Abstract
Interspecies somatic cell nuclear transfer (iSCNT) can be a solution for preservation of endangered species that have limited oocytes. It has been reported that blastocyst production by iSCNT is successful even if the genetic distances between donors and recipients are large. In particular, domestic pig oocytes can support the development of canine to porcine iSCNT embryos. Therefore, we examined whether porcine oocytes may be suitable recipient oocytes for Korean raccoon dog iSCNT. We investigated the effects of trichostatin A (TSA) treatment on iSCNT embryo developmental patterns and nucleolus formation. Enucleated porcine oocytes were fused with raccoon dog fibroblasts by electrofusion and cleavage, and blastocyst development and nucleolus formation were evaluated. To our knowledge, this study is the first in which raccoon dog iSCNT was performed using porcine oocytes; we found that 68.5% of 158 iSCNT embryos had the ability to cleave. However, these iSCNT embryos did not develop past the 4-cell stage. Treatment with TSA did not affect iSCNT embryonic development; moreover, the nuclei failed to form nucleoli at 48 and 72 h post-activation (hpa). In contrast, pig SCNT embryos of the control group showed 18.8% and 87.9% nucleolus formation at 48 and 72 hpa, respectively. Our results demonstrated that porcine cytoplasts efficiently supported the development of raccoon dog iSCNT embryos to the 4-cell stage, the stage of porcine embryonic genome activation (EGA); however, these embryos failed to reach the blastocyst stage and showed defects in nucleolus formation.
Keywords: embryonic genome activation (EGA), Interspecies somatic cell nuclear transfer (iSCNT), Nucleoli, Pig, Raccoon dog
Interspecies somatic cell nuclear transfer (iSCNT), which involves the use of oocytes obtained from different species, has been attempted to produce cloned embryos of exotic or endangered species from which sufficient oocytes cannot be obtained for research [1]. Various live offspring have been obtained by combining closely related species, such as cattle/gaur (Bos taurus/B. grunensis) [2] and domestic sheep/european mouflon (Ovis aries/O. orientalis musimon) [3]. It has been shown that bovine, porcine, and rabbit oocytes can support the remodeling and reprogramming of somatic cells from different species, and that these cybrid embryos can develop to the blastocyst stage [4]. This information suggests that it is possible to derive ES cells from iSCNT embryos even if these cybrid embryos have large genetic distances between donor and recipient species; this would eventually contribute genetically to the next-generation species by chimera production or induction of germ cell differentiation in vitro.
The raccoon dog (Nyctereutes procyonoides) is a canid indigenous to East Asia. Native East Asian raccoon dog populations have declined in recent years due to hunting, fur trade, and destruction of habitats by urbanization. In Gyeonggi, a province in the Republic of Korea, more than 100 Korean raccoon dogs (N. procyonoides koreensis) are rescued each year; nevertheless, more than half of them die due to disease and injury. We attempted to produce cloned Korean raccoon dogs through SCNT for conservation and management of the species; however, it is difficult to obtain large numbers of high-quality recipient oocytes from wild Korean raccoon dogs. Although in vitro maturation (IVM) is considered as an alternative approach for oocyte production, this technique is still too rudimentary for the production of high-quality, uniform oocytes in large numbers [5, 6]. Hence, we decided to use oocytes from other species as recipient oocytes. Domestic pig oocytes have been used for iSCNT for animals such as tigers and sheep; these embryos successfully developed to the blastocyst stage [7, 8]. Moreover, the success of iSCNT has been observed in the blastocyst development of canine and porcine cybrid embryos [9]. Therefore, we hypothesized that porcine oocytes may be suitable recipient oocytes for Korean raccoon dog iSCNT.
In iSCNT embryos, nucleoli precursor bodies (NPBs) originate from the oocyte [10, 11], while most proteins engaged in the formation of mature nucleoli should be transcribed from genes de novo in the donor nucleus [12]; thus, species compatibility of recipient oocytes is required for the success of nucleolus formation. It was observed that successfully developed iSCNT embryos showed nucleolus formation, whereas unsuccessfully developed iSCNT embryos had no nucleolus formation. Indeed, formation of nucleoli is essential for iSCNT embryo development [13].
In the present study, we produced cloned N. procyonoides koreensis embryos by iSCNT using pig oocytes as recipient cytoplasts. We initially analyzed the development of iSCNT embryos in vitro. Then, we investigated the effects of trichostatin A (TSA) treatment on the developmental competence of the embryos. Finally, we assessed nucleolus formation in iSCNT embryos in comparison with normal porcine SCNT embryos.
