Abstract
Objective
Insulin must move from the blood to the interstitium to initiate signaling, yet access to the interstitium may be impaired in cases of insulin resistance, such as obesity. We investigated whether consuming a short- and long-term high-fat diet (HFD) impairs insulin access to skeletal muscle, the major site of insulin-mediated glucose uptake.
Methods
Male mongrel dogs were divided into 3 groups consisting of control diet (n=16), short-term (n=8) and long-term HFD (n=8). Insulin sensitivity was measured with intravenous glucose tolerance tests. A hyperinsulinemic euglycemic clamp was performed in each animal at the conclusion of the study. During the clamp, lymph fluid was measured as a representation of the interstitial space to assess insulin access to muscle.
Results
Short- and long-term HFD induced obesity and reduced insulin sensitivity. Lymph insulin concentrations were approximately 50% of plasma insulin concentrations under control conditions. Long-term HFD caused fasting plasma hyperinsulinemia, however, interstitial insulin concentrations were not increased, suggesting impaired insulin access to muscle.
Conclusions
A HFD rapidly induces insulin resistance at the muscle and impairs insulin access under basal insulin concentrations. Hyperinsulinemia induced by a long-term HFD may be a compensatory mechanism necessary to maintain healthy insulin levels in muscle interstitium.
Keywords: insulin, obesity, interstitium, skeletal muscle, endothelium
Introduction
Insulin must move into the interstitial space to bind to its receptors and initiate glucose uptake in muscle 1,2, thus the access of insulin to muscle is required for appropriate insulin signaling. The transport of insulin to muscle relies on the endothelium, the function of which can be impaired by many aspects of the metabolic syndrome 5, including elevated plasma lipids 6, visceral obesity 7 and/or poor glucose control 8. Endothelial dysfunction has also been reported in pre-diabetes 9, in otherwise healthy insulin-sensitive individuals with obesity 10 and in people with a family history of diabetes 11, suggesting that endothelial dysfunction is an early complication of obesity12,13.
Findings from previous studies suggest that insulin access to metabolic tissues is reduced in disease states, but studies directly measuring insulin delivery to tissues under these conditions are limited. In one study, women with obesity were reported to have higher circulating levels of insulin than lean women, yet there were no differences in interstitial insulin levels in adipose tissue and skeletal muscle, suggesting impaired access of insulin to these tissues during obesity 14. Furthermore, a study in men with obesity reported slower transcapillary transport of insulin from the plasma to the interstitium as compared to lean men 15. However, it is unknown whether this impaired delivery is only evident in an established disease state, or whether it is an early alteration during the early development of obesity.
A previous study from our group reported an impairment in macromolecular delivery to the interstitium after a HFD, however no significant impairment in insulin access was detected at the supraphysiological insulin concentrations used 4. We have also previously shown that diet-induced obesity prevents the dispersion of insulin through skeletal muscle when it is injected directly into the interstitium 3. In both of these prior studies, however, it is not clear whether the delivery of insulin from the plasma to the interstitium at physiological insulin levels was impaired.
We therefore investigated the effects of physiological hyperinsulinemia due to short- and long-term diet-induced obesity on insulin access to skeletal muscle in a canine model. To test the hypothesis that insulin access to skeletal muscle is impaired by a short-term HFD, we measured lymph fluid as a representation of the interstitial space, and performed hyperinsulinemic euglycemic clamps and intravenous glucose tolerance tests to assess insulin sensitivity.
Methods
Animals
Male mongrel dogs (n=32) were housed in the University of Southern California Medical School Vivarium, or Cedars-Sinai Comparative Medicine facility under controlled kennel conditions (12h light:12h dark). Dogs were used for experiments only if judged to be in good health as determined by visual observation, body weight, hematocrit and body temperature. Protocols were conducted in conformity with the Public Health Service (PHS) Policy on Humane Care and Use of Laboratory Animals, and approved by the University of Southern California (USC) Institutional Animal Care and Use Committee, or the Cedars-Sinai Medical Center Institutional Animal Care and Use Committee, as appropriate. There was no significant effect of location on results (data not shown).
Protocol
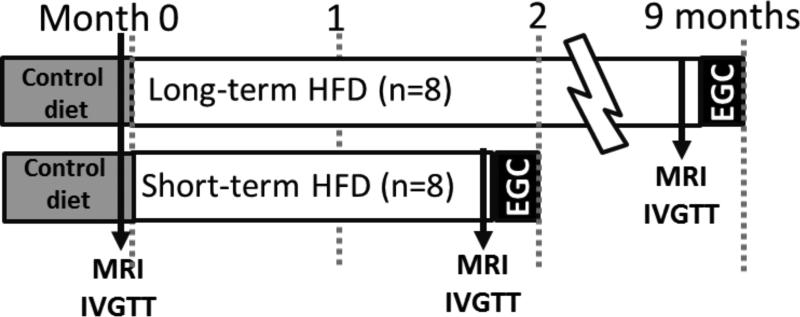
An experimental schematic is shown in Figure 1. A lean group (n=16, 28.6 ± 0.07 kg; data are mean ± SEM) underwent a hyperinsulinemic euglycemic clamp to determine whole body insulin sensitivity, muscle tissue insulin sensitivity and insulin access during control diet conditions. During short-term (n=8, 2.1 ± 0.1 months) or long-term HFD (n=8, 10.7 ± 1.7 months) MRIs and IVGTTs were performed at baseline and at the end of each dietary condition. A hyperinsulinemic euglycemic clamp was performed at the end of the protocol to assess whole body insulin sensitivity, muscle tissue insulin sensitivity and insulin access.
