Abstract
Aims
Several lines of evidence support a critical role of TLR4 in the neuroimmune responses associated with alcohol disorders and propose inhibitors of TLR4 signaling as potential treatments for alcoholism. In this work, we investigated the effect of T5342126 compound, a selective TLR4 inhibitor, on excessive drinking and microglial activation associated with ethanol dependence.
Methods
We used 2BC-CIE (two-bottle choice-chronic ethanol intermittent vapor exposure) paradigm to induce ethanol dependence in mice. After induction of the ethanol dependence, we injected T5342126 (i.p., 57 mg/kg) for 14 days while monitoring ethanol intake by 2BC (limited access to ethanol) method.
Results
T5342126 decreased ethanol drinking in both ethanol-dependent and non-dependent mice but T5342126 showed also dose-dependent non-specific effects represented by decreased animal locomotor activity, saccharine intake, and body core temperature. Six days after the last ethanol-drinking session, we examined the immunohistochemical staining of Iba-1 (ionized calcium-binding adapter molecule 1), a microglial activation marker, in the central nucleus of the amygdala (CeA) and dentate gyrus (DG) of the hippocampus. Notably, T5342126 reduced Iba-1 density in the CeA of both ethanol-dependent and non-dependent mice injected with T5342126. There were no significant differences in the DG Iba-1 density among the treatment groups.
Conclusions
Collectively, our data suggest that T5342126, via blocking TLR4 activation, contributes to the reduction of ethanol drinking and ethanol-induced neuroimmune responses. However, the non-specific effects of T5342126 may play a significant role in the T5342126 effects on ethanol drinking and thus, may limit its therapeutic potential for treatment of alcohol dependence.
Short summary
T5342126, an experimental TLR4 inhibitor, is effective in reducing ethanol drinking and inhibiting the activation and proliferation of microglia in both ethanol-dependent and non-dependent mice. However, T5342126's use as a potential candidate for the treatment of alcohol addiction may be limited due to its non-specific effects.
Introduction
The brain’s immune system plays an important role in the development and physiological function of the nervous system, and is also involved in the pathogenesis of neurodegenerative and psychiatric disorders, including alcohol and other substance use disorders (Bitzer-Quintero and Gonzalez-Burgos, 2012; Szabo and Lippai, 2014). The interaction between alcohol and the brain's immune system is bi-directional; alcohol exposure activates the brain’s immune response/system (He and Crews, 2008; Marshall et al., 2013) and alterations in the brain's immune system modulate alcohol-related behavior (Fernandez-Lizarbe et al., 2009; Alfonso-Loeches et al., 2010; Liu et al., 2011; Wu et al., 2011; Blednov et al., 2012; Wu et al., 2012). Innate immunity has emerged as a primary mediator of the alcohol-induced immune response and transition to alcohol dependence (Szabo and Lippai, 2014). TLRs (toll-like receptors such as TLR3 and TLR4), cytokines (IL-1β, TNF-α, IL-6), oxidative stress and RAGE (receptor for advanced glycation end products) have been considered key players mediating alcohol-induced immune response in the brain (Szabo and Lippai, 2014).
In the brain, TLR4 is expressed primarily by microglia and astrocytes (Trotta et al., 2014). Neuronal expression of TLR4 has been controversial but recent findings have implicated the expression of TLR4 in neurons (Liu et al., 2011; Okun et al., 2011). The critical activation step of the TLR4 signaling pathways is the binding of a TLR4 ligand to the TLR4–MD2 complex. The TLR4 ligands include a variety of molecules such as Microbial Associated Molecular Patterns [e.g. lipopolysaccharide (LPS)] and endogenous Danger Associated Molecular Patterns, also called alarmins (e.g. HMGB-1, HSP70, etc.) (Vezzani et al., 2011). Activation of TLR4 leads to the initiation of the innate immune response, classified by activation of microglia and astrocytes, release of inflammatory mediators, and production of NO and oxidative stress (Vezzani et al., 2011). Several lines of evidence support a critical role of TLR4 in the neuroimmune responses associated with alcohol use and abuse: (a) alcohol activates TLR4 in glial cells, leading to the initiation of the neuroimmune response (Fernandez-Lizarbe et al., 2009), (b) in TLR4 deficient mice, chronic alcohol does not activate glial cells and does not induce expression of inflammatory mediators, apoptosis or cognitive and anxiety-related behavioral impairments (Fernandez-Lizarbe et al., 2009; Alfonso-Loeches et al., 2010), (c) transgenic and pharmacological modulation of TLR4 signaling has been shown to reduce alcohol-induced sedation and motor impairment (Wu et al., 2012), as well as excessive alcohol drinking (Liu et al., 2011; Blednov et al., 2012), (d) alcohol exposure induces a release of endogenous TLR4 ligands (e.g. HMGB-1) in the brain (He and Crews, 2008), (e) a component of TLR4 signaling (CD14) plays a role in ethanol’s effect on GABAergic transmission in the central nucleus of the amygdala (CeA) (Bajo et al., 2014) and (f) chronic consumption of alcohol degrades the stomach lining and causes LPS to leak from the gut, activating proinflammatory processes that may contribute to neuroinflammation and neurodegeneration (Qin et al., 2007).
Because of the critical role of TLR4 in the initiation and maintenance of the immune response, several inhibitors of TLR4 signaling have been tested for treatments of TLR4-mediated inflammatory diseases including sepsis (Wang et al., 2015), pain (Liu et al., 2012) and substance use disorders (Bachtell et al., 2015). In this study, we evaluated the T5342126 compound, a selective TLR4 inhibitor (Chavez et al., 2011; Wang et al., 2012), for its effects on the escalated ethanol drinking in ethanol-dependent mice. We hypothesized that the effects of T5342126 would be: (a) a reduction of the escalation of ethanol drinking seen in our mouse model of alcohol dependence and (b) a blunting of the innate immune response and microglial activation.
Materials and Methods
Animals
Male C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME, USA and TSRI rodent breeding colony; n = 88) were received at 10 weeks of age and acclimated to a reverse light cycle (lights off 8:00AM, lights on 8:00PM) for 2 weeks prior to the initiation of the experiments. The mice were housed in a temperature and humidity-controlled room with food and water available ad libitum. All care procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and with the Institutional Animal Care and Use Committee policies of The Scripps Research Institute.
Two-bottle choice and chronic intermittent ethanol exposure
We used 56 male C57BL/6J mice (Jackson Laboratory) to examine the effects of T5342126 on ethanol intake in dependent and non-dependent mice using the mouse two-bottle choice and chronic intermittent ethanol (2BC-CIE) model (Becker and Lopez, 2004; Lopez and Becker, 2005). The remaining 48 mice were exposed to a limited access ethanol 2BC procedure followed by either CIE exposure in vapor chambers, to induce ethanol dependence, or air exposure in identical chambers (Fig. 1A). This CIE model results in escalated ethanol intake in ethanol-dependent mice (Becker and Lopez, 2004; Lopez and Becker, 2005).
