells to extravasate into the brain, whereas extravasation into the liver takes only 6 h for the same tumor cells (33). Breast cancer cells extravasate into the brain within 2–7 d, whereas melanoma cells were reported to take up to 14 d before reaching brain parenchyma (32,34). Consequently, arrested tumor cells have to survive within the brain vasculature for a significantly longer time than at other metastatic sites. Tumor cell survival in the cerebral microvasculature is therefore a particularly important step in the process of brain metastasis formation, and the ability of tumor cells to survive in such an environment greatly depends on the interactions between tumor cells and components of the vessel wall (32,35).
ARREST IN A NEW ORGAN
If tumor cells manage to survive in the circulation, they are carried by the flow until they get arrested in small capillaries in the new organ. A growing body of evidence suggests that the majority of tumor cells arrest in capillaries of a similar diameter, indicating that physical restriction initially plays the crucial role, and the stable attachments form in the following stage (27,34). Tumor cells metastasizing to the brain usually arrest in sites of slow flow within the capillary bed at vascular branch points (36).
EXTRAVASATION
At first, the vascular endothelium was considered to be a passive participant in the process of tumor cell extravasation; however, now it is clear that the endothelium activated by proinflammatory cytokines plays an essential role in modulating the adhesion of tumor cells and facilitating TEM (37). Adhesion to the endothelial cells requires the expression of cognate linkages and receptors on both tumor and endothelial cells. A wide range of ligands/linkages and receptors are involved in the process, including selectins, integrins, cadherins, CD44 and immunoglobulin superfamily receptors (25,27). A single tumor cell possesses multiple adhesion receptors, all of them facilitating the adhesion process and expressing slightly different functions. For instance, it has been demonstrated that endothelial selectin (E-selectin) is involved in melanoma cell extravasation to the brain (38). Several members of the immunoglobulin superfamily of cell adhesion molecules (in particular, intercellular adhesion molecule-1 [ICAM-1] and vascular cell adhesion molecule-1 [VCAM-1]) have also been identified as key participants of melanoma cell migration through the endothelial barrier (39,40). Thus, to completely halt the adhesion of tumor cells to the endothelium, several different receptors would need to be blocked (25). Additionally, the adhesion receptors may vary depending on the cancer type and the vascular bed. For example, the interaction between integrin α4β1 (VLA-4) and endothelial VCAM-1 seems to be exclusive for melanoma cells and has not been observed in metastatic carcinoma cells (40,41). Moreover, whereas tumor cells are attached to the endothelium, they interact with a wide variety of cell type circulating in the bloodstream, including platelets, monocytes, neutrophils and NK cells, and all of these cells can modulate the rate and efficiency of the extravasation process (25). The interaction between tumor cell ICAM-1 and β2 integrin on neutrophils has been shown to increase the levels of melanoma cell anchoring to the endothelium in vivo (42).
Several mechanisms facilitating the migration of tumor cells through the BBB have been identified. Endothelial cell junction opening can be stimulated by various factors secreted by tumor cells. For instance, angiopoietin-2 has been linked with early BBB breakdown and increased brain colonization by metastasizing breast cancer cells (43). Also cathepsin S has been shown to specifically mediate BBB transmigration of breast tumor cells via proteolytic processing of the junctional adhesion molecule (JAM)-B. Importantly, genetic or pharmacological inhibition of cathepsin S significantly impaired experimental brain metastasis (44). Specific adhesion molecules have also been found to facilitate tumor cell migration to the brain. The expression of integrin α3β1 has been associated with lung cancer brain metastasis, since tumor cells that preferably metastasize to the brain were shown to express high levels of this receptor (45). The interaction of the α3β1 integrin with laminin, which is implicated in tumor cell TEM and invasion, is suggested to also be critical (45). TEM of melanoma cells is facilitated by αVβ3 and α4β1 integrins (46). The binding of αVβ3 integrin to platelet endothelial cell adhesion molecule 1 (PECAM1) has been proposed to be involved in endothelial junction disruption (25). αB-crystallin, a molecular chaperone commonly expressed in aggressive tumors, has also been associated with increased adhesion of breast cancer cells to brain endothelium via α3β1 integrin-dependent mechanism (47). Recently, another mechanism of BBB breakdown, involving cancer-derived extracellular vesicles, was proposed. It was reported that tumor-derived miRNA-181c containing extracellular vesicles can impair the dynamics of intracellular actin by downregulating 3-phosphoinositide-dependent protein kinase 1 (PDPK1) in brain endothelial cells (48).
