Abstract
Background
A single-nucleotide polymorphism (SNP) in GABRA2 (rs279858) may moderate subjective response (SR) to alcohol. Results of studies in non-dependent drinkers examining this GABRA2 SNP on SR have been equivocal. This study examined this SNP’s direct and indirect effects on alcohol self-administration in dependent drinkers.
Method
The sample consisted of 63 Caucasian, non-treatment-seeking individuals with alcohol dependence. Subjective stimulation was assessed using the Biphasic Alcohol Effects Scale following consumption of an alcoholic priming drink (target breath alcohol content = 0.02 g%). Participants were subsequently offered the opportunity to self-administer up to eight additional drinks.
Results
Controlling for baseline stimulation, T-allele homozygotes, relative to individuals with at least one copy of the C-allele, reported greater initial stimulation, t(58) = 2.011, p = 0.049. Greater stimulation predicted greater subsequent alcohol self-administration, t(57) = 2.522, p = 0.015. Although rs279858 genotype did not directly impact self-administration (t(57) = −0.674, p = 0.503), it did have an indirect effect (95% confidence interval [0.068, 1.576]), such that T-allele homozygotes reported greater stimulation, which in turn predicted greater self-administration.
Conclusion
These results suggest that the influence of this SNP on SR differs depending on dose or stage of dependence. This study is the first to demonstrate an indirect effect of rs279858 genotype on drinking through SR. Although C-allele carriers have been shown to have an increased risk for alcohol dependence, in our dependent sample, greater stimulation was found among T-allele homozygotes, suggesting that the influence of SR on developing and maintaining dependence differs based on rs279858 genotype.
This study demonstrates an indirect effect of rs279858 genotype on drinking through SR. Although C-allele carriers have an increased risk for alcohol dependence, in our dependent sample, greater stimulation was found among T-allele homozygotes, suggesting that the influence of SR on developing dependence differs based on rs279858 genotype.
Introduction
Alcohol is one of the most commonly used addictive substances in the world (Rehm et al., 2009) and contributes to roughly one-third of all fatal car accidents and injury-related emergency room visits (MacLeod and Hungerford, 2011). Although research on the familial transmission of alcohol use disorders has long established the heritability of problematic drinking (McGue, 1997; Liu et al., 2004), understanding of the mechanisms through which genetic variation influences alcohol-related problems is limited. One promising genetic influence on the development of alcohol dependence is GABRA2, the gene that encodes the α2 subunit of the GABAA receptor (Reich et al., 1998; Enoch, 2008).
GABA is the primary inhibitory neurotransmitter in the central nervous system, and exerts its effects through binding at ionotropic (GABAA) and metabotropic (GABAB) receptors (Enoch, 2008). Alcohol’s primary mechanism of action is positive allosteric modulation of GABAA receptors (Korpi et al., 2007). Several of alcohol’s behavioral effects are potentiated by GABAA receptor functioning, including motor incoordination, anticonvulsant activity and preference for alcohol (Covault et al., 2004). Linkage studies have uncovered several genes responsible for encoding GABAA receptor subunits that are associated with alcohol-related phenotypes. Specifically, multiple single-nucleotide polymorphisms (SNPs) in GABRA2 were associated with alcohol dependence (Reich et al., 1998). One such SNP, rs279858, has received considerable attention and, in the Study of Addiction: Genetics and Environment (SAGE) genome-wide association study (Olfson and Bierut, 2012), was the only candidate locus that was at least moderately associated with alcohol dependence, with higher rates of dependence among individuals with one or more copy of the minor (C) allele. Variation in GABRA2 has also been associated with increases in heavy drinking/intoxication over time, with frequency of intoxication increasing disproportionately from adolescence to adulthood among individuals homozygous for the C-allele (Dick et al., 2013).
Given that modulation of GABAA receptors by alcohol is believed to underlie many of the pharmacological effects of alcohol and that GABRA2 has been linked to alcohol dependence, studies have begun to examine the relationship between subjective response (SR) to alcohol and GABRA2. Individual differences in response to alcohol may represent an endophenotype, or vulnerability marker, that is associated with a genetic risk for alcohol use disorders, though the pattern of response that confers greatest risk for alcohol-related problems is unclear (Morean and Corbin, 2010; Ray et al., 2010; Quinn and Fromme, 2011). While some studies have found that greater subjective stimulation and decreased impairment is related to increased levels of consumption, others have suggested that an attenuated response to the full range of pharmacological effects of alcohol is a more salient predictor of alcohol problems (Newlin and Thomson, 1990; Schuckit, 2004; Quinn and Fromme, 2011; King et al., 2014).