Figure 1.
Protocol schematic. Assessments of fat content by MRI and insulin sensitivity (IVGTT) were conducted before and after HFD. A terminal hyperinsulinemic euglycemic clamp was performed to assess insulin sensitivity and insulin access.
Diet
Animals in the lean group were fed dry chow ad libitum (42.4% carbohydrate, 297.7% protein, 29.9% fat, [mixture of Laboratory High Density Canine Diet and Prolab Canine 2000, Richmond, IN]). The HFD consisted of 825 grams dry chow, supplemented with 1 can of Hills Prescription Diet (415 g, 10% carbohydrate, 9% protein, 8% fat, 0.3% fiber, and 73% moisture [Hill's Pet Nutrition, Topeka, KS]) and 6 grams/kg lard, resulting in a diet of 5,392 kcal/day consisting of 27.9% carbohydrates, 20.1% protein and 52% fat presented to the dogs every day for the remainder of the study.
Magnetic resonance imaging (MRI)
Body fat distribution was assessed by MRI using a 3T Siemens Magnetom Verio. Total, subcutaneous and visceral fat mass were determined from abdominal axial images at the 20-cm region of the thorax, using the left renal artery branch from the abdominal aorta as a midpoint landmark. The abdominal image analysis was performed using Slice-O-Matic software (4.3 rev 10; Virtual Magic Inc., Montreal, Canada) by a single experienced observer. Visceral fat was defined as fat within the abdominal cavity. Subcutaneous fat was defined as fat under the skin.
Frequently sampled intravenous glucose tolerance test (IVGTT)
Animals were mildly restrained in a Pavlov sling as previously described 16 and an intracatheter was inserted into the saphenous or cephalic vein. Blood samples were drawn every 5 min for 15 min (3 baseline samples), at which time glucose was given as an intravenous bolus (0.3 g/kg body weight). Blood samples were collected at 2, 3, 4, 5, 6, 8, 10, 12, 14, 16, 19, 22, 23, 24, 25, 30, 40, 50, 60, 70, 90, 100, 120, 140, 160 and 180 min. At t=20, intravenous insulin (0.02 U/kg body weight) was administered. Insulin sensitivity (SI) was calculated using the minimal model of glucose kinetics using MINMOD Millennium software (MINMOD 6.02, MinMod, Los Angeles, CA). The acute insulin response to glucose (AIRg) was calculated as the area under the curve (AUC) of the insulin concentrations above the average of the basal values from 0 to 10 min. Disposition index is calculated as the product of insulin sensitivity and AIRg.
Anesthesia preparation for hyperinsulinemic euglycemic clamp
Animals were fasted overnight before the morning of the experiment. Dogs were sedated with acepromazine maleate (Prom-Ace, Aueco, Fort Dodge, IA; 0.22mg/kg) and atropine sulfate (Western Medical, Arcadia, CA; 0.11 mL/kg). Anesthesia was induced with sodium pentobarbital (Western Medical, Arcadia, CA; 0.5mL/kg) or propofol (Western Medical, Arcadia, CA; 6mg/kg) and maintained with isofluorane or sevoflurane (Western Medical, Arcadia, CA). Dogs were placed on heating pads to maintain body temperature. Intracatheters were inserted into the left cephalic vein for variable glucose infusion and the right cephalic vein for insulin, somatostatin, and tracer infusion. Saline was infused into the cephalic vein and maintained at a slow drip until the end of the experiment. Indwelling catheters were placed into both the left femoral artery and vein for sampling. The hindlimb lymphatic vessel was cannulated by placing a polyethylene catheter (PE10) into the afferent lymphatic vessel of the deep inguinal lymph node. Lymph was collected by gently massaging the leg directly above the popliteal area, which has been shown to instantaneously increase lymph drainage without affecting lymph or plasma oncotic pressures 17. Blood pressure (cuff on hind leg), heart rate, O2 saturation and CO2 were monitored continuously. At the conclusion of these experiments, animals were euthanized with an overdose of sodium pentobarbital (Eutha-6, Western Medical; 65mg/kg).
Hyperinsulinemic euglycemic clamp (EGC)
Immediately after the completion of the surgical procedures and sampling of basal fasting lymph and plasma, a basal insulin EGC was initiated (t=−180min). Somatostatin was infused to inhibit endogenous insulin secretion (1ug/min/kg; Bachem), and basal insulin was replaced systemically (0.2mU/min/kg; Novo Nordisk, Bagsvaerd, Denmark) and continuously for the remainder of the study. Exogenous 20% glucose was infused into the left cephalic vein at variable rates to clamp arterial glucose to basal levels throughout the experimental period. Plasma samples were taken from the femoral artery and femoral vein every 10-15 minutes. Lymph vessels were sampled by gently massaging the hindlimb distal to the site of catheterization. After 180 minutes of basal insulin replacement (t=0), the insulin concentration was increased by an infusion of 1.0mU/min/kg, which was continued for the remainder of the study. Insulin levels from the artery, vein and lymph were averaged over the last 45 minutes of the clamp to examine differences in insulin access at steady state.