Materials and Methods
All chemicals used in this study were purchased from Sigma Aldrich, St Louis, MO, USA, unless otherwise specified. This study was approved by the Committee on the Ethics of Animal Experiments (Chungbuk National University, Cheongju, Republic of Korea; permit number: CBNUA-584-13-01).
Ovary collection and IVM of porcine oocytes
Ovaries of prepubertal gilts were collected from a commercial abattoir. Porcine follicular fluid (pFF) and oocytes were aspirated from 3–6 mm follicles. Cumulus-oocyte complexes (COCs) were recovered under a stereomicroscope; those with at least three layers of compact cumulus cells and with homogenous cytoplasm were selected. The selected COCs were transferred to tissue culture medium 199 (Life Technologies, Rockville, MD, USA) supplemented with 26 mM sodium bicarbonate, 0.91 mM sodium pyruvate, 0.57 mM cysteine, 10 ng/ml epidermal growth factor, 0.5 IU/ml porcine luteinizing hormone, 0.5 IU/ml porcine follicle stimulating hormone, and 10% (v/v) pFF. COCs were cultured in a humidified atmosphere (39°C, 5% CO2). After 20–22 h of maturation with hormones, the oocytes were then washed and cultured for 20–22 h without hormone supplements.
Donor cell preparation
A 2-year-old male Korean raccoon dog was chosen as the donor animal. Fibroblasts were isolated from the ear skin. The seeded cells were cultured for 6–8 days with Dulbecco’s modified Eagle’s medium (DMEM) in a humidified atmosphere (39°C, 5% CO2). The attached cells were further cultured until confluence and subcultured at intervals of 5–7 days. The individual cells were retrieved from the monolayer by trypsinization for ~1 min and subsequently used for SCNT. Korean raccoon dog fibroblasts used as donor cells were analyzed for their chromosomal ploidy to verify that they had normal diploid (2n = 54 + Bs) chromosomes [14]. The karyotyping test was performed by Samkwang Medical Laboratories (Seoul, Korea); the test on the donor cells revealed normal diploid chromosomes (Fig. 1). Porcine fibroblasts were used as controls. Porcine fibroblasts were grown at 37°C in an atmosphere of 5% CO2 in DMEM containing 4.5 g/l glucose (Gibco BRL, Grand Island, NY, USA) and supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin, and 100 µg/ml streptomycin.
Fig. 1.
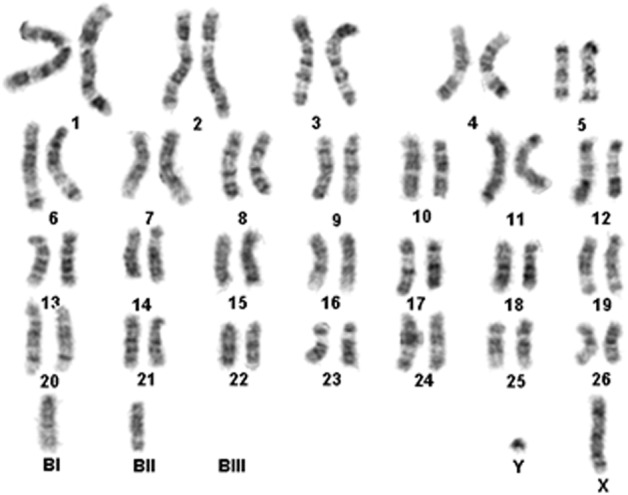
Karyotyping of raccoon dog fibroblasts used as donor cells to produce raccoon dog-porcine interspecies somatic cell nuclear transfer (iSCNT) embryos. The karyotype analysis revealed a set of normal diploid chromosomes (2n = 54 + Bs).
Micromanipulation for SCNT, fusion, and activation
After 40 h of IVM, denuded oocytes were incubated for 5 min in a manipulation medium (calcium-free TLH-BSA) containing 5 mg/ml Hoechst 33342. Oocytes were enucleated by aspirating the PB and MII chromosomes using a 16 μm glass pipette (Humagen, Charlottesville, VA). After enucleation, 12–15 μm donor cells with a smooth membrane surface were transferred into the perivitelline space of enucleated oocytes using a fine injecting pipette. The couplets were equilibrated with 0.28 M mannitol solution containing 0.5 mM HEPES, 0.1 mM CaCl2, and MgSO4 for 2–3 min and transferred to a fusion chamber containing two electrodes overlaid with mannitol solution. Membrane fusion was induced by applying an alternating current (AC) field of 2 V cycling at 1 MHz for 2 sec, followed by two pulses of 160 V/mm direct current (DC) for 60 µsec using a cell fusion generator (LF101; NepaGene, Chiba, Japan). Activated oocytes were washed 3–4 times with TLH-BSA. After 1 h, fused oocytes were cultured with PZM3 medium in an atmosphere of 5% O2, 5% CO2, and 90% N2 at 39°C. Cleavage and blastocyst formation were evaluated under a stereomicroscope at 48 and 168 h post-activation (hpa), respectively.