Analogically, as during intravasation, extravasating tumor cells can cross the endothelial barrier using the paracellular or transcellular route (31). Regardless of the exact mechanism, the process of extravasation requires dynamic changes to tumor cell shape, as well as formation of specific protrusive structures that facilitate the migration (37). It was demonstrated that cancer-specific protrusions called invadopodia are necessary for successful extravasation of tumor cells; therefore, modulating TEM by targeting these and potentially other metastatic protrusions for therapy may prove beneficial (29,49). In addition, it has been shown that RHO-family GTPases regulate the cytoskeleton and actomyosin contractility and play an important role in the extravasation of tumor cells to the tissue surrounding the vessel (25,37).
If tumor cells manage to overcome the endothelial barrier, the next obstacle on their way to the target organ is the basement membrane that surrounds the vasculature. Although, this step seems to share some similarities with the invasion of the basement membrane surrounding the primary tumor, the process of crossing the vascular basement membrane requires further investigation, since many aspects of it remain unknown. Neutrophils and monocytes have been shown to preferentially cross the vascular basement membrane at sites that are characterized by low levels of specific basement membrane proteins and, because of that, are more permissive to invasion. It remains unclear whether tumor cells use similar strategies to cross the basement membrane (25).
Reports in the literature differ on the matter whether tumor cells leave the brain endothelium intact or disrupt the vessel wall during TEM. TEM of breast cancer cells was reported to occur at sites of discontinuity of the vessel wall, without endothelial apoptosis or hypoxia (32). In vitro studies demonstrated that melanoma cells impaired the integrity of the brain endothelial monolayer, induced endothelial apoptosis and reduced transendothelial electrical resistance (22). Although it has been suggested that the barrier can be restored after tumor cell extravasation, intravascular proliferation, which characterizes some tumor cell lines with high affinity for the brain, may destroy a portion of the vessel leading to disruption of the BBB (15,31,32).
INITIATION AND MAINTENANCE OF GROWTH AT SECONDARY SITES
Most of the tumor cells undergo apoptosis within 24 h after the extravasation into the secondary organ (10,13). The ability to survive and initiate tumor growth in a distant organ is determined by specific features of metastasizing tumor cells, as well as molecular interactions with the new microenvironment. For example, tumor cell adhesion receptor integrin αVβ3 has been shown to induce continuous upregulation of VEGF and promote metastatic growth and recruitment of supporting blood vessels within the brain microenvironment (50). Importantly, the effects mediated by activated αVβ3 receptors seem to be strictly microenvironment dependent and have not been observed at other metastatic sites (50). In addition, increased activity of the PI3K-Akt pathway, a crucial regulator of cell survival and proliferation, has been reported in several tumor cell types, including melanoma and breast cancer cells metastasizing to the brain (51 –53). Recent studies demonstrated that inhibition of PI3K effectively controls metastatic growth of HER2-positive breast cancer cells in the brain (52).
If tumor cells manage to evade destruction by host defense mechanisms, they can exist in a secondary site in four alternative states: as dormant solitary cells, dormant micrometastases, pre-angiogenic micrometastases or fully developed vascularized metastatic lesions (Figure 1) (4,13). Dormant solitary cells are cells that are not proliferating or undergoing apoptosis, whereas dormant pre-angiogenic micrometastases refer to lesions in which cell proliferation is counterpoised by apoptosis, resulting in no change in tumor size. Solitary cells and micrometastases are usually clinically undetectable (13). Additionally, conventional anticancer therapeutics, which target actively proliferating cells, have little or no impact on dormant tumor cells and micrometastatic colonies (13).
Successful colonization and transformation into an actively growing macrometastatic lesion requires recruitment of necessary supporting stroma and development of a vascular network (4,12,13). Whereas a wide variety of molecular pathways are involved in the development of angiogenic vessels supporting secondary tumor growth, VEGF represents a crucial factor that modulates almost all aspects of neo-angiogenesis, including endothelial cell proliferation and assembly, lumen formation and the patterning of vascular networks. It was reported that tumor cells with high brain metastatic activity are characterized by increased VEGF secretion (54). Moreover, antisense VEGF transfectants of PC14-PE6 lung adenocarcinoma cells showed decreased incidence of experimental brain metastases, indicating that VEGF is required for tumor growth in the brain (55). The consequence of development of an adequate blood supply and successful colonization at the secondary site is a rapidly expanding macrometastatic lesion that can potentially serve as a new source for further metastatic dissemination (13).