Assays
Arterial, venous and lymph samples were collected in microtubes pre-coated with lithium-heparin (Becton Dickinson, Franklin Lakes, NJ). Arterial and venous tubes also contained 50uL EDTA (Sigma Chemicals, St Louis, MO). Blood samples were centrifuged immediately and the supernatant was transferred and stored at −20°C until further assay. Plasma and lymph samples were immediately assayed for glucose with an YSI 2700 autoanalyzer (Yellow Springs Instrument Co., Yellow Springs, OH) before freezing at −20°C. Insulin was measured in plasma and lymph with an ELISA for dog plasma (Alpco, Salem, NH). Steady state was defined as the final 40 minutes of the hyperinsulinemic clamp.
Tissue insulin sensitivity
Tissue insulin sensitivity (Stissue) reflects the insulin-mediated glucose uptake in response to the local, rather than systemic, insulin concentration and thus gives an indication of the ability of the muscle to respond to interstitial insulin. Stissue was calculated from the arterio-venous glucose difference (ΔAVGlu), the change in interstitial insulin (ΔInsI), and the glucose concentration (GlucSS) at steady state: ΔAVGlu/ΔInsI x GlucSS.
Statistical analyses
Experimental data are shown as mean ± SEM. Statistical analyses were performed with paired or unpaired Student's t tests or one-way ANOVAs with Tukey's pairwise comparisons, as appropriate (GraphPad Prism version 5.04 for Windows, GraphPad Software, San Diego, CA). One-tailed dependent t-tests were used to test planned comparisons due to the specific, directional nature of our a priori hypotheses. For example, it is well established that a high-fat diet in the canine model will lead to an increase in weight and a decrease in insulin sensitivity. All differences were considered statistically significant when p<0.05.
Results
High-fat feeding rapidly induces obesity, insulin resistance and hyperinsulinemic compensation
Short- and long-term high-fat feeding led to a significant increase in body weight (short-term: +4.8±1.3%, p<0.01; long-term: +13.2±3.3%, p<0.01), with increases in both visceral and subcutaneous fat depots (Table 1).
Table 1.
IVGTT characteristics before and after short- and long-term high-fat diet (HFD).
| SHORT-TERM HFD | Week 0 | Week 6 |
|---|---|---|
| Weight (kg) | 28.1±1.3 | 29.4±1.3* |
| Glucose (mg/dl) | 96.8±1.6 | 95.1±1.2 |
| Insulin (mU/L) | 9.0±1.8 | 10.8±1.8 |
| AIRg (mU/L).min | 542.6±45.0 | 588.9±50 |
| DI | 2373±517 | 1902±367 |
| SI (mU/L)−1.min−1 | 4.5±0.9 | 3.4±0.7* |
| Visc fat (%) | 11±1 | 16±1* |
| Subcut fat (%) | 10±2 | 13±2* |
| LONG-TERM HFD | Week 0 | Week 40 |
|---|---|---|
| Weight (kg) | 31.6±1.4 | 35.7±1.9* |
| Glucose (mg/dl) | 94.8±2.0 | 94.2±1.9 |
| Insulin (mU/L) | 8.8±1.6 | 12.7±2.2* |
| AIRg (mU/L).min | 494.9±70.3 | 623.0±72.9* |
| DI | 2291±240 | 2251±296 |
| SI (mU/L)−1.min−1 | 4.9±0.5 | 3.7±0.5* |
| Visc fat (%) | 13±1 | 17±2* |
| Subcut fat (%) | 11±1 | 13±2* |
Data are mean ± standard error of the mean.
p<0.05 vs Week 0.
Fasting insulin increased during the long-term HFD (8.77±1.6 vs 12.7±2.2 uU/ml, p<0.05), and showed a trend to increase during the short-term HFD, whereas fasting glucose levels remained unchanged (Table 1). Insulin sensitivity (SI) assessed by the IVGTT was significantly reduced after short- (4.5±0.9 vs 3.4±0.7 (mU/l)−1.min−1, p=0.04), and long-term HFD (4.9±0.4 vs 3.7±0.4 (mU/l)−1.min−1, p=0.02; Table 1). Hyperinsulinemic compensation was observed in the long-term HFD animals only, as indicated by an increased acute insulin response to glucose (AIRg) (494.9±70.3 to 623.0±72.9 mU/l.min, p=0.01; Table 1). The product of SI and AIRg, called the disposition index (DI), refers to the function of the beta cell over time 18. A reduction in DI indicates an inability of the beta cell to respond to insulin resistance and represents an increased risk for the future development of diabetes. However, due to hyperinsulinemic compensation in response to HFD, the DI remained unchanged (2373±517 vs 1902±367 short-term HFD, p=ns; 2291±240 vs 2251±296 long-term HFD, p=ns).
