Abstract
The P-element induced wimpy testis (Piwi) protein family is responsible for initiating spermatogenesis and maintaining the integrity of germ cells and stem cells, but little is known regarding its transcriptional regulation in poultry. Here, we characterized the methylation status of the Piwil1 promoter in five different spermatogenic cell lines using direct bisulfite pyrosequencing and determined that methylation correlates negatively with germ cell type-specific expression patterns of piwil1. We demonstrated that methylation of the −148 CpG site, which is the predicted binding site for the transcription factors TCF3 and NRF1, was differentially methylated in different spermatogenic cells. This site was completely methylated in PGCs (primordial germ cells), but was unmethylated in round spermatids. A similar result was obtained in the region from +121 to +139 CpG sites of the Piwil1 promoter CpG island, which was predicted to contain SOX2 binding sites. In addition, demethylation assays further demonstrated that DNA methylation indeed regulates Piwil1 expression during chicken spermatogenesis. Combined with transcription factor binding site prediction, we speculate that methylation influences the recruitment of corresponding transcription factors. Collectively, we show the negative correlation between promoter methylation and piwil1 expression and that the spatiotemporal expression of chicken Piwil1 from the PGC stage to the round spermatid stage is influenced by methylation-mediated transcription factor regulation.
Keywords: Chicken, DNA methylation, Piwil1, Spermatogenesis
Spermatogenesis is a complex developmental program by which male primordial germ cells (PGCs) differentiate into round spermatids through mitosis and meiosis. To become mature and functional sperm, male germ stem cells gradually lose their stem cell potential and undergo differentiation. During differentiation and spermatogenesis, orderly and precise spatiotemporal regulation of the expression of a variety of genes is essential to specify the germ cell fate [1, 2].
Many studies have reported that the protein Piwi is critical for both initiating spermatogenesis [3, 4] and maintaining the integrity of germ cells and stem cells in mice, zebrafish, and chicken [5,6,7]. Piwi genes encode Piwi proteins, which comprise a subfamily of Argonaute proteins.Argonaute proteins are known as PAZ Piwi domain (PPD) proteins and are a well-conserved family consisting of two subclasses: Ago and Piwi. There are four members of related Piwi subfamily genes termed Piwil1 (Piwi-like homolog 1), Piwil2, Piwil3, and Piwil4. The mouse genome encodes three members of the Piwi subfamily, Miwi (Piwil1), Miwi2 (Piwil4), and Mili (Piwil2), which are involved in the regulation of spermatogenesis [6,7,8]. The Drosophila genome also encodes three members: Piwi, aubergine (Aub), and argonaute 3 (Ago3) [9] which are each necessary for self-renewal of germ-line stem cells. Zebrafish has only two members of the Piwi family, Ziwi (zebrafish piwi/Piwil1) and Zili (Ziwi-like/Piwil2), of which Ziwi is responsible for maintenance of the germ line [7]. The chicken Piwi-like protein 1 (Ciwi) and 2 (Cili) were identified in 2012 [10]. Each of these Piwi proteins contributes to different stages of reproductive development, necessitating the precise spatiotemporal expression of different genes. The process of transcriptional regulation of Piwi in poultry spermatogenesis is poorly understood.
In this study, we analyzed the methylation status of the chicken Piwil1 promoter region in different spermatogenic cells. Furthermore, we used 5-aza-2′-deoxycytidine (5-aza) to inhibit DNA methyltransferase activity in PGCs in order to elucidate the requirements for methylation-mediated regulation of spatiotemporal expression of Piwil1 during spermatogenesis. In doing this, we hoped to enhance our understanding of how testis-specific expression of Piwil1 is regulated during chicken spermatogenesis.
Materials and Methods
Ethics statement
All experimental procedures were performed in accordance with the Regulations on The Administration of Experimental Animals issued by the Ministry of Science and Technology in 2001 (Beijing, China). All the animal experiment protocols were approved and guided by the Animal Care and Use Committee of Yangzhou University.