BRAIN METASTASIS
Brain metastases affect 10–30% of all cancer patients (31,32,56,57). The progressive growth of metastases in the brain tissue is usually associated with the terminal stage of disease, and the majority of patients exhibit multiple tumors at the time of the diagnosis (32). The localization of brain metastatic tumors usually correlates with the blood flow and tissue volume, with 80% of the tumors detected in the cerebral hemispheres, 15% in the cerebellum and 5% in the brainstem (58). The median survival for untreated patients is 1–2 months, which may be extended to 6 months with the appropriate treatment (58 –60).
The most common primary tumors metastasizing to the brain are lung (40–50%) and breast cancers (15–25%), followed by malignant melanoma (5–20%) (57,58). The main function of the BBB, located at the level of cerebral capillaries, is to provide a stable environment for the CNS (31,58). It is composed of capillary endothelial cells and pericytes surrounded by basal lamina, astrocytic end-feet and perivascular interneurons (58,60). The tight junctions formed between endothelial cells consist of transmembrane and cytoplasmic proteins that act as a highly selective barrier that allows the entry of necessary nutrients and protects the CNS from pathogens and potentially harmful small molecules circulating in the blood (31). To create a lesion in the brain, metastatic tumor cells have to migrate through the BBB. While the BBB blocks the entrance to the brain for most of the tumor cells, it remains unclear why some types of tumor cells can relatively easily pass through this highly selective barrier (31,61). It has been suggested that the brain endothelial cells can actively participate in metastatic progression and stimulate increased BBB permeability (58,62). Impairment of endothelial tight junctions that results in increased barrier permeability was reported to occur in a number of neurological disorders, including brain primary and metastatic tumors, multiple sclerosis and Alzheimer disease (63). Additionally, it was proposed that to take over the brain, disseminating tumor cells must express some specialized functions. In vitro studies have demonstrated that different types of melanoma cells reduced transendothelial electrical resistance of the endothelial cell monolayer, indicating that specific tumor cells can modulate junctional integrity (22). It was also reported that the ability of melanoma cells to pass through the BBB corresponds closely with melanotransferrin (MTf) expression (22,64). MTf is one of several antigens associated with the surface of melanoma cells. In addition to being an attractive target for strategies aimed at preventing brain metastasis formation, it was proposed to be a useful prognostic indicator for the development of brain metastases among patients with malignant melanoma (64). Another study reported that signal transducer and activator of transcription 3 (STAT3) activity was markedly elevated in human brain metastatic melanoma cells compared with cutaneous melanoma cells, suggesting close association between STAT3 expression and melanoma cell migration to the brain (19). Within metastatic breast cancer cells, several targets have been identified as mediators that promote tumor cell migration through the BBB, including cyclooxygenase-2 (COX2), the epidermal growth factor receptor (EGFR) ligand HBEGF and α-2,6-sialyltransferase 5 (ST6GALNAC5) (65,66). Whereas EGFR ligands and COX2 were previously linked to breast cancer infiltration of the lung, ST6GALNAC5 was recognized as a specific mediator of tumor cell passage through the BBB and thus may constitute a particularly valuable therapeutic target (65,67). Studies on primary tumors from lung cancer patients who developed metastases in the brain reported that the expression levels of three genes, namely CDH2, KIFC1, and FALZ, were highly predictive of brain metastases (68). Indeed, N-cadherin, coded by the CDH2 gene, is known to be involved in numerous processes associated with tumor progression, including tumor invasion and migration (69). It was also suggested that the expression of DCUN1D1, a squamous cell carcinoma-related oncogene, may play a role in tumor cell migration through the BBB and facilitate the development of brain metastasis in patients with non–small cell lung carcinoma (70).
Brain colonization by tumor cells is also largely regulated by cellular interactions with astrocytes, microglia cells and neurons (71). Astrocytes are the first brain cells encountered by extravasated tumor cells, and it has been observed that they can play both brain metastases–promoting as well as brain metastases–suppressing roles (71). Astrocytes support melanoma brain invasion by producing the ECM-degrading enzyme heparanase (72). The production of cytokines by astrocytes may also stimulate brain metastatic tumor growth by paracrine signaling (73). On the other hand, it was reported that that plasmin can convert membrane-bound astrocytic FasL into a paracrine death signal for extravasated lung and breast cancer cells. To prevent plasmin generation, metastasizing tumor cells express high levels of anti-plasminogen activator serpins (74). Microglia are the main immunocompetent cells in the CNS. Recently, it was reported that elevated expression of neurotrophin-3 (NT-3) in metastasizing breast tumor cells reduces the number of fully activated cytotoxic microglia and correlates with increased brain metastases formation (75). The role of neurons on tumor cell brain colonization is not fully understood. It was suggested that to metastasize to the brain, tumor cells may escape their normative genetic constraints by coinhabiting the neural niche. Indeed, breast tumor cells metastasizing to the brain were found to display a GABAergic phenotype analogous to that of neurons, indicating a metastasis-promoting effect of coinhabitation of the neuronal niche in the brain (76).