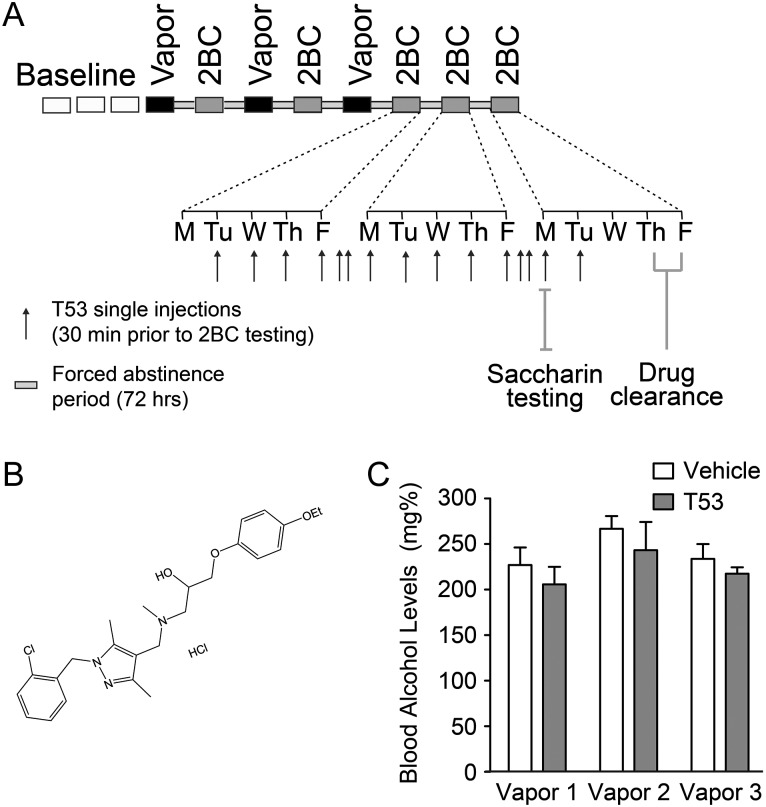
Fig. 1.
Experimental design and blood alcohol levels (BAL) in ethanol-dependent mice. (A) Overview of the 2BC-CIE treatment and the T5342126 injection paradigm. We injected T5342126 intra-peritoneally 30 min prior to 2BC testing for 15 consecutive days (injection 1 = 82 mg/kg; injections 2–15 = 57 mg/kg). (B) The chemical structure of T5342126. (C) The mean BAL were not significantly different between the mice selected for vehicle and T5342126 injections (two-way RM ANOVA, F(1,38) = 0.94; P = 0.34). The BAL varied significantly between the vapor bouts (F(2,38) = 3.88; P = 0.03).
For 20 days (5 days per week for 4 weeks), 30 min before the lights were turned off, mice were singly housed for 2 h with access to two drinking tubes, one containing 15% ethanol and the other containing water (i.e. 2BC). Ethanol and water consumption were recorded during this 2-h period. Subsequently, mice were divided into two balanced groups based on equal ethanol and water consumptions, while being exposed to CIE vapor or control air (CTL). The CIE group was injected i.p. with 1.75 g/kg ethanol + 68.1 mg/kg pyrazole (alcohol dehydrogenase inhibitor) and placed in vapor chambers for 4 days (16 h vapor on, 8 h off). On either the third or fourth day, tail blood was sampled to determine blood alcohol levels (BAL). Target BAL were between 200 and 250 mg/dl. The CTL group was injected with 68.1 mg/kg pyrazole in saline and placed in air chambers for the same intermittent time period as the CIE group. Following the fourth day of exposure, mice were allowed 72 h of undisturbed time, followed by 5 days of 2-h 2BC. This regiment, vapor/air exposure followed by 5 days of 2BC testing, was repeated two additional times for a total of three full rounds.
Based on drinking data from the first day of 2BC following the third round of vapor exposure, half of the mice in the CTL and CIE groups were assigned to receive T5342126 (Fig. 1B) and the remaining animals were assigned to receive saline injections based on comparable ethanol intakes. Mice were injected at 7:00AM (30 min prior to 2BC on Monday–Friday) starting with the second day of 2BC testing and continuing for 14 consecutive days. On the first day, 82 mg/kg T5342126 (in saline, i.p., volume 0.01 ml/g) was administered based on previous studies (Bevan et al., 2010; Wang et al., 2012); however, the mice developed robust non-specific effects (lethargic and cold on touch) so the dose was lowered to 57 mg/kg for the remaining 13 days. Performance on the 2BC test was assessed Tuesday–Friday and then Monday–Friday the following week. Final injections were given on the following Monday and two additional days were used to examine the drug-clearance period following T5342126 treatment. On the second Monday, mice were again injected with T5342126 (the 14th T5342126 injection) or saline based on group assignment and 2BC drinking was assessed with 0.033% saccharin and water using the same conditions as ethanol 2BC.
Tests for control behavior: open field test, body temperature and saccharin consumption
To examine the effects of T5342126 alone, we used 32 male C57BL/6J mice (10 weeks of age when received; TSRI rodent breeding colony) in three control tests separated by 1 week. After the 2-week acclimation period to a reverse light cycle (lights off 8:00AM, lights on 8:00PM), mice were assigned to receive 0, 14.25, 28.5, 42.75 and 57 mg/kg T5342126 (n = 6–7).
The open field test is used to examine anxiety-like responses of rodents exposed to stressful environmental stimuli (brightly illuminated open spaces) and to assess general activity levels (Crawley, 1999). For the test, we used a square white Plexiglas (50 × 50 cm) open field that was divided into 16 equal squares (12 outer and 4 inner). The walls of the open field were 22-cm high. Each animal was placed in the center of the field and several behavioral parameters [e.g. time spent in the inner squares (center), number of squares crossed, rearing and time spent grooming] were recorded during a 10-min observation period 30 min after T5342126 injection.
One week after conducting the open field test, basal body temperatures were taken before and 30 min after the mice were injected with T5342126. All temperature measurements were made to the nearest 0.1°C with a lubricated thermistor probe inserted 2 cm into the rectum of each animal.
Finally, the saccharin consumption test, serving as an index of anhedonia in mice (Schweizer et al., 2009), was performed 1 week later and was repeated in exactly the same way as described in 2BC experiment (see above).