Immunocytochemistry
At 48 and 72 hpa, SCNT and iSCNT embryos were fixed in 4% paraformaldehyde in PBS with 0.1% Triton X-100 at 4°C for 1 h and subsequently stained overnight with antibodies against nucleolin (C23, an indirect marker of RNA polymerase I activity; Santa Cruz Biotechnology, Santa Cruz, CA, USA, sc-13057). Embryos were washed in PBS/BSA and incubated with Fluorescein isothiocyanate (FITC)-conjugated secondary antibodies (Santa Cruz Biotechnology) at room temperature for 1 h. After incubation, embryos were then washed in PBS/BSA. Nuclei were counterstained with 5 μg/ml Hoechst 33342 for 10 min. Stained oocytes and embryos were mounted under a cover slip and examined using a fluorescence microscope.
Experiments
We evaluated the in vitro developmental competence of Korean raccoon dog iSCNT embryos generated using porcine oocytes as recipients. The embryos generated using fibroblasts from raccoon dog ear cells and porcine fibroblasts (passages three to seven) were cultured in PZM3 medium. Porcine SCNT embryos were subsequently used as quality and handling controls. The cleavage rate and blastocyst formation were analyzed.
The effects of TSA treatment on the in vitro developmental competence of Korean raccoon dog iSCNT embryos were investigated. iSCNT embryos and donor cells were treated with 5 nM TSA for 10 h. TSA treatment methods were based on those used by Yamanaka [15]. The rates of cleavage and in vitro development were calculated.
We evaluated the ability of porcine oocytes to support nucleolus formation in Korean raccoon dog iSCNT embryos. At 48 and 72 hpa, embryos that had developed to the four- to eight-cell stages were selected for evaluation of nucleolus formation. The formation of nucleoli was confirmed using the nucleolin (C23) immunocytochemistry method as described above.
Statistical analysis
Statistical analysis was conducted using the SPSS Inc. software (PASW Statistics 17). Embryo development was assessed by t-test and one-way analysis of variance with Duncan’s multiple-range test. All data are presented as mean ± SEM. Statistical differences at P < 0.05 were considered significant.
Results
The proportions of fused and cleaved oocytes were not significantly different between porcine SCNT embryos and Korean raccoon dog iSCNT embryos (Table 1). Blastomere number and blastocyst formation differed significantly. The percentage of eight-blastomere embryos was significantly reduced in raccoon dog iSCNT embryos (7.3%) compared with that in porcine SCNT embryos (23.8%). Some eight-blastomere raccoon dog iSCNT embryos did not have the same number of nuclei as blastomere cells; these embryos only had four nuclei, as shown by Hoechst 33342 staining (Fig. 2, Supplementary movies 1 and 2: online only).
Raccoon dog iSCNT embryos did not develop past the four-cell stage. Blastocysts formed only in porcine SCNT embryos.
Table 1. The in vitro development of raccoon dog-porcine iSCNT embryos.
| Embryo type | No. of used oocytes † |
No. of fused embryos |
No. (%) of embryos (48 hpa) at the stage of
|
No. (%) of cleaved embryos (48 hpa) |
No. (%) of blastocyst formation (168 hpa) |
|||||
| 1-cell | 2-cell | 4-cell | 6-cell | 8-cell | frag | |||||
| Porcine-porcine | 190 | 150 | 13 | 6 | 34 | 20 | 37 | 40 | 97 | 15 |
| (78.6 ± 2.9) | (8.0 ± 3.8) | (4.2 ± 1.4) | (23.6 ± 5.1) | (13.6 ± 1.7) | (23.8 ± 5.1) | (26.8 ± 2.2) | (65.2 ± 3.0) | (10.2 ± 1.6) | ||
| Raccoon dog-porcine | 204 | 158 | 15 | 23 | 55 | 20 | 11 | 34 | 109 | 0 |
| (77.6 ± 4.1) | (9.7 ± 1.7) | (14.4 ± 3.7) | (34.1 ± 5.8) | (12.7 ± 0.5) | (7.3 ± 2.8)* | (21.8 ± 2.0) | (68.5 ± 3.7) | (0.0)* | ||
† Three replicates. * indicate significant differences (P < 0.05). frag: Fragmented embryos that contain unequal sized blastomeres and multiple cellular fragments.