Fasting plasma insulin, but not interstitial lymph insulin, is elevated during high-fat feeding
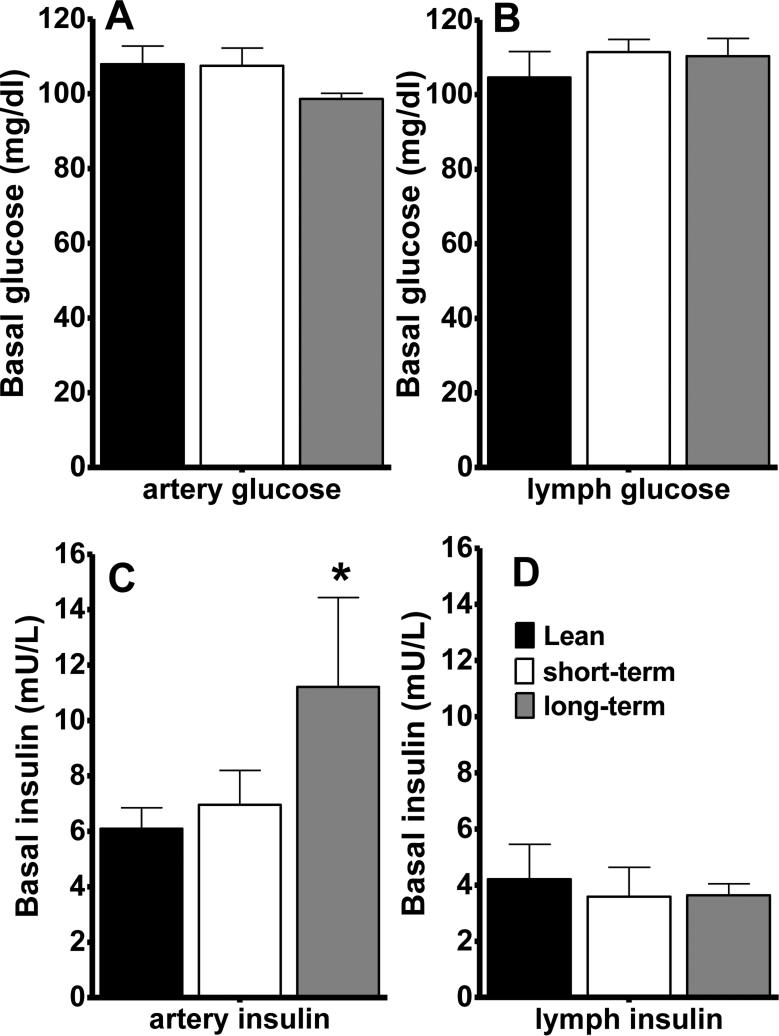
Fasting arterial and interstitial lymph glucose levels were similar, and were unaltered by HFD (Figure 2a and b). There was a significant elevation in fasting arterial insulin after long-, but not short-term HFD (Figure 2c), consistent with the fasting hyperinsulinemia and increased AIRg observed during the IVGTT (Table 1). However, we did not observe an increase in interstitial insulin levels in the lymph (Figure 2d), suggesting that plasma hyperinsulinemia associated with long-term HFD is not sufficient to increase interstitial insulin levels.
Figure 2.
Basal arterial glucose (A), lymph glucose (B), arterial insulin (C) and lymph insulin (D) concentrations during the hyperinsulinemic euglycemic clamp. Arterial insulin levels were significantly elevated after long-term HFD. Data are mean ± standard error of the mean. *p<0.05 vs lean animals.
High-fat feeding leads to reduced insulin sensitivity in skeletal muscle
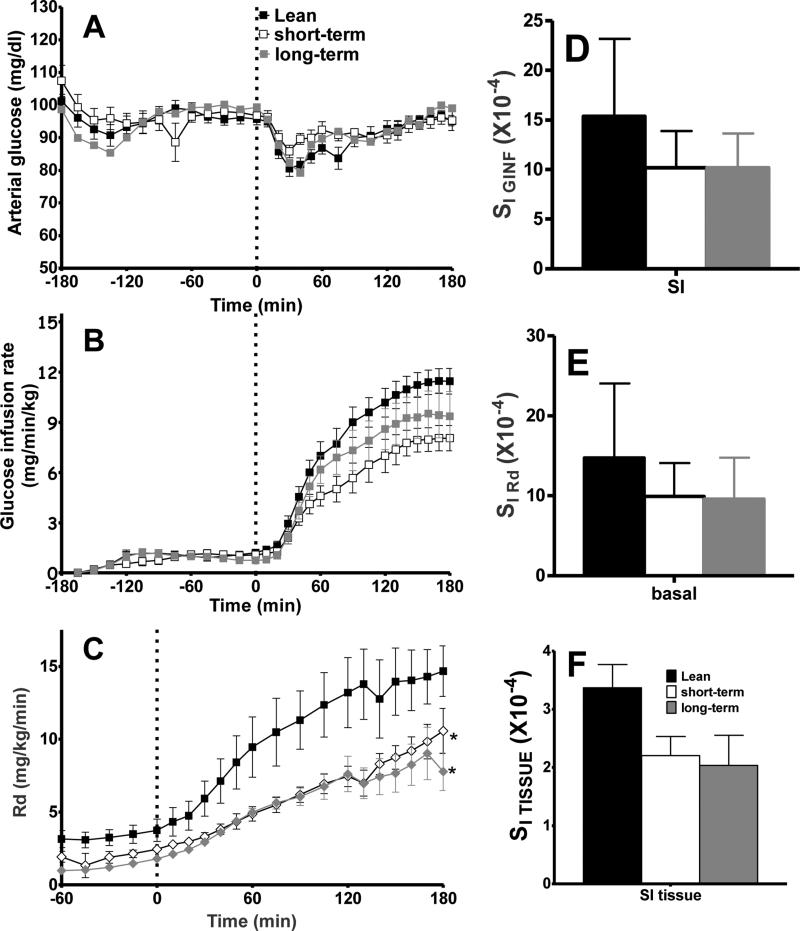
High-fat feeding led to a reduction in insulin sensitivity, as compared to lean controls, indicated by a reduction in the glucose infusion required to maintain plasma euglycemia (Figure 3a and b). Peripheral glucose uptake was reduced (Figure 3c), as was systemic and peripheral insulin sensitivity (Figure 3d and e). Local tissue insulin sensitivity, as measured by arterio-venous glucose difference in response to the interstitial insulin concentration, tended to be impaired by both short- and long-term HFD (Figure 3f).
