Abstract
The cervico-ocular reflex (COR) has a low gain in normal animals. In this study, we determined whether COR gain increases were specific to the low/midband frequency range, which is the range over which the angular vestibulo-ocular reflex (aVOR) is compromised by plugging. The gain and phase of the yaw and pitch COR and aVOR were compared in normal monkeys and those with all six semicircular canals or only the lateral canal plugged. During experiments animals sat with the body fixed to a chair and the head fixed in space. The body was oscillated about body-yaw and body-pitch axes over a frequency range of 0.05–6 Hz, with amplitude <10°. For normal animals, both yaw and pitch eye velocities were compensatory to the relative velocity of the head with respect to the body. The gains were 0.1–0.2 at frequencies below 1 Hz and decreased to zero as stimulus frequency increased above 1 Hz. Canal-plugged animals had COR gains close to 1.0 at low frequencies, decreasing to ≈0.6 at 0.5 Hz and to 0.2 for stimulus frequencies above 3 Hz. The phase of eye velocity was 180° relative to head-re-body velocity at frequencies below 0.5 Hz and shifted toward 270° as frequencies were increased to 4 Hz. This study demonstrates that adaptation of COR gain is tuned to a frequency range at which the aVOR is compromised by the canal plugging.
Keywords: cervico-ocular reflex, monkey, semicircular canal plugging, VOR
Introduction
When the head is rotated on the body, compensatory ocular rotation is driven by activation of the semicircular canals and neck pro-prioceptors, through the action of the angular vestibulo-ocular (aVOR) and cervico-ocular (COR) reflexes. There is a general agreement that in a wide range of species including human, the aVOR is the significant compensatory mechanism for head turning, with COR gains below 0.1.1–3 Vestibular pathologies, which compromise the gain of the aVOR, are associated with increases in the gain of the COR.4
Early studies of the COR indicated that it could augment the action of the aVOR to raise the overall gain of ocular responses to head movements relative to the body.4,5 These results have been challenged by some investigators, who, found that in addition to the gain of the COR being small, in human subjects there was no consistency in the phase.6,7 The majority of animal studies, however, support the idea that the COR is compensatory for head-on-body rotation. The action of the COR was partially addressed in studies following semicircular-canal plugging.3,4 Studies in the cat4 showed that there were increases in the gain of the horizontal COR over a wider frequency range (0.1 to 2.5 Hz) after canal plugging.4 In studies of lateral canal–plugged monkeys, the gain was higher at low frequencies when the head was rotated on the body (aVOR + COR activation) than during whole-body rotation (aVOR activation).3 This implies that there were increases in the COR at low frequencies. Since in the latter experiments, the head was rotated on a stationary body, the change in COR gain could not be fully evaluated, since the gain of the aVOR was substantial at higher frequencies.3
The question that remains is: how are the changes in COR gain related to the specific losses of the responses to semicircular canal activation? Canal plugging does not totally abolish the semicircular canal–related responses. Rather, it reduces the dominant time constant of the canal from 4–5 s to 0.025–0.070 s,8,23 depending on location of the plug in the canal duct. Thus, aVOR gains of all six canal-plugged animals are negligible for head oscillations below 1 Hz, but they gradually increase at higher frequency. In this study, we determined whether the summated action of the aVOR and COR after canal plugging could be considered compensatory over the whole range of frequencies of oscillation about yaw and pitch axes and whether COR gain changes are specific for the plane of the plugged canals.
Methods
Experiments, performed on two rhesus (Macaca mulatta) and five cynomolgus (Macaca fascicularis) monkeys conformed to the Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of Mount Sinai School of Medicine. Under anesthesia and in sterile conditions, a head mount was implanted on the skull to provide painless head fixation in stereotaxic coordinates during testing.9 Two weeks later, two coils were implanted on the left eye. One coil measured horizontal and vertical eye position. Another coil, placed approximately orthogonal to the frontal coil, measured torsional eye position. Later, the semicircular canals were plugged by opening each bony canal via the mastoid cavity and packing with fascia and bone chips.10,11 All six semicircular canals were plugged in two cynomolgus monkeys in 1998 (M9357) and 2006 (M17115), and both lateral canals were plugged in M98078 in 2001. The animals had completely recovered by the time of the experiments (2008).