Once tumor cells enter into the brain, they encounter environment that is more permissive to tumor growth than all other metastatic sites. The BBB provides the protection from immune surveillance, chemotherapeutics and other potentially harmful substances (31,71). Effective therapies for brain metastasis are therefore particularly difficult. Surgical excision along with whole-brain radiation is the most common treatment for a patient with a solitary metastatic lesion, whereas radiation, chemotherapy or a combination of both are used for multiple brain metastases. The role of chemotherapy in the treatment of brain metastatic tumors is limited for several reasons (58). First, large hydrophilic molecules, including many chemotherapeutic, are excluded from the CNS unless they can be actively transported by receptor-mediated transcytosis (58). Moreover, endothelial cells that constitute the BBB express enhanced levels of active drug efflux transporters of the ATP-binding cassette (ABC) gene family, which have been recognized as key determinants of drug distribution to, and elimination from, the CNS (58,77). The most widely characterized transporter of the ABC family is P-glycoprotein (P-gp), which has been a subject of numerous investigations and for years was considered a major obstacle in the delivery of therapeutics into the brain (78,79). Recent studies indicated P-gp function is largely supported by the breast cancer resistance protein (BCRP), and overexpression of BCRP is associated with resistance to a wide range of different anticancer agents including mitoxantrone, camptothecins, anthracyclines, flavopiridol and antifolates (80,81). P-gp and BCRP are considered to be the two dominant efflux transporters at the BBB (80). Importantly, BCRP can transport not only hydrophobic substrates, but also hydrophilic-conjugated organic anions, whereas P-gp transports mostly hydrophobic compounds (82). This overlap in substrate specificity between BCRP and P-gp leads to a synergistic effect of the transporters further limiting drug migration through the BBB (80,82,83). Additionally, astrocytes have been shown to protect tumor cells within the brain from cytotoxicity induced by chemotherapeutic drugs (31,58,60). For example, it was reported that in coculture experiments, the presence of astrocytes dramatically reduced 5-fluorouracil– and cisplatin-induced apoptosis in human tumor cells (58,60).
CONCLUSION
The incidence rates of brain metastasis are still increasing because of improved diagnostic methods and better control of primary tumors, resulting in longer patient survival. The limited therapeutic options for patients affected by the brain metastasis emphasize the urgent need for strategies designed to prevent the formation of metastatic tumors in the brain. Characterizing in detail the process of tumor cell migration through the BBB and the interactions between tumor cells and reactive brain cells has fundamental significance for developing effective preventive therapies that could be lifesaving for patients who are at risk for cancer spread to the brain. Recent discovery that tumor cell metastasizing to the brain express specific genes that promote their migration through the BBB may result in the identification of new therapeutic targets and the development of innovative therapeutic approaches.
ACKNOWLEDGMENTS
This work was supported by grants from the National Institutes of Health (CA133257) and Alltech Nutrigenomics.