Figure 3.
Insulin sensitivity as measured by the hyperinsulinemic euglycemic clamp. Arterial glucose (A) was maintained using a variable glucose infusion rate (B). Peripheral glucose uptake (C) was impaired by HFD-induced obesity. Obesity also caused reductions in insulin sensitivity, including SI GINF (D), SI RD (E), and SI tissue (F). Data are mean ± standard error of the mean. *p<0.05 vs lean animals.
Plasma insulin is elevated during the EGC following high-fat feeding
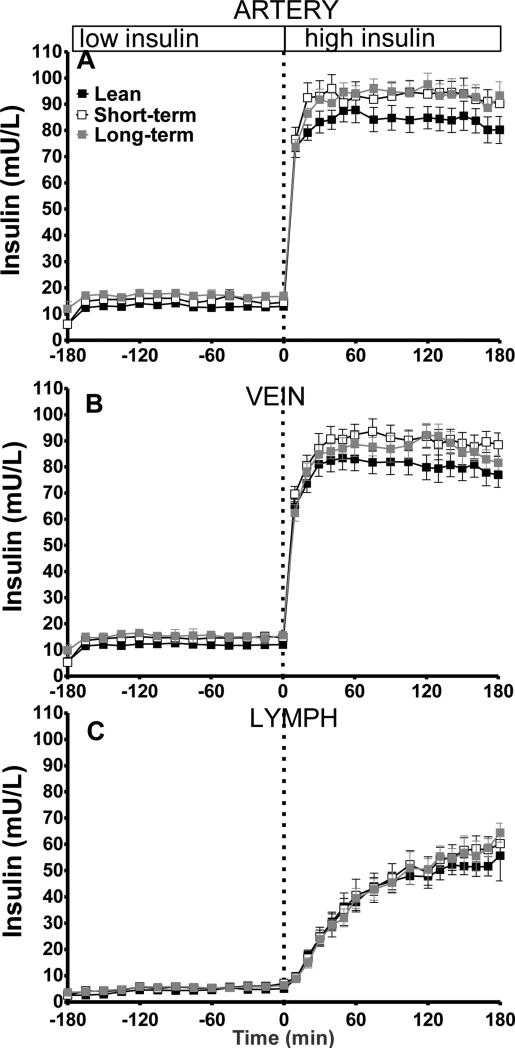
Immediately following the initiation of exogenous insulin infusion during the EGC, arterial and venous insulin levels began to increase (Figure 4a and b). Lymph insulin increased at a slower rate in response to insulin infusion. Arterial and venous insulin levels during the clamp were elevated in both short- and long-term HFD animals, as opposed to lean controls, despite matched insulin infusion (Figure 4). In contrast, insulin levels in the lymph were not significantly elevated and were not different from lean controls (Figure 4c), suggesting impaired insulin access during both short- and long-term HFD.
Figure 4.
Time course of insulin during the hyperinsulinemic euglycemic clamp in artery (A), vein (B) and lymph (C).
Discussion
The transport of appropriate levels of insulin from the plasma to the interstitium to bind insulin receptors and induce signaling in target metabolic tissues is an important component of insulin action. It has previously been shown that insulin resistance and obesity are associated with impaired insulin access to the interstitium, however this has typically been investigated after metabolic disease is well-established 14,25 .
Here we present data on the development of impaired insulin access during short-term, as well as long-term diet-induced insulin resistance and obesity. Following high-fat feeding, animals developed plasma hyperinsulinemia, however we observed no changes in interstitial insulin concentrations compared to healthy animals. Hyperinsulinemia without a change in lymph insulin indicates reduced insulin access to muscle, and may suggest that the hyperinsulinemia induced due to HFD may be required to maintain an appropriate level of insulin in the interstitium. Under higher plasma insulin concentrations during the clamp, there was no significant effect of diet or obesity to reduce insulin access, suggesting that the impaired insulin access to the interstitium detected under basal conditions can be overcome by infusing insulin at supraphysiological concentrations.
Our findings are consistent with previous studies that have shown that hyperinsulinemia is required to maintain appropriate interstitial insulin levels in the muscle and fat of women with obesity during an oral glucose tolerance test 14. Microdialysis has also been used to assess adipose interstitial insulin during clamps in lean subjects and those with obesity 25, and these data support the finding that reduced insulin access can be overcome at high insulin levels. However, neither of these studies investigated insulin access during basal conditions when insulin was in the physiological range.
A prior study from our laboratory demonstrated impaired access of macromolecules to the muscle interstitium after 12 weeks of a high fat diet 4. Consistent with our short-term HFD findings, insulin levels were not elevated in the plasma or interstitium of this study, thus in the present study we chose to employ a prolonged dietary intervention designed to induce plasma hyperinsulinemia. We further demonstrated that a short-term HFD for 2 months rapidly induces whole body and muscle-specific insulin resistance, prior to any detectable impairment in insulin access.
