Abstract
Proline-rich tyrosine kinase 2 (Pyk2), a non-receptor tyrosine kinase, is a member of the focal adhesion kinase family and is highly expressed in oocytes. Using a combination of confocal microscopy and RNAi, we localized and studied the function of both Pyk2 and tyrosine-phosphorylated Pyk2 (p-Pyk2) during mouse oocyte fertilization and early embryo development. At the onset of fertilization, Pyk2 and p-Pyk2 were detected predominantly in sperm heads and the oocyte cytoplasm. Upon formation of male and female pronuclei, Pyk2 and its activated form leave the cytoplasm and accumulate in the two pronuclei. We detected Pyk2 in blastomere nuclei and found both Pyk2 and p-Pyk2 in the pre-blastula cytoplasm. Pyk2 and its activated form then disappeared from the blastula nuclei and localized to the perinuclear regions, where blastula cells come into contact with each other. Pyk2 knockdown via microinjection of siRNA into the zygote did not inhibit early embryo development. Our results suggest that Pyk2 plays multiple functional roles in mouse oocyte fertilization as well as throughout early embryo development.
Keywords: Early-embryo development, Fertilization, Mouse oocyte, Pyk2
Proline-rich tyrosine kinase 2 (Pyk2) is a non-receptor tyrosine kinase belonging to the family of focal adhesion kinases (FAK) and is also known as cell adhesion kinase β (CAKβ), related adhesion focal tyrosine kinase (RAFTK), calcium-dependent tyrosine kinase (CADTK) and FAK2 [1,2,3,4]. Pyk2 contains a large N-terminal 4.1, ezrin, radixin, moesin (FERM) domain, a centrally located kinase domain, and a focal adhesion-targeting C-terminal domain. Pyk2 is activated through a range of stimuli, including growth factors, cytokines, integrin ligation, G-coupled receptor agonists, depolarization, UV irradiation, changes in osmolarity, stress-related signals, and increases in intracellular calcium concentration. During Pyk2 activation, the initial auto-phosphorylation of tyrosine 402 (Y402) occurs in response to cell surface stimuli and the resulting phosphorylated residue provides a docking site for the SH2 domains of c-Src and Fyn [1, 5]. Src activation causes Pyk2 phosphorylation at several other tyrosine residues, including tyrosines 579, 580, and 881 (Y579, Y580 and Y881). These phosphorylation events are essential for Pyk2 to achieve full kinase activity and to transduce signals to downstream effectors [6, 7]. Pyk2 has been shown to participate in the regulation of numerous downstream signal transducers, including the Src family tyrosine kinases [8], Rho GTPases [9], mitogen-activated protein kinase (MAPK) cascades [10, 11], PI3k/Akt [12, 13], and components of the NF-κB [14] pathways. Thus, Pyk2 is regarded as a convergence point for numerous central signaling pathways and has roles in cell adhesion, proliferation, and survival [8, 15]. In addition, Pyk2 regulates functions associated with the cytoskeleton, such as cell morphology, spreading, motility, and polarity. This is achieved either via binding to proteins that interact with the cytoskeleton, such as paxillin, p130cas, and the Arp2/3 complex, or via promoting Rho family signaling activity [15].
Although their amino acid sequences share about 65% identity, Pyk2 and FAK are found in different intracellular locations and show different cell and tissue expression. FAK is uniformly expressed throughout the body and in almost all cell types, while Pyk2 has a more restricted tissue expression pattern, with neuronal and hematopoietic tissues, fibroblasts, and epithelial and endothelial cells being the primary locations. However, oocytes express high levels of both Pyk2 and FAK [16]. Pyk2 expression has been reported in the oocytes of zebrafish [17], rat [18], and mouse [19]. In zebrafish oocytes, Pyk2 is activated in response to calcium transients. This activation is critical for sperm incorporation, as it promotes reorganization of cortical microfilaments, and is thus crucial for the formation of the fertilization cone [17]. Pyk2 co-localizes with microfilaments during several developmental stages of rat oocyte maturation, suggesting Pyk2 has an essential role in regulating actin filament organization [18]. Recently, Luo et al. reported Pyk2 activation during the period of anaphase resumption after fertilization. Furthermore, the study used Pyk2 suppression with a highly specific inhibitor, reducing the ability of mouse oocytes to incorporate sperm and proceed into anaphase [20]. These data suggest the involvement of Pyk2 in the both oocyte maturation and fertilization.
In this report, antibodies against Pyk2 and tyrosine 402-phosphorylated Pyk2 (p-Pyk2) were used to determine both the distribution and activation patterns of Pyk2 during mouse oocyte fertilization and early embryo development. Both Pyk2 and p-Pyk2 exhibited complex dynamic changes throughout the sperm head, the male and female pronucleus, the cytoplasm, the cell junctions, and the perinuclear region. However, a Pyk2 knockdown via injection of small-interfering RNA into the zygote did not inhibit early embryo development.
Materials and Methods
Reagents and antibodies
Media (M2, M16, and KSOM) were purchased from Sigma Chemical Company (St. Louis, MO). siRNA duplex oligoribonucleotides targeting the coding region of Pyk2 (GenBank Accession no: NM_001162365.1) and non-silencing siRNA were obtained from Invitrogen (Carlsbad, CA, USA). Polyclonal rabbit anti-mouse Pyk2 antibodies were purchased from Abcam (Cambidge, England). Polyclonal anti-phosphorylated Tyr402 Pyk2 antibodies were obtained from Sigma (St. Louis, MO) and Abcam. Mito-Tracker Green was obtained from Beyotime Biotechnology (NanTong, China). Pregnant mare serum gonadotropin (PMSG) and human chronic gonadotropin (hCG) were purchased from Sansheng Company (Ningbo, China). Unless otherwise mentioned, all other chemicals used in this study were purchased from Sigma Chemical Company.