Immunohistochemistry
Six days after finishing 2BC-CIE testing, 3–4 mice per treatment group were used for immunohistochemical analyses (n = 15). The mice were perfused with ice cold PBS and the brains were post-fixed with Z-Fix (Anatech Ltd, Battle Creek, MI, USA) for 72 h at room temperature. Sequential coronal sections (35 μm) were cut on cryostat and preserved in PBS and 0.1% sodium azide. Free-floating brain sections were rinsed multiple times with PBS, and endogenous alkaline phosphatase was quenched with BLOXALL (BLOXALL; Vector Laboratories, Burlingame, CA, USA) for 20 min. Sections were blocked for 1 h at room temperature in PBS containing 5% Normal Donkey Serum, 0.3% Triton X and 1 mg/ml BSA, and were incubated in a rabbit anti-Iba-1 antibody (#019-19741; Wako Chemicals USA, Richmond, VA, USA; dilution 1:1000) overnight at +4°C. Following PBS rinses, Iba-1 (ionized calcium-binding adapter molecule 1) staining was visualized using the ImmPRESS™-AP Anti-Rabbit IgG (alkaline phosphatase) Polymer Detection and ImmPACT Vector Red Substrate Kits (Vector Laboratories). All sections were rinsed thoroughly in 1x PBS between staining steps and after substrate reaction. Sections were mounted onto glass slides, dried overnight, dehydrated in increasing concentrations of ethanol, and permanently mounted with DPX. Sections were scanned with a Leica SCN400 scanner (Leica Microsystems, Buffalo Grove, IL, USA), and image analyses were conducted using Image Pro Premiere (Media Cybernetics, Inc., Rockville, MD, USA). The CeA and dentate gyrus (DG) of the hippocampus were selected after each image was converted into an 8-bit grayscale image for standardization purposes. We measured raw-integrated optical density, area of total Iba-1 staining in the region of the interest (ROI) and cell count in the CeA and DG. To specifically identify microglia-like cell bodies and exclude processes, we used five thresholding tools: a staining intensity filter (120–130), a region area filter (20 or 30) for cell body size, an optical density filter (3500–4000) for luminance, a circularity filter (0.1 minimum) for morphological structure of cell bodies and a width filter (3–5) to exclude intensely stained processes.
Data analysis and statistics
Statistical analysis of data acquired from all behavioral tests and immunohistochemistry was generated using GraphPad Prism 5.0 (GraphPad Software, San Diego, CA, USA). We used one- and two-way ANOVAs and accepted statistical significance at P < 0.05.
Results
Effects of T5342126 on ethanol intake in ethanol-dependent and non-dependent mice
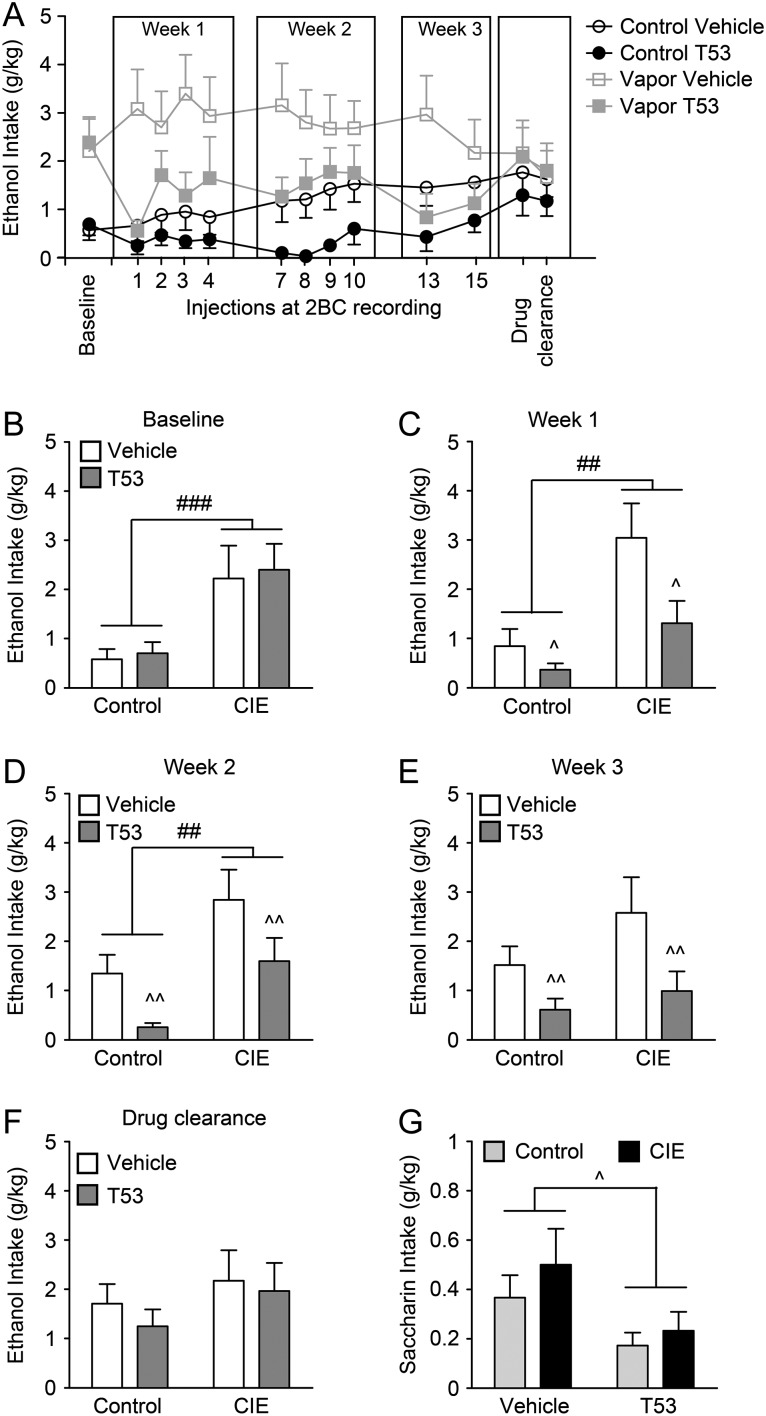
BAL of the CIE mice were 208.8 ± 10.8 mg/dl for round 1, 250.4 ± 15.8 mg/dl for round 2 and 221.1 ± 7.6 mg/dl for round 3 of ethanol-vapor exposure (Fig. 1C). CIE mice consumed significantly more ethanol than CTL mice on the day before T5342126 injections (baseline) were initiated (P < 0.001; Fig. 2A,B) confirming that the CIE conditions escalated ethanol-drinking behavior. During the injection phase of the study, this difference between CIE and CTL groups persisted and there was also a significant effect of T5342126 administration (57 mg/kg; P < 0.01; Fig. 2A–E). There was no significant interaction between the vapor exposure and T5342126 injections, suggesting that T5342126 decreased drinking in both ethanol-dependent and non-dependent mice. During the drug-clearance phase, the difference between CIE and CTL mice just missed significance (P = 0.05) due to an incomplete return to pre-injection drinking levels in CIE mice. However, there was no remaining effect of T5342126 (Fig. 2F).
Fig. 2.