Cell isolation, culture, and identification
Fertilized eggs from Rugao Yellow Chickens were provided by the Poultry Institute of the Chinese Academy of Agriculture Sciences and were incubated at 37.8°C and 70% relative humidity. PGCs were isolated from embryonic gonads at the Hamburger and Hamilton (H&H) Stage 27 (120 h post incubation) [11] under a stereomicroscope and then separated by density gradient equilibrium centrifugation and the differential adhesion method [12, 13]. Chicken spermatogonial stem cells (SSCs) were isolated following a previously published method [13] with slight modifications. Briefly, testes were aseptically dissected from 18-day-old embryos (H&H stage 44) and mechanically cut into small pieces using sterile surgical scissors. After digestion of testis tissue, SSCs were purified by density gradient centrifugation and the differential adhesion method. The different types of spermatogenic cells from testicular tissues were sorted by flow cytometry (FACSAria, BD Biosciences, San Jose, CA, USA) [14, 15]. Cells were dyed with 2-(4-amidinophenyl)-6-indolecarbamidine dihydrochloride (DAPI) (Beyotime Biotechnology, Shanghai, China) prior to flow cytometry to identify the haploid, diploid, and tetraploid cells according to the intensity of DNA staining. Haploid cells were round spermatids, diploid cells were spermatogonia, and tetraploid cells (4n) were primary spermatocytes that had completed DNA replication but not division during meiosis I. Until the separation and identification of different cells, PGCs and SSCs were cultured in knockout Dulbecco’s Modified Eagle’s Medium (KO-DMEM) (Gibco, NY, USA) supplemented with 10% fetal bovine serum (FBS) (Gibco), 2.5% chicken serum (Gibco), 2 mM L-glutamine (Gibco), 1× MEM nonessential amino acids (WISENT, St. Bruno, Quebec, Canada), 2 mM sodium pyruvate (Gibco), 1× HEPES (WISENT), 0.1 mM β-mercaptoethanol (Sigma Aldrich, St Louis, MO, USA), 5 ng/ml human stem cell factor (hSCF) (Sigma Aldrich), 10 ng/ml basic fibroblast growth factor (bFGF) (Sigma Aldrich), and 10 ng/ml mouse leukemia inhibitory factor (Sigma Aldrich). The cells were cultured in an incubator at 37°C with 5% atmospheric CO2 and 60–70% relative humidity.
PGC colonies were identified using the periodic acid-Schiff [16] stain (Jiancheng, Nanjing, China) and stage-specific embryonic antigen-1 (SSEA-1) staining (Santa Cruz, 100 Dallas, TX, USA); SSCs were identified using SSEA-1 staining according to the instructions provided.
DNA extraction and bisulfite conversion
Genomic DNA from different types of spermatogenic cells was extracted using QIAamp DNA Mini Kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. DNA quality and concentration were assessed with a Nanodrop-1000 spectrophotometer (Thermo Scientific, MA, USA). Bisulfite conversion of DNA was performed with the EpiTect Bisulfite Kit (Qiagen, Dusseldorf, Germany) according to the manufacturer’s instructions for bisulfite pyrosequencing (BP) analyses (50 ng DNA/sample).
Bioinformatics analyses
CpG island predictions for the chicken Piwil1 promoter were made with the MethPrimer software (http://www.urogene.org/methprimer/index1.html) using the following parameters: island size > 200, GC% > 50.0%, and Obs/Exp > 0.6. The JASPAR software (http://jaspar.genereg.net/cgi-bin/jaspar_db.pl) was used to predict transcription factor binding sites in the chicken Piwil1 promoter region using the JASPAR CORE Vertebrata database and a profile score threshold of ≥ 85%.
Direct bisulfite pyrosequencing
Bisulfite converted DNA was eluted in 20 μl of elution buffer and was subjected to polymerase chain reaction (PCR) amplification using primer sets targeting the specific region of the Piwil1 promoter. Four primer sets (Table 1) were designed to amplify four different domains of the Piwil1 promoter using the PyroMark Assay Design software (PSQ assay design software) (Qiagen). The PCR product was confirmed by 1.5% agarose gel electrophoresis to determine the size and quality of the products and to eliminate the possibility of primer dimers. The specific PCR products were then subjected to quantitative pyrosequencing analysis using a PyroMark Gold Q96 system (Qiagen) according to the manufacturer’s instructions. The results were analyzed with the PyroMark CpG software (Qiagen).
