Abstract
In vitro growth culture systems for oocytes are being developed in several mammalian species. In these growth culture systems, in vitro grown oocytes usually have lower blastocyst formation than in vivo grown oocytes after in vitro fertilization. Furthermore, there have been a few reports that investigated the fertilization ability of in vitro grown oocytes in large animals. The purpose of this study was to investigate the fertilization process and developmental competence of bovine oocytes grown in vitro. Oocyte-granulosa cell complexes collected from bovine early antral follicles (0.4−0.7 mm in diameter) were cultured for growth with 17β-estradiol and androstenedione for 14 days and matured in vitro. These oocytes were then inseminated for 6 or 12 h, and further cultured for development up to 8 days in vitro. After growth culture, oocytes grew from 95 µm to around 120 µm and acquired maturation competence (79%). Although fertilization rates of in vitro grown oocytes were low after 6 h of insemination, 34% of in vitro grown oocytes fertilized normally after 12 h of insemination, having two polar bodies and two pronuclei with a sperm tail, and 22% of these oocytes developed into blastocysts after 8 days of culture. The fertilization and blastocyst formation rates were similar to those of in vivo grown oocytes. In addition, blastocyst cell numbers were also similar between in vitro and in vivo grown oocytes. In conclusion, in vitro grown bovine oocytes are similar to in vivo grown oocytes in fertilization ability and can develop into blastocysts.
Keywords: Cow, In vitro fertilization, Oocyte growth, Oocyte maturation
In order to utilize the huge number of oocytes stored in mammalian ovaries, many technologies have been developed. Mammalian oocytes were fertilized in vitro for the first time in the late 1950s [1], and successful in vitro fertilizations in several mammals were reported in the 1960s and 1970s. Bovine oocyte in vitro fertilization was first reported in 1977 [2]; subsequently, the first offspring was produced from in vitro fertilized bovine oocytes [3]. These days, in vitro fertilization is a well-established tool and widely used in agricultural and medical fields [4]. More recently, many efforts have been made to develop in vitro growth culture systems for oocytes in a variety of species [5, 6]. Bovine live offspring have been produced from early antral follicle-derived oocytes after in vitro growth, maturation, and fertilization [7, 8, 9].
The in vivo or in vitro environment in which oocytes grow and mature is a major determinant factor of oocyte quality that represents fertilization ability and developmental competence [10, 11]. Although our previous reports [12, 13] showed that the culture system using steroid hormones supported bovine oocyte growth and promoted the acquisition of meiotic competence in vitro, oocyte growth culture systems still have a low yield of competent oocytes; thus, there have been a few reports that investigated the fertilization ability of in vitro grown oocytes in large animals. In addition, most of the reports have evaluated the fertilization ability of in vitro grown oocytes by observing cleavage and blastocyst formation after in vitro fertilization. Nevertheless, the fertilization process of in vitro grown oocytes remains unclear. In this study, we investigated the fertilization of in vitro grown bovine oocytes based on formation of pronuclei, second polar body extrusion, and sperm entry. The oocytes from bovine early antral follicles were cultured for growth, matured, and inseminated in vitro. The fertilization ability and subsequent developmental competence of in vitro grown oocytes were examined.
Materials and Methods
Chemicals
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise stated.
Collection of oocyte−granulosa cell complexes (OGCs)
Bovine ovaries from Japanese Black cattle were obtained from a local abattoir and transported to the laboratory. The ovaries were washed once in 0.2% (wt/vol) cetyltrimethylammonium bromide and three times in Dulbecco’s phosphate-buffered saline (PBS) containing 0.1% (wt/vol) polyvinyl alcohol (PVA) (PBS-PVA). OGCs consisting of oocytes and cumulus/granulosa cells were collected from antral follicles at two different sizes. For the collection of OGCs with fully grown oocytes, follicular fluids containing OGCs were obtained from antral follicles (4−6 mm in diameter) using needles (18 gauge; Terumo, Tokyo, Japan) and syringes; these OGCs served as the controls. For the collection of OGCs with growing oocytes, ovarian cortical slices (1−1.5 mm) were made using a surgical blade (No. 10; Feather Safety Razor, Tokyo, Japan) and forceps. Antral follicles (0.4−0.7 mm in diameter) were dissected from the cortices under a stereomicroscope (Leica MZ125; Leica Microsystems, Wetzlar, Germany). The follicles were opened with forceps and a blade (No. 10) to isolate OGCs in 25 mM HEPES-buffered medium 199 (HEPES-199; Nissui Pharmaceutical, Tokyo, Japan) containing 0.1% (wt/vol) PVA, 0.85 mg/ml sodium bicarbonate (Wako Pure Chemical Industries, Osaka, Japan), and 0.08 mg/ml kanamycin sulfate. After measuring oocyte diameter (excluding the zona pellucida) to the nearest 1 μm using an ocular micrometer attached to an inverted microscope, OGCs that contained oocytes of 90−100 μm in diameter were used for in vitro growth culture.