Oocyte and embryo collection
We conducted all experiments using protocols proved by and in accordance with the Animal Research Committee Guidelines of the Shandong Normal University. 10 IU PMSG was injected intraperitoneally into Kunming female mice aged 4 to 6 weeks (purchased from Shandong University experimental animal center, Jinan, China). 48 h later, 10 IU hCG was injected using the same procedure. After hCG injection, mice were killed and oviducts were removed at 14 to 16 h. The cumulus-oocyte complexes were collected from the oviducts into M2 medium, supplemented with 60 μg/ml penicillin, and 50 μg/ml streptomycin. To remove all cumulus cells, MII-arrested oocytes were briefly exposed to 300 IU/ml hyaluronidase followed by three subsequent washes in M2 medium.
In order to collect in vivo fertilized eggs and embryos, females were superovulated with PMSG and mated with Kunming mature male mice following hCG injection. 19 h after mating, fertilized eggs were obtained from oviducts. Embryos were collected at the following stages of development: time post-hCG: 22 h: zygote; 40 h: 2-cell; 60 h: 4-cell; 68 h: 8-cell; 80 h: morula; 96 h: blastocyst. Early-stage embryos were released from the oviduct and blastocysts were obtained from the uterus. Fertilized eggs were used for siRNA microinjection, while embryos were used for confocal microscopy.
In vitro fertilization
In vitro fertilization was conducted using 1 × 106/ml motile cauda epididymal sperm that was previously capacitated for 1 h with 2.5 mM taurine in M16 medium. We used ZP-free and MII-arrested oocytes to achieve a more synchronous time of fertilization. Oocytes were inseminated in a 50 μl drop of M16 medium at 37ºC and 5% CO2. The eggs were collected 0.5, 1, 1.5, 2, 4, and 6 h, respectively, after insemination and prepared for confocal microscopy.
Microinjection of Pyk2 siRNA
In order to investigate the role Pyk2 plays during the development of preimplantation embryos, small strings of siRNA were microinjected into the cytoplasm of the fertilized eggs (obtained as described above). A minimum of three repetitions was conducted per experiment, and 50 to 80 eggs were used per experimental group. To minimize damage to the eggs, the diameter of the microinjection needle was smaller than 1 μm. Throughout all experiments, we used a microinjection volume of about 10 pl diluted siRNA (10 μM) per egg. The same amount of non-silencing siRNA was injected as control. Microinjections were performed using an Olympus IX71 (Olympus, Tokyo, Japan) inverted microscope equipped with Narishige IM-9C hydraulic three-dimensional micromanipulators (Narishige, Tokyo, Japan). All microinjections were completed within 30 min. After microinjection, fertilized eggs of the RNAi treatment group and those of the control group were thoroughly washed and cultured in KSOM under mineral oil at 37ºC and with 5% CO2. Subsequently, some of the injected eggs were collected after 19 h for RNA extraction, reverse transcription, and real-time PCR to measure interference efficiency. Others were cultured for observation of embryo development. The Pyk2 siRNA sequences were as follows: (1) sense: UCUCCAGCACUCCGAUGACAUCCU, antisense: AGGAUGUCAUCGGAGUGCUGGAGA; (2) sense: UCUGAGGCAGGCUGUUCCUCUUCU, antisense: AGAAGAGGAACAGCCUGCCUCAGA; (3) sense: UUUCGUUCCAGGUAGUGUCCCAGU, antisense: AGCUGGGACACUACCUGGAACGAA. Non-silencing siRNA nucleotides (sense: GAACUCCAACUCUCCUCCCCGAGC, antisense: CUAAGAAACAAGACGGGGAGCCUC) were used as negative control. According to our results (data not shown), no.2 siRNA oligoribonucleotides were chosen for the interference experiments in the following sections.
RNA extraction, reverse transcription, and real-time PCR
Total RNA was extracted from 40 zygotes or embryos using an RNeasy micro purification kit (Qiagen, Düsseldorf, Germany). The first strand cDNA was synthesized with the first strand cDNA synthesis kit RevertAid™ (Fermentas, LTU) using oligo (dT) primers. We conducted real-time PCR analysis via SYBR® Green Realtime PCR Master Mix (TOYOBO, Osaka, Japan) using an iQ5 detection system (Bio-Rad, Hercules, CA, USA). Β-Actin mRNA levels in the same samples were used as an internal control. PCR primer sequences were:
Pyk2-forward: 5’-ATATTGGAGCCTACTACCTTTCAGG-3’,
Pyk2-reverse: 5’-CATAGGGCTGGTAAGCGTAGGA-3’;
β-actin-forward: 5’-CAGGTCATCACTATTGGCAACGAG-3’,
β-actin-reverse: 5’-GATGCCACAGGATTCCATACCC-3’.
The steps were as follows: 95˚C for 2 min, 42 cycles of 95˚C for 20 sec, 58˚C for 20 sec, and 72˚C for 30 sec. Relative gene expression was calculated using the 2-ΔΔCt method.
Western blot analysis
150 MII-stage oocytes were lysed in RIPA buffer and heated for 5min at 100°C. Protein separation was conducted via SDS-PAGE and proteins were transferred onto PVDF membranes (0.2 μm) in transfer buffer containing 0.1% SDS. After blocking in 5% nonfat dried milk TBST for 2 h at room temperature, the membranes were incubated overnight with a rabbit polyclonal anti-Pyk2 antibody (1:1000 diluted in the blocking buffer) and at a temperature of 4°C. The membranes were then incubated for 2 h with peroxidase-conjugated goat anti-rabbit IgG (1:5000) at room temperature. As the final step, the membranes were processed using the enhanced chemiluminescence detection system.