A manual 2-axis rotator was designed for this experiment, which allowed rotation of the body in yaw and in pitch about the C1–C3 level under a spatially fixed head. A turntable was mounted at the base of a U-shaped gimbal, which rotated about a spatial horizontal axis. The turntable could be positioned in any orientation relative to the spatial horizontal axis. The animals sat in a primate chair on the turntable. The head was fixed in an external head holder, providing stable head fixation in space as the body was oscillated beneath it. The animals’ chests were fixed by two straps, and their backs were supported to ensure that the long axis of the body would be aligned with the vertical axis of the head. Oscillation about each axis was manual at frequencies ranging from 0.05 to 6 Hz.
Yaw, pitch, and roll components of eye movement were recorded by a phase-detection system mounted on the head.12 Leftward yaw, downward pitch, and clockwise roll from the animal’s point of view were positive. Eye movements were calibrated off-line based on eye and head velocities, assuming that yaw and pitch aVOR gains of the canal-plugged monkeys in light were 1.0 and roll gain was 0.6.8,13 Chair yaw and pitch positions and the three components of eye position were stored with a resolution of 16 bits at 1 kHz for off-line analysis. Body rotation relative to spatially stationary head was inverted to represent relative head-re-body position. Data were digitally differentiated by a linear fit of 11 data points, and saccades were removed. Manual oscillation implies some variation in oscillation periods from cycle to cycle. When two or more cycles with similar periods were identified, data were fit with a sinusoid at this frequency. If the sinusoidal fit had an amplitude of oscillation that was significantly different from the average yaw velocity (F-statistic8), the gain was calculated. Gain was defined as (amplitude of the sine fit through eye velocity)/(amplitude of the sine fit through head velocity at the same frequency). Positive phase shifts indicate that eye velocity lagged head velocity. We define the out-of-phase relationship of eye and head velocity as 0° phase shift, since it was compensatory from the standpoint of the aVOR.
Results
Normal Monkeys
To provide a baseline, two normal rhesus and two normal cynomolgus monkeys were tested by body oscillation about yaw and pitch axes (Fig. 1). In agreement with previous studies, there was slight enhancement of gain of the yaw COR at the lowest frequencies (Fig. 1A, 1B). However, the gain remained at 0.1 or below at higher frequencies. The responses had a phase lag of ≈20° at higher frequencies (not shown). The pitch COR, which to our knowledge has not been investigated before, had similar characteristics. The gain approached 0.2 at the lowest frequencies, and decreased toward zero in 3 of the 4 animals as the frequency increased (Fig. 1C, 1D). One animal (M17088, Fig. 1C, open symbols) had pitch COR gain within 0.2–0.3 across all tested frequencies. The responses were out of phase with head velocity at lower frequencies, and slightly lagged head velocity at frequencies above 3 Hz (not shown). Thus, in general agreement with all previous studies, when the canal-induced aVOR was intact, the COR was relatively inert for yaw and pitch oscillation of the body on the head. The slight increase in the gain of the COR at the lowest frequencies could be considered as an augmentation of inappropriate gains and phases of the aVOR at these frequencies.
Figure 1.
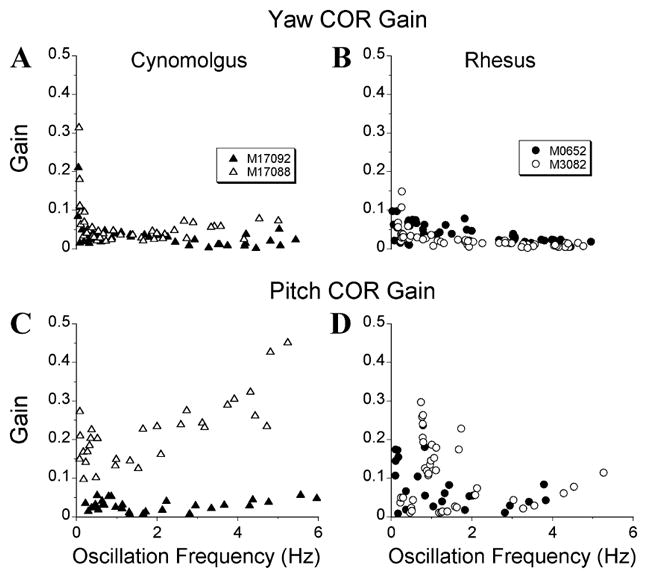
(A, B) Gains of yaw and (C, D) pitch cervico-ocular reflex (COR) induced in (A, C) naïve cynomolgus and (B, D) rhesus monkeys.