Fig. 2.
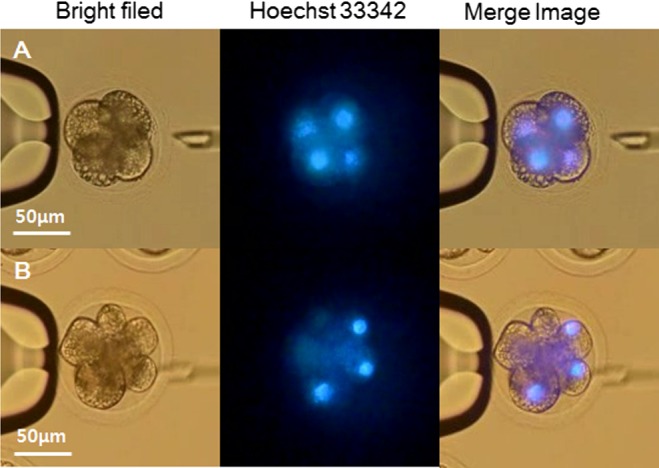
Photomicrographic images of cloned raccoon dog-porcine interspecies somatic cell nuclear transfer (iSCNT) embryos of (A) four-blastomere and (B) eight-blastomere embryos. (A) Four-blastomere raccoon dog iSCNT embryos have the same number of nuclei as blastomere cells. (B) However, some eight-blastomere raccoon dog iSCNT embryos did not have nuclei.
The effect of TSA treatment on the developmental competence of Korean raccoon dog iSCNT embryos was investigated (Table 2). Treatment with TSA did not have any effect on donor cells or during in vitro culture (IVC). Cleavage patterns and cleavage rates were not significantly different. Blastocysts were not formed in any of the raccoon dog iSCNT embryo groups.
Table 2. Effects of TSA treatment on in vitro development of raccoon dog-porcine iSCNT embryos.
| No. of cultured embryos* |
TSA (5 nM) treatment condition |
No. (%) of embryos (48 hpa) at the stage of
|
No. (%) of cleaved embryos (48 hpa) |
|||||
| 1-cell | 2-cell | 4-cell | 6-cell | 8-cell | frag | |||
| 104 | None | 12 | 17 | 35 | 11 | 8 | 21 | 71 |
| (11.5 ± 1.6) | (16.6 ± 2.7) | (33.4 ± 4.2) | (10.4 ± 3.9) | (7.8 ± 1.3) | (20.3 ± 1.0) | (68.2 ± 1.9) | ||
| 97 | IVC media | 13 | 12 | 34 | 10 | 6 | 22 | 62 |
| (13.5 ± 2.9) | (12.7 ± 4.2) | (35.0 ± 2.2) | (10.1 ± 2.0) | (6.3 ± 0.2) | (22.5 ± 1.0) | (64.0 ± 3.2) | ||
| 163 | Donor cell | 29 | 14 | 40 | 17 | 23 | 40 | 94 |
| (17.9 ± 2.8) | (8.8 ± 3.7) | (24.3 ± 6.7) | (10.4 ± 0.2) | (14.3 ± 3.2) | (24.2 ± 5.4) | (57.8 ± 6.5) | ||
* Three replicates. There were no significant differences in proportion for each stage and overall percentage of cleavage between all three groups: none (control), IVC (in vitro culture) media, and donor cell. frag: Fragmented embryos that contain unequal sized blastomeres and multiple cellular fragments.
