Abstract
Recently, the conception rates after artificial insemination have been pointed out to decline continuously. To overcome this problem, the control of frozen and thawed sperm quality is required. However, the mechanism of bovine sperm functional regulation is still largely unknown. In mammals, the ejaculated sperm are capable of showing fertilizing ability during migration in the female reproductive organs. It is well known that these female organs secrete several factors contributing to sperm capacitation. We previously reported that neurotensin (NT) secreted from the oviduct and cumulus cells enhanced sperm capacitation and acrosome reaction in mice. In this study, we confirmed the expression of the NT receptor (NTR1) in the bovine sperm neck region and the secretion of NT in the bovine uterus and oviduct. The similar expression patterns of NT and NTR1 suggests a conserved mechanism of sperm functional regulation between mouse and cattle. Thus, we examined the effects of exogenous NT on the bovine sperm functions. First, we showed that NT induced sperm protein tyrosine phosphorylation in a dose-dependent manner, suggesting that NT enhances sperm capacitation. Second, we showed that NT induced acrosome reactions of capacitated sperm in a dose-dependent manner, suggesting that NT facilitates acrosome reaction. Finally, we used a computer-aided sperm analysis system to show that NT did not have a great effect on sperm motility. These results suggest that NT acts as a facilitator of sperm capacitation and acrosome reaction in the female reproductive tracts in cattle, highlighting the importance of NT-mediated signaling to regulate sperm functions.
Keywords: Acrosome reaction, Bovine sperm, Capacitation, Neurotensin, Sperm motility
In the industrial framework of bovine reproduction, artificial insemination using frozen bovine semen is generally conducted. The trouble is that the conception rates after artificial insemination have declined continuously in Japan and other countries [1, 2]. The causes of this problem are not well known, but one possibility is inappropriate sperm quality for fertilization after artificial insemination [3]. In this regard, an adequate control of sperm quality after freezing and thawing can improve the efficiency of bovine production through artificial insemination.
Mammalian spermatozoa cannot fertilize an oocyte immediately after ejaculation. During migration, sperm are exposed to various factors in the female reproductive tract, such as hormones, signal transducing molecules, enzymes, ions and lipids secreted from female tissues [4]. It is through the interaction with these factors that sperm gain the fertilizing ability (i.e. capacitation) [5]. Consequently, only a limited number of sperm arriving at the oviductal ampulla undergo acrosomal exocytosis (i.e. acrosome reaction). Thus, numerous factors secreted from the female reproductive organs have an essential role in the fertilization process, by contributing to sperm function and, therefore, participating in the control of sperm quality.
Several studies have reported that receptors for some neurotransmitters, such as gamma aminobutyric acid [6], dopamine [7], or serotonin [8], are present in mammalian sperm. Binding of these receptors to their corresponding ligands is thought to regulate sperm function.
Neurotensin (NT) has been isolated from the bovine hypothalamus and characterized as a hypotensive neuropeptide. NT consists of 13 amino acids, and it is excised from an NT precursor [9]. In the brain, NT is believed to act as a modulator of the dopaminergic system [10, 11]. Intriguingly, NT expression is not only found in the central nervous system, but also in the small intestine and stomach where it participates in gastrointestinal motility and secretion [12]. Furthermore, the NT receptor is present in lymphocytes as well as in brain [13]. These reports together suggest that NT has multiple functions in several organs.
Three types of receptors (NTRs), named NTR1, NTR2, and NTR3 have been isolated from mouse and cattle. NTR1 and NTR2 belong to a family of receptors with seven transmembrane spanning domains and are coupled to G proteins (G protein-coupled receptors), whereas NTR3 belongs to a family of sorting receptors [14].
Previously, we reported that NTR1 expressed in mouse sperm, NT was secreted from reproductive tracts and cumulus cells, and NT enhanced sperm protein tyrosine phosphorylation and acrosome reaction in mice [15]. Moreover, a recent paper showed that the expression of mRNA for NT occurs in the bovine oviduct epithelium [16], suggesting a common reproductive mechanism between mouse and cattle. These findings motivated us to explore the possibility of using NT for controlling the sperm fertilization potential in bull. Thus, in this study, we investigated the expression of NT receptor in bovine sperm and examined the effects of NT on the bovine sperm.