T5342126 reduced ethanol and saccharin drinking in both control and ethanol-dependent mice. (A) The average daily ethanol intake of control and ethanol-dependent (CIE) mice injected with vehicle or T5342126 during measured by 2BC during limited 2 h access to ethanol (15%). (B) The baseline ethanol drinking, measured prior to T5342126 injections, was significantly increased in the ethanol-dependent mice compared to control mice (F(1,40) = 15.55, ###P = 0.0003). There were no significant differences in ethanol drinking between the mice selected for vehicle and T5342126 injections within the ethanol-vapor-treated groups (F(1,40) = 0.13, P > 0.05). (C–E) The average weekly ethanol intake of the control and ethanol-dependent (CIE) mice following T5342126 injections. Two-way ANOVA showed significant main effects of ethanol-vapor exposure and T5342126 injections in week 1 (vapor exposure: F(1,40) = 13.25, ##P = 0.0008; T5342126 injection: F(1,40) = 6.57, ^P = 0.014) and week 2 (vapor exposure: F(1,39) = 12.33, ##P = 0.0011; T5342126 injection: F(1,39) = 8.37, ^^P = 0.0062) that were not associated with significant interaction between the factors. In week 3 of the 2BC testing (E), we found significant main effect of the T5342126 injections (F(1,39) = 8.27, ^^P = 0.0065) but not of the ethanol-vapor exposure (F(1,39) = 2.76, P > 0.05). (F) Two days after the last T5342126 injection (drug clearance), we found no significant differences in ethanol intake between the vehicle and T5342126-injected control or ethanol-dependent mice. (G) The T5342126 injections significantly decreased saccharin intake in both control and ethanol-dependent mice (F(1,36) = 6.15, ^P = 0.018). We did not observe significant differences in saccharin intake between control and ethanol-dependent mice (F(1,36) = 1.09, P = 0.304). The data are presented as mean + SEM.
We also tested the effect of T5342126 injection on saccharin intake (T5342126 injection #14; Fig. 2A,G). Similarly to T5342126 effects on ethanol intake, we found a significant reduction in saccharin intake (P < 0.05) in both CIE and CTL mice.
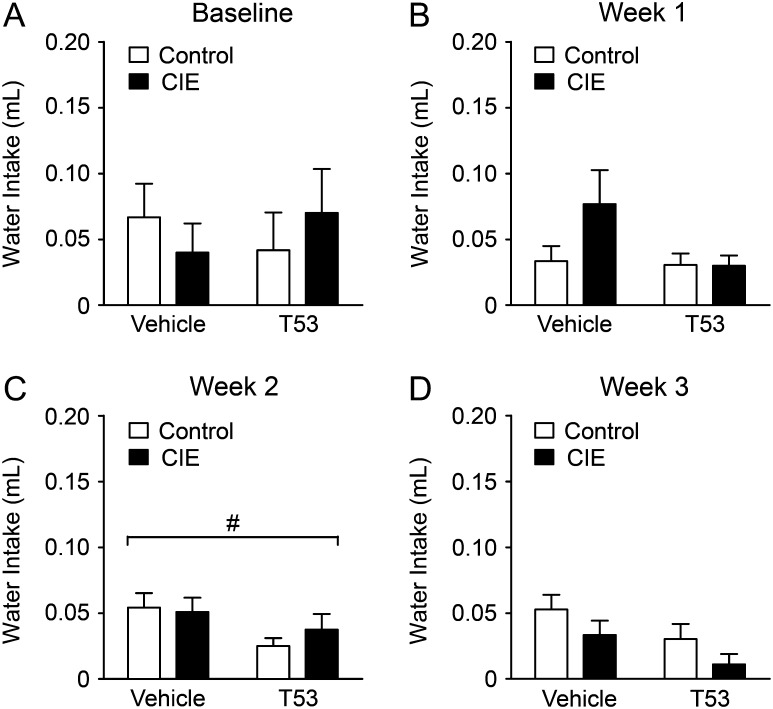
Baseline water intakes between the treatment groups were not significantly different indicating that CIE had no effect on water consumption (Fig. 3A). Over the injection period (2 weeks), CIE treatment had no significant (P > 0.05) effects on water intake (Fig. 3B,C). While T5342126 injections showed no significant effect on water intake in injection week 1 (P > 0.05), the injections significantly (P < 0.05) reduced water intake in both CIE and CTL mice in the subsequent weeks (Fig. 3B,C).
Fig. 3.
T5342126 reduced water consumption in both control and ethanol-dependent mice. The average daily water consumption measured by 2BC during 2 h testing period. (A) There was no significant difference in baseline water intake between the T5342126- and vehicle-injected ethanol-dependent (CIE) and non-dependent (control) mice (main effect of ethanol-vapor exposure: F(1,40) = 0.00; main effect of T5342126 injections: F(1,40) = 0.01). (B) Week 1 of the T5342126 injections: Neither ethanol-vapor exposure nor T5342126 have significant effect on the water consumption measured by 2BC testing in the first week T5342126 injections (main effect of ethanol-vapor exposure: F(1,40) = 2.14; main effect of T5342126 injections: F(1,40) = 2.86). (C) Week 2 of the T5342126 injections: T5342126 had significant main effects on the water consumption in both CIE and control mice (main effect of T5342126 injections: F(1,39) = 4.43, P = 0.042), while ethanol-vapor exposure had no significant impact on the water consumption (main effect of ethanol-vapor exposure: F(1,40) = 0.21). (D) Week 3 of T5342126 injections: The water consumption was decreased in T5342126-injected mice compared to vehicle-injected mice (F(1,36) = 4.17, P = 0.049). Ethanol-vapor exposure did not significantly altered the water consumption (F(1,36) = 3.12). The data are presented as mean + SEM and statistical significance # was set at P < 0.05.