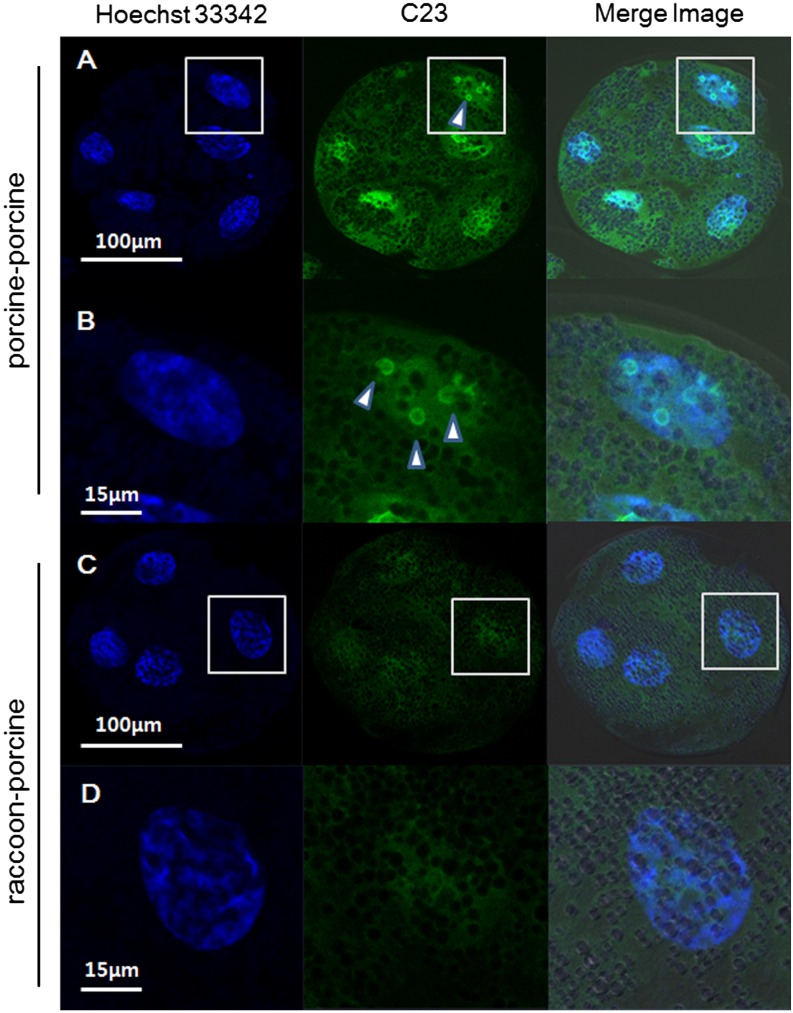
In this experiment, anti-nucleolin (C23) antibody labeling of nucleolin permitted visualization of active nucleoli in porcine and Korean raccoon dog fibroblasts (Fig. 3). At 48 hpa, nucleoplasmic nucleolin labeling was concentrated around presumptive NPBs in the form of shell-like structures in 18.8% of porcine SCNT embryos (Table 3), whereas raccoon dog iSCNT embryos failed to form nucleoli. During subsequent culture (72 h), the intensity of nucleolin staining of shell-like structures increased to 87.9% in porcine SCNT embryos (Fig. 4, Table 3), whereas nucleoli remained absent in raccoon dog iSCNT embryos.
Fig. 3.
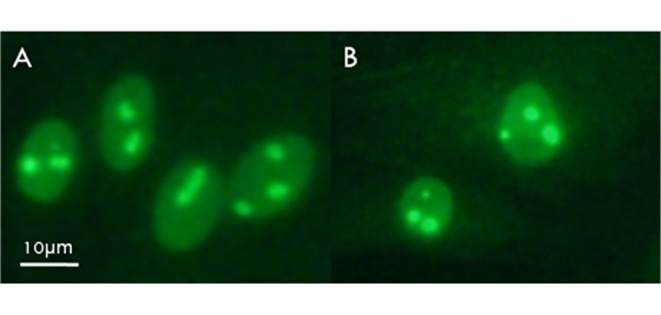
Anti-C23 (nucleolin) labeling of nucleoli in (A) porcine and (B) raccoon dog fibroblasts.
Table 3. Nucleolus formation in porcine somatic cell nuclear transfer embryo (SCNT) and raccoon dog-porcine interspecies somatic cell nuclear transfer (iSCNT) embryos.
| Embryo type | No. of cultured embryos* |
No. (%) of cleaved embryos |
No. of embryos with nucleoli/No. of embryos
analyzed |
||
| 48 h | 72 h | Total | |||
| Porcine-porcine | 101 | 65 (64.4) | 6/32 | 29/33 | 35/65 |
| Raccoon dog-porcine | 102 | 65 (63.7) | 0/31 | 0/34 | 0/65 |
* Three replicates.
Fig. 4.
Nucleolin (C23) in blastomeres of a porcine somatic cell nuclear transfer (SCNT) embryo and a raccoon dog-porcine interspecies somatic cell nuclear transfer (iSCNT) embryo. Nucleolin protein (arrowheads) was immunostained with anti-nucleolin (C23) (green) and DNA was counterstained with Hoechst (blue). A and B: porcine SCNT embryo, magnification × 200 and × 600. C and D: raccoon dog-porcine iSCNT embryo, magnification × 200 and × 600.