In vitro growth culture of oocytes
In vitro growth culture was performed according to the procedure described previously [8, 12] with modifications. OGCs were isolated from bovine antral follicles (0.4−0.7 mm in diameter) and the day of OGCs isolation was designated day 0. Groups of 10−20 OGCs were cultured in vitro for 14 days on Millicell inserts (30 mm diameter, 0.4 µm pore size; Cell Culture Inserts, Merck Millipore, Billerica, MA, USA) placed in Petri dishes (Falcon No. 351008, Becton Dickinson and Co., Bedford, MA, USA) at 38.5ºC in a controlled atmosphere (5% O2, 5% CO2, 90% N2) from day 0 to day 6, followed by an atmosphere of 5% CO2 in air from day 7 to day 14. We employed Millicell inserts in the present study to culture multiple OGCs at the same time and to reduce the time required for medium change, resulting in less contamination risk. In total, 2 ml of medium was placed in the dishes: 1 ml on the membrane and another 1 ml under the membrane. The culture medium for oocyte growth was Alpha Minimum Essential Medium (α-MEM; GIBCO, Invitrogen, Scotland, UK) supplemented with 5% (vol/vol) fetal bovine serum (FBS; ICN Biomedicals, Costa Mesa, CA, USA), 4% (wt/vol) polyvinylpyrrolidone (molecular weight 360,000), 4 mM hypoxanthine, 50 μg/ml ascorbic acid 2-glucoside (Hayashibara Biochemical Laboratories, Okayama, Japan), 55 μg/ml cysteine, 0.05 μM dexamethasone, 1 mM sodium pyruvate, 2.2 mg/ml sodium bicarbonate and 0.08 mg/ml kanamycin sulfate [8]. Based on our previous report [12], the medium was also supplemented with 10 ng/ml 17β-estradiol and 10 ng/ml androstenedione (Tokyo Chemical Industry, Tokyo, Japan). One-half volume of the culture medium was changed with fresh medium every other day after day 4. On day 7 and day 14, OGCs whose structures had collapsed—for example, those that exhibited complete detachment of granulosa cells from oocytes and/or contained oocytes that showed cytoplasmic degeneration—were classified as degenerative OGCs. After 14 days of culture, the diameters of oocytes that were enclosed by granulosa cells and showed no sign of degeneration were measured as described above and subjected to further experiments.
After in vitro growth culture, oocytes were denuded, fixed with acetic acid-ethanol (1:3), and stained with 1% (wt/vol) aceto-orcein to assess the stage of meiotic division with Nomarski interference microscopy. The oocytes were classified by morphology of the chromatin and nuclear envelope according to the classifications of Motlik et al. [14] and Hirao et al. [15]. The stages for oocytes with an intact germinal vesicle were classified as filamentous chromatin (FC), stringy chromatin (SC), and germinal vesicle I–IV (GV). After germinal vesicle breakdown, stages were classified as metaphase I (MI), anaphase I and telophase I (AI–TI), and metaphase II (MII).
In vitro maturation of oocytes
OGCs with growing oocytes were collected from 0.4−0.7 mm antral follicles and those with surviving oocytes after 14 days of in vitro growth culture were subjected to maturation culture. OGCs with fully grown oocytes collected from 4−6 mm antral follicles were also cultured for maturation as in vivo controls. The OGCs were cultured in 50 μl microdrops of the maturation medium covered with paraffin oil at 38.5ºC under an atmosphere of 5% CO2 in humidified air for 22 h. Each microdrop contained 4−5 OGCs. The maturation medium was bicarbonate-buffered medium 199 supplemented with 10% FBS, 0.1 mg/ml sodium pyruvate, 0.1 IU/ml human menopausal gonadotropin (hMG; Aska Pharmaceutical, Tokyo, Japan), 0.08 mg/ml kanamycin sulfate, and 2.2 mg/ml sodium bicarbonate. After culture, OGCs were subjected to subsequent in vitro fertilization. Selected oocytes were denuded mechanically using a small-bore pipette with the help of 0.1% (wt/vol) hyaluronidase (from bovine testes), then fixed and stained to assess the stage of meiotic division as described above.
