Abstract
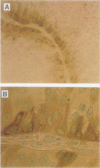
AIMS: To evaluate the expression of bcl-2 in transitional cell carcinoma (TCC) of the bladder; to compare bcl-2 expression with clinicopathological findings, p53 immunoreactivity, proliferating cell nuclear antigen (PCNA) expression, 2c deviation index (2cDI), 5c exceeding rate (5cER), and the mean nuclear area (MNA). METHODS: Cystectomy specimens from 77 patients with untreated, non-metastatic TCC of the bladder were studied. Expression of bcl-2, p53 and PCNA was detected immunohistochemically using the following monoclonal antibodies: bcl-2/124, DO-7 and PC10, respectively. Nuclear DNA content was analysed using static cytometry. RESULTS: Bcl-2 was expressed in 19 (24.7%) of 77 TCCs and in 74 (96.1%) of 77 normal samples of transitional epithelium (taken from normal tissue adjacent to the tumour in each case). In all cases, bcl-2 immunoreactivity was more intense in normal transitional epithelium than in TCC. In normal transitional epitehlium and superficial TCC bcl-2 immunoreactivity was observed at the basal layer, and not at the invasive front. Bcl-2 immunoreactivity was invesely correlated with histological grade and p53 immunoreactivity, and was not correlated with the pT category, disease progression, PCNA expression, 2cDI, 5cER, and the MNA. No significant correlation was found between bcl-2 expression and overall survival. CONCLUSIONS: Bcl-2 expression in TCC of the bladder seems to be associated with a less aggressive phenotype and does not play an important role in tumour progression.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auffermann W., Urquardt M., Rübben H., Wohltmann D., Böcking A. DNA grading of urothelial carcinoma of the bladder. Anticancer Res. 1986 Jan-Feb;6(1):27–32. [PubMed] [Google Scholar]
- Ben-Ezra J. M., Kornstein M. J., Grimes M. M., Krystal G. Small cell carcinomas of the lung express the Bcl-2 protein. Am J Pathol. 1994 Nov;145(5):1036–1040. [PMC free article] [PubMed] [Google Scholar]
- Bhargava V., Kell D. L., van de Rijn M., Warnke R. A. Bcl-2 immunoreactivity in breast carcinoma correlates with hormone receptor positivity. Am J Pathol. 1994 Sep;145(3):535–540. [PMC free article] [PubMed] [Google Scholar]
- Böcking A., Adler C. P., Common H. H., Hilgarth M., Granzen B., Auffermann W. Algorithm for a DNA-cytophotometric diagnosis and grading of malignancy. Anal Quant Cytol. 1984 Mar;6(1):1–8. [PubMed] [Google Scholar]
- Doglioni C., Dei Tos A. P., Laurino L., Chiarelli C., Barbareschi M., Viale G. The prevalence of BCL-2 immunoreactivity in breast carcinomas and its clinicopathological correlates, with particular reference to oestrogen receptor status. Virchows Arch. 1994;424(1):47–51. doi: 10.1007/BF00197392. [DOI] [PubMed] [Google Scholar]
- Finlay C. A., Hinds P. W., Levine A. J. The p53 proto-oncogene can act as a suppressor of transformation. Cell. 1989 Jun 30;57(7):1083–1093. doi: 10.1016/0092-8674(89)90045-7. [DOI] [PubMed] [Google Scholar]
- Fontanini G., Vignati S., Bigini D., Mussi A., Lucchi M., Angeletti C. A., Basolo F., Bevilacqua G. Bcl-2 protein: a prognostic factor inversely correlated to p53 in non-small-cell lung cancer. Br J Cancer. 1995 May;71(5):1003–1007. doi: 10.1038/bjc.1995.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemstreet G. P., 3rd, Rollins S., Jones P., Rao J. Y., Hurst R. E., Bonner R. B., Hewett T., Smith B. G. Identification of a high risk subgroup of grade 1 transitional cell carcinoma using image analysis based deoxyribonucleic acid ploidy analysis of tumor tissue. J Urol. 1991 Dec;146(6):1525–1529. doi: 10.1016/s0022-5347(17)38157-0. [DOI] [PubMed] [Google Scholar]