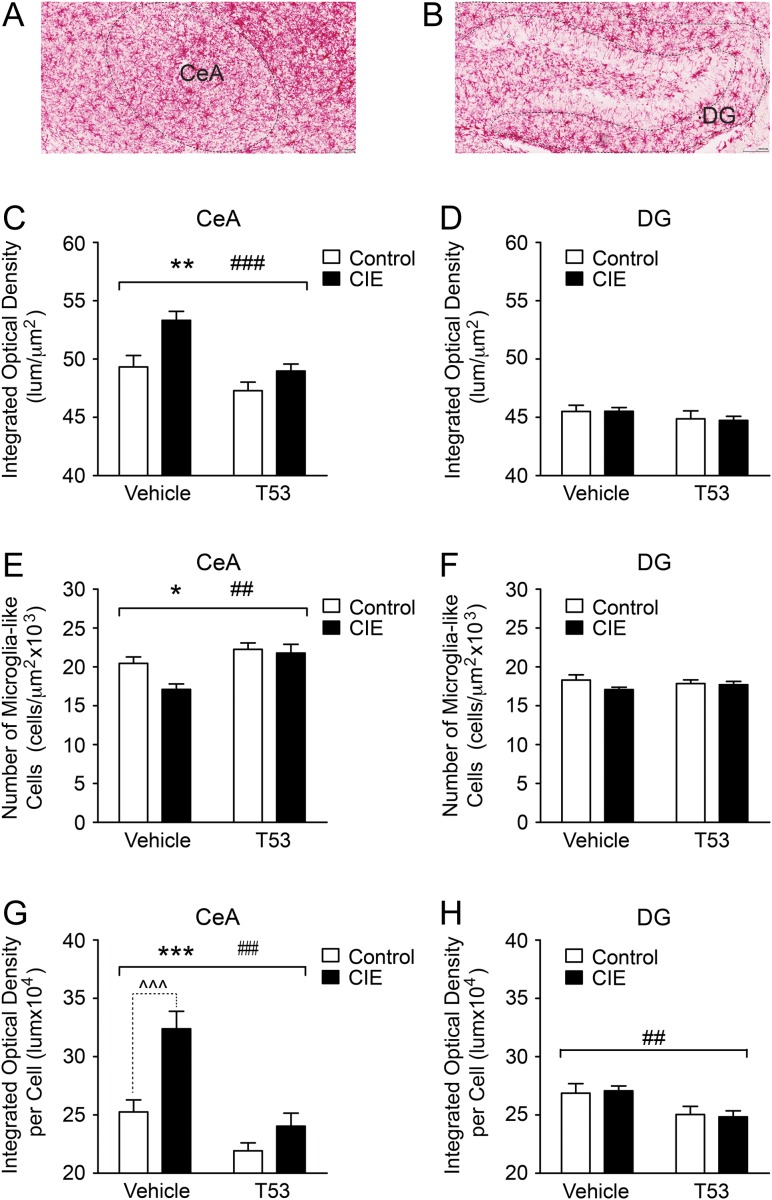
Effects of T5342126 on microglia activation
Six days after the last 2BC testing, we examined activation of microglia in the CIE and CTL mice tested for ethanol drinking by measuring differences in the abundance of Iba-1 in the CeA and DG. Iba-1 is a microglia marker and increased protein levels are indicative of microglial activation (Hovens et al., 2014). We found that both CIE and T5342126 injection had significant (P < 0.001) effects on the abundance of Iba-1 (optical density) in the CeA but not in the DG (Fig. 4C,D). While CIE increased Iba-1, T5342126 injections decreased Iba-1 levels in the CeA of ethanol-dependent and non-dependent mice. Both CIE and T5342126 injections affected the number of microglia-like cells in the CeA but not in DG (Fig. 4E,F). CIE decreased the number of microglia-like cells in the CeA compared to CTL mice, especially in the vehicle-injected group, and T5342126 injections normalized the number of microglia-like cells in CIE mice to the levels found in CTL mice.
Fig. 4.
T5342126 reduced activation of microglia in both control and ethanol-dependent mice. We used immunohistochemistry to determine changes in the expression of Iba-1 (a marker of microglia) in the CeA and DG. The mice were perfused 8 days after the last T5342126 injection. We determined optical density and counted microglia-like cells in the CeA and DG of the non-dependent (control) and ethanol-dependent (CIE) mice injected with vehicle or T5342126 (control-vehicle: n = 4; control-T5342126: n = 4; vapor-vehicle: n = 3; vapor-T5342126: n = 4). (A,B) Representative staining of Iba-1 in the CeA and DG, respectively. (C) In the CeA, we found significant main effects of the ethanol-vapor exposure (F(1,120) = 12.41, **P = 0.0006) and the T5342126 injections (F(1,120) = 15.65, ###P = 0.0001) on the overall abundance of Iba-1. There was no significant interaction between these factors. (D) In the DG, we did not observe significant alterations of Iba-1 abundance among the treatment groups (two-way ANOVA, P > 0.05). (E) We found significant main effects of the CIE (F(1,120) = 4.36, *P = 0.039) and T5342126 injections (F(1,120) = 12.52, ##P = 0.0006) on the number of the microglia-like cells in the CeA with no significant interactions between these factors (P > 0.05). (F) In the DG, neither chronic-vapor exposure nor T5342126 injections had significant main effects on the number of microglia-like cells (two-way ANOVA, P > 0.05). (G,H) We also calculated the abundance of Iba-1 per microglia-like cell. Both the chronic-vapor exposure (F(1,120) = 18.76, ***P < 0.0001) and T5342126 injections (F(1,120) = 29.96, ###P < 0.0001) had significant main effects on the Iba-1 abundance in a single microglia-like cell in the CeA, which were associated with the significant interaction (F(1,120) = 5.54, P = 0.0202) between these factors (G). Bonferroni post-hoc test showed significant (^^^P < 0.001) increase in the Iba-1 abundance in the microglia of the chronic ethanol-vapor exposure (CIE) mice injected with vehicle compared to vehicle-injected control mice but not between the T5342126-injected CIE and control mice (P > 0.05). (H) In the DG, we found significant reduction in the abundance of Iba-1 per microglia-like cell induced by T5342126 injections in both control and vapor-exposed mice (F(1,137) = 9.37, ##P = 0.0027). There were neither significant main effects of the chronic ethanol-vapor exposure nor interaction between the T5342126 injections and chronic-ethanol exposure. The data are presented as mean + SEM.
We also calculated the abundance of Iba-1 per microglia-like cell (normalizing the Iba-1 optical density with the number of microglia-like cells per ROI in a section) in the CeA and DG. T5342126 treatment had significant main effects, a reduction, on Iba-1 levels in a single microglia-like cell in both the CeA and DG (Fig. 4G,H). In the CeA, we also found significant main effects of CIE on Iba-1 abundance in a single microglia-like cell that was associated with significant interactions between T5342126 injections and CIE. Bonferroni post-hoc testing revealed a significant difference in Iba-1 levels in a single CeA microglia-like cell between CIE and CTL mice injected with vehicle but not the CIE and CTL mice injected with T5342126. These data indicate that T5342126, acting via inhibition of TLR4, is effective in ameliorating microglial activation. In addition, our data suggest that there is a brain-region-specific responsiveness of microglia to chronic-ethanol exposure.
