Abstract
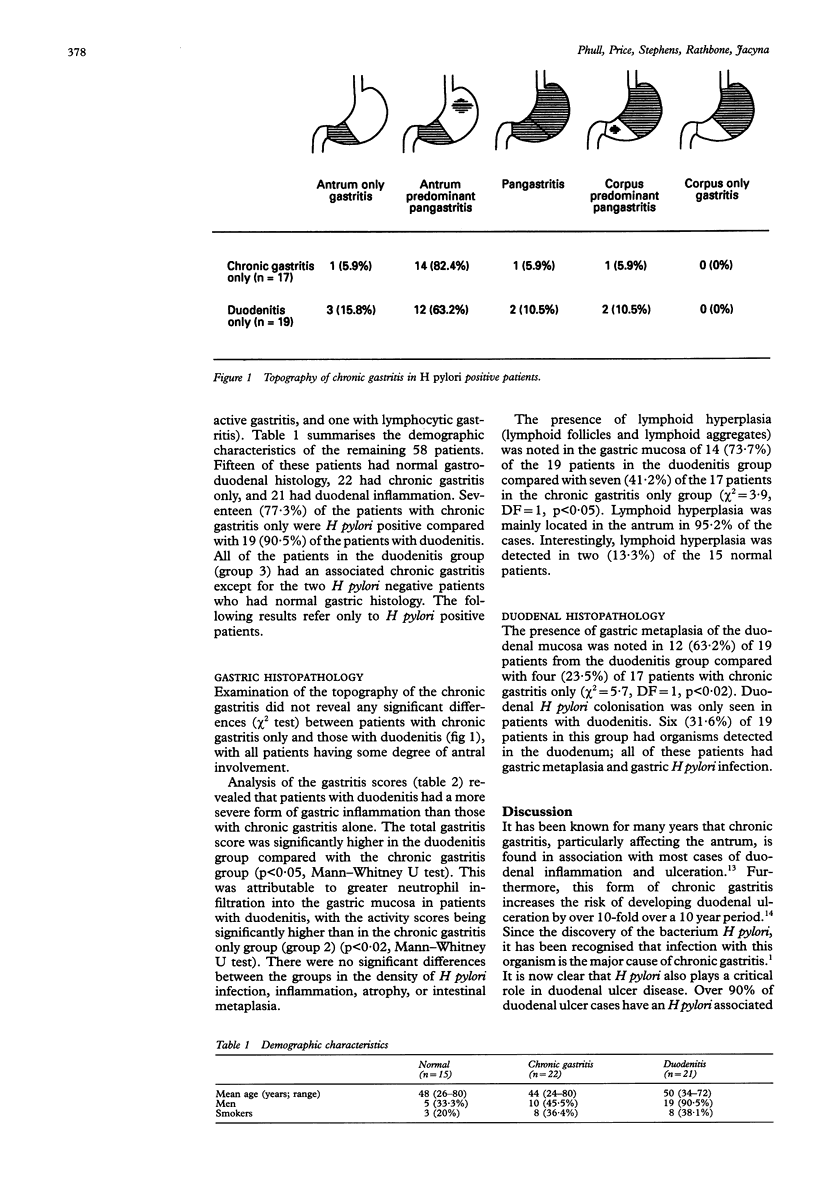
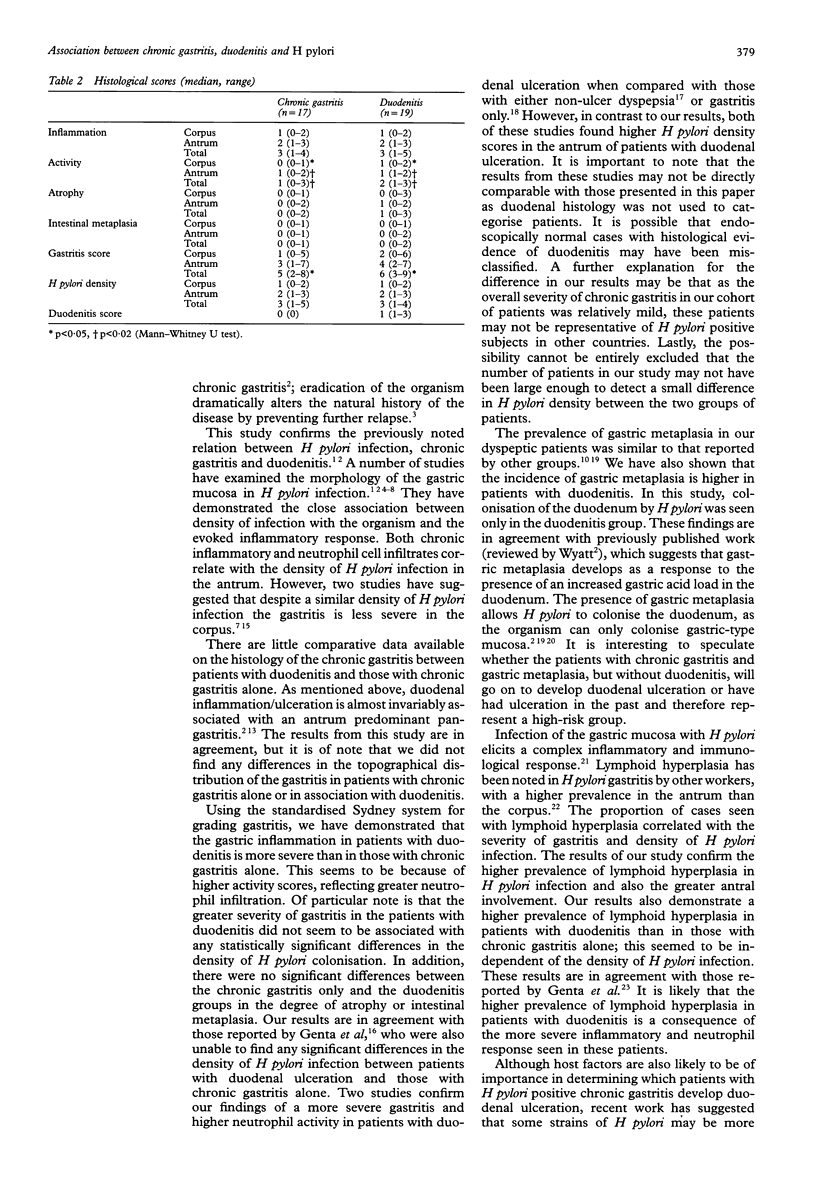
AIM: To compare the histological characteristics of Helicobacter pylori positive chronic gastritis in patients with and without associated duodenitis. METHODS: Gastric mucosal biopsy specimens were obtained from patients undergoing endoscopy for dyspepsia. Severity of gastritis and density of H pylori infection were graded according to the Sydney system. RESULTS: Of the 69 patients studied, 15 had normal histology, 22 had chronic gastritis only (77.3% H pylori positive), 21 had duodenitis (90.5% H pylori positive), and 11 had other diagnoses. In the H pylori positive patients, the median gastritis score was higher in the duodenitis group (6, range 3-9) than in the chronic gastritis only group (5, range 2-8), because of greater neutrophil activity scores in patients with duodenitis (median score 2 v 1). There were no differences in the density of H pylori infection, inflammation, atrophy, or intestinal metaplasia between patients with chronic gastritis only and those with duodenitis. CONCLUSIONS: These results suggest that H pylori positive patients with duodenitis have a more severe form of gastritis than those without associated duodenal inflammation. This is because of increased neutrophil activity, which seems to be independent of the density of H pylori infection.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayerdörffer E., Lehn N., Hatz R., Mannes G. A., Oertel H., Sauerbruch T., Stolte M. Difference in expression of Helicobacter pylori gastritis in antrum and body. Gastroenterology. 1992 May;102(5):1575–1582. doi: 10.1016/0016-5085(92)91716-h. [DOI] [PubMed] [Google Scholar]
- Bayerdörffer E., Oertel H., Lehn N., Kasper G., Mannes G. A., Sauerbruch T., Stolte M. Topographic association between active gastritis and Campylobacter pylori colonisation. J Clin Pathol. 1989 Aug;42(8):834–839. doi: 10.1136/jcp.42.8.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser M. J. Helicobacter pylori phenotypes associated with peptic ulceration. Scand J Gastroenterol Suppl. 1994;205:1–5. [PubMed] [Google Scholar]