Results of studies examining the effect of rs279858 on SR to alcohol in controlled situations have also been equivocal, with some indicating higher stimulation among C-allele carriers (Haughey et al., 2008; Kosobud et al., 2015) and others indicating lower stimulation (Pierucci-Lagha et al., 2005). The first study of this SNP on SR found that individuals homozygous for the more common T-allele endorsed greater stimulation and pleasurable effects due to alcohol than those who carried at least one copy of the C-allele (Pierucci-Lagha et al., 2005). Although this study did not utilize an alcohol-dependent sample, the authors concluded that the results were consistent with the ‘low-level response model’ of alcohol dependence, which suggests that C-allele carriers, purportedly at higher risk for developing alcohol use disorders, experience a diminished response to alcohol stimulation relative to lower risk T-allele homozygotes (Schuckit and Smith, 1996). Thus, the risk for alcohol dependence among C-allele carriers may be mediated by the decreased subjective stimulation response to alcohol (Pierucci-Lagha et al., 2005); however, the indirect effect of SR on later drinking and related consequences was not examined in that study.
More recent studies have failed to replicate this effect. In a sample of moderate to heavy social drinkers, Haughey et al. (2008) found that, across three different alcohol doses (0.02, 0.04 and 0.06 g%), C-allele homozygotes, as compared to T-allele carriers, reported greater alcohol ‘liking’. However, they found no genotype effects on self-reported stimulation. Among social drinkers, C-allele carriers have also been found to report lower sedative and aversive effects of alcohol (Uhart et al., 2013). Another study found that among non-dependent social drinkers, C-allele carriers reported greater stimulation following alcohol consumption, but only at a moderate alcohol dose (mean breath alcohol content ≈0.12 g% [men]; 0.08 g% [women]) (Arias et al., 2014).
Previous studies have been limited by their use of non-dependent social drinkers (who may or may not be at risk for developing alcohol dependence) and have failed to examine the potential mediating effect of SR on the relationship between GABRA2 genotype and drinking outcomes. This study examined both the direct and indirect effects (through SR to alcohol) of this SNP on drinking behavior in an alcohol-dependent sample.
Method
Participants were 81 non-treatment-seeking individuals with alcohol dependence randomized to placebo medication in two clinical laboratory experiments. We restricted the sample to Caucasian participants because allele frequencies for rs279858 differ substantially between racial groups and one of the studies did not recruit African-American participants. A total of 11 non-Caucasian participants were excluded from the analyses. One participant whose age (64 years) was greater than 9 SD above the mean age for the sample (24.73 years; SD = 4.24) was excluded from the sample, as were six participants who did not achieve a breath alcohol content (BrAC) ≥0.01 g% after the priming drink. Thus, the final sample for this analysis consisted of 63 participants. See Table 1 for demographic characteristics of the sample separated by genotype.
Table 1.
Demographics by GABRA2 genotype
| GABRA2 | |||||
|---|---|---|---|---|---|
| Total | Any C | T/T | Χ 2 | p | |
| N | 63 | 45 | 18 | ||
| Male | 45 (71%) | 32 (71%) | 13 (72%) | 0.008 | 0.930 |
| Family history positive | 19 (30%) | 14 (31%) | 5 (28%) | 0.068 | 0.795 |
| Mean (SD) | Mean (SD) | Mean (SD) | t(62) | p | |
| Age | 24.73 (4.24) | 24.47 (4.36) | 25.39 (3.96) | −0.777 | 0.440 |
| Education (years) | 14.60 (2.37) | 14.62 (2.62) | 14.52 (1.62) | 0.100 | 0.920 |
| Drinks/day | 5.99 (2.72) | 6.14 (3.00) | 5.63 (1.90) | 0.669 | 0.506 |
| Drinks/drinking day | 8.63 (3.34) | 8.94 (3.49) | 7.84 (1.90) | 1.183 | 0.241 |
| Binge drinking days | 51.77 (16.42) | 52.30 (15.76) | 50.45 (18.35) | −0.423 | 0.673 |
| % Binge drinking days | 83.71 (20.69) | 85.78 (19.88) | 78.53 (22.27) | −1.332 | 0.187 |
| Dependence severity | 11.58 (5.39) | 10.58 (5.78) | 12.02 (5.23) | 0.973 | 0.335 |
Notes: Drinking frequency data derived from a 90-day Timeline Followback Interview. Family history positive = 2 or more first or second degree relatives with probable alcoholism. Binge drinking = 5 or more drinks per drinking day for men (4 or more for women). Stimulant expectancy measured by a modified Biphasic Alcohol Effects Scale (BAES) assessed prior to beverage administration. Dependence severity measured by the Alcohol Dependence Scale.