Discussion
Recently, the rescue of endangered animals through SCNT has drawn considerable attention. Since the oocytes of endangered species are difficult to obtain, iSCNT methods have been attempted. In 2000, Lanza et al. reported the successful production of a cloned gaur by iSCNT; many other researchers have also reported the potential of iSCNT cloning [4]. Based on this concept, we performed Korean raccoon dog iSCNT using porcine oocytes. Raccoon dog cloning by iSCNT has not been previously reported. We investigated the possibility of using porcine oocytes as recipients in raccoon dog iSCNT with reference to previous studies of canine iSCNT [9, 16].
Raccoon dog-porcine iSCNT embryos and control porcine SCNT embryos were cleaved at similar rates (68.5% and 65.2%, respectively). Around 10% of control SCNT embryos had the ability to develop to the blastocyst stage, whereas blastocyst formation was not observed in iSCNT embryos. Certain iSCNT embryos seemed to cleave more than 4 blastomeres, but Hoechst 33342 staining revealed that some of the blastomeres did not enclose a nucleus. These results indicated that porcine oocytes could induce nuclear remodeling and reprogramming of Korean raccoon dog somatic cells; however, porcine oocyte support for nuclear remodeling and reprogramming was imperfect and led to arrest at the four-cell stage.
We investigated the potential of TSA treatment to improve incomplete nuclear remodeling and reprogramming processes in raccoon dog iSCNT embryos. These processes include chromatin remodeling, DNA methylation, and histone acetylation [17]. A low level of acetylated histone due to abnormal nucleus reprogramming is frequently observed in cloned embryos [18]. Hyperacetylation of histones increases the access of some transcription factors to nucleosomes [19, 20]; thus, increasing the histone acetylation level improves developmental competence in SCNT embryos. Histone deacetylase inhibitors such as TSA, scriptaid, and sodium butyrate can increase the histone acetylation level [21]. Furthermore, TSA treatment significantly improves developmental competence in porcine SCNT embryos [15] and canine-porcine iSCNT embryos [9]. However, TSA treatment did not affect the development of raccoon dog iSCNT embryos, as demonstrated by cleavage rate, cleavage pattern, and in vitro development. We suggest that factors other than histone acetylation caused the developmental arrest of raccoon dog iSCNT embryos.
It has been reported that, in some iSCNT embryos, nucleoli were not formed and development was arrested depending on the species of the cell donor and oocyte [13]. We examined the formation of nucleoli in raccoon dog iSCNT embryos by anti-nucleolin (C23) antibody labeling. This method can detect nucleolin in many mammalian species [12]. Nucleolin is a maternally inherited multifunctional nucleolar protein that has been implicated in chromatin structure, rDNA transcription, rRNA maturation, early stages of ribosome assembly, and nucleocytoplasmic transport [10, 12]. It is present primarily in nucleoli with active RNA polymerase I. All these attributes make nucleolin an ideal marker of nucleoli [13].
In the present study, nucleolin-labeled nucleoli were detected in porcine SCNT embryos, but they were not detected in raccoon dog iSCNT embryos. The inability of nucleoli to form in raccoon dog iSCNT embryos may be due to structural differences of promoter selectivity factors that play an important role in the formation of the RNA polymerase I complex on the promoter. These promoter selectivity factors are shown to be species specific [22]. In addition, demethylation capacity of ooplasm is a factor that can lead to absence of nucleoli in raccoon dog iSCNT embryos. Demethylation capacity of ooplasm is different between each species [23,24,25]. The demethylation of repetitive sequences of the donor genome is determined by recipient ooplasm and not by intrinsic properties of the donor nucleus [24, 25]. Ooplasms with different demethylation capacities have different abilities in supporting the initiation of nucleolus formation in various iSCNT embryos [13].
In zygotes and early embryos, nucleoli are maternally inherited and originate from the oocyte germinal vesicle. When oocytes are enucleated at the germinal vesicle stage, activated embryos do not develop. Furthermore, nucleoli originating from donor cell nuclei are not able to substitute maternal nucleoli and to support the development of nuclear transfer embryos derived from enucleated oocytes. Indeed, maternal nucleoli are essential for successful early embryonic development [26]. The nucleoli in developing embryos could also be formed by de novo synthesized materials [27]. Nucleoli are closely related to embryonic genome activation (EGA). Nucleolus formation in IVF bovine and porcine embryos occurs at the time of EGA [13].