Footnotes
Online address: http://www.molmed.org
DISCLOSURE
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
Cite this article as: Wrobel JK, Toborek M. (2016) Blood–brain barrier remodeling during brain metastasis formation Mol. Med. 22:32–40
REFERENCES
- 1.Macconaill LE, Garraway LA. Clinical implications of the cancer genome. J Clin Oncol. 2010;28:5219–28. doi: 10.1200/JCO.2009.27.4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farmer P, et al. Expansion of cancer care and control in countries of low and middle income a call to action. Lancet. 2010;376:1186–93. doi: 10.1016/S0140-6736(10)61152-X. [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D, Weinberg RA. Hallmarks of cancer the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–72. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 5.Valastyan S, Weinberg RA. Tumor metastasis molecular insights and evolving paradigms. Cell. 2011;147:275–92. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Labelle M, Hynes RO. The initial hours of metastasis the importance of cooperative host-tumor cell interactions during hematogenous dissemination. Cancer Discov. 2:1091–9. doi: 10.1158/2159-8290.CD-12-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Zijl F, Krupitza G, Mikulits W. Initial steps of metastasis cell invasion and endothelial transmigration. Mutat Res. 2011;728:23–34. doi: 10.1016/j.mrrev.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen DX, Bos PD, Massague J. Metastasis from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–84. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 9.Hunter K. The role of individual inheritance in tumor progression and metastasis. J Mol Med Berl. 2015;93:719–25. doi: 10.1007/s00109-015-1299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sahai E. Illuminating the metastatic process. Nat Rev Cancer. 2007;7:737–49. doi: 10.1038/nrc2229. [DOI] [PubMed] [Google Scholar]
- 11.Kim JW, et al. Rapid apoptosis in the pulmonary vasculature distinguishes non-metastatic from metastatic melanoma cells. Cancer Lett. 2004;213:203–12. doi: 10.1016/j.canlet.2004.03.042. [DOI] [PubMed] [Google Scholar]
- 12.Fidler IJ. The pathogenesis of cancer metastasis the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3:453–8. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 13.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559–8. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 14.Friedl P, Wolf K. Tumour-cell invasion and migration diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–8. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- 15.Friedl P, Alexander S. Cancer invasion and the microenvironment plasticity and reciprocity. Cell. 2011;147:992–8. doi: 10.1016/j.cell.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 16.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases regulators of the tumor microenvironment. Cell. 2010;141:52–8. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu L, et al. Biological characteristics of a specific brain metastatic cell line derived from human lung adenocarcinoma. Med. Oncol. 2010;27:708–14. doi: 10.1007/s12032-009-9273-1. [DOI] [PubMed] [Google Scholar]
- 18.Mendes O, Kim HT, Stoica G. Expression of MMP2, MMP9 and MMP3 in breast cancer brain metastasis in a rat model. Clin Exp Metastasis. 2005;22:237–8. doi: 10.1007/s10585-005-8115-6. [DOI] [PubMed] [Google Scholar]
- 19.Xie TX, et al. Activation of stat3 in human melanoma promotes brain metastasis. Cancer Res. 2006;66:3188–8. doi: 10.1158/0008-5472.CAN-05-2674. [DOI] [PubMed] [Google Scholar]
- 20.Feng S, et al. Matrix metalloproteinase-2 and -9 secreted by leukemic cells increase the permeability of blood-brain barrier by disrupting tight junction proteins. PLoS One. 2011;6:e20599. doi: 10.1371/journal.pone.0020599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang Y, Rosenberg GA. MMP-mediated disruption of claudin-5 in the blood-brain barrier of rat brain after cerebral ischemia. Methods Mol Biol. 2011;762:333–8. doi: 10.1007/978-1-61779-185-7_24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fazakas C, et al. Transmigration of melanoma cells through the blood-brain barrier role of endothelial tight junctions and melanoma-released serine proteases. PLoS One. 2011;6:e20758. doi: 10.1371/journal.pone.0020758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–8. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deryugina EI, Quigley JP. Tumor angiogenesis MMP-mediated induction of intravasation- and metastasis-sustaining neovasculature. Matrix Biol. 2015;44(46C):94–112. doi: 10.1016/j.matbio.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reymond N, d’Agua BB Ridley AJ. Crossing the endothelial barrier during metastasis. Nat Rev Cancer. 2013;13:858–8. doi: 10.1038/nrc3628. [DOI] [PubMed] [Google Scholar]