Subjects were recruited via media advertisements and administered a brief phone screen to determine eligibility. All subjects were required to meet DSM-IV (American Psychiatric Association, 2000) diagnostic criteria for current alcohol dependence, as assessed by the Structured Clinical Interview for DSM-IV (First and Gibbon, 2004), and to deny currently seeking treatment for their alcohol use. Exclusion criteria were current DSM-IV diagnosis of dependence on any other substance except nicotine; the use of any psychoactive medication or substance except nicotine or marijuana in the past 30 days, as confirmed by a urine drug screen (which had to be negative for all drugs, including Δ9-tetrahydrocannabinol, prior to participation); current DSM-IV Axis I diagnosis other than alcohol dependence; current suicidal/homicidal ideation; history of significant medical illness or liver enzymes three times or more than normal.
The Medical University of South Carolina Institutional Review Board approved all procedures. All participants provided informed consent prior to participation. The parent studies were clinical experiments that involved subacute medication dosing, in which subjects were randomized to active medication or placebo; the latter group made up the sample for this analysis. The procedures for both studies were identical and consisted of a bar laboratory session after 8 days of study drug. Prior to completing the bar laboratory session, subjects provided a blood sample for genetic analysis. GABRA2 rs279858 genotyping was conducted post hoc after all data were collected but blind to any subject data using a TaqMan 5′ nuclease assay, run with three known controls for each genotype. Genotype frequencies (C/C = 10; C/T = 35; T/T = 18) were consistent with the expected frequencies for individuals of European descent and in Hardy–Weinberg equilibrium. Preliminary analyses revealed no significant differences between the C/C and C/T groups in terms of pre-study key drinking variables, thus the two groups were combined.
The bar laboratory session took place in a simulated bar laboratory that included a bar stocked with liquor bottles, alcohol advertisements and bar stools (see Drobes et al., 2003 for further description). After baseline assessment and consumption of a standardized light lunch, participants were administered a ‘priming drink’ (80-proof liquor mixed with fruit juice) adjusted for their sex and weight to produce a target BrAC of 0.02–0.03 g%, and were instructed to consume it within 5 min. Participants who failed to achieve a peak BrAC of 0.01 g% or higher were excluded from analyses. A peak BrAC of 0.02–0.03 g% is equivalent to roughly two standard drinks, an amount that has consistently shown increases in BAES stimulation rating in past studies conducted in our laboratory (Anton et al., 2004; Voronin et al., 2008).
SR was assessed using the BAES (Martin et al., 1993), which was administered three times following consumption (10, 20 and 30 min). SR measured at the 10-min time point was utilized in this study as blood alcohol levels were still rising for all participants. The BAES (Martin et al., 1993) is a 14-item questionnaire comprising two sub-scales that assess subjective experiences of alcohol stimulation (e.g. energized, talkative) and sedation (e.g. heavy head, slow thoughts). Participants rated the extent to which they experienced each effect on 11-point Likert-type scales from not at all (0) to extremely (10). Whereas stimulation is more pronounced on the ascending limb of the blood alcohol concentration curve, sedation is more evident as blood alcohol levels decline (Earleywine and Martin, 1993; Erblich et al., 2003; King et al., 2011). Preliminary analyses revealed no differences in subjective sedation by GABRA2 genotype, and sedation was not significantly associated with free-choice alcohol consumption (Table 2); therefore, analyses focused on stimulation.
Table 2.
Bar laboratory drinking characteristics by GABRA2 genotype
| GABRA2 | |||||
|---|---|---|---|---|---|
| Total | Any C | T/T | |||
| Mean (SD) | Mean (SD) | Mean (SD) | t(61) | p | |
| Pre-drink | |||||
| Stimulation expectancy | 37.89 (15.04) | 38.24 (16.04) | 37.00 (12.59) | 0.294 | 0.769 |
| Sedation expectancy | 18.60 (11.71) | 19.76 (11.25) | 15.72 (12.65) | 1.241 | 0.219 |
| Post-drink | |||||
| Subjective stimulation | 10.81 (11.60) | 9.20 (11.10) | 14.83 (12.17) | −1.771 | 0.082 |
| Subjective sedation | 5.33 (8.98) | 4.84 (9.12) | 6.56 (8.77) | −0.680 | 0.499 |
| Free-choice alcohol consumption | 3.92 (3.16) | 3.93 (3.22) | 3.88 (3.08) | 0.050 | 0.960 |
Note: Subjective stimulation was included as a covariate in subsequent regression analyses.