After fertilization, embryos drive their development in the initial cycles using accumulated molecules originating from oocytes, which are gradually replaced by molecules of embryonic origin. These changes occur through changes in chromatin structure and chromatin protein content, particularly histone proteins [28]; these changes are collectively known as EGA, which includes RNA polymerase I activation, nucleolus formation, and activation of genes engaged in morula and blastocyst formation. The formation of nucleoli indicates at least partial EGA; thus, raccoon dog-porcine iSCNT embryos might fail to reach EGA. The suitability of nucleoli originating from oocytes with xeno-nuclei is an important factor in iSCNT embryonic development.
In summary, we produced raccoon-dog iSCNT embryos using porcine oocytes. The porcine oocytes partly supported the remodeling and reprogramming of raccoon-dog somatic cell nuclei, but did not fully support the development of embryos to the blastocyst stage. Moreover, the materials from porcine cytoplasts did not support EGA, leading to failure of raccoon dog nucleolus formation and subsequent developmental failure of raccoon dog iSCNT embryos.
Acknowledgments
This work was supported, in part, by a grant from the “Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ011077, PJ011288), Rural Development Administration” and “Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through the Advanced Production Technology Development Program, funded by the Ministry of Agriculture, Food and Rural Affairs (MAFRA) (Grant number: 115103-02)”, Republic of Korea.
References
- 1.Lee E, Bhuiyan MM, Watanabe H, Matsuoka K, Fujise Y, Ishikawa H, Fukui Y. Production of cloned sei whale (Balaenoptera borealis) embryos by interspecies somatic cell nuclear transfer using enucleated pig oocytes. J Vet Sci 2009; 10: 285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lanza RP, Cibelli JB, Diaz F, Moraes CT, Farin PW, Farin CE, Hammer CJ, West MD, Damiani P. Cloning of an endangered species (Bos gaurus) using interspecies nuclear transfer. Cloning 2000; 2: 79–90. [DOI] [PubMed] [Google Scholar]
- 3.Loi P, Ptak G, Barboni B, Fulka J, Jr, Cappai P, Clinton M. Genetic rescue of an endangered mammal by cross-species nuclear transfer using post-mortem somatic cells. Nat Biotechnol 2001; 19: 962–964. [DOI] [PubMed] [Google Scholar]
- 4.Beyhan Z, Iager AE, Cibelli JB. Interspecies nuclear transfer: implications for embryonic stem cell biology. Cell Stem Cell 2007; 1: 502–512. [DOI] [PubMed] [Google Scholar]
- 5.Farstad W. Assisted reproductive technology in canid species. Theriogenology 2000; 53: 175–186. [DOI] [PubMed] [Google Scholar]
- 6.Saikhun J, Sriussadaporn S, Thongtip N, Pinyopummin A, Kitiyanant Y. Nuclear maturation and development of IVM/IVF canine embryos in synthetic oviductal fluid or in co-culture with buffalo rat liver cells. Theriogenology 2008; 69: 1104–1110. [DOI] [PubMed] [Google Scholar]
- 7.Hashem MA, Bhandari DP, Kang SK, Lee BC. Cell cycle analysis and interspecies nuclear transfer of in vitro cultured skin fibroblasts of the Siberian tiger (Panthera tigris Altaica). Mol Reprod Dev 2007; 74: 403–411. [DOI] [PubMed] [Google Scholar]
- 8.Uhm SJ, Gupta MK, Kim T, Lee HT. Expression of enhanced green fluorescent protein in porcine- and bovine-cloned embryos following interspecies somatic cell nuclear transfer of fibroblasts transfected by retrovirus vector. Mol Reprod Dev 2007; 74: 1538–1547. [DOI] [PubMed] [Google Scholar]
- 9.Sugimura S, Narita K, Yamashiro H, Sugawara A, Shoji T, Terashita Y, Nishimori K, Konno T, Yoshida M, Sato E. Interspecies somatic cell nucleus transfer with porcine oocytes as recipients: A novel bioassay system for assessing the competence of canine somatic cells to develop into embryos. Theriogenology 2009; 72: 549–559. [DOI] [PubMed] [Google Scholar]
- 10.Rickards B, Flint SJ, Cole MD, LeRoy G. Nucleolin is required for RNA polymerase I transcription in vivo. Mol Cell Biol 2007; 27: 937–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Svarcova O, Laurincik J, Avery B, Mlyncek M, Niemann H, Maddox-Hyttel P. Nucleolar development and allocation of key nucleolar proteins require de novo transcription in bovine embryos. Mol Reprod Dev 2007; 74: 1428–1435. [DOI] [PubMed] [Google Scholar]