Canal-Plugged Monkeys
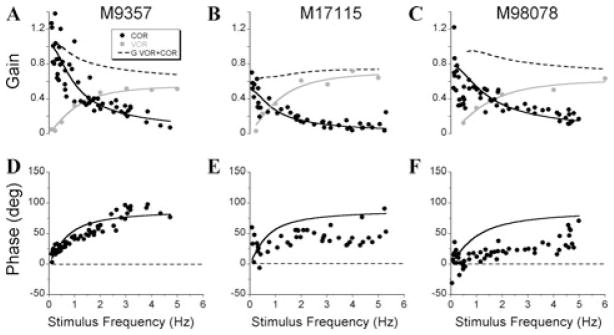
There was a substantial difference in the function of the COR in the six canal-plugged animals in both yaw and pitch. Body yaw oscillation at 0.5 Hz about a spatial vertical axis induced yaw eye velocities that were out of phase with head-re-body velocity (Fig. 2A). The COR gain was close to unity and was ≈180° out of phase with head-re-body velocity relative to the body, at this frequency (Fig. 2A). Thus, following canal plugging, the COR alone was generating perfect compensation for the ≈0 aVOR gain at this frequency.8 When the body was oscillated under a stationary head at 2 Hz, the evoked eye velocities lagged contralateral head-re-body velocity by 90° (Fig. 2B), with a gain of ≈0.4. Thus, the COR gain declined as the expected gain of the aVOR following canal plugging increased and its phase shifted toward head velocity.8 This conclusion is supported by the behavior of the yaw gains of the COR of the two of the six canal-plugged animals (Fig. 3A, 3B) and one lateral canal-plugged animal (Fig. 3C) when they were tested in the plane of the lateral canals. In all three animals, the COR gains were close to unity at lower frequencies and fell to about 0.2 at the highest tested frequencies (Fig. 3A–3C, black circles). The phase was 0°, that is, out-of-phase with head velocity at lower frequencies, but lagged by 90°, toward head position, at high frequencies. To determine gain and phase of the COR, data were fit with a simple integrator model with the following transfer function:
where gCOR is gain, TCOR is time constant of the COR, and f is the frequency of the body oscillation. Fits of the gain values (Fig. 3A–3C, black lines) indicate that the COR gain (gCOR) was 1.02 in M9357, 0.51 in M17115, and 0.79 in M98078. The time constants of the COR were 0.68 s in M9357, 0.64 s in M17115, and 0.96 s in M98079. When the same sets of parameters were implemented to fit the phase of the COR, there was a reasonable match with the data of M9357 (Fig. 3D, cf. black symbols and line). The data had the same shape in M17115 and M98078 (Fig. 3E, 3F, black symbols), but the fits were farther from the data.
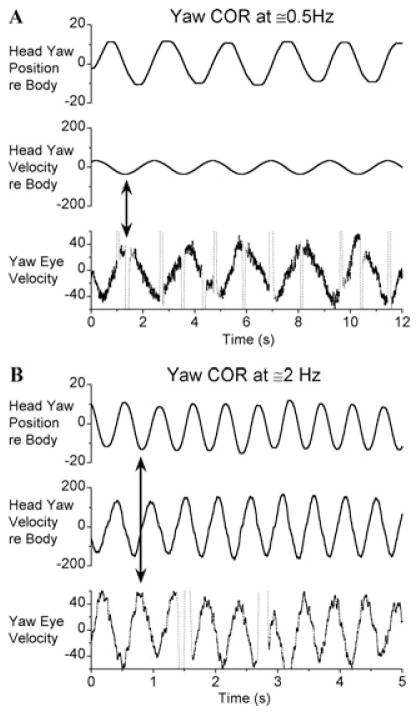
Figure 2.
