Abstract
AIMS: To analyse the frequency of loss of heterozygosity (allele loss, LOH) in a large sample of colorectal carcinomas using highly informative markers along chromosome 11q. METHODS: One hundred paired samples of colorectal cancer and normal tissue were genotyped at six microsatellite markers on chromosome 11q (cen-D11S1313-D11S901-DRD2/NCAM-D11S29- D11S968-tel). The high levels of heterozygosity at these markers allow allele loss to be determined in about 80% of cases at any one locus. The frequency of replication errors (RERs, microsatellite instability) has also been determined. RESULTS: LOH was found at frequencies of 25% and 29% at the distal D11S968 (11qter) and D11S29 (11q23.3) loci, slightly above the accepted baseline of 0-20%. Allele loss at NCAM, DRD2, D11S901, and D11S1313 was not raised above baseline levels. The probable genetic mechanism of allele loss--chromosomal non-disjunction, mitotic recombination, deletion, or gene conversion--seemed to vary between tumours and no consistent mechanism of mutation was found. Microsatellite instability was found in 23 (23%) tumours. No associations were found between LOH and clinical data (patient sex, age at presentation, tumour site, and Duke's stage). CONCLUSIONS: Although gene(s) on 11q may have a role in the development of a minority of colorectal carcinomas, this study provides evidence against the general importance of allele loss on chromosome 11q in the pathogenesis of colorectal cancer. The results also have implications for the importance of 11q in other cancers: it seems less likely that a single tumour supressor gene at this location promotes the growth of all types of tumour when lost. Rather, one or more genes with tissue specific effects may be involved.
Full text
PDF

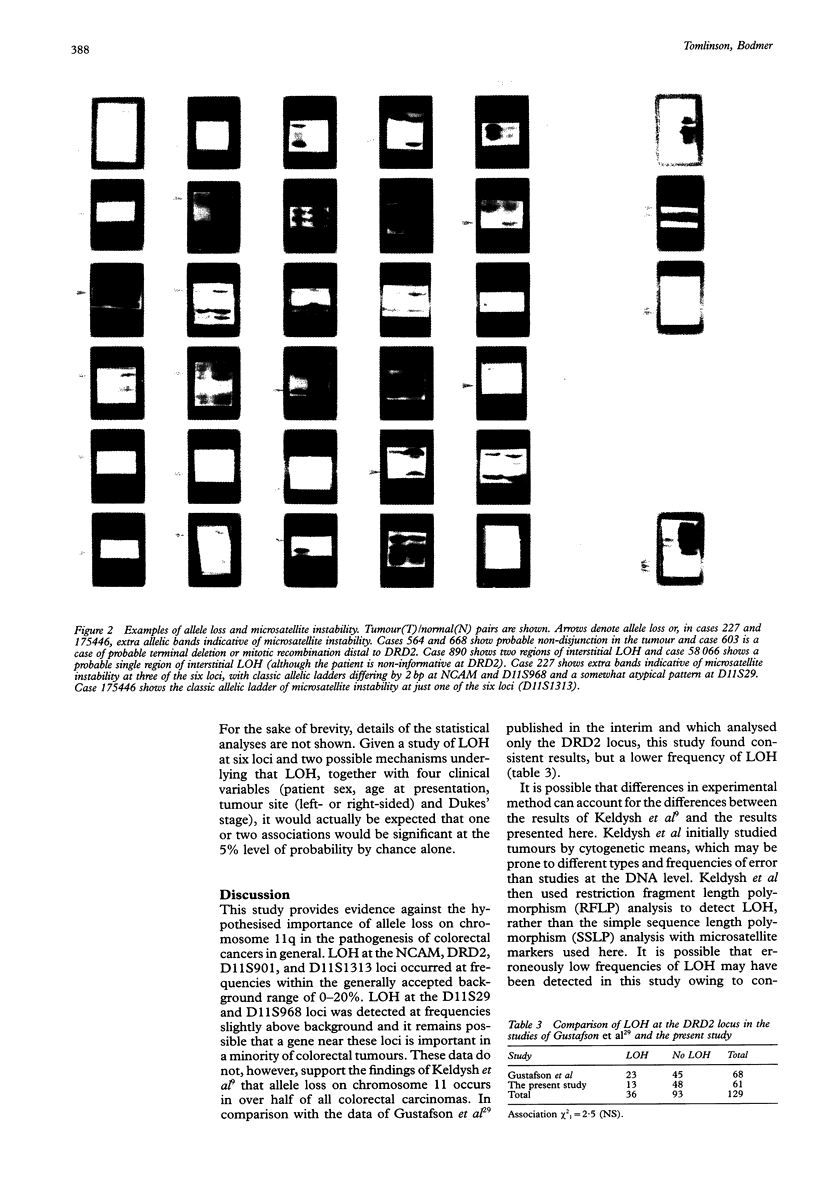


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaltonen L. A., Peltomäki P., Leach F. S., Sistonen P., Pylkkänen L., Mecklin J. P., Järvinen H., Powell S. M., Jen J., Hamilton S. R. Clues to the pathogenesis of familial colorectal cancer. Science. 1993 May 7;260(5109):812–816. doi: 10.1126/science.8484121. [DOI] [PubMed] [Google Scholar]
- Baker S. J., Fearon E. R., Nigro J. M., Hamilton S. R., Preisinger A. C., Jessup J. M., vanTuinen P., Ledbetter D. H., Barker D. F., Nakamura Y. Chromosome 17 deletions and p53 gene mutations in colorectal carcinomas. Science. 1989 Apr 14;244(4901):217–221. doi: 10.1126/science.2649981. [DOI] [PubMed] [Google Scholar]
- Bethwaite P. B., Koreth J., Herrington C. S., McGee J. O. Loss of heterozygosity occurs at the D11S29 locus on chromosome 11q23 in invasive cervical carcinoma. Br J Cancer. 1995 Apr;71(4):814–818. doi: 10.1038/bjc.1995.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter S. L., Negrini M., Baffa R., Gillum D. R., Rosenberg A. L., Schwartz G. F., Croce C. M. Loss of heterozygosity at 11q22-q23 in breast cancer. Cancer Res. 1994 Dec 1;54(23):6270–6274. [PubMed] [Google Scholar]