Table 1. Primers sets used for amplification of four Piwil1 promoter specific regions.
| Primer names | Primer sequences (5’–3’) | Amplicon length (bp) | Annealing temperature (°C) |
|---|---|---|---|
| PIWIL1-1 | F: Btn-GTAAGATTTGGAGAGAGAATGGAATTTTAT | 279 (–263~+15) | 58 |
| R: CCCTACCAAACTCTCTTCAACCTACAC | |||
| S: AGGAAAATTTTTTTATAAAAGTTA | |||
| PIWIL1-2 | F: Btn-AAGATTTGGAGAGAGAATGGAATTTTA | 277 (–261~+15) | 58 |
| R: CCCTACCAAACTCTCTTCAACCTACAC | |||
| S: AAAACCCACCAAACCCTCTAATT | |||
| PIWIL1-3 | F: Btn-GGTTTTAGGGAGTTAATTAGAGGG | 237 (–67~+169) | 58 |
| R: CCCTCAACATTACCTCAATCAC | |||
| S: CTACCAAACTCTCTTCAACCT | |||
| PIWIL1-4 | F: Btn-GTGTAGGTTGAAGAGAGTTT | 182 (–11~+170) | 58 |
| R: ACCCTCAACATTACCTCAATC | |||
| S: CAACATTACCTCAATCAC |
F, forward primer; R, reverse primer; S, sequencing primer.
5-aza-2′-deoxycytidine treatment
To inhibit DNA-methyltransferase, PGCs were seeded in 12-well plates and allowed to attach for 24 h. The cells were then treated with or without 5-aza-2′-deoxycytidine (Sigma Aldrich) at a concentration of 0.05 μM and 0.08 μM for 24 h, as described under the section “Cell isolation, culture, and identification”. At 24 h, the cells were harvested and counted. Cell viability was assessed using a CCK-8 assay (Vazyme Biotech, Piscataway, USA), which is a modified method based on the classical MTT reduction assay [17]. Two biological replicates were performed with three technical replicates each.
RNA isolation and qRT-PCR analysis
Total RNA was extracted from different spermatogenic cells and PGCs treated with or without 5-aza at 0.05 μM, using TRIzol reagent [18] and quantified with a Nanodrop-1000 spectrophotometer. The RNA (1 μg) isolated for each condition was reverse transcribed to complementary DNA (cDNA) using the FastQuant RT Kit (with gDNase) (Tiangen, Beijing, China) following the manufacturer’s instructions. The cDNA was stored at −20°C until required. Quantitative real time PCR (qRT-PCR) was performed for Piwil1 and gene expression was normalized against GAPDH. The primers were designed with the Oligo7.0 software according to the sequences included in the GenBank database and were synthesized by the Shanghai Sangon Biotechnology (Shanghai, China) (Table 2). The experiment was performed on an ABI 7500 Real-time PCR Detection System (Applied Biosystems, Carlsbad, CA) using the UltraSYBR Mixture (with ROX) (CoWin Biotechnology, Beijing, China), and each sample was analyzed in triplicate.
Table 2. Primers sets used for qRT-PCR.
| Gene symbol | Primer sequences (5’–3’) | Amplicon length (bp) | Annealing temperature (°C) |
|---|---|---|---|
| PIWIL1 | F: TCACCTGAGCAAAGACAAC | 126 | 60 |
| R: TCCCGTAAAGGACAGTAAG | |||
| GAPDH | F: CGATCTGAACTACATGGTTTAC | 151 | 60 |
| R: 5’TCTGCCCATTTGATGTTGC |
F, forward primer; R, reverse primer.
Statistical analyses
All results are expressed as mean values. Statistical analyses and graphical representations were performed with the GraphPad Prism 6 software (San Diego, CA). A P-value less than 0.05 was considered as significant and a P-value less than 0.01 was considered as extremely significant. The statistical tests used are indicated in the figure legends.