Our conclusions rely on the assumption that the lymph is an accurate representation of the interstitial environment. Other methods to sample the interstitium directly have proven problematic, since they can induce local inflammation and often result in a limited number of samples. Microdialysis is an indirect method used to measure interstitial insulin, however this method has a low recovery rate. Sampling the lymph fluid has been identified as an effective method since it provides a direct measurement of interstitial fluid with complete recovery, and can be used to examine temporal changes as multiple samples can be obtained over time 30. Lymph sampling of insulin has been shown to match results provided by microdialysis 14,15,25,30, and can therefore provide an unadulterated sample of the interstitial space. However, studies directly comparing lymph sampling and microdialysis for interstitial measurements are necessary to fully validate this technique.
There are various aspects of endothelial function that are involved in insulin access to the muscle interstitium, and targeting the endothelium has been proposed for the treatment of diabetes 33. The endothelium functions as a barrier, and changes in permeability have been shown to occur with endothelial dysfunction 26,27. Therefore, it is likely that impaired endothelial function directly impairs insulin access, thus impacting insulin action at the tissue level. Measuring endothelial function by changes in bulk blood flow can provide information on macrovascular endothelial function, but only provides an indirect measure of microvascular function. Therefore, methods such as tracer techniques to assess transendothelial transport and microvascular imaging will be important in future studies for assessing endothelial permeability and transport.
Many factors may contribute to the development of endothelial dysfunction observed with insulin resistance and obesity. Both glucose and lipids are often elevated in populations of individuals with obesity, and are involved in endothelial dysfunction during the development of type 2 diabetes 12. However, neither fasting hyperglycemia nor hyperlipidemia was found in the present study following a HFD. We have previously demonstrated that nocturnal free fatty acids are elevated after a high-fat diet 16, and may play a role in reduced insulin access in our model. Although nocturnal free fatty acids were not measured in this study, results from our previous lipid infusion studies would suggest that hyperlipidemia alone is not sufficient to impair insulin access 28. We have also demonstrated that time spent in the laboratory and/or aging are not responsible for any metabolic impairments in our model 29.
Finally, inflammation has been proposed as a link between obesity, the metabolic syndrome and endothelial dysfunction 32, and it will be important for future studies to investigate the role of inflammation and its effects on endothelial function and insulin access in this model. In addition, future studies must examine changes in cellular insulin signaling in the muscle—for example, whether phosphorylation of the insulin receptor is reduced following a HFD in this model. The present studies concluded under conditions of hyperinsulinemia, therefore any reductions in cellular insulin resistance would likely be masked by the large insulin stimulation immediately prior to tissues collection. Indeed, we observed that at high insulin doses, impaired access was overcome.
Further, it is possible that tissue samples from the present study would be affected by muscle inflammation following the long surgery and the experimental procedure. Future analyses examining levels of adhesion molecules such as ICAM1 and VCAM1 are necessary to directly relate inflammation and endothelial function to impaired insulin access.
The function of the lymphatic system in metabolic disease has been questioned, since remodeling of the system has been shown to occur in rats with metabolic syndrome 31, suggesting that lymph may not accurately reflect the interstitium due to alterations in vessel structure in the later stages of obesity. Though our animals develop insulin resistance, they do not develop diabetes and therefore are unlikely to have experienced the lymphatic system remodeling associated with late-stage obesity and metabolic syndrome. It is possible that a longer exposure to HFD or a second physiological insult, such as β-cell insufficiency, pronounced endothelial dysfunction, or a separate intervention such as a high-salt or high-carbohydrate diet is necessary for the development of diabetes and subsequent lymphatic remodeling in this model.
In conclusion, we have demonstrated that reduced insulin access is an early defect during the development of obesity and insulin resistance and that plasma hyperinsulinemia associated with long-term high-fat feeding may serve as a compensatory mechanism to maintain interstitial insulin levels. Further research is required to investigate the longer-term implications of reduced transendothelial transport, endothelial function and interstitial insulin levels on the development and progression of diabetes, obesity and cardiovascular disease.
What is already known about this subject?
Transport of insulin to muscle relies on the endothelium.
Endothelial dysfunction is suggested to be an early complication of obesity.
Long-term obesity is associated with impaired insulin delivery to muscle; however, whether insulin access is impaired during the early stages of obesity development is unknown.
What does this study add?
Diet-induced obesity rapidly induces plasma hyperinsulinemia, but does not increase interstitial insulin concentrations, suggesting reduced insulin access to muscle.
Impaired insulin access can be overcome by the infusion of high levels of exogenous insulin into circulation.
Cellular insulin sensitivity is rapidly impaired by a high-fat diet, but does not decrease further with prolonged obesity and continued fat feeding.
ACKNOWLEDGMENTS
The authors would like to thank Rita Thomas, CSMC, for performing the insulin, cortisol and catecholamine assays and Edgardo Paredes, CSMC, and the Cedars-Sinai Medical Center Comparative Medicine staff for their assistance with and care for our animals. We thank Stella P. Kim for comments on the manuscript. C.M.K. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding: This work was supported by NIH grants DK29867 and DK27619 and Society in Science, The Branco Weiss Fellowship, administered by the ETH Zürich (to JLB).
Footnotes
Disclosure: The authors declare no conflict of interest.