- Collins J. S., Sloan J. M., Hamilton P. W., Watt P. C., Love A. H. Investigation of the relationship between gastric antral inflammation and Campylobacter pylori using graphic tablet planimetry. J Pathol. 1989 Dec;159(4):281–285. doi: 10.1002/path.1711590404. [DOI] [PubMed] [Google Scholar]
- Covacci A., Censini S., Bugnoli M., Petracca R., Burroni D., Macchia G., Massone A., Papini E., Xiang Z., Figura N. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5791–5795. doi: 10.1073/pnas.90.12.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cover T. L., Dooley C. P., Blaser M. J. Characterization of and human serologic response to proteins in Helicobacter pylori broth culture supernatants with vacuolizing cytotoxin activity. Infect Immun. 1990 Mar;58(3):603–610. doi: 10.1128/iai.58.3.603-610.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree J. E., Farmery S. M., Lindley I. J., Figura N., Peichl P., Tompkins D. S. CagA/cytotoxic strains of Helicobacter pylori and interleukin-8 in gastric epithelial cell lines. J Clin Pathol. 1994 Oct;47(10):945–950. doi: 10.1136/jcp.47.10.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree J. E., Figura N., Taylor J. D., Bugnoli M., Armellini D., Tompkins D. S. Expression of 120 kilodalton protein and cytotoxicity in Helicobacter pylori. J Clin Pathol. 1992 Aug;45(8):733–734. doi: 10.1136/jcp.45.8.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree J. E., Taylor J. D., Wyatt J. I., Heatley R. V., Shallcross T. M., Tompkins D. S., Rathbone B. J. Mucosal IgA recognition of Helicobacter pylori 120 kDa protein, peptic ulceration, and gastric pathology. Lancet. 1991 Aug 10;338(8763):332–335. doi: 10.1016/0140-6736(91)90477-7. [DOI] [PubMed] [Google Scholar]
- Digestive Disease Week and the 95th Annual Meeting of the American Gastroenterological Association. New Orleans, Louisiana, May 15-18, 1994. Abstracts. Gastroenterology. 1994 Apr;106(4 Suppl):A1–1198. [PubMed] [Google Scholar]
- Eidt S., Stolte M. Prevalence of lymphoid follicles and aggregates in Helicobacter pylori gastritis in antral and body mucosa. J Clin Pathol. 1993 Sep;46(9):832–835. doi: 10.1136/jcp.46.9.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figura N., Guglielmetti P., Rossolini A., Barberi A., Cusi G., Musmanno R. A., Russi M., Quaranta S. Cytotoxin production by Campylobacter pylori strains isolated from patients with peptic ulcers and from patients with chronic gastritis only. J Clin Microbiol. 