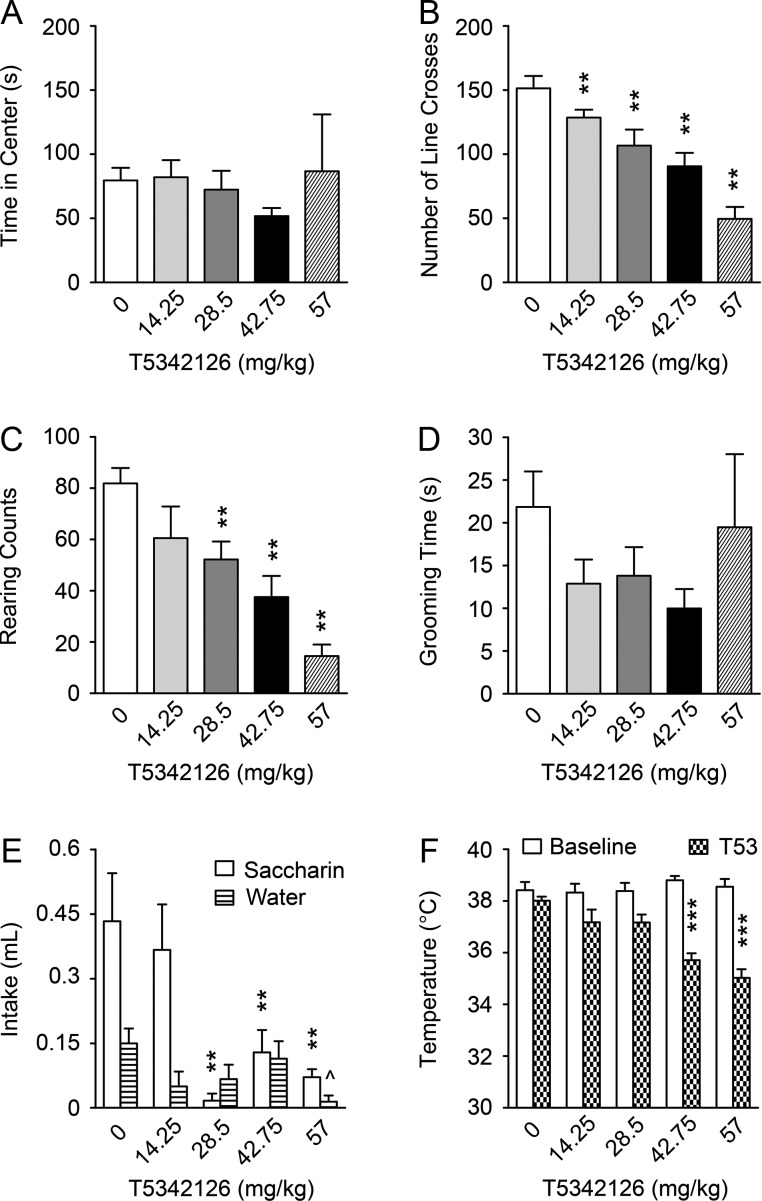
Effects of a single T5342126 injection on activity, anxiety, body temperature and saccharin consumption in ethanol naive mice
Based on T5342126-induced reduction in ethanol and water consumption found in both CIE and CTL mice and non-specific effects of the initial dose (87 mg/kg) of T5342126, we suspected that the effects of T5342126 on the ethanol drinking may involve its effects on other basal behaviors. Therefore, we assessed the effect of several T5342126 doses (14.25, 28.5, 47.75 and 57 mg/kg) on activity, anxiety and reward-related behavior by examining open field behavior, body temperature and saccharin intake. In the open field test, all the tested doses of T5342126, except of the lowest one (14.25 mg/kg), significantly decreased the number of line crosses (P < 0.0001) and rearing behavior (P < 0.0001), suggesting a reduction in the activity of the mice. However, T5342126 had no significant effects on the other activity and anxiety measurements such as the time spent in the center of the open field and time spent grooming (Fig. 5A–D). T5342126 at doses 28.5, 42.75 and 57 mg/kg significantly decreased saccharin intake (P < 0.001; Fig. 5E), suggesting that T5342126 affects reward-related behavior. Finally, while there were no significant differences in baseline body core temperature among the tested mice, 30 min after the injection with the higher doses of T5342126 (42.75 and 57 mg/kg) core temperature was significantly decreased (P < 0.001; Fig. 5F). Thus, these data suggest that the tested doses of T5342126 impacts several mouse behaviors including activity, temperature and reward-related behavior that may contribute to T5342126-induced reduction in ethanol and water intake observed in ethanol-dependent and non-dependent mice.
Fig. 5.
The dose-dependent effects of T5342126 on anxiety and activity in the ethanol-naïve mice. The activity and anxiety were examined by the open field test. The anxiety behavior was measured by the time spent in the center of the open field and by the number of crosses the lines: (A) None of the T5342126 doses altered significantly the time spent in the center of the testing field (one-way ANOVA, F(4,27) = 0.352, P = 0.840). (B) Except the lowest T5342126 dose (14.25 mg/kg; P = 0.127), all the higher T5342126 doses significantly reduced the number of line crosses by the naïve mice (F(4,27) = 15.551, P < 0.0001; Fisher PLSD **P < 0.01). The effects of T5342126 on activity of the ethanol naive mice were assessed by changes in rearing counts and grooming time: (C) The higher doses of T5342126 (28.5–57 mg/kg) significantly reduced activity measured by rearing counts (F(4,27) = 10.277, P < 0.0001; Fisher PLSD **P < 0.01). (D) T5342126 had no significant effects on the grooming of the naïve mice (F(4,27) = 0.969, P = 0.4409). The data are presented as mean + SEM. (E) The ethanol-naïve mice drank less saccharin after a single T5342126 injection at the doses 28.5–57 mg/kg (F(4,27) = 6.770, P < 0.0007; Fisher PLSD **P < 0.01). T5342126 decreased significantly water intake (F(4,27) = 2.776, P = 0.0472) but only at the highest T5342126 dose (57 mg/kg; Tukey post-hoc test ^P < 0.05). (F) While there were no significant differences in baseline body temperature among the naïve mice (F(4,27) = 0.442, P = 0.777), the T5342126 injections significantly reduced body temperature at 42.75 and 57 mg/kg (F(4,27) = 13.977, P < 0.0001; Fisher PLSD ***P < 0.0001).
Discussion
In this study, we evaluated a selective TLR4 inhibitor, T5342126, for its ability to reduce ethanol-drinking and ameliorate neuroimmune responses induced by ethanol dependence. We found that T5342126 decreased ethanol drinking in both dependent and non-dependent mice; however, it was associated with a dose-dependent reduction in activity, saccharin intake, and body temperature. In both groups, T5342126 also reduced the immunohistochemical staining of Iba-1, a microglial activation marker, in the CeA with no significant effects seen in the DG. Thus, our data suggest that T5342126, via blockade of TLR4 activation, is able to reduce ethanol drinking and neuroimmune responses. However, the non-specific effects of T5342126 may play a significant role in its effects on ethanol drinking and thus limit its therapeutic potential.
We used the 2BC-CIE method to induce ethanol dependence, characterized by an escalation in voluntary ethanol drinking (Becker and Lopez, 2004) that lasted for 2 weeks after the final vapor exposure, consistent with previous findings (Lopez and Becker, 2005). T5342126 had a non-specific effect on ethanol drinking and we cannot rule out the possibility that this was due, at least in part, to the compound’s side effects. There was also evidence that tolerance develops to T5342126 as ethanol drinking in both of the T5342126-injected groups increased across injections. While a single injection of (+)-naloxone, a TLR4 inhibitor acting via TLR4-MyD88 was effective in reducing acute alcohol-induced sedation and motor impairment in mice (Wu et al., 2012), prolonged inhibition of TLR4 by T5342126 may not be effective in completely reducing ethanol drinking, due to its non-specific effects and potential tolerance post-ethanol dependence. Previous studies demonstrating a critical role of TLR4 in chronic alcohol actions used TLR4 KO mice or brain-region-specific knock-down of TLR4 and showed decreased neuroimmune responses and behavioral and cognitive impairments, rather than amelioration of ethanol-dependence-induced changes (Fernandez-Lizarbe et al., 2009; Alfonso-Loeches et al., 2010; Liu et al., 2011; Pascual et al., 2011; June et al., 2015). There are a few cases showing that targeting a single pathway leads to decreased excessive drinking, for instance inhibition of TLR4-MyD88 (Wu et al., 2012) or IL-1R1 signaling (Wu et al., 2011). Notably, FDA-approved drugs having anti-inflammatory properties such as PPAR agonists (fenofibrate, pioglitazone, tesaglitazar, bezafibrate) (Stopponi et al., 2011; Blednov et al., 2015), phosphodiesterase inhibitors (e.g. ibudilast, rolipram) (Blednov et al., 2014; Bell et al., 2015) and naloxone/naltrexone (Kamdar et al., 2007) also have shown efficacy in mitigating alcohol-dependence-related behaviors. These drugs simultaneously target several inflammatory pathways as well as other brain systems. Thus, we speculate that multi-target anti-inflammatory drugs (or a combination of single-target drugs) may be more beneficial for treatment of alcoholism. Importantly, different inflammatory pathways seem to be critical for different stages of alcohol addiction and for different alcohol-related behaviors (Robinson et al., 2014).