REFERENCES
- 1.Castillo C, Bogardus C, Bergman R, Thuillez P, Lillioja S. Interstitial insulin concentrations determine glucose uptake rates but not insulin resistance in lean and obese men. J Clin Invest. 1994;93:10–16. doi: 10.1172/JCI116932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang YJ, Hope ID, Ader M, Bergman RN. Insulin transport across capillaries is rate limiting for insulin action in dogs. J Clin Invest. 1989;84:1620–1628. doi: 10.1172/JCI114339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kolka CM, Harrison LN, Lottati M, Chiu JD, Kirkman EL, Bergman RN. Diet- induced obesity prevents interstitial dispersion of insulin in skeletal muscle. Diabetes. 2009;59:619–626. doi: 10.2337/db09-0839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ellmerer M, Hamilton-Wessler M, Kim SP, Huecking K, Kirkman E, Chiu J, Richey J, Bergman RN. Reduced access to insulin-sensitive tissues in dogs with obesity secondary to increased fat intake. Diabetes. 2006;55:1769–1775. doi: 10.2337/db05-1509. [DOI] [PubMed] [Google Scholar]
- 5.Dell'Omo G, Penno G, Pucci L, Mariani M, Del Prato S, Pedrinelli R. Abnormal capillary permeability and endothelial dysfunction in hypertension with comorbid Metabolic Syndrome. Atherosclerosis. 2004;172:383–389. doi: 10.1016/j.atherosclerosis.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 6.Symons JD, Abel ED. Lipotoxicity contributes to endothelial dysfunction: a focus on the contribution from ceramide. Rev Endocr Metab Disord. 2013;14:59–68. doi: 10.1007/s11154-012-9235-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sturm W, Sandhofer A, Engl J, Laimer M, Molnar C, Kaser S, Weiss H, Tilg H, Ebenbichler CF, Patsch JR. Influence of Visceral Obesity and Liver Fat on Vascular Structure and Function in Obese Subjects. Obesity (Silver Spring) 2009 doi: 10.1038/oby.2009.81. [DOI] [PubMed] [Google Scholar]
- 8.Ceriello A, Esposito K, Piconi L, Ihnat MA, Thorpe JE, Testa R, Boemi M, Giugliano D. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes. 2008;57:1349–1354. doi: 10.2337/db08-0063. [DOI] [PubMed] [Google Scholar]
- 9.Eringa EC, Serne EH, Meijer RI, Schalkwijk CG, Houben AJ, Stehouwer CD, Smulders YM, van Hinsbergh VW. Endothelial dysfunction in (pre)diabetes: characteristics, causative mechanisms and pathogenic role in type 2 diabetes. Rev Endocr Metab Disord. 2013;14:39–48. doi: 10.1007/s11154-013-9239-7. [DOI] [PubMed] [Google Scholar]
- 10.Czernichow S, Greenfield JR, Galan P, Bastard JP, Charnaux N, Samaras K, Safar ME, Blacher J, Hercberg S, Levy BI. Microvascular dysfunction in healthy insulin-sensitive overweight individuals. J Hypertens. 2010;28:325–332. doi: 10.1097/HJH.0b013e328333d1fc. [DOI] [PubMed] [Google Scholar]
- 11.Goldfine AB, Beckman JA, Betensky RA, Devlin H, Hurley S, Varo N, Schonbeck U, Patti ME, Creager MA. Family history of diabetes is a major determinant of endothelial function. J Am Coll Cardiol. 2006;47:2456–2461. doi: 10.1016/j.jacc.2006.02.045. %20. [DOI] [PubMed] [Google Scholar]
- 12.Okon EB, Chung AW, Zhang H, Laher I, van Breemen C. Hyperglycemia and hyperlipidemia are associated with endothelial dysfunction during the development of type 2 diabetes. Can J Physiol Pharmacol. 2007;85:562–567. doi: 10.1139/y07-026. [DOI] [PubMed] [Google Scholar]
- 13.Schaefer C, Biermann T, Schroeder M, Fuhrhop I, Niemeier A, Ruther W, Algenstaedt P, Hansen-Algenstaedt N. Early microvascular complications of prediabetes in mice with impaired glucose tolerance and dyslipidemia. Acta Diabetol. 2009;47:19–27. doi: 10.1007/s00592-009-0114-7. [DOI] [PubMed] [Google Scholar]
- 14.Sandqvist M, Strindberg L, Schmelz M, Lonnroth P, Jansson PA. Impaired delivery of insulin to adipose tissue and skeletal muscle in obese women with postprandial hyperglycemia. J Clin Endocrinol Metab. 2011;96:E1320–E1324. doi: 10.1210/jc.2011-0233. [DOI] [PubMed] [Google Scholar]
- 15.Sjostrand M, Gudbjornsdottir S, Holmang A, Lonn L, Strindberg L, Lonnroth P. Delayed transcapillary transport of insulin to muscle interstitial fluid in obese subjects. Diabetes. 2002;51:2742–2748. doi: 10.2337/diabetes.51.9.2742. [DOI] [PubMed] [Google Scholar]
- 16.Kim SP, Catalano KJ, Hsu IR, Chiu JD, Richey JM, Bergman RN. Nocturnal free fatty acids are uniquely elevated in the longitudinal development of diet-induced insulin resistance and hyperinsulinemia. Am J Physiol Endocrinol Metab. 2007;292:E1590–E1598. doi: 10.1152/ajpendo.00669.2006. [DOI] [PubMed] [Google Scholar]