Confocal microscopy
Both the fertilized eggs and embryos at the required stage were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS). The PBS was supplemented with 100 μM sodium ortho-vanadate to inhibit phosphatase activity for 30 min and permeabilized for 30 min in the incubation buffer (0.5% TritonX-100 in 20 mM Hepes, pH 7.4, 3 mM MgCl2, 50 mM NaCl, 300 mM sucrose, 0.02% NaN3). The blastocysts were fixed and permeabilized for 40 min. After permeabilization, all eggs and embryos were washed three times in PBS with 0.1% Tween 20 and blocked in 1% BSA-supplemented PBS at room temperature. The samples were then incubated with either polyclonal rabbit anti-mouse Pyk2 antibodies or anti-Tyr402 phospho-Pyk2 antibodies diluted 1:200 at room temperature for 1 h. The eggs and embryos were rinsed three times and incubated for 1 h with 1:200 fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit IgG (Zhongshan, Peking, China), followed by staining with 10 μg/ml propidium iodide. Finally, the samples were mounted on glass slides and evaluated using a TCSSP8 DLS laser scanning confocal microscope (Leica Microsystems, Wetzlar, Germany). For the negative control, we replaced the first antibody with rabbit IgG.
To study mitochondria distribution in early embryos, 8-cell stage embryos were collected from the oviducts of mated female mice and cultured for 30 min in KSOM with 100 nM Mito-Tracker Green at 37ºC and 5% CO2. The embryos were then washed thoroughly with fresh KSOM and mounted on glass slides for further investigation, as described above.
Statistical analysis
Pyk2 mRNA expression in 2-cell and 4-cell stage embryos was determined relative to that in the 1-cell stage embryos. Statistical significance was analyzed via multiple comparisons. We calculated the development rates of embryos at 2-, 4-, and 8-cell stages after injection of Pyk2 siRNA or control siRNA and analyzed the data using independent sample T-test. P < 0.05 was considered to indicate a statistically significant result. The data are given as mean ± SD. All statistical analysis was performed using SPSS 16.0.
Results
Pyk2 expression in MII oocytes and early embryonic stages
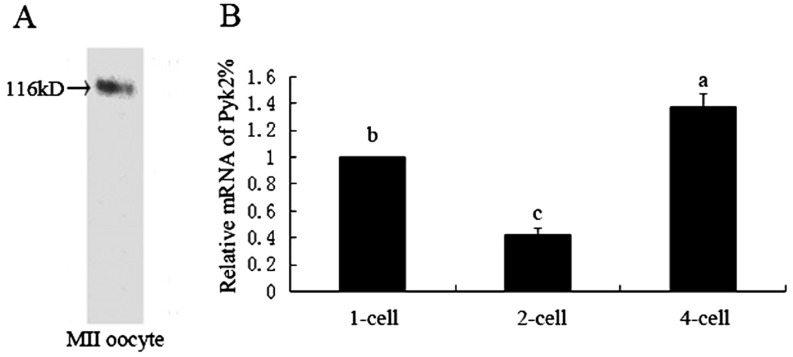
To examine whether Pyk2 was expressed in mouse oocytes, MII oocytes were subjected to western blot analysis. As shown in Fig. 1-A, we found a single band at 116 kDa as predicted in the MII oocyte sample. We used real-time PCR analysis to investigate the relative mRNA levels in embryos at the 1-, 2-, and 4-cell stages. As shown in Fig. 1-B, Pyk2 mRNA expression levels were lower in 2-cell embryos but higher in 4-cell embryos compared with that in the zygotes.
Fig. 1.
Detection of Pyk2 expression in oocytes and early embryos of mice. A: The sample (200 MII stage oocytes) was analyzed by western blot and probed with a Pyk2-specific antibody. A single band at about 116 kDa was detected. B: Pyk2 mRNA expression in 1-, 2-, and 4-cell stages was examined by real-time PCR. The expression rates were normalized to those in the 1-cell stage. Statistical analysis was performed using multiple comparison. Different letters in the columns represent significant difference.
Subcellular localization of Pyk2 and p-Pyk2 during mouse oocyte fertilization
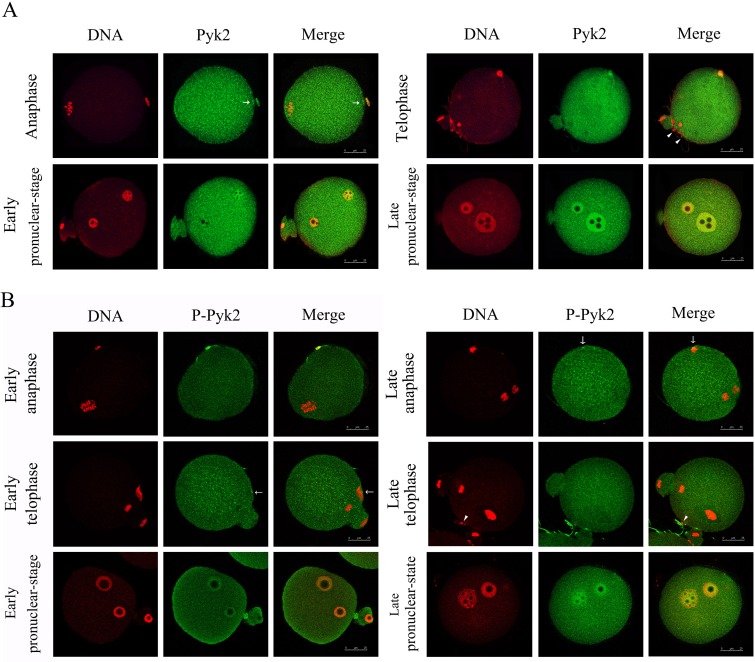
To understand the distribution and activation patterns of Pyk2 during fertilization, we examined the subcellular localization of Pyk2 during in-vitro fertilization using either polyclonal anti-Pyk2 or anti-tyrosine402-phosphorylated Pyk2 antibodies. As shown in Fig. 2-A, when sperm attachment triggers the separation of sister chromatids, Pyk2 is distributed throughout the cytoplasm with one signal in the head of the sperm and the other signal in the oocyte cortex close to the sperm head (see arrows). During telophase, a distinct Pyk2 signal was detected in both the sperm head and the cytoplasm. No Pyk2 signal was found in those the sperm heads that were attached to the oocyte, but did not fertilize it (indicated with arrowheads in Fig. 2-A; telophase). After sperm chromosome deconcentration, Pyk2 was prominently found in the male and female pronuclei with the exception of its uniform cytoplasm localization (Fig. 2-A; early and late pronucleus stage).