Results
CpG island prediction
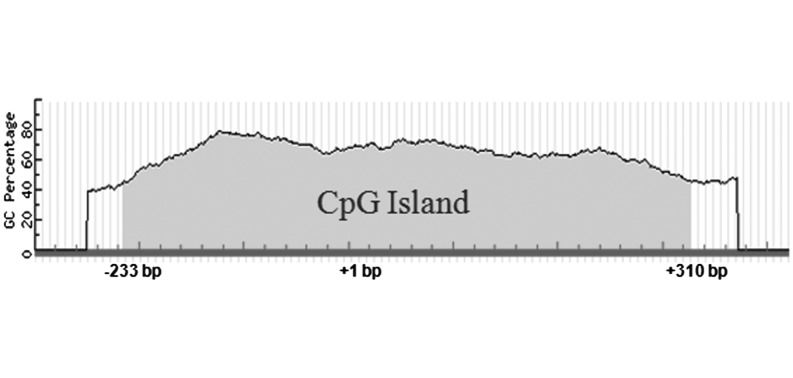
To determine whether DNA methylation influences Piwil1 expression and regulation during chicken spermatogenesis, we first performed a CpG island prediction analysis in the 2 kb region upstream of the Piwil1 start codon revealing a large CpG island (543 bp, –233 to +310 bp, TSS = +1) in the 5′-flanking region of Piwil1 (Fig. 1).
Fig. 1.
CpG island (543 bp, –233 to +310 bp, TSS = +1) found in the 2-kb region upstream of Piwil1 ATG.
Promoter methylation status of Piwil1 promoter in different spermatogenic cell types
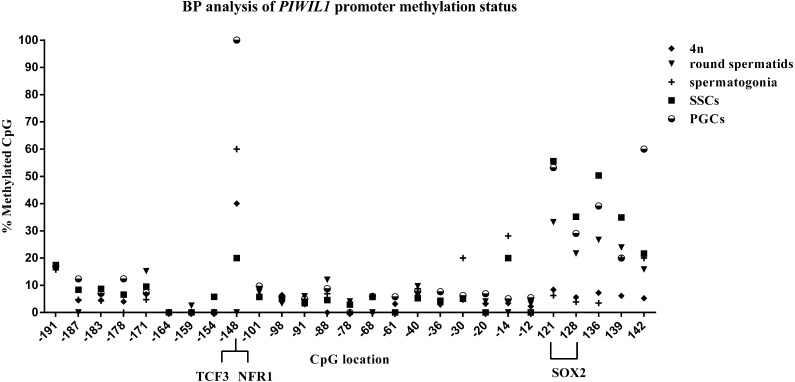
To determine the spatial and temporal methylation status of the predicted CpG island in the Piwil1 promoter region and its association with the expression and regulation of chicken Piwil1 during spermatogenesis, we analyzed bisulfite-treated genomic DNA isolated from different spermatogenic cells, including PGCs, SSCs, spermatogonia, 4n cells, and round spermatids. The mean methylation values of these CpG sites showed striking differences among different spermatogenic cells (16.21% in PGCs, 12.44% in SSCs, 8.42% in spermatogonia, 8.48% in round spermatids, and 5.62% in 4n cells). The mean methylation values of the Piwil1 promoter CpG island in five different types of spermatogenic cell populations are shown in Fig. 2. Our assays indicated the decreasing methylation of this region as spermatogenesis progressed. To compare this with the expression of Piwil1, we measured Piwil1 mRNA expression in each of the cell types mentioned. Piwil1 was highly expressed in round spermatids whereas its expression in PGCs and SSCs was low (Fig. 3). These results indicate that Piwil1 expression and Piwil1 promoter methylation are inversely correlated in chicken spermatogenic cells. The CpG sites from +121 to +139 of the Piwil1 promoter CpG island in almost all spermatogenic cells were highly methylated except those in 4n cells and spermatogonia. The +121 and +128 CpG sites showed a similar methylation pattern across cell types: high methylation in SSCs and PGCs and low methylation in 4n and spermatogonia. Computational prediction of transcription factor binding sites in the Piwil1 promoter CpG island indicated a potential binding site for SOX2 (+121 to +128 bp, CGTTTGTC). In addition, the −148 site of the Piwil1 promoter region in spermatogenic cells exhibited CpG-specific methylation, where this site was highly methylated in PGCs, SSCs, spermatogonia and 4n (probability = 100%, 20%, 60% and 40%, respectively), but was not methylated (probability = 0%) in round spermatids. This site was predicted to be a putative binding site for the transcription factors TCF3 and NRF1.