- 26.Khuon S, et al. Myosin light chain kinase mediates transcellular intravasation of breast cancer cells through the underlying endothelial cells a three-dimensional FRET study. J Cell Sci. 2010;123:431–8. doi: 10.1242/jcs.053793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goubran HA, Stakiw J, Radosevic M, Burnouf T. Platelets effects on tumor growth. Semin Oncol. 2014;41:359–8. doi: 10.1053/j.seminoncol.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 28.Lee TH, Avraham HK, Jiang S, Avraham S. Vascular endothelial growth factor modulates the transendothelial migration of MDA-MB-231 breast cancer cells through regulation of brain microvascular endothelial cell permeability. J Biol Chem. 2003;278:5277–8. doi: 10.1074/jbc.M210063200. [DOI] [PubMed] [Google Scholar]
- 29.Leong HS, et al. Invadopodia are required for cancer cell extravasation and are a therapeutic target for metastasis. Cell Rep. 2014;8:1558–8. doi: 10.1016/j.celrep.2014.07.050. [DOI] [PubMed] [Google Scholar]
- 30.Wels J, Kaplan RN, Rafii S, Lyden D. Migratory neighbors and distant invaders: tumor-associated niche cells. Genes Dev. 2008;22:559–8. doi: 10.1101/gad.1636908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilhelm I, Molnar J, Fazakas C, Hasko J, Krizbai IA. Role of the blood-brain barrier in the formation of brain metastases. Int J Mol Sci. 2013;14:1383–8. doi: 10.3390/ijms14011383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lorger M, Felding-Habermann B. Capturing changes in the brain microenvironment during initial steps of breast cancer brain metastasis. Am J Pathol. 2010;176:2958–8. doi: 10.2353/ajpath.2010.090838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paku S, Dome B, Toth R, Timar J. Organ-specificity of the extravasation process an ultrastructural study. Clin Exp Metastasis. 2000;18:481–8. doi: 10.1023/a:1011858925376. [DOI] [PubMed] [Google Scholar]
- 34.Kienast Y, et al. Real-time imaging reveals the single steps of brain metastasis formation. Nat Med. 2010;16:116–8. doi: 10.1038/nm.2072. [DOI] [PubMed] [Google Scholar]
- 35.JuanYin J, et al. Noninvasive imaging of the functional effects of anti-VEGF therapy on tumor cell extravasation and regional blood volume in an experimental brain metastasis model. Clin Exp Metastasis. 2009;26:403–8. doi: 10.1007/s10585-009-9238-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hanibuchi M, Kim SJ, Fidler IJ, Nishioka Y. The molecular biology of lung cancer brain metastasis an overview of current comprehensions and future perspectives. J Med Invest. 2014;61:241–8. doi: 10.2152/jmi.61.241. [DOI] [PubMed] [Google Scholar]
- 37.Miles FL, Pruitt FL, van Golen KL Cooper CR. Stepping out of the flow capillary extravasation in cancer metastasis. Clin Exp Metastasis. 2008;25:305–8. doi: 10.1007/s10585-007-9098-2. [DOI] [PubMed] [Google Scholar]
- 38.Ravindranath MH, et al. Endothelial-selectin ligands sialyl Lewis(x) and sialyl Lewis(a) are differentiation antigens immunogenic in human melanoma. Cancer. 1997;79:1686–8. doi: 10.1002/(sici)1097-0142(19970501)79:9<1686::aid-cncr8>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 39.Ghislin S, et al. LFA-1 and ICAM-1 expression induced during melanoma-endothelial cell co-culture favors the transendothelial migration of melanoma cell lines in vitro. BMC Cancer. 2012;12:455. doi: 10.1186/1471-2407-12-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klemke M, Weschenfelder T, Konstandin MH, Samstag Y. High affinity interaction of integrin alpha4beta1 (VLA-4) and vascular cell adhesion molecule 1 (VCAM-1) enhances migration of human melanoma cells across activated endothelial cell layers. J Cell Physiol. 2007;212:368–8. doi: 10.1002/jcp.21029. [DOI] [PubMed] [Google Scholar]
- 41.Fritzsche J, Simonis D, Bendas G. Melanoma cell adhesion can be blocked by heparin in vitro suggestion of VLA-4 as a novel target for antimetastatic approaches. Thromb Haemost. 2008;100:1166–8. [PubMed] [Google Scholar]
- 42.Huh SJ, Liang S, Sharma A, Dong C, Robertson GP. Transiently entrapped circulating tumor cells interact with neutrophils to facilitate lung metastasis development. Cancer Res. 2010;70:6071–8. doi: 10.1158/0008-5472.CAN-09-4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Avraham HK, et al. Angiopoietin-2 mediates blood-brain barrier impairment and colonization of triple-negative breast cancer cells in brain. J Pathol. 2014;232:369–8. doi: 10.1002/path.4304. [DOI] [PubMed] [Google Scholar]