1989 Jan;27(1):225–226. doi: 10.1128/jcm.27.1.225-226.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiocca R., Villani L., Luinetti O., Gianatti A., Perego M., Alvisi C., Turpini F., Solcia E. Helicobacter colonization and histopathological profile of chronic gastritis in patients with or without dyspepsia, mucosal erosion and peptic ulcer: a morphological approach to the study of ulcerogenesis in man. Virchows Arch A Pathol Anat Histopathol. 1992;420(6):489–498. doi: 10.1007/BF01600253. [DOI] [PubMed] [Google Scholar]
- Genta R. M., Hamner H. W., Graham D. Y. Gastric lymphoid follicles in Helicobacter pylori infection: frequency, distribution, and response to triple therapy. Hum Pathol. 1993 Jun;24(6):577–583. doi: 10.1016/0046-8177(93)90235-9. [DOI] [PubMed] [Google Scholar]
- Hasan M., Sircus W., Ferguson A. Duodenal mucosal architecture in non-specific and ulcer-associated duodenitis. Gut. 1981 Aug;22(8):637–641. doi: 10.1136/gut.22.8.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kekki M., Sipponen P., Siurala M. Progression of antral and body gastritis in patients with active and healed duodenal ulcer and duodenitis. Scand J Gastroenterol. 1984 May;19(3):382–388. [PubMed] [Google Scholar]
- Leunk R. D., Johnson P. T., David B. C., Kraft W. G., Morgan D. R. Cytotoxic activity in broth-culture filtrates of Campylobacter pylori. J Med Microbiol. 1988 Jun;26(2):93–99. doi: 10.1099/00222615-26-2-93. [DOI] [PubMed] [Google Scholar]
- Phadnis S. H., Ilver D., Janzon L., Normark S., Westblom T. U. Pathological significance and molecular characterization of the vacuolating toxin gene of Helicobacter pylori. Infect Immun. 1994 May;62(5):1557–1565. doi: 10.1128/iai.62.5.1557-1565.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price A. B., Levi J., Dolby J. M., Dunscombe P. L., Smith A., Clark J., Stephenson M. L. Campylobacter pyloridis in peptic ulcer disease: microbiology, pathology, and scanning electron microscopy. Gut. 1985 Nov;26(11):1183–1188. doi: 10.1136/gut.26.11.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price A. B. The Sydney System: histological division. J Gastroenterol Hepatol. 1991 May-Jun;6(3):209–222. doi: 10.1111/j.1440-1746.1991.tb01468.x. [DOI] [PubMed] [Google Scholar]