Materials and Methods
Sperm and female reproductive tissue preparations
For sperm preparation, four semen straws from different mature Japanese Black cattle bulls were used in this study. Four of the bulls were of known fertility and each semen sample contained at least 60% of progressively motile sperm after thawing. Each frozen semen sample was thawed in a water bath at 38°C for 15 sec and transferred to 1.5 ml capped plastic tubes with 1000 μl of phosphate-buffered saline (PBS, pH 7.4). After centrifugation of the tubes at 1000 rpm for 5 min at room temperature, the supernatant was discarded and the sediment was resuspended in BGM-1 (bovine gamete medium 1) at a concentration of 5 × 107 cells/ml [17]. Each sperm suspension (from four different bulls) was used for each of the performed experiments. For uterus and oviduct preparation, three female reproductive organs from different Japanese Black cattle cows or heifers, at an unknown estrous cycle phase, were obtained from a local slaughterhouse, transferred to laboratory, and dissected into small pieces using fine forceps.
Western blot and immunocytochemistry
Western blot analysis was performed to determine the expression of NTR1 in bovine sperm and the NT expression in the female uterus and oviduct. Samples (sperm pellets or shreds of uterus/oviduct) were homogenized in ice-cold RIPA buffer (50 mM Tris-HCl [pH 7.6], 150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, and 1% protease inhibitor) (Nacalai Tesque, Kyoto, Japan) and sonicated on ice, to extract total cell or tissue proteins. The samples were then centrifuged, and the supernatants were collected. Each supernatant was resuspended in the same volume of 2 × sample buffer (Nacalai Tesque), and the extracted solutions were boiled for 5 min. The proteins were separated by 12% SDS-PAGE and transferred to polyvinylidene difluoride membranes. Membrane blocking with Blocking One (Nacalai Tesque) was performed for 60 min at room temperature. After three washes with PBS containing 0.1% Tween 20, membranes were treated with mouse monoclonal anti-NTR1 antibody (sc-374492, 1:1000; Santa Cruz Biotechnology, Dallas, TX, USA) or rabbit polyclonal anti-NT precursor antibody (sc-20806, 1:1000; Santa Cruz Biotechnology), overnight at 4°C, and washed again three times with PBS containing 0.1% Tween 20. Then, membranes were treated with horseradish peroxidase (HRP)-conjugated anti-mouse immunoglobulin M antibody (1:1000; Santa Cruz Biotechnology) or HRP-conjugated anti-rabbit immunoglobulin G antibody (1:2000; Promega, Madison, WI, USA), for 2 h at room temperature. After three more washes, the membrane was reacted with Chemilumi-One (Nacalai Tesque), and images were obtained using a LAS-3000-mini Lumino Image Analyzer (Fujifilm, Tokyo, Japan).
Immunocytochemical analysis was performed to investigate the localization of NTR1 in sperm. The sperm samples were collected by centrifugation at 1000 rpm for 5 min, and fixed with 2% paraformaldehyde and 1% Triton-X in PBS for 15 min at 4°C. They were then washed twice with PBS, and blocked with Blocking One (Nacalai Tesque) for 60 min at 4°C. Next, the sperm suspensions were incubated with anti-NTR1 antibody (1:100) overnight at 4°C. After being washed three times with PBS, the suspensions were incubated for 60 min at room temperature with Alexa Fluor 488-conjugated anti-mouse immunoglobulin M (1:500; Thermo Fisher Scientific, Waltham, MA, USA) and Hoechst 33342 (1:5000; Thermo Fisher Scientific). The treated samples were then washed, suspended in PBS, mounted on glass slides, and finally covered with glass. Images were obtained by using a LSM-710 confocal laser microscope (Carl Zeiss, Jena, Germany).