In our study, we used a lower dose of T5342126 compared to the effective dose (82 mg/kg) reported in rats and mice (Bevan et al., 2010; Wang et al., 2012). The main reason for adjusting the T5342126 dose was due to the observation of confounding non-specific effects (reduced activity, body temperature, etc.) in both dependent and non-dependent mice that were not evident in rats and Balb/c mice (Bevan et al., 2010; Wang et al., 2012). Our findings indicate potential species and strain differences in sensitivity and/or pharmacokinetics of T5342126. Although T5342126’s non-specific effects may preclude its clinical use as a potential therapeutic candidate for the treatment of alcoholism, T5342126-induced decreases in body temperature may be beneficial in some medical situations, such as fever management and management of a hyperthermia observed in humans following psychostimulant overdose (Callaway and Clark, 1994) and alcohol withdrawal (Brust, 2014).
To determine the effectiveness of T5342126 in reducing alcohol-induced neuroimmune response, we used immunohistochemistry and staining for a marker for microglia/macrophages, Iba-1. It has been shown that activation of TLR4 signaling is critical for activation of microglia (Fernandez-Lizarbe et al., 2009; Alfonso-Loeches et al., 2010), which, in turn, leads to an increase in the abundance of Iba-1 in the brain (Chung et al., 2010). We focused on two brain regions: CeA and DG. The CeA is recruited during the transition to alcohol dependence and plays a critical role in the withdrawal/negative affective stage of alcohol addiction including ethanol drinking (Gilpin et al., 2015). However, the neuroimmune response to chronic alcohol seems to be limited in the CeA (He and Crews, 2008). In the DG of animals treated chronically with ethanol, the activation of microglia associated with an increase in Iba-1 staining or number of Iba-1- labeled microglia has been reported (Qin and Crews, 2012; Contet et al., 2014). Therefore, we predicted limited changes in Iba-1 staining in the CeA, especially between vehicle-injected-dependent and non-dependent mice. In addition, we expected T5342126-induced normalization of the increased Iba-1 staining in the DG in dependent mice compared to non-dependent mice. Surprisingly, we observed comparable levels of Iba-1 staining and number of microglia-like cells in the DG of the mice from all the treatment groups. Our findings suggest that the activation of microglia in the DG, usually found after chronic-ethanol exposure, diminished after 6 days of ethanol withdrawal. In contrast, we observed an increase in Iba-1 staining in the CeA of ethanol-dependent mice injected with vehicle that was accompanied with a decrease in the total number of microglia-like cells. As anticipated, T5342126 injections reduced Iba-1 staining in both dependent and non-dependent mice, and normalized the number of microglia-like cells in the CeA of dependent mice compared to the non-dependent group. The absence of morphological changes in the CeA suggests that there is early stage activation of the CeA microglia during the ethanol-withdrawal period that is reversed by inhibition of TLR4 signaling. Our data suggest that TLR4 signaling shows brain-region and potentially addiction-stage specificity in microglia and neuroimmune responses induced by chronic-ethanol exposure.
Previous studies demonstrating a critical role of TLR4 in chronic alcohol actions used TLR4 KO mice or brain-region-specific knock-down of TLR4. These studies showed prevention of development of neuroimmune responses, and behavioral and cognitive impairments (Fernandez-Lizarbe et al., 2009; Alfonso-Loeches et al., 2010; Liu et al., 2011; Pascual et al., 2011; June et al., 2015), rather than amelioration of ethanol-dependence-induced changes, the primary focus of this study. Importantly, different inflammatory pathways seem to be critical for different stages of alcohol addiction and for different alcohol-related behaviors (Robinson et al., 2014). Therefore, we speculate that TLR4 inhibitors, including T5342126, may be more effective in prevention of the development of ethanol dependence rather than amelioration of neurobiological and behavioral changes associated with the ethanol dependence.
In conclusion, our study showed that T5342126, a TLR4 inhibitor, is effective in reducing ethanol drinking and inhibiting the activation and proliferation of microglia in both ethanol-dependent and non-dependent mice. Most importantly, T5342126 as a potential candidate for the treatment of alcohol addiction may be limited due to its non-specific effects.
Acknowledgments
We thank Dr. Kiosses (Core Microscopy Facility at The Scripps Research Institute) for his help with and advice on immunohistochemical experiments. This is manuscript #29307 from The Scripps Research Institute.
Funding
Support for this study was provided by the National Institute on Alcohol Abuse and Alcoholism grants as part of INIA West Consortium [NIH AA013498 to M.R., AA013517 to M.B. and M.R., and NIH AA020893 to A.J.R.], and the National Institute of General Medical Sciences [NIH GM 101279 to H.Y.].
Conflict of interest Statement
None declared.