- 44.Sevenich L, et al. Analysis of tumour- and stroma-supplied proteolytic networks reveals a brain-metastasis-promoting role for cathepsin S. Nat Cell Biol. 2014;16:876–8. doi: 10.1038/ncb3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoshimasu T, et al. Increased expression of integrin alpha3beta1 in highly brain metastatic subclone of a human non-small cell lung cancer cell line. Cancer Sci. 2004;95:142–8. doi: 10.1111/j.1349-7006.2004.tb03195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Voura EB, Ramjeesingh RA, Montgomery AM, Siu CH. Involvement of integrin alpha(v)beta 3 and cell adhesion molecule L1 in transendothelial migration of melanoma cells. Mol Biol Cell. 2001;12:2699–8. doi: 10.1091/mbc.12.9.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Malin D, et al. alphaB-crystallin a novel regulator of breast cancer metastasis to the brain. Clin Cancer Res. 2014;20:56–8. doi: 10.1158/1078-0432.CCR-13-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tominaga N, et al. Brain metastatic cancer cells release microRNA-181c-containing extracellular vesicles capable of destructing blood-brain barrier. Nat Commun. 2015;6:6716. doi: 10.1038/ncomms7716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gligorijevic B, et al. N-WASP-mediated invadopodium formation is involved in intravasation and lung metastasis of mammary tumors. J Cell Sci. 2012;125:724–8. doi: 10.1242/jcs.092726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lorger M, Krueger JS, O’Neal M, Staflin K, Felding-Habermann B. Activation of tumor cell integrin alphavbeta3 controls angiogenesis and metastatic growth in the brain. Proc Natl Acad Sci U S A. 2009;106:10666–8. doi: 10.1073/pnas.0903035106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cho JH, et al. AKT1 activation promotes development of melanoma metastases. Cell Rep. 2015;13:898–8. doi: 10.1016/j.celrep.2015.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nanni P, et al. Multiorgan metastasis of human HER-2+ breast cancer in Rag2-/-; Il2rg-/- mice and treatment with PI3K inhibitor. PLoS One. 2012;7:e39626. doi: 10.1371/journal.pone.0039626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Molnar J, et al. Transmigration characteristics of breast cancer and melanoma cells through the brain endothelium role of Rac and PI3K. Cell Adh Migr 8 December 2015 [Epub ahead of print] 2015 doi: 10.1080/19336918.2015.1122156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oku T, et al. Tumor growth modulation by sense and antisense vascular endothelial growth factor gene expression: effects on angiogenesis, vascular permeability, blood volume, blood flow, fluorodeoxyglucose uptake, and proliferation of human melanoma intracerebral xenografts. Cancer Res. 1998;58:4185–8. [PubMed] [Google Scholar]
- 55.Yano S, et al. Expression of vascular endothelial growth factor is necessary but not sufficient for production and growth of brain metastasis. Cancer Res. 2000;60:4959–8. [PubMed] [Google Scholar]
- 56.Ba JL, Jandial R, Nesbit A, Badie B, Chen M. Current and emerging treatments for brain metastases. Oncology (Williston Park) 2015;29:250–8. [PubMed] [Google Scholar]
- 57.Soto MS, Serres S, Anthony DC, Sibson NR. Functional role of endothelial adhesion molecules in the early stages of brain metastasis. Neuro Oncol. 2014;16:540–8. doi: 10.1093/neuonc/not222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eichler AF, et al. The biology of brain metastases translation to new therapies. Nat Rev Clin Oncol. 2011;8:344–8. doi: 10.1038/nrclinonc.2011.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fidler IJ, Yano S, Zhang RD, Fujimaki T, Bucana CD. The seed and soil hypothesis vascularisation and brain metastases. Lancet Oncol. 2002;3:53–8. doi: 10.1016/s1470-2045(01)00622-2. [DOI] [PubMed] [Google Scholar]
- 60.Fidler IJ. The role of the organ microenvironment in brain metastasis. Semin Cancer Biol. 2011;21:107–8. doi: 10.1016/j.semcancer.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 61.Jia W, Martin TA, Zhang G, Jiang WG. Junctional adhesion molecules in cerebral endothelial tight junction and brain metastasis. Anticancer Res. 2013;33:2353–8. [PubMed] [Google Scholar]
- 62.Huber JD, Egleton RD, Davis TP. Molecular physiology and pathophysiology of tight junctions in the blood-brain barrier. Trends Neurosci. 2001;24:719–8. doi: 10.1016/s0166-2236(00)02004-x. [DOI] [PubMed] [Google Scholar]
- 63.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–8. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 64.Rolland Y, Demeule M, Fenart L, Beliveau R. Inhibition of melanoma brain metastasis by targeting melanotransferrin at the cell surface. Pigment Cell Melanoma Res. 2009;22:86–8. doi: 10.1111/j.1755-148X.2008.00525.x. [DOI] [PubMed] [Google Scholar]