Sperm protein tyrosine phosphorylation analysis
To detect sperm protein tyrosine phosphorylation, equivalent volumes of the suspensions were divided into microtubules. PBS (control) or NT (Merck Millipore, Darmstadt, Germany) dissolved in saline, to a final concentration of 0.1, 1 and 10 µM, were added to the sample. The suspensions were cultured for 4 h under 5% CO2 in air at 38°C. After incubation, the sperm pellets were collected by centrifugation at 10,000 rpm for 5 min at 4°C. The obtained sperm pellets were washed with PBS and centrifuged again at 10,000 rpm. After the supernatants were discarded, Laemmli sample buffer was added to extract the proteins. The obtained proteins were separated by 12% SDS-PAGE and transferred to polyvinylidene membranes. After 60 min of blocking with 1% bovine serum albumin (BSA) in Tris-buffered saline containing 0.1% Tween 20 (TBS-T, pH 7.5–7.8), the membrane was treated overnight at 4°C with a mouse monoclonal anti-phosphotyrosine 4G10 antibody (05-321, 1:20000; Merck Millipore) or with anti-α-tubulin (T9026, 1:10000; Merck Millipore) as an internal control. After three washes with TBS-T, the membrane was treated with HRP-conjugated anti-mouse immunoglobulin G antibody (1:2000; Promega) for 2 h at room temperature. Detection was performed with Chemilumi One and images were obtained using the Lumino Image Analyzer. Densitometric analyses were performed using the Image Gauge v4.22 analysis software (Fujifilm).
Acrosome reaction assay by lectin staining
After equal volumes of the suspension were divided into microtubes, sperm capacitation was induced using the heparin treatment described in Parrish et al. [18]. Briefly, the sperm samples were incubated in BGM-1 medium, and added to 10 μg/ml heparin for 4 h at 38°C under 5% CO2 in air. After incubation, the NT solution was added to each suspension at the final concentrations of 0.1, 1, 10, and 100 µM, and the samples were incubated for 30 min at 38°C under 5% CO2 in air. Then, each sample was smeared onto glass slides and air-dried. After 60 min of blocking using Blocking One, the acrosome reaction was assessed by staining with fluorescein isothiocyanate-conjugated peanut agglutinin lectin (FITC-PNA) (J Oil Mills, Tokyo, Japan) [19]. FITC-PNA was diluted 1:500 in a light-shielded humidity chamber. After washing with PBS, the slides were covered with mounting medium and glass. The acrosome-reacted spermatozoa were quantified under a fluorescence microscope. Duplicate counting of at least 100 sperm cells was performed. The percentage of sperm with no fluorescence over the acrosomal region was calculated as the number of PNA-negative sperm cells per total counted sperm.
Sperm motility assay
After capacitation treatment, NT was added to each suspension at a final concentration of 1, 10, and 100 µM. After 0, 10, 30, and 60 min, 4 μl of the samples was placed onto 2-chamber slides with a depth of 12 μm (SC 12-01-C; Leja, Nieuw-Vennep, Netherlands). At least 200 sperm cells in five fields (at least 200 sperm) of a chamber were divided into motile and dead sperm, and both the percentage of motile sperm and the sperm motility parameters were evaluated using a computer assisted sperm analysis (CASA) system (SMAS, DITECT, Tokyo, Japan). Films were taken for 1 sec, at an interval of once per 1/60 sec. The evaluated sperm motility parameters were straight-line velocity (VSL, μm/sec), curvilinear velocity (VCL, μm/sec), linearity (LIN = VSL/VCL × 100, %), amplitude of lateral head displacement (ALH, μm), and beat-cross frequency (BCF, Hz). The trajectories of sperm were automatically extracted from the movie and overlaid on the last frame, by using the CASA system.
Statistical analysis
All experiments were replicated at least three times. Data are presented as means ± standard error (SE). Statistical analyses were carried out using analysis of variance (ANOVA) and the Fisher’s protected least significant difference test. A P value < 0.01 was considered to indicate significant differences (** P < 0.01, * P < 0.05).