In vitro fertilization and development
For in vitro fertilization, straws with frozen semen (Japanese Black, P706, Livestock Improvement Association of Japan, Tokyo, Japan) were thawed in a water bath at 37ºC. The spermatozoa were washed twice by centrifugation. First, they were washed (760 × g) for 20 min over a two-step gradient with 2 ml of 90% and 2 ml of 45% Percoll (GE Healthcare UK, Buckinghamshire, England, UK) in PBS, then washed (372 × g) in 3 ml of insemination medium (IVF100, Research Institute for the Functional Peptides, Yamagata, Japan) for 5 min. The final concentration of spermatozoa was adjusted to 2.8−4.8 × 106 sperm/ml by dilution with the insemination medium. Depending on the available number of OGCs, they were then transferred to 50 or 100 μl microdrops of sperm suspension (50 and 100 μl microdrops contained 10−13 and 20−25 OGCs, respectively) and incubated for 6 or 12 h at 38.5ºC in a 5% CO2 humidified atmosphere. After insemination, some OGCs were subjected to evaluation of fertilization, while others were subjected to evaluation of development. In instances when the number of OGCs were insufficient for both experiments, they were subjected to either of the two experiments.
For evaluation of fertilization, the mean (±SEM) concentrations of spermatozoa in insemination medium for in vivo and in vitro grown OGCs were 3.4 ± 0.1 × 106 sperm/ml (n = 3) and 3.3 ± 0.3 × 106 sperm/ml (n = 4), respectively. For evaluation of development, the mean (± SEM) concentrations of spermatozoa for in vivo and in vitro grown OGCs were 3.3 ± 0.2 × 106 sperm/ml (n = 6) and 3.5 ± 0.3 × 106 sperm/ml (n = 7), respectively. There were no significant differences between the sperm concentrations of in vivo and in vitro grown OGCs (unpaired t test, P > 0.05).
To assess fertilization, some of the oocytes were denuded using a small-bore pipette after 6 or 12 h of insemination, followed by in vitro development (10 or 4 h, respectively) for a total of 16 h after insemination. The oocytes were then fixed with acetic acid-ethanol (1:3) and stained with 1% (wt/vol) aceto-orcein. Oocytes that had two polar bodies and two pronuclei with a sperm tail were classified as normal fertilization. Fertilization other than normal fertilization―for example, oocytes that had an enlarged sperm head with anaphase/telophase I chromosomes, two pronuclei with a sperm tail and one polar body, or more than two sperm heads or pronuclei―were classified as abnormal fertilization. MI, AI−TI, or MII oocytes and oocytes with one or two pronuclei without sperm tails were classified as unfertilized oocytes. Oocytes that showed cytoplasmic degeneration were classified as degenerated oocytes.
After in vitro fertilization, remaining oocytes were denuded mechanically and subjected to development culture. The day of in vitro fertilization was designated day 0. Oocytes (presumptive zygotes) were cultured in 50 µl microdrops of serum free medium (IVD101, Research Institute for the Functional Peptides) for up to 8 days. Each microdrop contained 5−7 oocytes. The rates of blastocyst formation on day 8 were recorded. After 7 days of development culture, several randomly selected blastocysts from in vivo grown oocytes and in vitro grown oocytes were washed twice in PBS−PVA for 15 min each and fixed in 4% (wt/vol) paraformaldehyde in PBS−PVA for 60 min. Fixed blastocysts were washed twice in PBS−PVA for 15 min each and blocked in PBS−PVA containing 1 mg/ml bovine serum albumin for 60 min. Blastocysts were then stained with ProLong Gold Antifade Regent with DAPI (P36931; Molecular Probes, Invitrogen, Carlsbad, CA, USA) and observed under a fluorescence microscope (BX53F; Olympus, Tokyo, Japan) for cell counting.
Statistical analysis
The frequencies of oocytes at each stage of the meiotic division were analyzed using Chi-square test. Differences among the mean (± SEM) diameters of oocytes were analyzed using one-way ANOVA followed by the Tukey-Kramer multiple range test (Excel software with the add-in Ekuseru-Toukei 2010; Social Survey Research Information, Tokyo, Japan). For statistical analyses of fertilization rate and blastocyst formation, data were subjected to one-way ANOVA followed by the Tukey-Kramer multiple range test. Differences in cell numbers of blastocysts were analyzed by an unpaired t test. Values of P < 0.05 were considered significant.