This study was primarily interested in the impact of GABRA2 genotype on the interpretation of the pharmacological effects of alcohol. Non-pharmacological factors such as environmental and expectancy effect, also contribute to alcohol’s SR. To more carefully control the drinking environment to minimize contextual influences on subjective stimulation, all participants consumed the alcoholic beverages alone in a simulated bar laboratory. Additionally, a modified version of the BAES that assessed expected response to alcohol was administered prior to alcohol administration to account for expectancy effects and to more finely focus the analysis on the immediate pharmacological effects of alcohol during the bar laboratory session.
After the priming drink, participants were offered the opportunity to consume up to eight additional alcoholic beverages, each calibrated by weight and gender to produce a target BrAC of ~0.015 g%. Participants were told that they had a ‘bar tab’ credit of $16 with which they could purchase drinks over the next 2 h. A tray of four drinks was placed on the bar 40 min after the initial priming dose, and participants were informed that they could consume as many of the drinks as they desired during a 1-h period at the cost of $2 per drink. After 1 h passed, another tray of four drinks was placed on the bar with the same instruction. The total number of drinks (up to 8) consumed during the limited-access drinking period served as the primary outcome variable. The day following the study, participants were debriefed, counseled regarding the detrimental effects of their drinking and compensated $300 for completing the study, as well as any remaining ‘bar tab’ money not spent on drinks.
Statistical analyses
Differences in initial SR to alcohol by GABRA2 genotype were analyzed using the PROCESS macro (Hayes, 2013) in SPSS version 22. Stimulant expectancy was entered as a covariate. The dichotomous GABRA2 genotype variable was entered as the independent variable, subjective stimulation as the mediator and free-choice alcohol consumption as the dependent variable. The statistical significance of the indirect effect of GABRA2 genotype on within-session drinking through subjective stimulation was assessed using 10,000 bootstrap samples to calculate the bias-corrected bootstrap confidence interval (CI) of the indirect effect (Hayes, 2013). Bias-corrected bootstrapping is recommended for models assessing multiple comparisons (such as mediation models) as the approach achieves higher power while maintaining reasonable control over Type I errors (MacKinnon et al., 2002).
Results
Subject demographics are presented in Table 1. Participants obtained a mean peak BrAC of 0.019 g% (SD = 0.005). T-allele homozygotes did not differ from C-allele carriers in terms of peak BrAC (t(61) = −1.289, p = 0.202), or stimulant expectancies (t(61) = 0.294, p = 0.769) (Table 2).
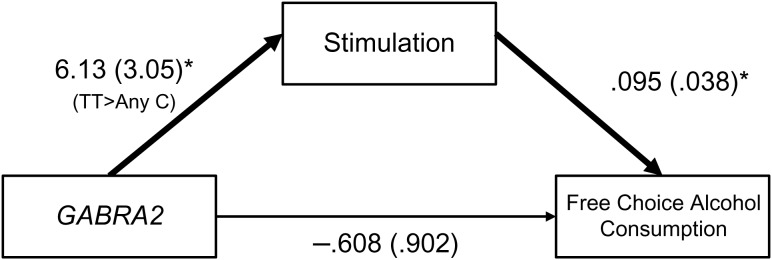
Individuals homozygous for the T-allele, relative to C-allele carriers, reported greater stimulation after controlling for age, family history of alcoholism and baseline stimulation expectancy scores, t(58) = 2.01, p = 0.049 (see Table 3 for summary of results). Greater stimulation predicted greater alcohol consumption, t(58) = 2.52, p = 0.015. Although genotype did not directly impact drinking (t(59) = −0.673, p = 0.503), it did have an indirect effect (95% CI [0.068, 1.576]), such that T-allele homozygotes, relative to C-allele carriers, reported greater stimulation, which in turn predicted heavier drinking (Fig. 1).
Table 3.
Summary of regression analyses
| B | SE | t | |
|---|---|---|---|
| Model 1: DV = Post priming drink stimulation | |||
| Age | −0.114 | 0.331 | −0.345 |
| Family history of alcoholism | 1.99 | 3.01 | 0.663 |
| Stimulation expectancy | 0.257 | 0.094 | 2.748** |
| GABRA2 genotype (TT > CT/CC) | 6.126 | 3.046 | 2.011* |
| Model 2: DV = Free-choice alcohol consumption | |||
| Age | −0.036 | 0.095 | −0.380 |
| Family history of alcoholism | −0.401 | 0.865 | −0.464 |
| Stimulation expectancy | −0.039 | 0.029 | −1.373 |
| Subjective stimulation | 0.095 | 0.038 | 2.522* |
| GABRA2 genotype (TT = CT/CC) | −0.608 | 0.902 | −0.674 |
Notes: *p < 0.05; **p < 0.01.