Results
Expression of NTR1 in spermatozoa
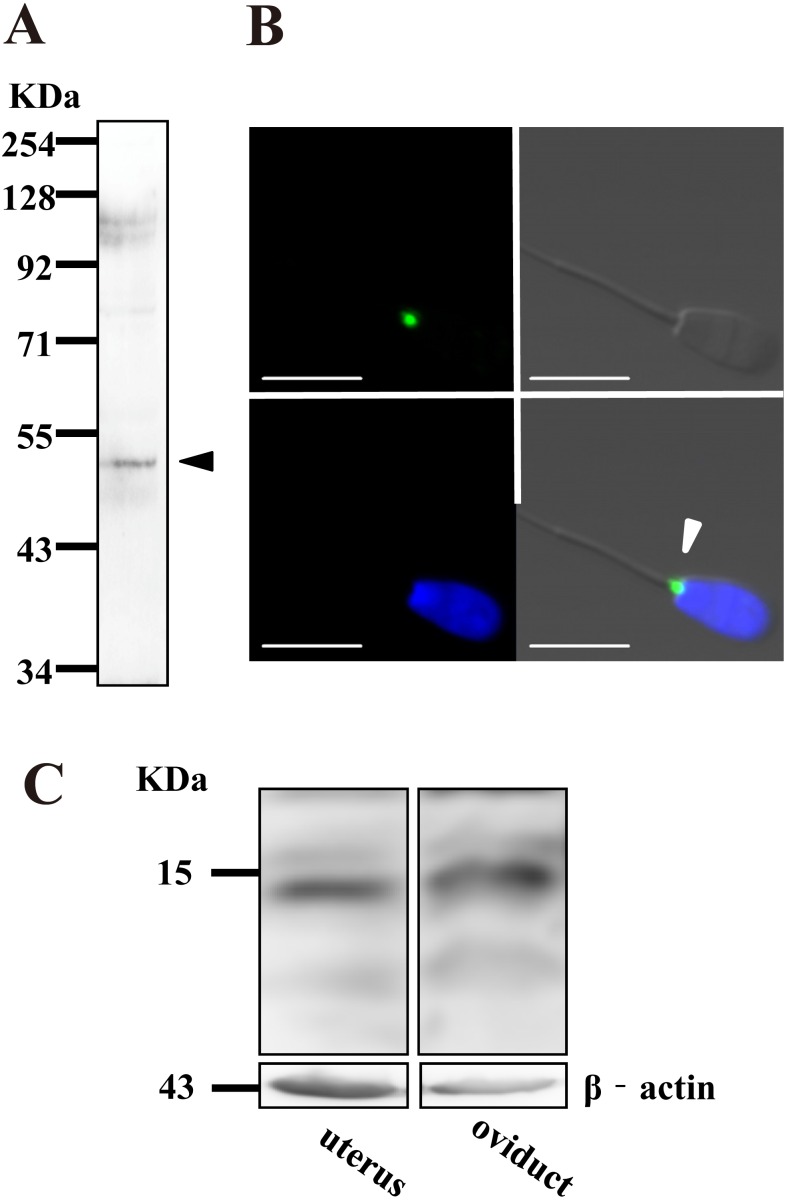
We performed western blot and immunocytochemical analyses using the anti-NTR1 antibody, to determine the expression and localization of NTR1 in bovine sperm. By western blot analysis, we detected a specific band around 48 kDa, which coincided with the molecular size of NTR1 (Fig. 1A). In addition, immunocytochemical analysis showed spot-like immunoreactivity of NTR1 at the neck region of bovine sperm (Fig. 1B), which was abolished by incubation without the NTR1 antibody (data not shown). Importantly, we also found expression of the NT precursor in bovine uterus and oviduct (Fig. 1C). Together, these findings suggest that bovine sperm have competence to bind the NT molecule secreted from female reproductive organs.
Fig. 1.
Detection of NTR1 expression in bovine sperm. (A) NTR1 expression in bovine sperm was revealed by western blot analysis. (B) Representative immunocytochemical image showing NTR1 localization in bovine sperm. Note the spot like immunoreactivity, which was detected at the neck region of sperm (arrowhead). Blue: nuclear (Hoechst 33342), Green: NTR1, Bars = 10 μm. (C) NT precursor expression in a bovine uterus (left) and oviduct (right) was revealed by western-blot analysis. β-actin was used as a control.