- Scheppach W. Effects of short chain fatty acids on gut morphology and function. Gut. 1994 Jan;35(1 Suppl):S35–S38. doi: 10.1136/gut.35.1_suppl.s35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipponen P., Varis K., Fräki O., Korri U. M., Seppälä K., Siurala M. Cumulative 10-year risk of symptomatic duodenal and gastric ulcer in patients with or without chronic gastritis. A clinical follow-up study of 454 outpatients. Scand J Gastroenterol. 1990 Oct;25(10):966–973. doi: 10.3109/00365529008997621. [DOI] [PubMed] [Google Scholar]
- Siurala M., Sipponen P., Kekki M. Campylobacter pylori in a sample of Finnish population: relations to morphology and functions of the gastric mucosa. Gut. 1988 Jul;29(7):909–915. doi: 10.1136/gut.29.7.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolte M., Eidt S., Ohnsmann A. Differences in Helicobacter pylori associated gastritis in the antrum and body of the stomach. Z Gastroenterol. 1990 May;28(5):229–233. [PubMed] [Google Scholar]
- Telford J. L., Ghiara P., Dell'Orco M., Comanducci M., Burroni D., Bugnoli M., Tecce M. F., Censini S., Covacci A., Xiang Z. Gene structure of the Helicobacter pylori cytotoxin and evidence of its key role in gastric disease. J Exp Med. 1994 May 1;179(5):1653–1658. doi: 10.1084/jem.179.5.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tummuru M. K., Cover T. L., Blaser M. J. Cloning and expression of a high-molecular-mass major antigen of Helicobacter pylori: evidence of linkage to cytotoxin production. Infect Immun. 1993 May;61(5):1799–1809. doi: 10.1128/iai.61.5.1799-1809.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt J. I., Rathbone B. J., Dixon M. F., Heatley R. V. Campylobacter pyloridis and acid induced gastric metaplasia in the pathogenesis of duodenitis. J Clin Pathol. 1987 Aug;40(8):841–848. doi: 10.1136/jcp.40.8.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt J. I., Rathbone B. J., Sobala G. M., Shallcross T., Heatley R. V., Axon A. T., Dixon M. F. Gastric epithelium in the duodenum: its association with Helicobacter pylori and inflammation. J Clin Pathol. 1990 Dec;43(12):981–986. doi: 10.1136/jcp.43.12.981. [DOI] [PMC free article] [PubMed] [Google Scholar]