Results
In vitro growth and maturation of oocytes
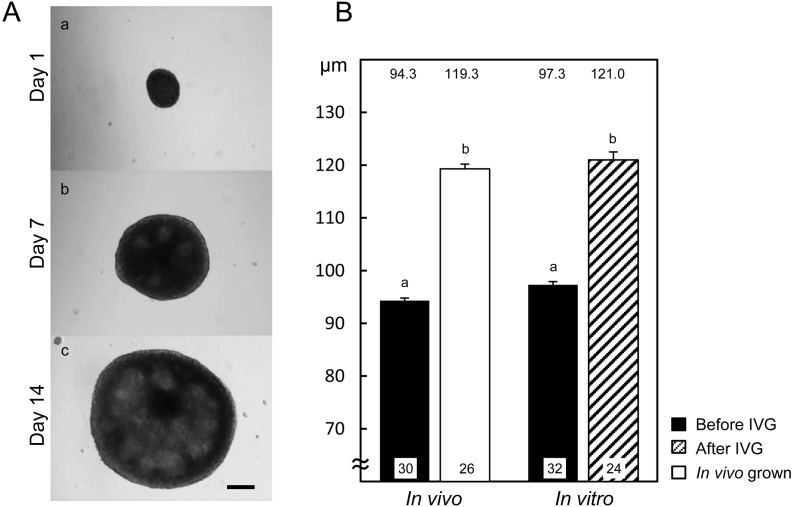
Figure 1A shows the morphology of OGCs during growth culture. On day 1, each complex attached to the membrane and started to grow, and antrum-like cavities were formed in the granulosa cell layers by day 7. The dome-like structures were further developed during culture. On day 14, 75% of OGCs maintained structures that contained viable oocytes. The mean diameters of oocytes collected from 0.4−0.7 mm antral follicles were around 95 µm (Fig. 1B). After 14 days of growth culture, the mean diameter of oocytes was increased to 121.0 ± 1.3 µm, and the diameter was comparable to that of in vivo grown oocytes (119.3 ± 1.0 µm).
Fig. 1.
Typical morphology of bovine OGCs during growth culture (A) and the diameters of oocytes after growth culture (B). (A) OGCs were cultured for 14 days. A scale bar represents 200 μm. (B) The black bars indicate the diameters of oocytes isolated from 0.4−0.7 mm antral follicles before in vitro growth culture (IVG). The blank bar and striped bars represent the diameters of oocytes collected from 4−6 mm antral follicles (In vivo) and after 14 days of growth culture (In vitro), respectively. The numbers of oocytes examined are shown at the bottom of each bar, and the numbers above the bars indicate the mean diameters of oocytes (µm). a, b Values differ significantly (P < 0.05).
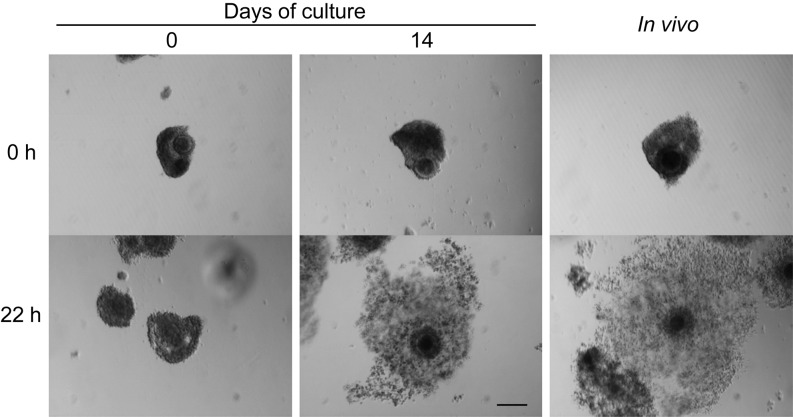
Table 1 shows the meiotic competence of in vitro grown oocytes from 0.4−0.7 mm antral follicles. After 22 h of maturation culture, all oocytes without growth culture remained at the FC (57%) or SC (43%) stage, whereas all oocytes with growth culture resumed meiosis. Both in vivo and in vitro OGCs underwent cumulus expansion during the maturation culture (Fig. 2), and some of the oocytes reached MII after culture; The MII rate of in vitro grown oocytes (79%) was comparable to that of in vivo grown oocytes (81%). When the total number of oocytes initially used for growth culture was taken into consideration, 59% of oocytes matured to MII after maturation culture.
Table 1. Meiotic competence of in vitro grown bovine oocytes.
| In vitro growth (day) 1 | No. of oocytes used 2 | No. (%) of oocytes at the stage of 3 | ||||||
|---|---|---|---|---|---|---|---|---|
| IVG | IVM | FC | SC | GV | MI | AI−TI | MII4 | |
| 0 | — | 30 | 17 (57) | 13 (43) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 14 | 32 | 24 | 0 (0) | 0 (0) | 0 (0) | 2 (8) | 3 (13) | 19 (79) [59] |
| In vivo | — | 26 | 0 (0) | 0 (0) | 0 (0) | 5 (19) | 0 (0) | 21 (81) |
1 OGCs collected from 0.4−0.7 mm antral follicles were subjected to in vitro maturation culture before (0) or after 14 days of in vitro growth culture (14). OGCs from 4−6 mm antral follicles were subjected to in vitro maturation culture as in vivo controls (In vivo). 2 After in vitro growth culture (IVG), OGCs with surviving oocytes were transferred to in vitro maturation culture (IVM). 3 FC: filamentous chromatin, SC: stringy chromatin, GV: germinal vesicle I−IV, MI: metaphase I, AI−TI: anaphase I and telophase I, MII: metaphase II. 4 The number in [ ] indicates the percentage of MII oocytes from the oocytes initially used for IVG.