Typical example of yaw eye velocities induced by body oscillation of a six canal-plugged animal (M9357) at frequencies of (A) ≈0.5 Hz and (B) ≈2.0 Hz. Vertical dotted lines in the eye-velocity traces are saccades that were marked for exclusion from analysis. Vertical double-headed arrows indicate that the peaks of yaw eye velocity are (A) 180° out of phase with peak yaw head-re-body velocity at 0.5 Hz, but (B) 180° out of phase with peak yaw head-re-body position at 2 Hz.
Figure 3.
(A–C) Yaw cervico-ocular reflex (COR) (black symbols) and angular vestibulo-ocular reflex (aVOR) (gray symbols) obtained by rotation about spatial vertical axis in a six canal-plugged (M9357 and M17115) and lateral canal-plugged (M98078) animals. The black line is the fit of the COR gains with COR transfer function. The gray line is the fit of aVOR gains with a model-based aVOR transfer function. The dashed line is the summation of COR and aVOR transfer functions. (D–F) COR phases. The black line is the prediction of the COR phases based on the COR gain transfer function determined for each animal.
To determine whether the changes in COR gain were compensatory, the gains of the aVOR were determined (Fig. 3, gray symbols) and combined with the COR gains over the same frequency range. As in previous studies, the aVOR gains were negligible at low frequencies, but increased at higher frequencies (Fig. 3A–3C), while phases shifted toward acceleration at lower frequencies and toward velocity at higher frequencies (not shown). To characterize the frequency response of the aVOR, the gains were fitted with the following transfer function:
where g1 is a direct pathway gain, Tc is a time constant of the plugged canal and go and To are the coupling and time constant of the velocity storage integrator, respectively.14 To simplify comparison between the animals for the purpose of the present study we assumed that the time constant of velocity storage was 12 s, based on the pooled time constants of the normal animals. The time constants of the plugged canals were 101 ms in M9357, 94 ms in M17115, and 77 ms in M98078, consistent with previous values.8 The direct pathway gain for these animals was 0.56, 0.72, and 0.63, respectively.
When the gains of the aVOR and COR were summed (Fig. 3A–3C, dashed lines), the gain was ≈0.8 across the entire frequency range. Thus, the COR and aVOR acted together in the canal-plugged animals to maintain almost perfect compensatory gains and phases during head movement. Since the aVOR gain is close to unity in the normal animal except at low frequencies, the same conclusion can be drawn for the COR–aVOR interaction in the normal animal.
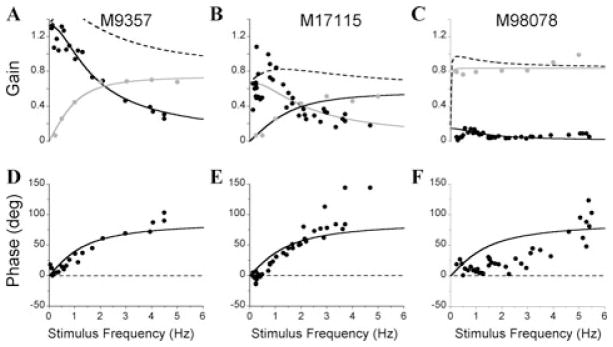
We also compared the pitch aVOR and COR gains and phases from the same animals. Of particular interest was whether the pitch COR would be increased in the two animals with plugged vertical canals and would be unaffected in the third animal in which only the lateral canals were plugged. The time constants of the pitch COR were 1.2 s and 1.6 s in M9357 and M17115, respectively (Fig. 4A and 4B). The pitch COR gain of the animal with intact vertical canals was close to that of the normal animals (Fig. 4C). Despite small gain values across all tested frequencies, the data were well fit with the COR gain function, where the time constant of the COR pathway was 1.3 s (Fig. 4C). The direct COR pathway gain in the lateral canal–plugged animal was 0.85, which was close to that of the animals with all six canals plugged (1.3 and 0.68 for M9357 and M17115, respectively).