Fig. 2.
Localization of Pyk2 and p-Pyk2 during fertilization. In vitro fertilized eggs at different stages of development were collected and fixed for immunofluorescence analysis. To make the DNA visible and to confirm the stages of the fertilization process, the specimens were stained with propidium iodide. Laser-scanning confocal microscopy was used to locate Pyk2 (A, green), p-Pyk2 (B, green), and DNA (PI, red). A: Pyk2 (green) distribution in fertilized eggs at anaphase, telophase, early pronuclear stage, and late pronuclear stage. B: Distribution of Tyr402 p-Pyk2 during fertilization of early and late anaphase, telophase, and pronuclear stage. Bar, 25 μm. All experiments were repeated at least three times and representative images are shown.
As shown in Fig. 2-B, shortly after fertilization, the well-aligned chromosomes were separated, and active Pyk2 was found in distinct areas in both the sperm head and the cytoplasm (Fig. 2-B; early anaphase). At the late anaphase stage, when the sperms were incorporated into the oocyte, the activated Pyk2 signal disappeared from the sperm head and appeared in the cytoplasm. In addition, we also observed p-Pyk2 in the cortex adjacent to the sperm in half-fertilized oocytes (50%, 9/18). In the early and late telophase, when the second polar bodies were extruded, phosphorylated Pyk2 was distributed evenly throughout the cytoplasm. In the early telophase, we detected activated Pyk2 in the cortex close to the sperm in minority zygotes (29.6%, 8/27). Matching the result for Pyk2, p-Pyk2 was not detected in the non-incorporated sperm head that remained attached to the oocyte (indicated by the arrowhead, Fig. 2-B; late telophase). At the early pronuclear stage, accumulation of activated Pyk2 was detected in the newly-formed female and male pronuclei. The fluorescence from the pronuclei became more intense during the late pronuclear stage.
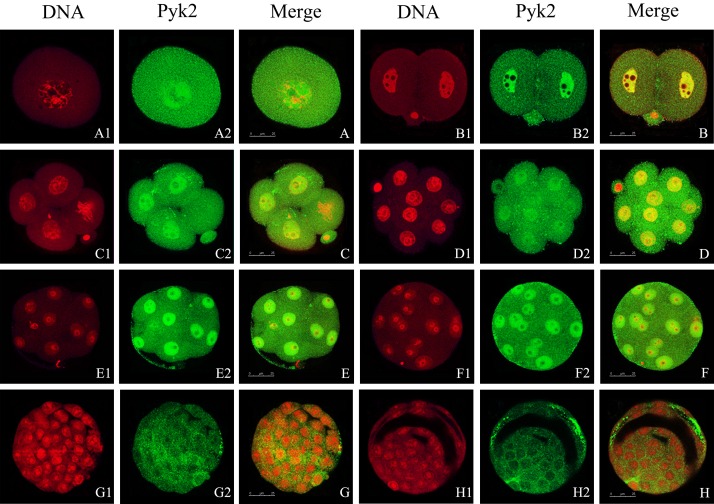
Expression and localization of Pyk2 during early embryo development
When the male and female pronuclei fuse, meiosis is complete and zygotes reach the stage prior to mitosis. We found Pyk2 in the nucleus and cytoplasm (Fig. 3). We observed the same distribution pattern in cleavage-stage embryos, up until the morulastage (Fig. 3-B–F). In the 2-cell stage, Pyk2 signals were also detected at the cell junctions (Fig. 3-B). In the blastocyst, Pyk2 was no longer detected in the cell nuclei and was instead distributed throughout the cytoplasm (Fig. 3-G2, -G, -H2 and -H). With the expansion of blastocoele, the Pyk2 fluorescence signal started to appear in the gaps of the trophoblast cells (Fig. 3-H2, -H). During early embryonic development, aggregation of Pyk2 in the cell nucleus is a common feature of embryos at different stages. However, when the blastomere enters the mitotic stage, Pyk2 localized uniformly throughout the cytoplasm (Fig. 3-C, -C1, and -C2). In addition, Pyk2 detected in the cytoplasm of the early embryonic cleavage cells was not evenly distributed, but aggregated as many tiny spots (Fig. 3-A2–H2).
Fig. 3.
Location of Pyk2 during early embryo development. Embryos at different stages were collected and stained with antibodies against total Pyk2. DNA was stained by PI. Laser scanning confocal microscopy was used to locate Pyk2 (green) and DNA (red). A, A1, and A2: zygote; B, B1, and B2: 2-cell embryo; C, C1, and C2: 4-cell embryo; D, D1, and D2: 8-cell embryo; E, E1, E2, F, F1, and F2: morula; G, G1, and G2: early blastula; H, H1, and H2: expanded blastula. Scale bar, 25 μm. At least 30 embryos per stage were scanned and representative images are shown.