Fig. 1.
Model results of GABRA2 genotype predicting subjective stimulation and ad lib consumption. Values are unstandardized regression coefficients (standard errors). Bold lines indicate significant coefficients, p < 0.05. Covariate not shown: stimulation expectancy.
Discussion
To the best of our knowledge, this was the first study to evaluate the effect of a GABRA2 SNP (rs279858) on SR to alcohol in an alcohol-dependent sample. This study is also novel in that it assessed the potential effect of this polymorphism on within-session drinking through its influence on subjective stimulation and sedation. Since GABRA2 genotype did not affect alcohol-induced sedation, but did influence alcohol-induced stimulation, the results may be viewed as somewhat contrary to the ‘low level of response’ model of alcohol dependence (Schuckit et al., 2004). However, the current findings are more consistent with data from our past studies in this population (Anton et al., 2004; Voronin et al., 2008) and those of others (Newlin and Thomson, 1990; King et al., 2011) that suggest a relationship between a more pronounced stimulant response to alcohol and subsequent consumption. This finding also accords with recent evidence that greater alcohol-induced stimulation predicts heavier future consumption and alcohol problems (King et al., 2015). Although C-allele carriers have been shown to have an increased risk for alcohol dependence, in our dependent sample, greater stimulation was found among T-allele homozygotes, suggesting that the influence of stimulation on developing and maintaining alcohol dependence may differ based on rs279858 genotype.
While GABRA2 genotype did not directly predict within-session alcohol consumption, it influenced drinking indirectly through its effect on subjective stimulation. These results suggest that the higher initial stimulant response among T-allele homozygotes may mediate the influence of this SNP on later drinking. The results of this study are partially consistent with those of Roh et al. (2011), who found greater response to alcohol on the Alcohol Sensation Scale among rs279858 T-allele carriers in a moderate drinking Japanese sample; however, the study failed to find an association between rs279858 genotype and subjective stimulation as measured by the BAES, suggesting either drinking population differences or epistatic genetic influences. The attenuated stimulant response among C-allele carriers in our alcohol-dependent sample differs from recent research that found a higher stimulant response among non-dependent C-allele carriers administered a moderate alcohol dose (Arias et al., 2014). This inconsistency may be explained by the lower dose of alcohol administered in this study. However, results from a study utilizing a comparable alcohol dose to this study (~0.02 g%) with non-dependent social drinkers, found no differences in SR by GABRA2 genotype (Haughey et al., 2008). Greater subjective stimulation among C-allele carriers in previous studies of non-dependent social drinkers has been suggested to be associated with vulnerability to alcohol dependence, but because subjects in these studies were not alcohol dependent and were not followed longitudinally, this hypothesis is arguably speculative. Blunted subjective stimulation among T-allele homozygotes in these studies may have been protective against the development of heavier alcohol use. Alternatively, the influence of this SNP on alcohol-induced stimulation may change as individuals transition from social drinking through heavy use to alcohol dependence, implying a gene by environment (alcohol consumption or age) interaction. In any case, further study of the effects of GABRA2 genotype on SR to alcohol among individuals with a range of drinking behavior is needed.
The major finding of this study was that GABRA2 genotype impacts drinking via SR to alcohol. The rs279858 polymorphism might also interact with other genetic variants that have been shown to influence SR, including SNPs in OPRM1, the gene that encodes the mu-opioid receptor, and ALDH, the gene that encodes the alcohol-metabolizing enzyme aldehyde dehydrogenase. Individuals with at least one copy of the G allele of the OPRM1 A118G SNP have been found to endorse greater subjective craving and ‘high’ after alcohol consumption (Ray and Hutchison, 2004). While the GABAergic system is believed to primarily affect the sedative, ataxic and anxiolytic alcohol effects of alcohol (Enoch, 2008), it may also impact stimulation. Individuals homozygous for the rs279858 T-allele who also carry at least one OPRM1 A118G G allele may endorse higher levels of stimulation relative to their peers without this genetic profile, which would positively reinforce continued use. SR may also be influenced by the pharmacokinetic properties of alcohol. Individuals who carry the ALDH2-2 allele, which is commonly found in individuals of East Asian ancestry, are less able to metabolize acetaldehyde to acetate, resulting in aversive effects, including flushing, headache and nausea (Luczak et al., 2006). Genes that modulate GABA receptors, such as GABRA2, may interact with ALDH and ADH to produce greater stimulation across the blood alcohol curve, thus positively reinforcing continued use. Interactions between GABRA2 rs279858 genotype and other genetic variants that affect SR are worthy of future study.