References
- Alfonso-Loeches S, Pascual-Lucas M, Blanco AM, et al. (2010) Pivotal role of TLR4 receptors in alcohol-induced neuroinflammation and brain damage. J Neurosci 30:8285–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachtell R, Hutchinson MR, Wang X, et al. (2015) Targeting the Toll of drug abuse: the translational potential of toll-like receptor 4. CNS Neurol Disord Drug Targets 14:692–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajo M, Madamba SG, Roberto M, et al. (2014) Innate immune factors modulate ethanol interaction with GABAergic transmission in mouse central amygdala. Brain Behav Immun 40:191–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker HC, Lopez MF. (2004) Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol Clin Exp Res 28:1829–38. [DOI] [PubMed] [Google Scholar]
- Bell RL, Lopez MF, Cui C, et al. (2015) Ibudilast reduces alcohol drinking in multiple animal models of alcohol dependence. Addict Biol 20:38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan DE, Martinko AJ, Loram LC, et al. (2010) Selection, preparation, and evaluation of small-molecule inhibitors of Toll-like receptor 4. ACS Med Chem Lett 1:194–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitzer-Quintero OK, Gonzalez-Burgos I. (2012) Immune system in the brain: a modulatory role on dendritic spine morphophysiology. Neural Plast 2012:348642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Benavidez JM, Black M, et al. (2014) Inhibition of phosphodiesterase 4 reduces ethanol intake and preference in C57BL/6J mice. Front Neurosci 8:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Benavidez JM, Black M, et al. (2015) Peroxisome proliferator-activated receptors alpha and gamma are linked with alcohol consumption in mice and withdrawal and dependence in humans. Alcohol Clin Exp Res 39:136–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Ponomarev I, Geil C, et al. (2012) Neuroimmune regulation of alcohol consumption: behavioral validation of genes obtained from genomic studies. Addict Biol 17:108–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brust JC. (2014) Acute withdrawal: diagnosis and treatment. Handb Clin Neurol 125:123–31. [DOI] [PubMed] [Google Scholar]
- Callaway CW, Clark RF. (1994) Hyperthermia in psychostimulant overdose. Ann Emerg Med 24:68–76. [DOI] [PubMed] [Google Scholar]
- Chavez SA, Martinko AJ, Lau C, et al. (2011) Development of beta-amino alcohol derivatives that inhibit Toll-like receptor 4 mediated inflammatory response as potential antiseptics. J Med Chem 54:4659–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung DW, Yoo KY, Hwang IK, et al. (2010) Systemic administration of lipopolysaccharide induces cyclooxygenase-2 immunoreactivity in endothelium and increases microglia in the mouse hippocampus. Cell Mol Neurobiol 30:531–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contet C, Kim A, Le D, et al. (2014) mu-Opioid receptors mediate the effects of chronic ethanol binge drinking on the hippocampal neurogenic niche. Addict Biol 19:770–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN. (1999) Behavioral phenotyping of transgenic and knockout mice: experimental design and evaluation of general health, sensory functions, motor abilities, and specific behavioral tests. Brain Res 835:18–26. [DOI] [PubMed] [Google Scholar]
- Fernandez-Lizarbe S, Pascual M, Guerri C. (2009) Critical role of TLR4 response in the activation of microglia induced by ethanol. J Immunol 183:4733–44. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Herman MA, Roberto M. (2015) The central amygdala as an integrative hub for anxiety and alcohol use disorders. Biol Psychiatry 77:859–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Crews FT. (2008) Increased MCP-1 and microglia in various regions of the human alcoholic brain. Exp Neurol 210:349–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovens IB, Nyakas C, Schoemaker RG. (2014) A novel method for evaluating microglial activation using ionized calcium-binding adaptor protein-1 staining: cell body to cell size ratio. Neuroimmunol Neuroinflamm 1:82–8. [Google Scholar]
- June HL, Liu J, Warnock KT, et al. (2015) CRF-amplified neuronal TLR4/MCP-1 signaling regulates alcohol self-administration. Neuropsychopharmacology 40:1549–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamdar NK, Miller SA, Syed YM, et al. (2007) Acute effects of naltrexone and GBR 12909 on ethanol drinking-in-the-dark in C57BL/6J mice. Psychopharmacology (Berl) 192:207–17. [DOI] [PubMed] [Google Scholar]
- Liu J, Yang AR, Kelly T, et al. (2011) Binge alcohol drinking is associated with GABAA alpha2-regulated Toll-like receptor 4 (TLR4) expression in the central amygdala. Proc Natl Acad Sci USA 108:4465–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Gao YJ, Ji RR. (2012) Emerging role of Toll-like receptors in the control of pain and itch. Neurosci Bull 28:131–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MF, Becker HC. (2005) Effect of pattern and number of chronic ethanol exposures on subsequent voluntary ethanol intake in C57BL/6J mice. Psychopharmacology (Berl) 181:688–96. [DOI] [PubMed] [Google Scholar]
- Marshall SA, Mcclain JA, Kelso ML, et al. (2013) Microglial activation is not equivalent to neuroinflammation in alcohol-induced neurodegeneration: the importance of microglia phenotype. Neurobiol Dis 54:239–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun E, Griffioen KJ, Mattson MP. (2011) Toll-like receptor signaling in neural plasticity and disease. Trends Neurosci 34:269–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual M, Balino P, Alfonso-Loeches S, et al. (2011) Impact of TLR4 on behavioral and cognitive dysfunctions associated with alcohol-induced neuroinflammatory damage. Brain Behav Immun 25:S80–91. [DOI] [PubMed] [Google Scholar]
- Qin L, Crews FT. (2012) NADPH oxidase and reactive oxygen species contribute to alcohol-induced microglial activation and neurodegeneration. J Neuroinflammation 9:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, Wu X, Block ML, et al. (2007) Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 55:453–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson G, Most D, Ferguson LB, et al. (2014) Neuroimmune pathways in alcohol consumption: evidence from behavioral and genetic studies in rodents and humans. Int Rev Neurobiol 118:13–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer MC, Henniger MS, Sillaber I. (2009) Chronic mild stress (CMS) in mice: of anhedonia, ‘anomalous anxiolysis’ and activity. PLoS One 4:e4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopponi S, Somaini L, Cippitelli A, et al. (2011) Activation of nuclear PPARgamma receptors by the antidiabetic agent pioglitazone suppresses alcohol drinking and relapse to alcohol seeking. Biol Psychiatry 69:642–9. [DOI] [PubMed] [Google Scholar]
- Szabo G, Lippai D. (2014) Converging actions of alcohol on liver and brain immune signaling. Int Rev Neurobiol 118:359–80. [DOI] [PubMed] [Google Scholar]
- Trotta T, Porro C, Calvello R, et al. (2014) Biological role of Toll-like receptor-4 in the brain. J Neuroimmunol 268:1–12. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Maroso M, Balosso S, et al. (2011) IL-1 receptor/Toll-like receptor signaling in infection, inflammation, stress and neurodegeneration couples hyperexcitability and seizures. Brain Behav Immun 25:1281–9. [DOI] [PubMed] [Google Scholar]
- Wang X, Loram LC, Ramos K, et al. (2012) Morphine activates neuroinflammation in a manner parallel to endotoxin. Proc Natl Acad Sci USA 109:6325–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Shan X, Dai Y, et al. (2015). Curcumin analog L48H37 prevents lipopolysaccharide-induced TLR4 signaling pathway activation and sepsis via targeting MD2. J Pharmacol Exp Ther 353:539–50. [DOI] [PubMed] [Google Scholar]
- Wu Y, Lousberg EL, Moldenhauer LM, et al. (2011) Attenuation of microglial and IL-1 signaling protects mice from acute alcohol-induced sedation and/or motor impairment. Brain Behav Immun 25:S155–64. [DOI] [PubMed] [Google Scholar]
- Wu Y, Lousberg EL, Moldenhauer LM, et al. (2012) Inhibiting the TLR4-MyD88 signalling cascade by genetic or pharmacological strategies reduces acute alcohol-induced sedation and motor impairment in mice. Br J Pharmacol 165:1319–29. [DOI] [PMC free article] [PubMed] [Google Scholar]