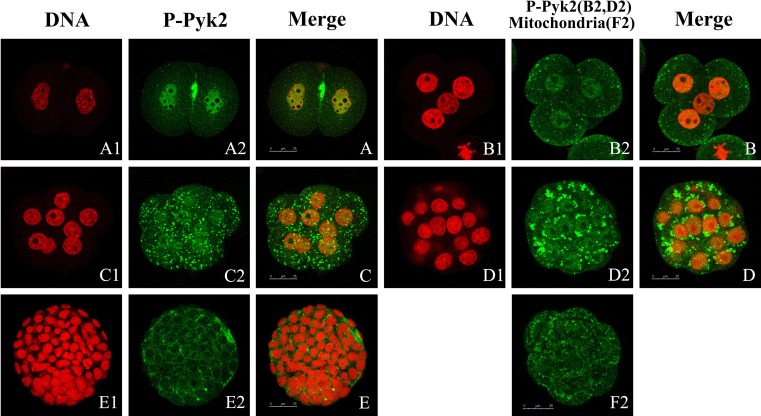
In the 2-cell stage, we found p-Pyk2 particularly in the joints of the two cells and the nuclei, but not in the nucleoli, which did not stain (Fig. 4-A2, -A). During the cleavage stage, the fluorescence signals in the nuclei increasingly weakened until the morula stage (Fig. 4-B, -C, and -D). Pyk2 was also present throughout the cytoplasm (Fig. 4-B, -C) and in the perinuclear region (Fig. 4-D), where the fluorescence appeared to have a punctuate distribution, presented as many points or peaches (Fig. 4-B2, -C2 and -D2). In the blastula stage, p-Pyk2 disappeared from the nuclei and appeared in the perinuclear or in the joint of the cells (Fig. 4-E2, -E). In this experiment, the localization of mitochondria in early mouse embryos was achieved via Mito Tracker Green. As shown in Fig. 4-F2, we detected strong immunoreactivity in the cytoplasm, especially in the perinuclear region with a typical punctuate pattern characteristic for p-Pyk2 distribution.
Fig. 4.
Tyr402 phosphorylation Pyk2 during embryonic development. Embryos at the 2- (A1, A2, and A), 4- (B1, B2, and B), and 8-cell (C1, C2, and C) stage; morula (D1, D2, and D); and blastula (E1, E2, and E) were collected and stained using specific antibody against phosphorylated tyrosine402 Pyk2. Primary antibodies were detected via FITC-labeled secondary antibodies. DNA was stained with PI. Laser scanning confocal microscopy was used to locate Pyk2 (green) and DNA (red). Distribution of mitochondria (green) was analyzed by laser scanning confocal microscopy (F2). Scale bar, 25 μm. At least 30 embryos were scanned perstage and representative images are shown.
Functional requirement of Pyk2 in early embryo development
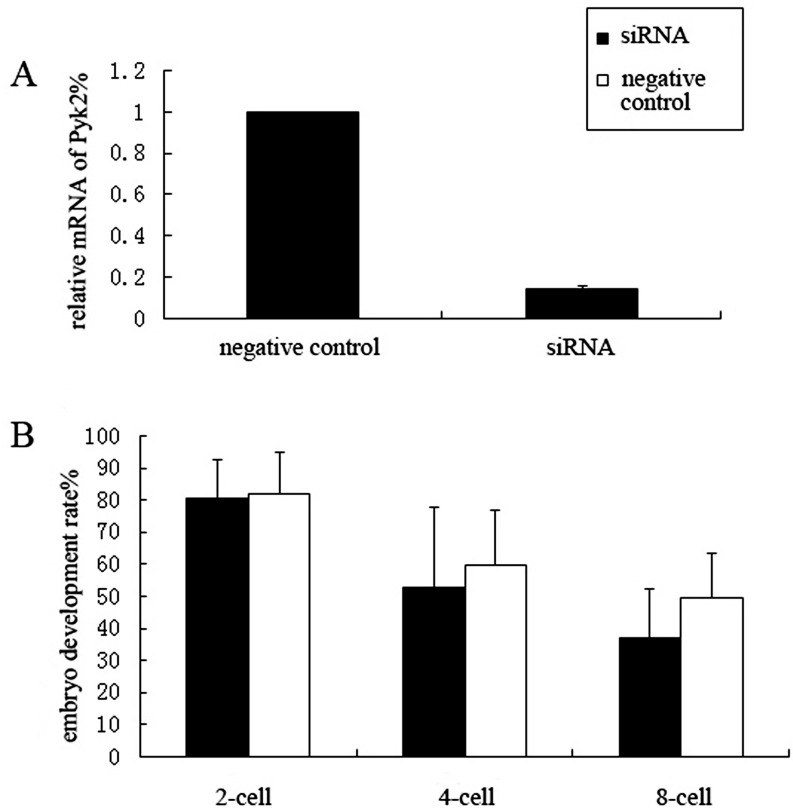
To determine whether Pyk2 plays an important role in the development of early mouse embryos, we microinjected siRNA into the cytoplasm of fertilized mouse eggs and observed the subsequent embryo development of Pyk2 knockdown zygotes. We first verified the knockdown efficiency. As shown in Fig. 5-A, interference led to a Pyk2 mRNA expression of 18.9% in siRNA-injected zygotes relative to that in the negative control zygotes. After microinjection, the zygotes were cultured in KSOM and embryo development at the 2-, 4-, and 8-cell stages were observed and recorded. The 2-cell rates of both Pyk2 siRNA and negative siRNA injected group were 77.34% (215/278) and 80.45% (214/266), respectively; the 4-cell rate was 51.67% (62/120) and 58.97% (46/78), respectively. These data show no difference between the Pyk2 siRNA group and the negative siRNA group in both 2-cell and 4-cell development. The 8-cell rate decreased after Pyk2 siRNA microinjection (37.86%, 39/103) compared to that of negative siRNA microinjection (50.00%, 39/78); however the difference between these data was non-significant (P = 0.086, Fig. 5-B).
Fig. 5.