Figure 4.
(A–C) Pitch cervico-ocular reflex (COR) (black symbols) and angular vestibule-ocular reflex (aVOR) (gray symbols) obtained by rotation about spatial vertical axis in a six canal-plugged (M9357 and M17115) and lateral canal-plugged (M98078) animals. The black line is the fit of COR gains with a first-order COR transfer function. The gray line is a fit of aVOR gains with model-based transfer function. The dashed line is the summation of COR and aVOR transfer functions. (D–F) COR phases. The black line is the prediction of the COR phases based on the COR gain transfer function determined for each animal.
The phase characteristics of the COR for the six canal-plugged animals were well fit by the model with values determined from the COR distribution (Fig. 4D, 4E, black symbols and lines). The fit of the actual data was less consistent for the third animal (Fig. 4F, black symbols and line). Regardless, in every case, COR-evoked eye velocities were in phase with head velocity at low frequencies, but shifted toward head position at higher frequencies, where COR gain values became smaller. Similar to the yaw aVOR gains, the pitch aVOR gain was negligible and advanced toward head acceleration at low frequencies, but became substantial with appropriate phases at higher frequencies. The dominant time constant of the plugged vertical canals was 119 ms in M9357 and 79 ms in M17115, and the direct pathway gains were 0.74 and 0.56 for these two animals, assuming that the time constant of velocity storage was 12 s. Monkey M9878 (Fig. 4C and 4F) had intact vertical canals and its COR gain remained close to that of the normal animals. The summed gain of the pitch aVOR and COR across all tested frequencies was above unity at low frequencies in M9357, while it was close to 1.0 above 3 Hz (Fig. 4A–4C, dashed lines). The summated gain for M17115 was close to 0.8 at all tested frequencies. The COR gain in pitch for the lateral canal-plugged animal was negligible, as in the normal animals (Fig. 4C, black symbols), showing that the plugged canal was the stimulus for producing the increase in COR gain.
Discussion
This study shows that the yaw and pitch COR adapts to compensate for deficiencies in aVOR produced by canal plugging. This adaptation occurred in a frequency-dependent manner and was specific to the plane of the affected canals. In accord with previous studies,1,2 we found that COR gain is negligible in normal animals (below 0.1), although it was higher at low frequencies (≈0.05 Hz). This is consistent with the hypothesis that the COR gain compensates for aVOR deficiencies,5 as the gain of the aVOR falls off at low frequencies.15 Our conclusions are also consistent with previous findings that there is an inverse relationship between COR and aVOR gains with age.16 The COR gain could also be adapted by immobilization of the neck over several hours.17 It is not clear whether neck proprioceptive signals remain the same during COR gain adaptation; however, increase in COR gain due to deficits of the canal input2,7,18,19 and inability to increase COR gain in patients with cerebellar lesions indicate that COR gain adaptation occurs centrally rather than in the periphery.20
To obtain the aVOR gain and phase characteristics, we utilized a transfer function derived from the velocity-storage model of the aVOR.14 This model processes the head-velocity signal by a first-order transfer function composed of a canal (cupula) time constant. This is cascaded with a transfer function, which is a summation of velocity storage and a direct pathway.14 The transfer function associated with the COR was assumed to be a first-order system (an integrator) having a single time constant. When the resulting aVOR and COR gains determined by these models were summed for each animal, the resultant curves had stable gains across all tested frequencies, although there was variation among animals. The time constant of the COR integrator was about 1 s for both yaw and pitch, indicating that this system does not utilize a path through velocity storage as does the visual system14,21 or proprioception during locomotion.22 The fits to the gain versus frequency plots for the canal-plugged animals in the present study gave a time constant of ≈100 ms, which is close to the 70 ms previously obtained by step responses.8 The time constant for the other two animals based on the gain values are expected to be smaller8,23 and were consistently smaller in the present study. Thus, our model, which now includes velocity storage, is consistent with the simplified approach utilized previously.