Although numerous pharmacological interventions have been approved for the treatment of alcohol dependence, those with the most promise have been shown to reduce the reinforcing properties (e.g. subjective stimulation) and cue-induced craving for the drug (Drobes et al., 2003; Anton et al., 2004; Anton et al., 2012). The effectiveness of such pharmacological treatments for alcohol dependence is moderate at best, and there is considerable need for the identification of factors that may indicate who would benefit most from medications that reduce the rewarding effects of alcohol. Stimulant dampening pharmacological interventions may be more effective for those more prone to reporting higher levels of stimulation following an initial dose of alcohol, such as those homozygous for the rs279858 T-allele.
The results of this study should be viewed in the context of its limitations. As noted previously, participants were drawn from the placebo condition of two clinical laboratory experiments, which were designed to assess the effectiveness of medications in reducing alcohol consumption. The belief that one is receiving a medication believed to reduce use may have impacted both the SR following alcohol consumption and drinking decisions; however, there is no rationale for GABRA2 genotype to affect any such placebo effects. Due to GABRA2 rs279858 allele frequency considerations, the sample was constrained to Caucasians; thus, results may not generalize to other racial and ethnic groups. When examining SR to alcohol, it is beneficial to obtain measurements in settings that are consistent with typical drinking environments, as response to alcohol has been shown to vary according to the drinking environment (Corbin et al., 2015). This study is strengthened by the use of a simulated bar that is more consistent with an environment in which drinking typically occurs. Nonetheless, results of this study may not generalize to other drinking contexts, such as drinking in non-bar settings.
This study sought to examine the role of GABRA2 genotype on the interpretation of pharmacological alcohol effects, by controlling for non-pharmacological factors (e.g. cognitive expectancies about alcohol effects). As alcohol expectancies are a complex phenomenon, they are likely influenced by multiple genes and therefore we sought to isolate the pharmacological effect under controlled conditions in that setting (e.g. free of social interaction). Efforts were made to carefully control the drinking environment to minimize contextual influences on subjective stimulation. However, it is also possible that differences in alcohol expectancies may be part of the phenotype attributable to the GABRA2 SNP. Future studies are needed to examine the potential influence of genes on alcohol-related expectancies, and how these factors may interact to influence drinking.
While the peak BAC of 20–30 mg% might be considered low, it is equivalent to two standard drinks, an amount that has consistently shown increases in BAES stimulation rating in our hands (Voronin et al., 2008; Anton et al., 2012). In fact, we had previously reported more stimulation at 20–30 mg% BAC than at higher concentrations (Thomas et al., 2004) and have previously reported a relationship between stimulation at this BAC and free-choice drinking in the bar laboratory (Anton et al., 2004, Voronin et al., 2008). Furthermore, we have reported that stimulation caused by this BAC is influenced by variation in other genes (Anton et al., 2012). Finally, individuals who consume greater quantities of alcohol must first pass through this level of BAC on their way to heavier drinking, and stimulation early in drinking is likely to predict further drinking, as our previous data show.
Despite these limitations, the results of this study provide additional support for the hypothesis that GABRA2 rs279858 genotype moderates the stimulating effects of alcohol, and that this effect mediates drinking. Individuals homozygous for the rs279858 T-allele endorsed greater initial stimulation following a low dose of alcohol, which in turn, led to increased alcohol consumption. To the best of our knowledge, this is the first study to examine the effects of this GABRA2 SNP on subjective stimulation using an alcohol-dependent sample and its effect on within-session drinking. Future research is needed to replicate this finding in larger diverse samples. Larger samples would also allow for the examination of other genetic variants believed to impact SR, and how these alleles interact with GABRA2 to impact subjective stimulation.
Acknowledgements
Ms. Yeongbin Im, MS, performed the GABRA2 SNP assays for this project. Patrick Randall, PhD, assisted in data management and randomization protocols. The authors would like to recognize Mark Ghent, BA, for his hard work in evaluating the subjects and performing the bar laboratory experiments.
Funding
Supported by NIAAA grants K05 AA017435 (R.F.A.), K99 AA021419 (J.P.S.), K23 AA020842 (J.J.P.) and P50 AA010761.
Conflict of interest statement
None declared.