- Fearon E. R., Cho K. R., Nigro J. M., Kern S. E., Simons J. W., Ruppert J. M., Hamilton S. R., Preisinger A. C., Thomas G., Kinzler K. W. Identification of a chromosome 18q gene that is altered in colorectal cancers. Science. 1990 Jan 5;247(4938):49–56. doi: 10.1126/science.2294591. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990 Jun 1;61(5):759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- Foulkes W. D., Campbell I. G., Stamp G. W., Trowsdale J. Loss of heterozygosity and amplification on chromosome 11q in human ovarian cancer. Br J Cancer. 1993 Feb;67(2):268–273. doi: 10.1038/bjc.1993.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara Y., Emi M., Ohata H., Kato Y., Nakajima T., Mori T., Nakamura Y. Evidence for the presence of two tumor suppressor genes on chromosome 8p for colorectal carcinoma. Cancer Res. 1993 Mar 1;53(5):1172–1174. [PubMed] [Google Scholar]
- Gustafson C. E., Young J., Leggett B., Searle J., Chenevix-Trench G. Loss of heterozygosity on the long arm of chromosome 11 in colorectal tumours. Br J Cancer. 1994 Sep;70(3):395–397. doi: 10.1038/bjc.1994.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyapay G., Morissette J., Vignal A., Dib C., Fizames C., Millasseau P., Marc S., Bernardi G., Lathrop M., Weissenbach J. The 1993-94 Généthon human genetic linkage map. Nat Genet. 1994 Jun;7(2 Spec No):246–339. doi: 10.1038/ng0694supp-246. [DOI] [PubMed] [Google Scholar]
- Hampton G. M., Mannermaa A., Winqvist R., Alavaikko M., Blanco G., Taskinen P. J., Kiviniemi H., Newsham I., Cavenee W. K., Evans G. A. Loss of heterozygosity in sporadic human breast carcinoma: a common region between 11q22 and 11q23.3. Cancer Res. 1994 Sep 1;54(17):4586–4589. [PubMed] [Google Scholar]
- Hampton G. M., Penny L. A., Baergen R. N., Larson A., Brewer C., Liao S., Busby-Earle R. M., Williams A. W., Steel C. M., Bird C. C. Loss of heterozygosity in cervical carcinoma: subchromosomal localization of a putative tumor-suppressor gene to chromosome 11q22-q24. Proc Natl Acad Sci U S A. 1994 Jul 19;91(15):6953–6957. doi: 10.1073/pnas.91.15.6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauge X. Y., Grandy D. K., Eubanks J. H., Evans G. A., Civelli O., Litt M. Detection and characterization of additional DNA polymorphisms in the dopamine D2 receptor gene. Genomics. 1991 Jul;10(3):527–530. doi: 10.1016/0888-7543(91)90431-d. [DOI] [PubMed] [Google Scholar]
- Healy E., Rehman I., Angus B., Rees J. L. Loss of heterozygosity in sporadic primary cutaneous melanoma. Genes Chromosomes Cancer. 1995 Feb;12(2):152–156. doi: 10.1002/gcc.2870120211. [DOI] [PubMed] [Google Scholar]
- Ionov Y., Peinado M. A., Malkhosyan S., Shibata D., Perucho M. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature. 1993 Jun 10;363(6429):558–561. doi: 10.1038/363558a0. [DOI] [PubMed] [Google Scholar]
- Keldysh P. L., Dragani T. A., Fleischman E. W., Konstantinova L. N., Perevoschikov A. G., Pierotti M. A., Della Porta G., Kopnin B. P. 11q deletions in human colorectal carcinomas: cytogenetics and restriction fragment length polymorphism analysis. Genes Chromosomes Cancer. 1993 Jan;6(1):45–50. doi: 10.1002/gcc.2870060109. [DOI] [PubMed] [Google Scholar]
- Kenmotsu M., Gouchi A., Maruo Y., Murashima N., Hiramoto Y., Iwagaki H., Orita K. [The expression of neural cell adhesion molecule (NCAM), neural invasion and recurrence patterns in rectal cancer--a study using anti-NACM (neural cell adhesion molecule) antibody]. Nihon Geka Gakkai Zasshi. 1994 Feb;95(2):66–70. [PubMed] [Google Scholar]