The signals and the central pathways that adapt the COR are not clear. Visual–vestibular mismatch using pursuit stimuli can adapt the COR within 10 min,1,24 while full-field visual–vestibular mismatch does not.1 This suggests that the flocculus, which subserves pursuit,25 and suppression of nystagmus26 may be important for COR adaptation.27 Thus, both visual following and COR mechanisms are adapted at low frequencies following plugging, which inactivates the canals at low frequencies. Thus, synergy between visual following and the aVOR, such that pursuit is enhanced after injuries that compromise the aVOR, is also present for the COR. A major difference between the two is that visual following is an important mechanism in its own right, while the COR appears to be a separate auxiliary compensatory system that contributes little to normal compensation, but has a profound impact when the normal functioning of the canals is compromised.
Acknowledgments
This work was supported by grants from the NIH EY11812, EY04148, DC04996, DC05204, DC2390, DC09255 & EY01867. We thank Dmitri Ogorodnikov, Sergey Tarasenko, and Juan Martinez for technical support.
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Mandellos D, Anastasopoulos D, Becker W. Smooth pursuit rather than visual signals mediate short-term adaptation of the cervico-ocular reflex in humans. Exp Brain Res. 2006;169:153–161. doi: 10.1007/s00221-005-0134-7. [DOI] [PubMed] [Google Scholar]
- 2.Huygen PL, Verhagen WI, Nicolasen MG. Cervico-ocular reflex enhancement in labyrinthine-defective and normal subjects. Exp Brain Res. 1991;87:457–464. doi: 10.1007/BF00231863. [DOI] [PubMed] [Google Scholar]
- 3.Böhmer A, Henn V. Compensatory eye movements during high angular accelerations in the monkey. Adv Otorhinolaryngol. 1983;30:174–176. doi: 10.1159/000407633. [DOI] [PubMed] [Google Scholar]
- 4.Baker J, et al. Oculomotor reflexes after semicircular canal plugging in cats. Brain Res. 1982;252:151–155. doi: 10.1016/0006-8993(82)90989-1. [DOI] [PubMed] [Google Scholar]
- 5.Bárány R. Augenbewegungen durch Thoraxbewegungen ausgelöst. Zentralbl Physiol. 1906;20:298–302. [Google Scholar]
- 6.Mergner T, et al. Eye movements evoked by proprioceptive stimulation along the body axis in humans. Exp Brain Res. 1998;120:450–460. doi: 10.1007/s002210050418. [DOI] [PubMed] [Google Scholar]
- 7.Bronstein AM, Hood JD. The cervico-ocular reflex in normal subjects and patients with absent vestibular function. Brain Res. 1986;373:399–408. doi: 10.1016/0006-8993(86)90355-0. [DOI] [PubMed] [Google Scholar]
- 8.Yakushin SB, et al. Dynamics and kinematics of the angular vestibulo-ocular reflex in monkey: effects of canal plugging. J Neurophysiol. 1998;80:3077–3099. doi: 10.1152/jn.1998.80.6.3077. [DOI] [PubMed] [Google Scholar]
- 9.Yakushin SB, et al. Functions of the nucleus of the optic tract (NOT). I. Adaptation of the gain of the horizontal vestibulo-ocular reflex. Exp Brain Res. 2000;131:416–432. doi: 10.1007/s002219900303. [DOI] [PubMed] [Google Scholar]
- 10.Money KE, Scott JW. Functions of separate sensory receptors of non-auditory labyrinth of the cat. Am J Physiol. 1962;202:1211–1220. doi: 10.1152/ajplegacy.1962.202.6.1211. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki JI, et al. Canal-plugging in the rhesus monkey: a tool to study the contribution of individual canals to nystagmus generation. Acta Otolaryngol (Suppl) 1991;481:91–93. doi: 10.3109/00016489109131354. [DOI] [PubMed] [Google Scholar]