Effects of Pyk2 RNAi on early embryonic development of mice. Pyk2 siRNA and negative control siRNA was microinjected into fertilized eggs. A: interference efficiency was determined by RT-qPCR. Pyk2 mRNA levels in zygotes after RNAi was 18.9% relative to the negative control. B: Embryonic development after Pyk2 RNAi. The 2-, 4-, and 8-cell rates determined using an independent sample T-test; no significant difference was found between the siRNA and the negative control groups.
Discussion
Fertilization triggers egg activation, transforming the quiescent egg into a zygote capable of undergoing the complex cell division cycle and differentiation to ultimately form an embryo. The numerous control mechanisms involved in this activation process and early embryo development include a series of protein kinase cascades required for successful development. In this report, we examined the expression patterns and localization of Pyk2 (a non-receptor tyrosine kinase of the FAK family) in fertilized eggs and preimplantation embryos. Our results reflect that Pyk2 may play a vital role during mammalian oocyte fertilization and early embryo development.
We determined the expression of Pyk2 in mouse MII oocytes and the Pyk2 mRNA level in early developmental embryos using a combination of westernblot and real-time PCR analysis. We expressed Pyk2 in mouse MII oocytes. Pyk2 expression has previously been reported in mouse, rat, and zebrafish oocytes, and it functions to either organize or reorganize the actin cytoskeleton and participates in the process of oocyte maturation and fertilization [17,18,19]. The Pyk2 mRNA levels in 2-cell embryos were significantly lower than that in the zygotes, which might be due to maternal gene degradation in the 2-cell stage. The increased expression of Pyk2 mRNA in 4-cell embryos detected in our experiment might be a result of zygotic gene expression. This expression pattern shows that Pyk2 is a maternal gene and that it is expressed at a higher level in the early embryos after the activation of zygotic genes.
Studies using a variety of model systems have found tyrosine kinase to play a critical role during fertilization. Src family kinases (SFKs) are activated quickly after fertilization in species with external fertilization, such as marine invertebrates, fish, and frogs. These kinases trigger the sperm-induced calcium oscillations that initiate the egg activation process [21,22,23,24]. In mammals, however, SFKs are not required for these calcium oscillations, which are triggered by a sperm-borne phospholipase [25]. In this experiment, we examined the location of both Pyk2 and tyrosine 402 phosphorylated Pyk2 during fertilization. Immunofluorescence analysis revealed Pyk2 accumulation in the sperm head and the oocyte cytoplasm shortly after fertilization. We detected the earliest Pyk2 or p-Pyk2 signals when the sister chromatids were just separated and the sperm was attached to but not incorporated into the oocyte. We detected no Pyk2 signal in the unfertilized sperm head that was bound to the surface of the oocyte, so we deduced that the accumulated Pyk2 in the sperm head originated from the ooplasm at the moment when the sperm head fused with the oocyte membrane. Luo et al. [20] recently detected Pyk2 activation shortly after fertilization of mouse oocytes and artificial inhibition of the Pyk2 activity blocked the incorporation of sperm and the activity of the oocyte. Their analysis in combination with our findings implies that Pyk2 and its activity are important for the incorporation of the sperm into the mouse oocyte. In a subset of the fertilized eggs, we located Pyk2 in the actin filament-enriched cortex above the sperm. In zebrafish, Pyk2 regulates sperm incorporation via organization of the fertilization cone microfilament [17]. Hence, we speculate that in mouse oocytes, Pyk2 plays a role in activating the cortex actin filaments that incorporate the sperm into oocyte. After successful incorporation of the sperm into the oocyte, activated Pyk2 was no longer co-localized with the sperm head. The total Pyk2 staining was still observed in the sperm head and the early female pronucleus. Following pronucleus formation, Pyk2 was gradually activated in the pronucleus. These results showed that the inactivated Pyk2 might participate in the de-condensation of accumulated chromosomes, while pronucleus formation is required for Pyk2 activation.
Localization of Pyk2 in cells was complicated. The kinase was detected in the perinuclear region and cytoplasm in addition to the cell-to-cell border [26]. Pyk2 has been detected with the podosomes of osteoclasts and macrophages [27, 28]. A point mutant of Pyk2, P859A (which had lost one of its PXXP motifs) was exclusively localized in the nucleus of many cell types. Notably, overexpressed wild-type Pyk2 also accumulates in the nucleus of cells treated with a nuclear protein export inhibitor [29]. These observations indicate that Pyk2 shuttles between the cytoplasm and the nucleus. Pyk2 has also been detected in the nucleus of human epidermal keratinocytes and chicken epiphyseal chondrocytes [30, 31]. Depolarization induced Pyk2 translocation to the nucleus of neurons and PC12 cells [32]. These findings suggest a function of Pyk2 in the cell nucleus. In this study, Pyk2 was mainly accumulated in the nucleus of the blastomeres before the blastula stage. p-Pyk2 immunofluorescence tended to be weaker in the nuclei after the 2-cell stage. Furthermore, Pyk2 exited the nucleus in the blastula instead of localizing to the cytoplasm. Our data suggests that inactivated Pyk2 plays a role in the blastomere nuclei during the cleavage process. Studies from embryonic, somatic, and tumor cells indicate both nuclear FAK and Pyk2-promoted cell proliferation and survival through enhanced p53 ubiquitination and degradation. Degradation of p53 occurs in a kinase-independent manner when the FERM domain acts as a scaffold to enhance Mdm2-dependent p53 ubiquitination [33, 34]. The early embryo undergoes rapid cell division that divides the cytoplasm of the fertilized egg into numerous cells until the blastula stage. These cells build the foundation for further development through gastrulation and organ formation. Thus, Pyk2 might participate in regulating early embryo cell proliferation and survival through a kinase-independent and p53-associated pathway. The export of Pyk2 from the nuclei might be a necessity for further development. In this study, we found strong immunoreactivity of p-Pyk2 in the cytoplasm. This immunoreactivity had a punctuate pattern and was found especially the perinuclear region. The distribution pattern was similar to the location of mitochondria. p-Pyk2 co-localized with the mitochondria in chicken epiphyseal chondrocytes [31]. Whether p-Pyk2 is essential for mitochondrial function in mouse embryo development needs further investigation. In the blastocyst stage, Pyk2 and p-Pyk2 were detected in the perinuclear region or cell borders. It is possible that Pyk2 regulates the formation of cell junctions and/or signal transduction in mouse blastocysts.