- 12.Ginisty H, Sicard H, Roger B, Bouvet P. Structure and functions of nucleolin. J Cell Sci 1999; 112: 761–772. [DOI] [PubMed] [Google Scholar]
- 13.Lagutina I, Zakhartchenko V, Fulka H, Colleoni S, Wolf E, Fulka J, Jr, Lazzari G, Galli C. Formation of nucleoli in interspecies nuclear transfer embryos derived from bovine, porcine, and rabbit oocytes and nuclear donor cells of various species. Reproduction 2011; 141: 453–465. [DOI] [PubMed] [Google Scholar]
- 14.Wada MY, Lim Y, Wurster-Hill DH. Banded karyotype of a wild-caught male Korean raccoon dog, Nyctereutes procyonoides koreensis. Genome 1991; 34: 302–306. [Google Scholar]
- 15.Yamanaka K, Sugimura S, Wakai T, Kawahara M, Sato E. Acetylation level of histone H3 in early embryonic stages affects subsequent development of miniature pig somatic cell nuclear transfer embryos. J Reprod Dev 2009; 55: 638–644. [DOI] [PubMed] [Google Scholar]
- 16.Lee E, Kim JH, Park SM, Jeong YI, Lee JY, Park SW, Choi J, Kim HS, Jeong YW, Kim S, Hyun SH, Hwang WS. The analysis of chromatin remodeling and the staining for DNA methylation and histone acetylation do not provide definitive indicators of the developmental ability of inter-species cloned embryos. Anim Reprod Sci 2008; 105: 438–450. [DOI] [PubMed] [Google Scholar]
- 17.Latham KE. Early and delayed aspects of nuclear reprogramming during cloning. Biol Cell 2005; 97: 119–132. [DOI] [PubMed] [Google Scholar]
- 18.Wee G, Koo DB, Song BS, Kim JS, Kang MJ, Moon SJ, Kang YK, Lee KK, Han YM. Inheritable histone H4 acetylation of somatic chromatins in cloned embryos. J Biol Chem 2006; 281: 6048–6057. [DOI] [PubMed] [Google Scholar]
- 19.Lee DY, Hayes JJ, Pruss D, Wolffe AP. A positive role for histone acetylation in transcription factor access to nucleosomal DNA. Cell 1993; 72: 73–84. [DOI] [PubMed] [Google Scholar]
- 20.Li E. Chromatin modification and epigenetic reprogramming in mammalian development. Nat Rev Genet 2002; 3: 662–673. [DOI] [PubMed] [Google Scholar]
- 21.Enright BP, Kubota C, Yang X, Tian XC. Epigenetic characteristics and development of embryos cloned from donor cells treated by trichostatin A or 5-aza-2′-deoxycytidine. Biol Reprod 2003; 69: 896–901. [DOI] [PubMed] [Google Scholar]
- 22.Heix J, Zomerdijk JC, Ravanpay A, Tjian R, Grummt I. Cloning of murine RNA polymerase I-specific TAF factors: conserved interactions between the subunits of the species-specific transcription initiation factor TIF-IB/SL1. Proc Natl Acad Sci USA 1997; 94: 1733–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beaujean N, Taylor JE, McGarry M, Gardner JO, Wilmut I, Loi P, Ptak G, Galli C, Lazzari G, Bird A, Young LE, Meehan RR. The effect of interspecific oocytes on demethylation of sperm DNA. Proc Natl Acad Sci USA 2004; 101: 7636–7640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen T, Zhang YL, Jiang Y, Liu JH, Schatten H, Chen DY, Sun QY. Interspecies nuclear transfer reveals that demethylation of specific repetitive sequences is determined by recipient ooplasm but not by donor intrinsic property in cloned embryos. Mol Reprod Dev 2006; 73: 313–317. [DOI] [PubMed] [Google Scholar]
- 25.Chen T, Zhang YL, Jiang Y, Liu SZ, Schatten H, Chen DY, Sun QY. The DNA methylation events in normal and cloned rabbit embryos. FEBS Lett 2004; 578: 69–72. [DOI] [PubMed] [Google Scholar]
- 26.Ogushi S, Palmieri C, Fulka H, Saitou M, Miyano T, Fulka J., Jr The maternal nucleolus is essential for early embryonic development in mammals. Science 2008; 319: 613–616. [DOI] [PubMed] [Google Scholar]
- 27.Kyogoku H, Fulka J, Jr, Wakayama T, Miyano T. De novo formation of nucleoli in developing mouse embryos originating from enucleolated zygotes. Development 2014; 141: 2255–2259. [DOI] [PubMed] [Google Scholar]
- 28.Latham KE, Schultz RM. Embryonic genome activation. Front Biosci 2001; 6: D748–D759. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.