Effect of neurotensin on sperm protein tyrosine phosphorylation
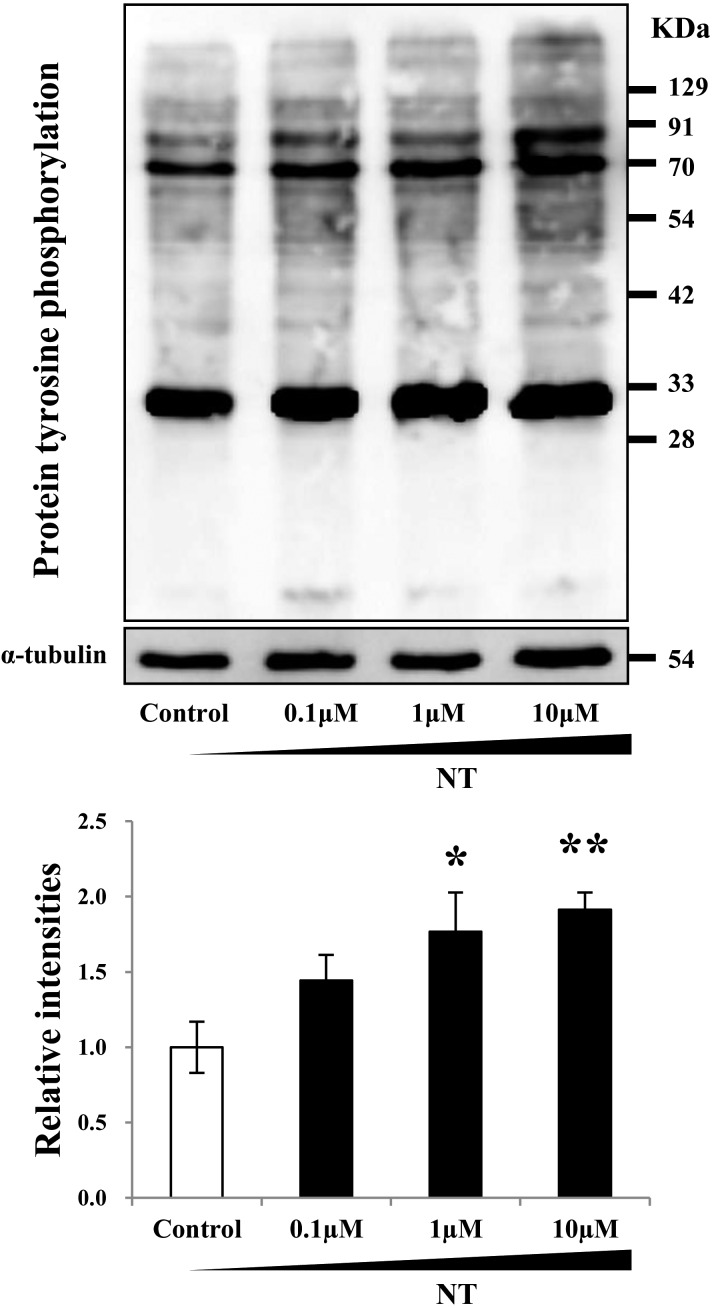
We asked whether NT induces sperm protein tyrosine phosphorylation, which is the major indicator of sperm capacitation [20, 21]. After sperm incubation in BGM-1 medium supplemented with NT at various concentrations for 4 h, total tyrosine phosphorylation level was increased in a concentration-dependent manner (Fig. 2). In particular, tyrosine phosphorylation of sperm proteins around 30, 70, and 90 kDa were increased remarkably. These results suggest that NT exposure to bovine sperm enhances capacitation, probably through the interaction between NT and NTR1.
Fig. 2.
Effect of NT on sperm protein tyrosine phosphorylation. Protein tyrosine phosphorylation in bovine sperm was revealed by western blotting. Upper panel shows the representative blotting image of at least five repeated experiments, with independent sample preparations. Note that bands around 30, 70, and 90 kDa increased in a dose-dependent manner by NT addition. The lower panel shows quantification and comparison of the total amount of tyrosine phosphorylated proteins between control and NT treated sperm. The control protein bands were assigned a base value of 1. α-Tubulin was used as a loading control. Data are shown as the means ± SE (*: P < 0.05, **: P < 0.01).