Knocking down Pyk2 via siRNA injection into zygotes did not inhibit the development of the 8-cell stage. Pyk2-/- mice are fertile and do not show grossanatomical abnormalities compared with wild-type littermates [9]. This could reflect the possibility of one or more alternative molecules to compensate the function of Pyk2 during early embryo development. FAK may be a candidate molecule due to its homologous structure and similar localization during early embryo development (data not shown). In conclusion, Pyk2 is both expressed and activated during mouse oocyte fertilization and early embryo development. Pyk2 distribution changes dynamically during this process. However, in vitro knockdown of Pyk2 via siRNA injection did not inhibit further development beyond the 8-cell stage. We therefore conclude that Pyk2 in combination with other molecules play a key role in mouse oocyte fertilization and early embryo development.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (NSFC, number: 31101034). We thank Dr Dong Cheng and Huilyu for providing technical guidance.
References
- 1.Lev S, Moreno H, Martinez R, Canoll P, Peles E, Musacchio JM, Plowman GD, Rudy B, Schlessinger J. Protein tyrosine kinase PYK2 involved in Ca(2+)-induced regulation of ion channel and MAP kinase functions. Nature 1995; 376: 737–745. [DOI] [PubMed] [Google Scholar]
- 2.Avraham S, London R, Fu Y, Ota S, Hiregowdara D, Li J, Jiang S, Pasztor LM, White RA, Groopman JE, et al. Identification and characterization of a novel related adhesion focal tyrosine kinase (RAFTK) from megakaryocytes and brain. J Biol Chem 1995; 270: 27742–27751. [DOI] [PubMed] [Google Scholar]
- 3.Sasaki H, Nagura K, Ishino M, Tobioka H, Kotani K, Sasaki T. Cloning and characterization of cell adhesion kinase beta, a novel protein-tyrosine kinase of the focal adhesion kinase subfamily. J Biol Chem 1995; 270: 21206–21219. [DOI] [PubMed] [Google Scholar]
- 4.Yu H, Li X, Marchetto GS, Dy R, Hunter D, Calvo B, Dawson TL, Wilm M, Anderegg RJ, Graves LM, Earp HS. Activation of a novel calcium-dependent protein-tyrosine kinase. Correlation with c-Jun N-terminal kinase but not mitogen-activated protein kinase activation. J Biol Chem 1996; 271: 29993–29998. [DOI] [PubMed] [Google Scholar]
- 5.Dikic I, Tokiwa G, Lev S, Courtneidge SA, Schlessinger J. A role for Pyk2 and Src in linking G-protein-coupled receptors with MAP kinase activation. Nature 1996; 383: 547–550. [DOI] [PubMed] [Google Scholar]
- 6.Wu SS, Jácamo RO, Vong SK, Rozengurt E. Differential regulation of Pyk2 phosphorylation at Tyr-402 and Tyr-580 in intestinal epithelial cells: roles of calcium, Src, Rho kinase, and the cytoskeleton. Cell Signal 2006; 18: 1932–1940. [DOI] [PubMed] [Google Scholar]
- 7.Kohno T, Matsuda E, Sasaki H, Sasaki T. Protein-tyrosine kinase CAKbeta/PYK2 is activated by binding Ca2+/calmodulin to FERM F2 α2 helix and thus forming its dimer. Biochem J 2008; 410: 513–523. [DOI] [PubMed] [Google Scholar]
- 8.Avraham H, Park SY, Schinkmann K, Avraham S. RAFTK/Pyk2-mediated cellular signalling. Cell Signal 2000; 12: 123–133. [DOI] [PubMed] [Google Scholar]
- 9.Okigaki M, Davis C, Falasca M, Harroch S, Felsenfeld DP, Sheetz MP, Schlessinger J. Pyk2 regulates multiple signaling events crucial for macrophage morphology and migration. Proc Natl Acad Sci USA 2003; 100: 10740–10745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blaukat A, Ivankovic-Dikic I, Grönroos E, Dolfi F, Tokiwa G, Vuori K, Dikic I. Adaptor proteins Grb2 and Crk couple Pyk2 with activation of specific mitogen-activated protein kinase cascades. J Biol Chem 1999; 274: 14893–14901. [DOI] [PubMed] [Google Scholar]
- 11.Pandey P, Avraham S, Kumar S, Nakazawa A, Place A, Ghanem L, Rana A, Kumar V, Majumder PK, Avraham H, Davis RJ, Kharbanda S. Activation of p38 mitogen-activated protein kinase by PYK2/related adhesion focal tyrosine kinase-dependent mechanism. J Biol Chem 1999; 274: 10140–10144. [DOI] [PubMed] [Google Scholar]
- 12.Banno Y, Ohguchi K, Matsumoto N, Koda M, Ueda M, Hara A, Dikic I, Nozawa Y. Implication of phospholipase D2 in oxidant-induced phosphoinositide 3-kinase signaling via Pyk2 activation in PC12 cells. J Biol Chem 2005; 280: 16319–16324. [DOI] [PubMed] [Google Scholar]
- 13.Basile JR, Afkhami T, Gutkind JS. Semaphorin 4D/plexin-B1 induces endothelial cell migration through the activation of PYK2, Src, and the phosphatidylinositol 3-kinase-Akt pathway. Mol Cell Biol 2005; 25: 6889–6898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi CS, Kehrl JH. PYK2 links G(q)alpha and G(13)alpha signaling to NF-kappa B activation. J Biol Chem 2001; 276: 31845–31850. [DOI] [PubMed] [Google Scholar]