- 12.Ogorodnikov D, et al. Head fixed field coil system for measuring eye movements in freely moving monkeys. Conf Proc IEEE Eng Med Biol Soc. 2006;1:5567–5570. doi: 10.1109/IEMBS.2006.260407. [DOI] [PubMed] [Google Scholar]
- 13.Yakushin SB, et al. Normalization effects of vision on the compensatory VOR after canal plugging. In: Highstein SM, Cohen B, Büttner-Ennever JA, editors. Ann NY Acad Sci: New Directions in Vestibular Research. Vol. 781. New York Academy of Sciences; New York: 1996. pp. 713–717. [DOI] [PubMed] [Google Scholar]
- 14.Raphan T, Matsuo V, Cohen B. Velocity storage in the vestibulo-ocular reflex arc (VOR) Exp Brain Res. 1979;35:229–248. doi: 10.1007/BF00236613. [DOI] [PubMed] [Google Scholar]
- 15.Melvill Jones G, Barry W, Kowalsky N. Dynamics of the semicircular canals compared in yaw, pitch and roll. Aerosp Med. 1964;35:984–989. [PubMed] [Google Scholar]
- 16.Kelders WP, et al. Compensatory increase of the cervico-ocular reflex with age in healthy humans. J Physiol. 2003;553:311–317. doi: 10.1113/jphysiol.2003.049338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montfoort I, et al. Adaptation of the cervico-and vestibulo-ocular reflex in whiplash injury patients. J Neurotrauma. 2008;25:687–693. doi: 10.1089/neu.2007.0314. [DOI] [PubMed] [Google Scholar]
- 18.Kasai T, Zee DS. Eye-head coordination in labyrinthine-defective human beings. Brain Res. 1978;144:123–141. doi: 10.1016/0006-8993(78)90439-0. [DOI] [PubMed] [Google Scholar]
- 19.Heimbrand S, et al. Optically induced plasticity of the cervico-ocular reflex in patients with bilateral absence of vestibular function. Exp Brain Res. 1996;112:372–380. doi: 10.1007/BF00227943. [DOI] [PubMed] [Google Scholar]
- 20.Bronstein AM, Mossman S, Luxon LM. The neck-eye reflex in patients with reduced vestibular and optokinetic function. Brain. 1991;114:1–11. [PubMed] [Google Scholar]
- 21.Cohen B, Matsuo V, Raphan T. Quantitative analysis of the velocity characteristics of optokinetic nystagmus and optokinetic after-nystagmus. J Physiol. 1977;270:321–344. doi: 10.1113/jphysiol.1977.sp011955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Solomon D, Cohen B. Gaze compensatory nystagmus during circular locomotion in darkness: vestibular and somatosensory contributions to eye and head movements in the running monkey. J Neurophysiol. 1992;67:1158–1170. doi: 10.1152/jn.1992.67.5.1158. [DOI] [PubMed] [Google Scholar]
- 23.Rabbitt RD, Boyle R, Highstein SM. Influence of surgical plugging on horizontal semicircular canal mechanics and afferent response dynamics. J Neurophysiol. 1999;82:1033–1053. doi: 10.1152/jn.1999.82.2.1033. [DOI] [PubMed] [Google Scholar]
- 24.Rijkaart DC, et al. Short-term adaptation of the cervico-ocular reflex. Exp Brain Res. 2004;156:124–128. doi: 10.1007/s00221-004-1878-1. [DOI] [PubMed] [Google Scholar]
- 25.Lisberger SG, Miles FA, Zee DS. Signals used to compute errors in monkey vestibuloocular reflex: possible role of flocculus. J Neurophysiol. 1984;52:1140–1153. doi: 10.1152/jn.1984.52.6.1140. [DOI] [PubMed] [Google Scholar]
- 26.Takemori S, Cohen B. Loss of suppression of vestibular nystagmus after flocculus lesions. Brain Res. 1974;72:213–224. doi: 10.1016/0006-8993(74)90860-9. [DOI] [PubMed] [Google Scholar]
- 27.Wiksten B. The central cervical nucleus in the cat. II. The cerebellar connections studied with retrograde transport of horseradish peroxidase. Exp Brain Res. 1979;36:155–173. doi: 10.1007/BF00238475. [DOI] [PubMed] [Google Scholar]