Fig. 2.
Bisulfite pyrosequencing (BP) analysis of Piwil1 promoter methylation status in different spermatogenic cells. The position of each CpG is indicated (TSS = +1). The binding region for the transcription factors TCF3 and NRF1 includes the –148 CpG site and the SOX2 (from +121 to +128 bp, CGTTTGTC) binding region includes the +121 and +128 CpG sites.
Fig. 3.
Relative Piwil1 mRNA expression in different spermatogenic cells of chicken. * P < 0.05; ** P < 0.01 compared with the expression level in round spermatids. PGCs: primordial germ cells; SSCs: spermatogonia stem cells; 4n: tetraploid cells.
Effect of 5-aza-2′-deoxycytidine on cell proliferation and viability
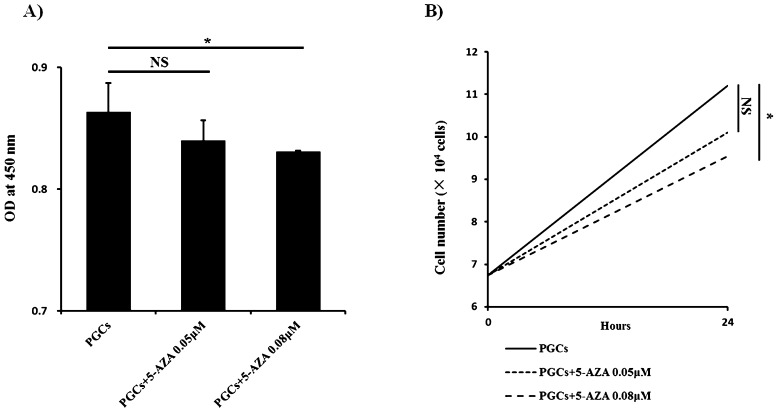
To evaluate the potential relationship between Piwil1 promoter methylation and expression, PGCs were treated with 5-aza. We first assessed the impact of 5-aza on cell viability and proliferation using a CCK-8 assay. As shown in Fig. 4A, 0.08 μM 5-aza significantly decreased PGC viability (P < 0.05 vs. untreated cells), whereas 0.05 μM 5-aza had no significant effect on cell viability (P = NS). Similar results were observed for cell proliferation as well (Fig. 4B).
Fig. 4.
Effect of 5-aza on cell proliferation and viability. A) Cell viability was measured with the CCK-8 kit. NS, not significant; * P < 0.05 (Student-Newman-Keuls test) vs. untreated cells. B) Cell proliferation. The results are representative of 3 independent experiments.
Expression of Piwil1 after treatment with 5-aza- 2′-deoxycytidine
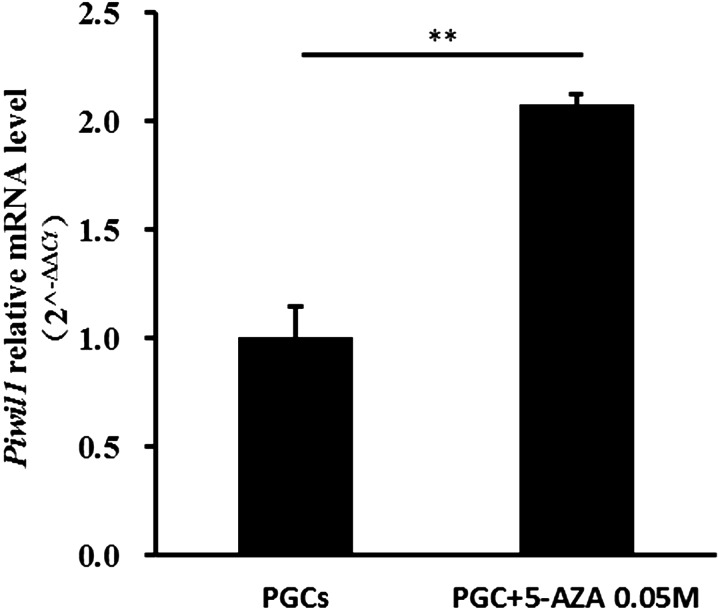
After assessing the influence of 5-aza on cell proliferation and viability, we treated PGCs with 0.05 µM 5-aza for 24 h and checked the Piwil1 mRNA expression level. As shown in Fig. 5, treatment with 5-aza induced nearly two-fold increase in Piwil1 mRNA expression compared to untreated cells (P < 0.05).