Fig. 2.
Representative morphology of bovine OGCs before and after maturation culture. After 14 days of growth culture, OGCs with surviving oocytes were subjected to maturation culture for 22 h. OGCs collected from 0.4−0.7 mm antral follicles (0) and 4−6 mm antral follicles (In vivo) were cultured as for in vivo control. A scale bar represents 200 µm.
Fertilization ability of oocytes
After maturation culture, oocytes grown in vivo and in vitro were fertilized in vitro to assess their fertilization ability. After 6 and 12 h of insemination, oocytes grown in vivo were fertilized normally (38 and 37%, respectively) (Table 2). All of these oocytes had two polar bodies and two pronuclei with a sperm tail (Fig. 3A). There were no significant differences in rates of total unfertilized in vivo grown oocytes between 6 and 12 h of insemination (52 and 41%, respectively); however, the rate of unfertilized MII oocytes significantly decreased, and the rate of oocytes that formed pronuclei without corresponding sperm tail (classified as Others in Table 2) increased after 12 h of insemination. Among in vitro grown oocytes, only 3% of oocytes showed normal fertilization and most of the oocytes (74%) were unfertilized after 6 h of insemination (Table 2). After 12 h of insemination, the rate of unfertilized oocytes significantly decreased, especially for MII oocytes; conversely the normal fertilization rate increased to 34% and was similar to that of in vivo grown oocytes. Normal fertilized oocytes had two polar bodies and two pronuclei with a sperm tail (Fig. 3B). Abnormal fertilization rates of in vitro grown oocytes were slightly higher than those of in vivo grown oocytes for both 6 and 12 h of insemination.
Table 2. Fertilization ability of in vitro grown bovine oocytes after in vitro maturation.
| Growth 1 | Insemination (h) |
No. of oocytes examined (replicates) |
Fertilized oocytes (%) 2 | Unfertilized oocytes (%) 3 | DG 4 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal | Abnormal fertilization | ||||||||||
| 2PN2PB | Polyspermy | Others | Total | MI | MII | Others | Total | ||||
| In vitro | 6 | 31 (4) | 1 (3)a | 4 (13)a | 3 (9)ab | 7 (23)ab | 3 (10) | 11 (35)a | 9 (29)ab | 23 (74)a | 0 (0) |
| 12 | 32 (4) | 11 (34)b | 5 (16)a | 6 (19)b | 11 (34)b | 4 (13) | 2 (6)b | 4 (13)a | 10 (31)b | 0 (0) | |
| In vivo | 6 | 50 (3) | 19 (38)b | 1 (2)b | 2 (4)a | 3 (6)a | 4 (8) | 16 (32)a | 6 (12)a | 26 (52)ab | 2 (4) |
| 12 | 49 (3) | 18 (37)b | 3 (6)ab | 6 (12)ab | 9 (18)ab | 1 (2) | 2 (4)b | 17 (35)b | 20 (41)ab | 2 (4) | |
1 OGCs collected from 0.4−0.7 mm antral follicles were subjected to in vitro growth culture with 10 ng/ml 17β-estradiol (E2) and 10 ng/ml androstenedione (A4) for 14 days (In vitro). Examined oocytes from 4−6 mm antral follicles were used as in vivo controls (In vivo). 2 Normal fertilization: the oocytes that have two polar bodies and two pronuclei with a sperm tail (2PN2PB). Abnormal fertilization: fertilization other than normal fertilization; for example, the oocytes have an enlarged sperm head with anaphase/telophase I chromosomes, two pronuclei with a sperm tail and one polar body, or more than two sperm heads or pronuclei (polyspermy). 3 Metaphase I (MI), or metaphase II (MII) oocytes and oocytes that have one or two pronuclei without sperm tails (Others) were classified as unfertilized oocytes. 4 Degenerated oocytes. a, b Values with different superscripts in the same column differ significantly (P < 0.05).
Fig. 3.