References
- American Psychiatric Association (2000) Diagnostic and statistical manual of mental disorders (4th ed, text rev.). Washington, DC: doi:10.1002/jps.3080051129 [Google Scholar]
- Anton R, Drobes D, Voronin K, et al. (2004) Naltrexone effects on alcohol consumption in a clinical laboratory paradigm: temporal effects of drinking. Psychopharmacology 173:32–40. Retrieved from http://link.springer.com/article/10.1007/s00213-003-1720-7 [DOI] [PubMed] [Google Scholar]
- Anton RF, Voronin KK, Randall PK, et al. (2012) Naltrexone modification of drinking effects in a subacute treatment and bar-lab paradigm: influence of OPRM1 and dopamine transporter (SLC6A3) genes. Alcohol Clin Exp Res 36:2000–7. doi:10.1111/j.1530-0277.2012.01807.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias AJ, Covault J, Feinn R, et al. (2014) A GABRA2 variant is associated with increased stimulation and ‘high’ following alcohol administration. Alcohol Alcohol 49:1–9. doi:10.1093/alcalc/agt163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin WR, Scott C, Boyd SJ, et al. (2015) Contextual influences on subjective and behavioral responses to alcohol. Exp Clin Psychopharmacol 23:59–70. [DOI] [PubMed] [Google Scholar]
- Covault J, Gelernter J, Hasselbrock V, et al. (2004) Allelic and haplotypic association of GABRA2 with alcohol dependence. Am J Med Genet B (Neuropsychiatric Genetics) 129B:104–9. Retrieved from http://onlinelibrary.wiley.com/doi/10.1002/ajmg.b.30091/full [DOI] [PubMed] [Google Scholar]
- Dick D, Cho S, Latendresse S, et al. (2013) Genetic influences on alcohol use across stages of development: GABRA2 and longitudinal trajectories of drunkenness from adolescence to young adulthood. Addict Biol doi:10.1111/adb.12066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobes DJ, Anton RF, Thomas SE, et al. (2003) A clinical laboratory paradigm for evaluating medication effects on alcohol consumption: Naltrexone and nalmefene. Neuropsychopharmacology 28:755–64. [DOI] [PubMed] [Google Scholar]
- Earleywine M, Martin CS (1993) Anticipated stimulant and sedative effects of alcohol vary with dosage and limb of the blood alcohol curve. Alcohol Clin Exp Res 17:135–9. [DOI] [PubMed] [Google Scholar]
- Enoch M. (2008) The role of GABA A receptors in the development of alcoholism. Pharmacol Biochem Behav 90:95–104. Retrieved from http://www.sciencedirect.com/science/article/pii/S0091305708000725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erblich J, Earleywine M, Erblich B, et al. (2003) Biphasic stimulant and sedative effects of ethanol. Addict Behav 28:1129–39. doi:10.1016/S0306-4603(02)00221-6 [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M (2004) The Structured Clinical Interview for DSM-IV axis I Disorders (SCID-I) and the Structured Clinical Interview for DSM-IV Axis II Disorders (SCID-II). In H. J. Hilsenroth & D. L. Segal (Eds.) Comprehensive handbook of psychological assessment, Vol. 2: Personality assessment (pp. 134–43). Hoboken, NJ: John Wiley & Sons; doi:10.1002/cpp [Google Scholar]
- Haughey HM, Ray L, Finan P, et al. (2008) Human gamma-aminobutyric acid A receptor alpha2 gene moderates the acute effects of alcohol and brain mRNA expression. Genes Brain Behav 7:447–54. doi:10.1111/j.1601-183X.2007.00369.x [DOI] [PubMed] [Google Scholar]
- Hayes A. (2013) Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. New York: Guilford Press. [Google Scholar]
- King AC, de Wit H, McNamara PJ, et al. (2011) Rewarding, stimulant, and sedative alcohol responses and relationship to future binge drinking. Arch Gen Psychiatry 68:389–99. doi:10.1001/archgenpsychiatry.2011.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, Hasin D, O’Connor SJ, et al. (2015) A Prospective 5-year re-examination of alcohol response in heavy drinkers progressing in alcohol use disorder. Biol Psychiatry 1–10. doi:10.1016/j.biopsych.2015.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, McNamara PJ, Hasin DS, et al. (2014) Alcohol challenge responses predict future alcohol use disorder symptoms: A 6-year prospective study. Biol Psychiatry 75:798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korpi ER, Debus F, Linden AM, et al. (2007) Does ethanol act preferentially via selected brain GABAA receptor subtypes? The current evidence is ambiguous. Alcohol 41:163–76. [DOI] [PubMed] [Google Scholar]