Fig. 5.
Impact of 5-aza on Piwil1 expression in PGCs. Results are representative of 2 independent experiments. ** P < 0.01 (Student-Newman-Keuls test) vs. untreated cells.
Discussion
CpG islands are regions with enriched CG content and are usually associated with promoter regions. The methylation of one CpG dinucleotide within the transcriptional regulatory element of a promoter region can alter the accessibility of DNA to specific transcription factors, and thus cause gene silencing or decreased expression. In contrast, hypomethylation of CpG sites in the gene promoter regions is generally associated with increased gene expression.
In this study, we used direct bisulfite pyrosequencing to determine the methylation status of a predicted CpG island in the 5′-flanking region of the chicken Piwil1 promoter in different spermatogenic cells, including PGCs, SSCs, spermatogonia, 4n cells, and round spermatids. The results showed that the −148 CpG site and the CpG sites in the posterior fraction of the Piwil1 promoter region CpG island were differentially methylated within different spermatogenic cells. The mean methylation degree of the CpG island in the Piwil1 promoter region was higher in PGCs and SSCs and lower in round spermatids (probability = 16.21%, 12.44%, and 8.48%, respectively), whereas Piwil1 was highly expressed in round spermatids and showed low expression in PGCs and SSCs. This suggests a negative correlation between methylation of the Piwil1 promoter and Piwil1 expression in chicken spermatogenic cells, which is similar to the reports by Chang et al. in the chicken and findings in other animals [5, 19]. Another finding of this study is that the −148 CpG methylation site containing predicted binding sites for the transcription factors TCF3 and NRF1 is differentially methylated in the spermatogenic cells types. PGCs showed complete methylation and round spermatids showed absence of methylation at this site, which suggests that TCF3 and NFR1 regulate Piwil1 expression in germ cells. The +121 and +128 CpG sites located in the predicted SOX2 binding region (+121 to +128 bp) were also differentially methylated, and showed a similar methylation trend, that is, higher methylation in PGCs and SSCs, and lower methylation in round spermatids. This, too, suggests that SOX2 might be involved in regulation of Piwil1 during chicken spermatogenesis.
To confirm that methylation affects the expression of Piwil1 in chicken spermatogenic cells, we used 5-aza to inhibit DNA methylation in chicken PGCs. When methylation was inhibited, the expression of Piwil1 in PGCs increased by nearly two-fold relative to that in untreated cells, further supporting the negative relationship between Piwil1 promoter methylation and Piwil1 expression. Methylation of CpG sites in the Piwil1 promoter might inhibit binding of the transcription factors TCF3 and NRF1, thereby repressing Piwil1 expression. Thus, when demethylation occurs, these transcription factors bind to the Piwil1 promoter and activate its expression.
To date, much of the research on the regulation of Piwi gene transcription has focused on the transcription factors NF-Y, USF, and A-MYB. Chang et al. and Sohn et al. reported that DNA methylation and NF-Y co-regulate Piwil1 expression in chickens [20, 21]. Hou et al. found that methylation-mediated USF controls Miwi expression from the midpachytene spermatocyte stage to the round spermatid stage in mice [5]. Li et al. found an evolutionarily conserved A-MYB feed-forward loop in which A-MYB regulates the expression of Piwil1 by binding its promoter in both rooster and mouse testis [22]. However, in this study, we discovered additional transcription factors that might be involved in regulating Piwil1 expression during chicken spermatogenesis. TCF3 was reported to be involved in mouse embryonic stem cell self-renewal by repressing Nanog transcription [23,24,25]. NRF1 mediates the transcriptional regulation of key metabolic genes related to cellular growth and development [26,27,28]. Further studies on the mechanism of how these transcription factors regulate Piwil1 expression in chickens will provide a better understanding of spermatogenesis, germ-cell development, and differentiation.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (31372297) and the Agricultural Science & Technology Pillar Program of Jiangsu Province, China (BE2013392).