- Hockenbery D. M., Zutter M., Hickey W., Nahm M., Korsmeyer S. J. BCL2 protein is topographically restricted in tissues characterized by apoptotic cell death. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):6961–6965. doi: 10.1073/pnas.88.16.6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockenbery D., Nuñez G., Milliman C., Schreiber R. D., Korsmeyer S. J. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990 Nov 22;348(6299):334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- Hollstein M., Sidransky D., Vogelstein B., Harris C. C. p53 mutations in human cancers. Science. 1991 Jul 5;253(5015):49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- Korsmeyer S. J. Bcl-2 initiates a new category of oncogenes: regulators of cell death. Blood. 1992 Aug 15;80(4):879–886. [PubMed] [Google Scholar]
- Lane D. P. Cancer. p53, guardian of the genome. Nature. 1992 Jul 2;358(6381):15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- Levine A. J., Momand J., Finlay C. A. The p53 tumour suppressor gene. Nature. 1991 Jun 6;351(6326):453–456. doi: 10.1038/351453a0. [DOI] [PubMed] [Google Scholar]
- Levine A. J. The p53 tumor-suppressor gene. N Engl J Med. 1992 May 14;326(20):1350–1352. doi: 10.1056/NEJM199205143262008. [DOI] [PubMed] [Google Scholar]
- Lipponen P. K., Aaltomaa S. Apoptosis in bladder cancer as related to standard prognostic factors and prognosis. J Pathol. 1994 Aug;173(4):333–339. doi: 10.1002/path.1711730408. [DOI] [PubMed] [Google Scholar]
- Lipponen P. K., Eskelinen M. J., Jauhiainen K., Harju E., Terho R., Haapasalo H. Independent clinical, histological and quantitative prognostic factors in transitional-cell bladder tumours, with special reference to mitotic frequency. Int J Cancer. 1992 May 28;51(3):396–403. doi: 10.1002/ijc.2910510311. [DOI] [PubMed] [Google Scholar]
- Lipponen P. K., Nordling S., Eskelinen M. J., Jauhiainen K., Terho R., Harju E. Flow cytometry in comparison with mitotic index in predicting disease outcome in transitional-cell bladder cancer. Int J Cancer. 1993 Jan 2;53(1):42–47. doi: 10.1002/ijc.2910530109. [DOI] [PubMed] [Google Scholar]
- Lipponen P. K. Over-expression of p53 nuclear oncoprotein in transitional-cell bladder cancer and its prognostic value. Int J Cancer. 1993 Feb 1;53(3):365–370. doi: 10.1002/ijc.2910530304. [DOI] [PubMed] [Google Scholar]
- McDonnell T. J., Troncoso P., Brisbay S. M., Logothetis C., Chung L. W., Hsieh J. T., Tu S. M., Campbell M. L. Expression of the protooncogene bcl-2 in the prostate and its association with emergence of androgen-independent prostate cancer. Cancer Res. 1992 Dec 15;52(24):6940–6944. [PubMed] [Google Scholar]
- O'Connor P. M., Jackman J., Jondle D., Bhatia K., Magrath I., Kohn K. W. Role of the p53 tumor suppressor gene in cell cycle arrest and radiosensitivity of Burkitt's lymphoma cell lines. Cancer Res. 1993 Oct 15;53(20):4776–4780. [PubMed] [Google Scholar]
- Pilotti S., Collini P., Rilke F., Cattoretti G., Del Bo R., Pierotti M. A. Bcl-2 protein expression in carcinomas originating from the follicular epithelium of the thyroid gland. J Pathol. 1994 Apr;172(4):337–342. doi: 10.1002/path.1711720408. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y., Croce C. M. Analysis of the structure, transcripts, and protein products of bcl-2, the gene involved in human follicular lymphoma. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5214–5218. doi: 10.1073/pnas.83.14.5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waseem N. H., Lane D. P. Monoclonal antibody analysis of the proliferating cell nuclear antigen (PCNA). Structural conservation and the detection of a nucleolar form. J Cell Sci. 1990 May;96(Pt 1):121–129. doi: 10.1242/jcs.96.1.121. [DOI] [PubMed] [Google Scholar]
- van den Oord J. J., Vandeghinste N., De Ley M., De Wolf-Peeters C. Bcl-2 expression in human melanocytes and melanocytic tumors. Am J Pathol. 1994 Aug;145(2):294–300. [PMC free article] [PubMed] [Google Scholar]