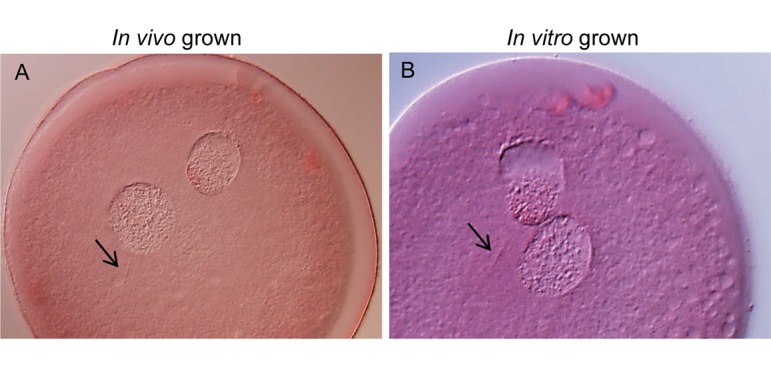
Morphology of in vivo and in vitro grown bovine oocytes after in vitro fertilization. After 16 h of insemination, both in vivo (A) and in vitro (B) grown oocytes had two pronuclei and two polar bodies. The arrows indicate sperm tails.
Developmental competence of oocytes
The developmental competences of in vivo and in vitro grown oocytes were assessed by development culture up to 8 days (Table 3). After insemination for 6 and 12 h, oocytes grown in vivo developed into blastocysts (23% and 22%, respectively) following development culture for 8 days. Although almost none of the in vitro grown oocytes that were inseminated for 6 h developed into blastocysts after 8 days of culture (1%), 22% of the oocytes developed into blastocysts when they were inseminated for 12 h. This blastocyst formation rate was comparable to that of in vivo grown oocytes.
Table 3. Developmental competence of in vitro grown bovine oocytes after in vitro maturation and fertilization.
| Growth 1 | Insemination (h) | No. of oocytes used 2
(replicates) |
No. of blastocysts (%) 3 |
|---|---|---|---|
| In vitro | 6 | 95 (7) | 1 (1)a |
| 12 | 98 (7) | 22 (22)b | |
| In vivo | 6 | 136 (6) | 31 (23)b |
| 12 | 126 (6) | 28 (22)b |
1 OGCs collected from 0.4−0.7 mm antral follicles were subjected to in vitro growth culture for 14 days followed by in vitro maturation and fertilization (In vitro). Examined oocytes from 4−6 mm antral follicles were used as in vivo controls (In vivo). 2 Number of oocytes used for in vitro fertilization. 3 After in vitro fertilization, oocytes were further cultured for development up to 8 days. a, b Values with different superscripts in the same column differ significantly (P < 0.05).
To assess the quality of blastocysts derived from in vitro grown oocytes, cell numbers of randomly selected blastocysts were counted on day 7 of in vitro development (Fig. 4). The mean numbers of blastocyst cells derived from in vivo and in vitro grown oocytes were 90.5 ± 4.0 (n = 9) and 90.8 ± 5.3 (n = 8), respectively; these numbers were comparable with each other.
Fig. 4.
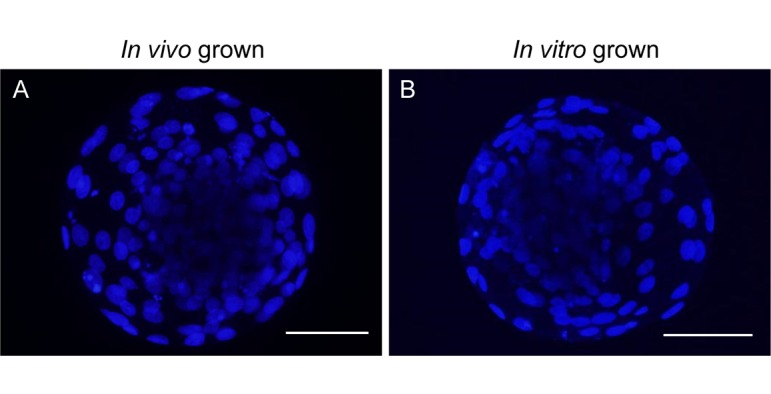
Bovine blastocysts derived from in vivo and in vitro grown oocytes. Blastocysts (Day 7 of in vitro development) derived from in vivo grown oocytes isolated from 4−6 mm antral follicles (A) and in vitro grown oocytes growth-cultured with E2 and A4 (B) were stained with DAPI. Scale bars represent 100 µm.
Discussion
During oocyte growth phase in the ovary, oocytes increase their volume, acquire maturation competence, and accumulate the materials necessary for prospective fertilization and development. In this study, we showed that in vitro growth culture supported the acquisition of fertilization ability and developmental competence, as well as complete oocyte growth and meiotic competence during in vitro growth.