- 17.Ikomi F, Hunt J, Hanna G, Schmid-Schonbein GW. Interstitial fluid, plasma protein, colloid, and leukocyte uptake into initial lymphatics. J Appl Physiol (1985 ) 1996;81:2060–2067. doi: 10.1152/jappl.1996.81.5.2060. [DOI] [PubMed] [Google Scholar]
- 18.Bergman RN. Lilly lecture 1989. Toward physiological understanding of glucose tolerance. Minimal-model approach. Diabetes. 1989;38:1512–1527. doi: 10.2337/diab.38.12.1512. [DOI] [PubMed] [Google Scholar]
- 19.Barrett EJ, Eggleston EM, Inyard AC, Wang H, Li G, Chai W, Liu Z. The vascular actions of insulin control its delivery to muscle and regulate the rate-limiting step in skeletal muscle insulin action. Diabetologia. 2009;52:752–764. doi: 10.1007/s00125-009-1313-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kubota T, Kubota N, Kadowaki T. The role of endothelial insulin signaling in the regulation of glucose metabolism. Rev Endocr Metab Disord. 2013;14:207–216. doi: 10.1007/s11154-013-9242-z. [DOI] [PubMed] [Google Scholar]
- 21.Clark MG, Wallis MG, Barrett EJ, Vincent MA, Richards SM, Clerk LH, Rattigan S. Blood flow and muscle metabolism: a focus on insulin action. Am J Physiol Endocrinol Metab. 2003;284:E241–E258. doi: 10.1152/ajpendo.00408.2002. [DOI] [PubMed] [Google Scholar]
- 22.Rattigan S, Zhang L, Mahajan H, Kolka CM, Richards SM, Clark MG. Factors influencing the hemodynamic and metabolic effects of insulin in muscle. Curr Diabetes Rev. 2006;2:61–70. doi: 10.2174/157339906775473653. [DOI] [PubMed] [Google Scholar]
- 23.Villela NR, Kramer-Aguiar LG, Bottino DA, Wiernsperger N, Bouskela E. Metabolic disturbances linked to obesity: the role of impaired tissue perfusion. Arq Bras Endocrinol Metabol. 2009;53:238–245. doi: 10.1590/s0004-27302009000200015. [DOI] [PubMed] [Google Scholar]
- 24.Vincent MA, Barrett EJ, Lindner JR, Clark MG, Rattigan S. Inhibiting NOS blocks microvascular recruitment and blunts muscle glucose uptake in response to insulin. Am J Physiol Endocrinol Metab. 2003;285:E123–E129. doi: 10.1152/ajpendo.00021.2003. [DOI] [PubMed] [Google Scholar]
- 25.Mokshagundam SP, Peiris AN, Stagner JI, Gingerich RL, Samols E. Interstitial insulin during euglycemic-hyperinsulinemic clamp in obese and lean individuals. Metabolism. 1996;45:951–956. doi: 10.1016/s0026-0495(96)90261-9. [DOI] [PubMed] [Google Scholar]
- 26.Scalia R, Gong Y, Berzins B, Zhao LJ, Sharma K. Hyperglycemia is a major determinant of albumin permeability in diabetic microcirculation: the role of mu-calpain. Diabetes. 2007;56:1842–1849. doi: 10.2337/db06-1198. [DOI] [PubMed] [Google Scholar]
- 27.Yoon N, Dang TQ, Chasiotis H, Kelly SP, Sweeney G. Altered transendothelial transport of hormones as a contributor to diabetes. Diabetes Metab J. 2014;38:92–99. doi: 10.4093/dmj.2014.38.2.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kolka CM, Richey JM, Castro AV, Broussard JL, Ionut V, Bergman RN. Lipid- induced insulin resistance does not impair insulin access to skeletal muscle. Am J Physiol Endocrinol Metab. 2015;308:E1001–E1009. doi: 10.1152/ajpendo.00015.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Broussard JL, Nelson MD, Kolka CM, Bediako IA, Paszkiewicz RL, Smith L, Szczepaniak EW, Stefanovski D, Szczepaniak LS, Bergman RN. Rapid development of cardiac dysfunction in a canine model of insulin resistance and moderate obesity. Diabetologia. 2015 doi: 10.1007/s00125-015-3767-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wiig H, Swartz MA. Interstitial fluid and lymph formation and transport: physiological regulation and roles in inflammation and cancer. Physiol Rev. 2012;92:1005–1060. doi: 10.1152/physrev.00037.2011. [DOI] [PubMed] [Google Scholar]
- 31.Zawieja SD, Wang W, Wu X, Nepiyushchikh ZV, Zawieja DC, Muthuchamy M. Impairments in the intrinsic contractility of mesenteric collecting lymphatics in a rat model of metabolic syndrome. Am J Physiol Heart Circ Physiol. 2012;302:H643–H653. doi: 10.1152/ajpheart.00606.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yudkin JS. Inflammation, obesity, and the metabolic syndrome. Horm Metab Res. 2007;39:707–709. doi: 10.1055/s-2007-985898. [DOI] [PubMed] [Google Scholar]
- 33.Sharma A, Bernatchez PN, de Haan JB. Targeting endothelial dysfunction in vascular complications associated with diabetes. Int J Vasc Med. 2012;2012:750126. doi: 10.1155/2012/750126. Epub;%2011 Oct 13.:750126. [DOI] [PMC free article] [PubMed] [Google Scholar]