- 15.Schaller MD. Cellular functions of FAK kinases: insight into molecular mechanisms and novel functions. J Cell Sci 2010; 123: 1007–1013. [DOI] [PubMed] [Google Scholar]
- 16.Kinsey WH. Intersecting roles of protein tyrosine kinase and calcium signaling during fertilization. Cell Calcium 2013; 53: 32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma D, Kinsey WH. PYK2: a calcium-sensitive protein tyrosine kinase activated in response to fertilization of the zebrafish oocyte. Dev Biol 2013; 373: 130–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meng XQ, Zheng KG, Yang Y, Jiang MX, Zhang YL, Sun QY, Li YL. Proline-rich tyrosine kinase2 is involved in F-actin organization during in vitro maturation of rat oocyte. Reproduction 2006; 132: 859–867. [DOI] [PubMed] [Google Scholar]
- 19.McGinnis LK, Luo J, Kinsey WH. Protein tyrosine kinase signaling in the mouse oocyte cortex during sperm-egg interactions and anaphase resumption. Mol Reprod Dev 2013; 80: 260–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo J, McGinnis LK, Carlton C, Beggs HE, Kinsey WH. PTK2b function during fertilization of the mouse oocyte. Biochem Biophys Res Commun 2014; 450: 1212–1217. (BBRC). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carroll DJ, Albay DT, Terasaki M, Jaffe LA, Foltz KR. Identification of PLCgamma-dependent and -independent events during fertilization of sea urchin eggs. Dev Biol 1999; 206: 232–247. [DOI] [PubMed] [Google Scholar]
- 22.Giusti AF, Carroll DJ, Abassi YA, Terasaki M, Foltz KR, Jaffe LA. Requirement of a Src family kinase for initiating calcium release at fertilization in starfish eggs. J Biol Chem 1999; 274: 29318–29322. [DOI] [PubMed] [Google Scholar]
- 23.Giusti AF, O’Neill FJ, Yamasu K, Foltz KR, Jaffe LA. Function of a sea urchin egg Src family kinase in initiating Ca2+ release at fertilization. Dev Biol 2003; 256: 367–378. [DOI] [PubMed] [Google Scholar]
- 24.Sato K, Tokmakov AA, Iwasaki T, Fukami Y. Tyrosine kinase-dependent activation of phospholipase Cgamma is required for calcium transient in Xenopus egg fertilization. Dev Biol 2000; 224: 453–469. [DOI] [PubMed] [Google Scholar]
- 25.Kurokawa M, Sato K, Smyth J, Wu H, Fukami K, Takenawa T, Fissore RA. Evidence that activation of Src family kinase is not required for fertilization-associated [Ca2+]i oscillations in mouse eggs. Reproduction 2004; 127: 441–454. [DOI] [PubMed] [Google Scholar]
- 26.Matsuya M, Sasaki H, Aoto H, Mitaka T, Nagura K, Ohba T, Ishino M, Takahashi S, Suzuki R, Sasaki T. Cell adhesion kinase β forms a complex with a new member, Hic-5, of proteins localized at focal adhesions. J Biol Chem 1998; 273: 1003–1014. [DOI] [PubMed] [Google Scholar]
- 27.Duong LT, Lakkakorpi PT, Nakamura I, Machwate M, Nagy RM, Rodan GA. PYK2 in osteoclasts is an adhesion kinase, localized in the sealing zone, activated by ligation of αvβ3 integrin, and phosphorylated by src kinase. J Clin Invest 1998; 102: 881–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duong LT, Rodan GA. PYK2 is an adhesion kinase in macrophages, localized in podosomes and activated by beta(2)-integrin ligation. Cell Motil Cytoskeleton 2000; 47: 174–188. [DOI] [PubMed] [Google Scholar]
- 29.Aoto H, Sasaki H, Ishino M, Sasaki T. Nuclear translocation of cell adhesion kinase β/proline-rich tyrosine kinase 2. Cell Struct Funct 2002; 27: 47–61. [DOI] [PubMed] [Google Scholar]
- 30.Schindler EM, Baumgartner M, Gribben EM, Li L, Efimova T. The role of proline-rich protein tyrosine kinase 2 in differentiation-dependent signaling in human epidermal keratinocytes. J Invest Dermatol 2007; 127: 1094–1106. [DOI] [PubMed] [Google Scholar]
- 31.Arcucci A, Montagnani S, Gionti E. Expression and intracellular localization of Pyk2 in normal and v-src transformed chicken epiphyseal chondrocytes. Biochimie 2006; 88: 77–84. [DOI] [PubMed] [Google Scholar]
- 32.Faure C, Corvol JC, Toutant M, Valjent E, Hvalby O, Jensen V, El Messari S, Corsi JM, Kadaré G, Girault JA. Calcineurin is essential for depolarization-induced nuclear translocation and tyrosine phosphorylation of PYK2 in neurons. J Cell Sci 2007; 120: 3034–3044. [DOI] [PubMed] [Google Scholar]
- 33.Lim ST, Chen XL, Lim Y, Hanson DA, Vo TT, Howerton K, Larocque N, Fisher SJ, Schlaepfer DD, Ilic D. Nuclear FAK promotes cell proliferation and survival through FERM-enhanced p53 degradation. Mol Cell 2008; 29: 9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lim ST, Miller NL, Nam JO, Chen XL, Lim Y, Schlaepfer DD. Pyk2 inhibition of p53 as an adaptive and intrinsic mechanism facilitating cell proliferation and survival. J Biol Chem 2010; 285: 1743–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]