After in vitro maturation and fertilization, 22% of in vitro grown oocytes inseminated for 12 h developed into blastocysts following development culture for 8 days. This blastocyst formation rate was similar to that of in vivo grown oocytes. Since blastocyst cell numbers were also similar between in vitro and in vivo grown oocytes, the quality of blastocysts derived from in vitro grown oocytes appeared to be comparable to that from in vivo grown oocytes. Moreover, more than one third of oocytes grown in vivo and in vitro were fertilized normally after 16 h of insemination, having two pronuclei and two polar bodies. These results indicated that in vitro grown oocytes have normal fertilization ability and are competent to develop into blastocysts.
However, few in vitro grown oocytes were fertilized normally after 6 h of insemination, and almost none of them developed into blastocysts after 8 days of culture. The rate of unfertilized oocytes decreased and normal fertilized in vitro grown oocytes increased to 34% only after 12 h of insemination. Meanwhile, there were no significant changes in rates of normal fertilized and total unfertilized in vivo grown oocytes between 6 and 12 h of insemination. Although the rate of unfertilized MII oocytes decreased, the normal fertilization rate was not increased even after 12 h of insemination. Unfertilized oocytes seemed to undergo parthenogenetic activation, since they formed pronuclei without corresponding sperm tail. These results suggested that fertilization of in vitro grown oocytes was delayed compared with that of in vivo grown oocytes.
Since spermatozoa have to penetrate cumulus cell layers and the zona pellucida to reach the oocyte plasma membrane, cumulus cells and the zona pellucida are key factors of fertilization. Cumulus expansion during oocyte maturation is essential for normal fertilization [16], and the cumulus cells in expanded cumulus induce sperm capacitation [17]. Since these abilities are considered to be regulated by oocyte secreted paracrine factors during oocyte growth [18], it is suggested that in vitro cultured cumulus cells might not acquire enough ability to expand completely or to induce the sperm capacitation needed for fertilization. After penetration cumulus cell layers, spermatozoa hydrolyze and penetrate the zona pellucida to reach the oocyte plasma membrane. The zona pellucida is formed during oocyte growth and increase in thickness as oocytes increase in diameter [19]. Although zona pellucida glycoproteins are exclusively synthesized by oocytes in mice [20], it is considered that both oocytes and granulosa cells synthesize the zona pellucida in bovine follicles [21, 22]. Long-term growth culture may influence the zona pellucida glycoprotein production in oocytes and/or granulosa cells and thus, influence the morphology or function of the zona pellucida. Although we did not examine the zona pellucida properties of in vitro grown oocytes in this experiment, it is inferred that in vitro grown oocytes took longer to be penetrated by spermatozoa than in vivo grown oocytes due to specific factors (cumulus cells and the zona pellucida). Alternatively, the oocyte itself could also be a factor that influences the fertilization of in vitro grown oocytes. For successful fertilization and subsequent development, both nuclear maturation and cytoplasmic maturation of oocytes are important [23], and oocytes acquire these competences during their growth phase. Since abnormal fertilization rates of in vitro grown oocytes were generally higher than those of in vivo grown oocytes, it is thought that some of the in vitro grown oocytes might not accomplish cytoplasmic maturation during in vitro maturation culture despite of the resulting MII percentage that was similar to that of in vivo grown oocytes.
Oocytes ultimately formed two polar bodies and two pronuclei with a sperm tail at 16 h of insemination after fertilization and developed normally into blastocysts after 8 days of culture. The cell number of blastocysts was also normal. In conclusion, in vitro grown bovine oocytes are similar to in vivo grown oocytes in fertilization ability and are competent to develop into blastocysts.
Acknowledgments
We are grateful to the staff of Kobe-Branch, Animal Biotechnology Center, Livestock Improvement Association of Japan, Inc., for supplying ovaries and the staff of Okayama A.I. Center, Livestock Improvement Association of Japan, Inc., for supplying frozen semen. This work was supported in part by Japan Society for the Promotion of Science KAKENHI Grant Number 25292192 (to TM).