- 65.Bos PD, et al. Genes that mediate breast cancer metastasis to the brain. Nature. 2009;459:1005–8. doi: 10.1038/nature08021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gabos Z, et al. Prognostic significance of human epidermal growth factor receptor positivity for the development of brain metastasis after newly diagnosed breast cancer. J Clin Oncol. 2006;24:5658–8. doi: 10.1200/JCO.2006.07.0250. [DOI] [PubMed] [Google Scholar]
- 67.Minn AJ, et al. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436:518–8. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Grinberg-Rashi H, et al. The expression of three genes in primary non-small cell lung cancer is associated with metastatic spread to the brain. Clin Cancer Res. 2009;15:1755–8. doi: 10.1158/1078-0432.CCR-08-2124. [DOI] [PubMed] [Google Scholar]
- 69.Nakashima T, et al. Neural-cadherin expression associated with angiogenesis in non-small-cell lung cancer patients. Br J Cancer. 2003;88:1727–8. doi: 10.1038/sj.bjc.6600955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yoo J, et al. Immunohistochemical expression of DCUN1D1 in non-small cell lung carcinoma its relation to brain metastasis. Cancer Res Treat. 2012;44:57–8. doi: 10.4143/crt.2012.44.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Winkler F. The brain metastatic niche. J Mol Med Berl. 2015;93:1213–8. doi: 10.1007/s00109-015-1357-0. [DOI] [PubMed] [Google Scholar]
- 72.Marchetti D, Li J, Shen R. Astrocytes contribute to the brain-metastatic specificity of melanoma cells by producing heparanase. Cancer Res. 2000;60:4767–8. [PubMed] [Google Scholar]
- 73.Seike T, et al. Interaction between lung cancer cells and astrocytes via specific inflammatory cytokines in the microenvironment of brain metastasis. Clin Exp Metastasis. 2011;28:13–8. doi: 10.1007/s10585-010-9354-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Valiente M, et al. Serpins promote cancer cell survival and vascular co-option in brain metastasis. Cell. 2014;156:1002–8. doi: 10.1016/j.cell.2014.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Louie E, et al. Neurotrophin-3 modulates breast cancer cells and the microenvironment to promote the growth of breast cancer brain metastasis. Oncogene. 2013;32:4064–8. doi: 10.1038/onc.2012.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Neman J, et al. Human breast cancer metastases to the brain display GABAergic properties in the neural niche. Proc. Natl. Acad. Sci. U. S. A. 2014;111:984–8. doi: 10.1073/pnas.1322098111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yonemori K, et al. Disruption of the blood brain barrier by brain metastases of triple-negative and basal-type breast cancer but not HER2/neu-positive breast cancer. Cancer. 2010;116:302–8. doi: 10.1002/cncr.24735. [DOI] [PubMed] [Google Scholar]
- 78.Wanek T, et al. Factors governing P-glycoprotein-mediated drug-drug interactions at the blood-brain barrier measured with positron emission tomography. Mol Pharm. 2015;12:3214–8. doi: 10.1021/acs.molpharmaceut.5b00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cannon RE, Peart JC, Hawkins BT, Campos CR, Miller DS. Targeting blood-brain barrier sphingolipid signaling reduces basal P-glycoprotein activity and improves drug delivery to the brain. Proc Natl Acad Sci U S A. 2012;109:15930–8. doi: 10.1073/pnas.1203534109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.de Vries NA, et al. P-glycoprotein and breast cancer resistance protein: two dominant transporters working together in limiting the brain penetration of topotecan. Clin Cancer Res. 2007;13:6440–8. doi: 10.1158/1078-0432.CCR-07-1335. [DOI] [PubMed] [Google Scholar]
- 81.Lemos C, Jansen G, Peters GJ. Drug transporters: recent advances concerning BCRP and tyrosine kinase inhibitors. Br. J. Cancer. 2008;98:857–8. doi: 10.1038/sj.bjc.6604213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ni Z, Bikadi Z, Rosenberg MF, Mao Q. Structure and function of the human breast cancer resistance protein (BCRP/ABCG2) Curr. Drug Metab. 2010;11:603–8. doi: 10.2174/138920010792927325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kodaira H, Kusuhara H, Ushiki J, Fuse E, Sugiyama Y. Kinetic analysis of the cooperation of P-glycoprotein (P-gp/Abcb1) and breast cancer resistance protein (Bcrp/Abcg2) in limiting the brain and testis penetration of erlotinib, flavopiridol, and mitoxantrone. J. Pharmacol. Exp. Ther. 2010;333:788–8. doi: 10.1124/jpet.109.162321. [DOI] [PubMed] [Google Scholar]