References
- 1.Loveland KL, Hogarth C, Mendis S, Efthymiadis A, Ly J, Itman C, Meachem S, Brown CW, Jans DA. Drivers of germ cell maturation. Ann N Y Acad Sci 2005; 1061: 173–182. [DOI] [PubMed] [Google Scholar]
- 2.Tanaka H, Baba T. Gene expression in spermiogenesis. Cell Mol Life Sci 2005; 62: 344–354. [DOI] [PubMed] [Google Scholar]
- 3.Cox DN, Chao A, Lin H. piwi encodes a nucleoplasmic factor whose activity modulates the number and division rate of germline stem cells. Development 2000; 127: 503–514. [DOI] [PubMed] [Google Scholar]
- 4.Kuramochi-Miyagawa S, Kimura T, Ijiri TW, Isobe T, Asada N, Fujita Y, Ikawa M, Iwai N, Okabe M, Deng W, Lin H, Matsuda Y, Nakano T. Mili, a mammalian member of piwi family gene, is essential for spermatogenesis. Development 2004; 131: 839–849. [DOI] [PubMed] [Google Scholar]
- 5.Hou Y, Yuan J, Zhou X, Fu X, Cheng H, Zhou R. DNA demethylation and USF regulate the meiosis-specific expression of the mouse Miwi. PLoS Genet 2012; 8: e1002716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deng W, Lin H. miwi, a murine homolog of piwi, encodes a cytoplasmic protein essential for spermatogenesis. Dev Cell 2002; 2: 819–830. [DOI] [PubMed] [Google Scholar]
- 7.Houwing S, Berezikov E, Ketting RF. Zili is required for germ cell differentiation and meiosis in zebrafish. EMBO J 2008; 27: 2702–2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuramochi-Miyagawa S, Watanabe T, Gotoh K, Totoki Y, Toyoda A, Ikawa M, Asada N, Kojima K, Yamaguchi Y, Ijiri TW, Hata K, Li E, Matsuda Y, Kimura T, Okabe M, Sakaki Y, Sasaki H, Nakano T. DNA methylation of retrotransposon genes is regulated by Piwi family members MILI and MIWI2 in murine fetal testes. Genes Dev 2008; 22: 908–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gunawardane LS, Saito K, Nishida KM, Miyoshi K, Kawamura Y, Nagami T, Siomi H, Siomi MC. A slicer-mediated mechanism for repeat-associated siRNA 5′ end formation in Drosophila. Science 2007; 315: 1587–1590. [DOI] [PubMed] [Google Scholar]
- 10.Kim TH, Yun TW, Rengaraj D, Lee SI, Lim SM, Seo HW, Park TS, Han JY. Conserved functional characteristics of the PIWI family members in chicken germ cell lineage. Theriogenology 2012; 78: 1948–1959. [DOI] [PubMed] [Google Scholar]
- 11.Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol 1951; 88: 49–92. [PubMed] [Google Scholar]
- 12.Yu F, Ding LJ, Sun GB, Sun PX, He XH, Ni LG, Li BC. Transgenic sperm produced by electrotransfection and allogeneic transplantation of chicken fetal spermatogonial stem cells. Mol Reprod Dev 2010; 77: 340–347. [DOI] [PubMed] [Google Scholar]
- 13.Li B, Wang XY, Tian Z, Xiao XJ, Xu Q, Wei CX, Y F, Sun HC, Chen GH. Directional differentiation of chicken spermatogonial stem cells in vitro. Cytotherapy 2010; 12: 326–331. [DOI] [PubMed] [Google Scholar]
- 14.Mays-Hoopes LL, Bolen J, Riggs AD, Singer-Sam J. Preparation of spermatogonia, spermatocytes, and round spermatids for analysis of gene expression using fluorescence-activated cell sorting. Biol Reprod 1995; 53: 1003–1011. [DOI] [PubMed] [Google Scholar]