References
- 1.Chang MC. Fertilization of rabbit ova in vitro. Nature 1959; 184(Suppl 7): 466–467. [DOI] [PubMed] [Google Scholar]
- 2.Iritani A, Niwa K. Capacitation of bull spermatozoa and fertilization in vitro of cattle follicular oocytes matured in culture. J Reprod Fertil 1977; 50: 119–121. [DOI] [PubMed] [Google Scholar]
- 3.Brackett BG, Bousquet D, Boice ML, Donawick WJ, Evans JF, Dressel MA. Normal development following in vitro fertilization in the cow. Biol Reprod 1982; 27: 147–158. [DOI] [PubMed] [Google Scholar]
- 4.Bavister BD. Early history of in vitro fertilization. Reproduction 2002; 124: 181–196. [DOI] [PubMed] [Google Scholar]
- 5.Miyano T. In vitro growth of mammalian oocytes. J Reprod Dev 2005; 51: 169–176. [DOI] [PubMed] [Google Scholar]
- 6.Picton HM, Harris SE, Muruvi W, Chambers EL. The in vitro growth and maturation of follicles. Reproduction 2008; 136: 703–715. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto K, Otoi T, Koyama N, Horikita N, Tachikawa S, Miyano T. Development to live young from bovine small oocytes after growth, maturation and fertilization in vitro. Theriogenology 1999; 52: 81–89. [DOI] [PubMed] [Google Scholar]
- 8.Hirao Y, Itoh T, Shimizu M, Iga K, Aoyagi K, Kobayashi M, Kacchi M, Hoshi H, Takenouchi N. In vitro growth and development of bovine oocyte-granulosa cell complexes on the flat substratum: effects of high polyvinylpyrrolidone concentration in culture medium. Biol Reprod 2004; 70: 83–91. [DOI] [PubMed] [Google Scholar]
- 9.Huang W, Kang SS, Nagai K, Yanagawa Y, Takahashi Y, Nagano M. Mitochondrial activity during pre-maturational culture in in vitro-grown bovine oocytes is related to maturational and developmental competences. Reprod Fertil Dev 2014; 28: 349–356. [DOI] [PubMed] [Google Scholar]
- 10.Lequarre AS, Vigneron C, Ribaucour F, Holm P, Donnay I, Dalbiès-Tran R, Callesen H, Mermillod P. Influence of antral follicle size on oocyte characteristics and embryo development in the bovine. Theriogenology 2005; 63: 841–859. [DOI] [PubMed] [Google Scholar]
- 11.Krisher RL. In vivo and in vitro environmental effects on mammalian oocyte quality. Annu Rev Anim Biosci 2013; 1: 393–417. [DOI] [PubMed] [Google Scholar]
- 12.Makita M, Miyano T. Steroid hormones promote bovine oocyte growth and connection with granulosa cells. Theriogenology 2014; 82: 605–612. [DOI] [PubMed] [Google Scholar]
- 13.Makita M, Miyano T. Androgens promote the acquisition of maturation competence in bovine oocytes. J Reprod Dev 2015; 61: 211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Motlík J, Koefoed-Johnsen HH, Fulka J. Breakdown of the germinal vesicle in bovine oocytes cultivated in vitro. J Exp Zool 1978; 205: 377–383. [DOI] [PubMed] [Google Scholar]
- 15.Hirao Y, Tsuji Y, Miyano T, Okano A, Miyake M, Kato S, Moor RM. Association between p34cdc2 levels and meiotic arrest in pig oocytes during early growth. Zygote 1995; 3: 325–332. [DOI] [PubMed] [Google Scholar]
- 16.Chen L, Russell PT, Larsen WJ. Functional significance of cumulus expansion in the mouse: roles for the preovulatory synthesis of hyaluronic acid within the cumulus mass. Mol Reprod Dev 1993; 34: 87–93. [DOI] [PubMed] [Google Scholar]
- 17.Shimada M, Yanai Y, Okazaki T, Noma N, Kawashima I, Mori T, Richards JS. Hyaluronan fragments generated by sperm-secreted hyaluronidase stimulate cytokine/chemokine production via the TLR2 and TLR4 pathway in cumulus cells of ovulated COCs, which may enhance fertilization. Development 2008; 135: 2001–2011. [DOI] [PubMed] [Google Scholar]
- 18.Elvin JA, Clark AT, Wang P, Wolfman NM, Matzuk MM. Paracrine actions of growth differentiation factor-9 in the mammalian ovary. Mol Endocrinol 1999; 13: 1035–1048. [DOI] [PubMed] [Google Scholar]
- 19.Wassarman PM. Zona pellucida glycoproteins. J Biol Chem 2008; 283: 24285–24289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wassarman PM, Litscher ES. Mammalian fertilization: the egg’s multifunctional zona pellucida. Int J Dev Biol 2008; 52: 665–676. [DOI] [PubMed] [Google Scholar]
- 21.Kölle S, Sinowatz F, Boie G, Palma G. Differential expression of ZPC in the bovine ovary, oocyte, and embryo. Mol Reprod Dev 1998; 49: 435–443. [DOI] [PubMed] [Google Scholar]
- 22.Sinowatz F, Kölle S, Töpfer-Petersen E. Biosynthesis and expression of zona pellucida glycoproteins in mammals. Cells Tissues Organs 2001; 168: 24–35. [DOI] [PubMed] [Google Scholar]
- 23.Ferreira EM, Vireque AA, Adona PR, Meirelles FV, Ferriani RA, Navarro PA. Cytoplasmic maturation of bovine oocytes: structural and biochemical modifications and acquisition of developmental competence. Theriogenology 2009; 71: 836–848. [DOI] [PubMed] [Google Scholar]