- Kosobud AEK, Wetherill L, Plawecki MH, et al. (2015) Adaptation of subjective responses to alcohol is affected by an interaction of GABRA2 genotype and recent drinking. Alcohol Clin Exp Res 39:1148–57. doi:10.1111/acer.12749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu I-C, Blacker DL, Xu R, et al. (2004) Genetic and environmental contributions to the development of alcohol dependence in male twins. Arch Gen Psychiatry 61:897–903. doi:10.1001/archpsyc.61.9.897 [DOI] [PubMed] [Google Scholar]
- Luczak S, Glatt S, Wall T (2006) Meta-analyses of ALDH2 and ADH1B with alcohol dependence in Asians. Psychol Bull 132:607–21. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP, Lockwood CM, Hoffman JM, et al. (2002) A comparison of methods to test mediation and other intervening variable effects. Psychol Methods 7:83–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod JBA., Hungerford DW (2011) Alcohol-related injury visits: do we know the true prevalence in U.S. trauma centres? Injury 42:922–6. doi:10.1016/j.injury.2010.01.098 [DOI] [PubMed] [Google Scholar]
- Martin C, Earleywine M, Musty R, et al. (1993) Development and validation of the biphasic alcohol effects scale. Alcohol Clin Exp Res 17:140–6. [DOI] [PubMed] [Google Scholar]
- McGue M. (1997) A behavioral-genetic perspective on children of alcoholics. Alcohol Health Res World 21:210–7. [PMC free article] [PubMed] [Google Scholar]
- Morean ME, Corbin WR (2010) Subjective response to alcohol: a critical review of the literature. Alcohol Clin Exp Res 34:385–95. [DOI] [PubMed] [Google Scholar]
- Newlin DB, Thomson JB (1990) Alcohol challenge with sons of alcoholics: A critical review and analysis. Psychol Bull 108:383–402. [DOI] [PubMed] [Google Scholar]
- Olfson E, Bierut L (2012) Convergence of genome-wide association and candidate gene studies for alcoholism. Alcohol Clin Exp Res 36:2086–94. doi:10.1111/j.1530-0277.2012.01843.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierucci-Lagha A, Covault J, Feinn R, et al. (2005) GABRA2 alleles moderate the subjective effects of alcohol, which are attenuated by finasteride. Neuropsychopharmacology 30:1193–1203. doi:10.1038/sj.npp.1300688 [DOI] [PubMed] [Google Scholar]
- Quinn PD, Fromme K (2011) Subjective response to alcohol challenge: a quantitative review. Alcohol Clin Exp Res 35:1759–70. doi:10.1111/j.1530-0277.2011.01521.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Hutchison KE (2004) A polymorphism of the mu-opioid receptor gene (OPRM1) and sensitivity to the effects of alcohol in humans. Alcohol Clin Exp Res 28:1789–95. doi:10.1097/01.ALC.0000148114.34000.B9 [DOI] [PubMed] [Google Scholar]
- Ray LA, Mackillop J, Monti PM (2010) Subjective responses to alcohol consumption as endophenotypes: Advancing behavioral genetics in etiological and treatment models of alcoholism. Subst Use Misuse 45:1742–65. doi:10.3109/10826084.2010.482427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm J, Mathers C, Popova S, et al. (2009) Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet 373:2223–33. doi:10.1016/S0140-6736(09)60746-7 [DOI] [PubMed] [Google Scholar]
- Reich T, Edenberg HJ, Goate A, et al. (1998) Genome-wide search for genes affecting the risk for alcohol dependence. Am J Med Genet Neuropsychiatr Genet 81:207–15. [PubMed] [Google Scholar]
- Roh S, Matsushita S, Hara S, et al. (2011) Role of GABRA2 in moderating subjective responses to alcohol. Alcohol Clin Exp Res 35:400–7. doi:10.1111/j.1530-0277.2010.01357.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit M. (2004) Testing the level of response to alcohol: Social information processing model of alcoholism risk—A 20 year prospective study. Alcohol Clin Exp Res 28:1881–9. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL (1996) An 8-year follow-up of 450 sons of alcoholic and control subjects. Arch Gen Psychiatry 53:202–10. [DOI] [PubMed] [Google Scholar]
- Thomas SE, Drobes DJ, Voronin K, et al. (2004) Following alcohol consumption, nontreatment-seeking alcoholics report greater stimulation but similar sedation compared with social drinkers. J Stud Alcohol 65:330–5. [DOI] [PubMed] [Google Scholar]
- Uhart M, Weerts EM, McCaul ME, et al. (2013) GABRA2 markers moderate the subjective effects of alcohol. Addict Biol 18:357–69. doi:10.1111/j.1369-1600.2012.00457.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voronin K, Randall P, Myrick H, et al. (2008) Aripiprazole effects on alcohol consumption and subjective reports in a clinical laboratory paradigm - Possible influence of self-control. Alcohol Clin Exp Res 32:1954–61. doi:10.1111/j.1530-0277.2008.00783.x [DOI] [PMC free article] [PubMed] [Google Scholar]