- 15.Mozdziak PE, Angerman-Stewart J, Rushton B, Pardue SL, Petitte JN. Isolation of chicken primordial germ cells using fluorescence-activated cell sorting. Poult Sci 2005; 84: 594–600. [DOI] [PubMed] [Google Scholar]
- 16.Riesco MF, Martínez-Pastor F, Chereguini O, Robles V. Evaluation of zebrafish (Danio rerio) PGCs viability and DNA damage using different cryopreservation protocols. Theriogenology 2012; 77: 122–130.e2. [DOI] [PubMed] [Google Scholar]
- 17.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983; 65: 55–63. [DOI] [PubMed] [Google Scholar]
- 18.Connolly MA, Clausen PA, Lazar JG. Purification of RNA from animal cells using trizol. CSH Protoc 2006; 2006: 10–15. [DOI] [PubMed] [Google Scholar]
- 19.Gu Y, Li Q, Pan Z, Li M, Luo H, Xie Z. Molecular cloning, gene expression and methylation status analysis of PIWIL1 in cattle-yaks and the parental generation. Anim Reprod Sci 2013; 140: 131–137. [DOI] [PubMed] [Google Scholar]
- 20.Sohn YA, Lee SI, Choi HJ, Kim HJ, Kim KH, Park TS, Han JY. The CCAAT element in the CIWI promoter regulates transcriptional initiation in chicken primordial germ cells. Mol Reprod Dev 2014; 81: 871–882. [DOI] [PubMed] [Google Scholar]
- 21.Chang G, Chen R, Xu L, Ma T, Wang H, Chen J, Zhang Y, Li Z, Wan F, Guo X, Xu Q, Zhao W, Chen G. DNA methylation and NF-Y regulate Piwil1 expression during chicken spermatogenesis. Anim Reprod Sci 2015; 162: 95–103. [DOI] [PubMed] [Google Scholar]
- 22.Li XZ, Roy CK, Dong X, Bolcun-Filas E, Wang J, Han BW, Xu J, Moore MJ, Schimenti JC, Weng Z, Zamore PD. An ancient transcription factor initiates the burst of piRNA production during early meiosis in mouse testes. Mol Cell 2013; 50: 67–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pereira L, Yi F, Merrill BJ. Repression of Nanog gene transcription by Tcf3 limits embryonic stem cell self-renewal. Mol Cell Biol 2006; 26: 7479–7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cole MF, Johnstone SE, Newman JJ, Kagey MH, Young RA. Tcf3 is an integral component of the core regulatory circuitry of embryonic stem cells. Genes Dev 2008; 22: 746–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yi F, Pereira L, Hoffman JA, Shy BR, Yuen CM, Liu DR, Merrill BJ. Opposing effects of Tcf3 and Tcf1 control Wnt stimulation of embryonic stem cell self-renewal. Nat Cell Biol 2011; 13: 762–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Virbasius CA, Virbasius JV, Scarpulla RC. NRF-1, an activator involved in nuclear-mitochondrial interactions, utilizes a new DNA-binding domain conserved in a family of developmental regulators. Genes Dev 1993; 7(12A): 2431–2445. [DOI] [PubMed] [Google Scholar]
- 27.Blesa JR, Prieto-Ruiz JA, Abraham BA, Harrison BL, Hegde AA, Hernández-Yago J. NRF-1 is the major transcription factor regulating the expression of the human TOMM34 gene. Biochem Cell Biol 2008; 86: 46–56. [DOI] [PubMed] [Google Scholar]
- 28.Efiok BJ, Chiorini JA, Safer B. A key transcription factor for eukaryotic initiation factor-2 alpha is strongly homologous to developmental transcription factors and may link metabolic genes to cellular growth and development. J Biol Chem 1994; 269: 18921–18930. [PubMed] [Google Scholar]