- Kobayashi H., Espinosa R., 3rd, Fernald A. A., Begy C., Diaz M. O., Le Beau M. M., Rowley J. D. Analysis of deletions of the long arm of chromosome 11 in hematologic malignancies with fluorescence in situ hybridization. Genes Chromosomes Cancer. 1993 Dec;8(4):246–252. doi: 10.1002/gcc.2870080407. [DOI] [PubMed] [Google Scholar]
- Konstantinova L. N., Fleischman E. W., Knisch V. I., Perevozchikov A. G., Kopnin B. P. Karyotype peculiarities of human colorectal adenocarcinomas. Hum Genet. 1991 Mar;86(5):491–496. doi: 10.1007/BF00194640. [DOI] [PubMed] [Google Scholar]
- Lothe R. A., Peltomäki P., Meling G. I., Aaltonen L. A., Nyström-Lahti M., Pylkkänen L., Heimdal K., Andersen T. I., Møller P., Rognum T. O. Genomic instability in colorectal cancer: relationship to clinicopathological variables and family history. Cancer Res. 1993 Dec 15;53(24):5849–5852. [PubMed] [Google Scholar]
- Muleris M., Salmon R. J., Dutrillaux B. Cytogenetics of colorectal adenocarcinomas. Cancer Genet Cytogenet. 1990 Jun;46(2):143–156. doi: 10.1016/0165-4608(90)90100-o. [DOI] [PubMed] [Google Scholar]
- Solomon E., Voss R., Hall V., Bodmer W. F., Jass J. R., Jeffreys A. J., Lucibello F. C., Patel I., Rider S. H. Chromosome 5 allele loss in human colorectal carcinomas. Nature. 1987 Aug 13;328(6131):616–619. doi: 10.1038/328616a0. [DOI] [PubMed] [Google Scholar]
- Telatar M., Concannon P., Tolun A. Dinucleotide repeat polymorphism at 11q23. Hum Genet. 1994 Jul;94(1):109–109. doi: 10.1007/BF02272856. [DOI] [PubMed] [Google Scholar]
- Thibodeau S. N., Bren G., Schaid D. Microsatellite instability in cancer of the proximal colon. Science. 1993 May 7;260(5109):816–819. doi: 10.1126/science.8484122. [DOI] [PubMed] [Google Scholar]
- Tomlinson I. P., Gammack A. J., Stickland J. E., Mann G. J., MacKie R. M., Kefford R. F., McGee J. O. Loss of heterozygosity in malignant melanoma at loci on chromosome 11 and 17 implicated in the pathogenesis of other cancers. Genes Chromosomes Cancer. 1993 Jul;7(3):169–172. doi: 10.1002/gcc.2870070310. [DOI] [PubMed] [Google Scholar]
- Tomlinson I. P., Strickland J. E., Lee A. S., Bromley L., Evans M. F., Morton J., McGee J. O. Loss of heterozygosity on chromosome 11 q in breast cancer. J Clin Pathol. 1995 May;48(5):424–428. doi: 10.1136/jcp.48.5.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelstein B., Fearon E. R., Hamilton S. R., Kern S. E., Preisinger A. C., Leppert M., Nakamura Y., White R., Smits A. M., Bos J. L. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988 Sep 1;319(9):525–532. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- Walker G. J., Palmer J. M., Walters M. K., Hayward N. K. A genetic model of melanoma tumorigenesis based on allelic losses. Genes Chromosomes Cancer. 1995 Feb;12(2):134–141. doi: 10.1002/gcc.2870120208. [DOI] [PubMed] [Google Scholar]
- Warnich L., Groenewald I., Theart L., Retief A. E. Highly informative dinucleotide repeat polymorphism at the D11S29 locus on chromosome 11q23. Hum Genet. 1992 May;89(3):357–359. doi: 10.1007/BF00220560. [DOI] [PubMed] [Google Scholar]
- Young J., Leggett B., Gustafson C., Ward M., Searle J., Thomas L., Buttenshaw R., Chenevix-Trench G. Genomic instability occurs in colorectal carcinomas but not in adenomas. Hum Mutat. 1993;2(5):351–354. doi: 10.1002/humu.1380020505. [DOI] [PubMed] [Google Scholar]