Effect of neurotensin on acrosome reaction
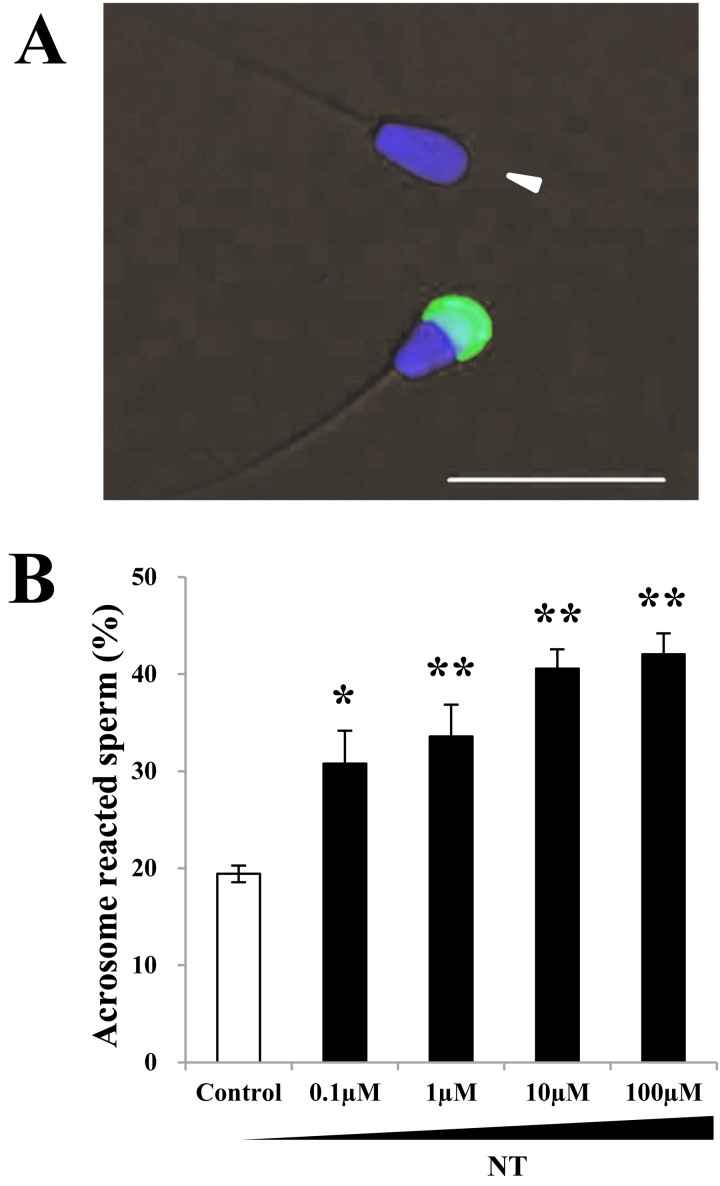
We evaluated the effect of NT on the acrosomal disappearance in capacitated sperm. After 4 h of sperm incubation in heparin-containing BGM-1 medium, to induce capacitation, the acrosome status of sperm was determined by staining with FITC-PNA lectin [19] (Fig. 3A). The percentages of sperm without PNA-positive acrosome after administration of 0 (control), 0.1, 1, 10 and 100 µM of NT were 19.4 ± 0.9, 30.8 ± 3.4, 33.6 ± 3.3, 40.6 ± 2.0 and 42.1 ± 2.1, respectively (Fig. 3B). Dose dependent increase of sperm acrosomal disappearance suggests that NT is likely to facilitate acrosome reaction of bovine capacitated sperm.
Fig. 3.
Effect of NT on the acrosome reaction. (A) Representative image of PNA staining for bovine sperm. Sperm with no green fluorescence over the acrosomal region were scored as PNA-negative sperm (arrowhead). Blue: nuclear (Hoechst 33342), Green: PNA, Bar = 50 μm. (B) Fraction of acrosome reacted (PNA-negative) sperm at various doses of NT (0, 0.1, 1, 10, 100 μM). Sperm acrosomal disappearance rates were evaluated by calculating the number of PNA-negative sperm out of total sperm. Each experiment was replicated four times. Data are shown as the means ± SE (*: P < 0.05, **: P < 0.01).
Effect of neurotensin on sperm motility
Finally, we evaluated the effects of NT treatment on sperm motility, which is a critical sperm function for normal fertilization. Before NT treatment, around half of the bovine sperm were motile and showed a symmetrical flagellar bend and beat pattern that drives sperm in a straight line movement, which is called activated motility [22, 23] (Fig. 4A, Table 1). When sperm movement shifts to hyperactivated motility, flagellar bend and beat pattern are changed to asymmetric and acquire a higher amplitude, which reflects in the motility parameters as follows: increase of VCL and ALH, and decrease of LIN [26, 27]. By using the CASA system, we found that the average of each of the sperm motility parameters, including motility rate (Motile), VSL, VCL, LIN, ALH and BCF, is not significantly changed by any dose of NT treatment at any time point (Table 1). Interestingly, while the vast majority of sperm did not show a significant change in the motility patterns, a small number of sperm cells showed a hyperactivated motility pattern (Fig. 4B). This suggests that the effect of NT on sperm motility is small, while cellular responses can be heterogeneous. Taken together, these results suggest that NT does not have a great effect on sperm motility.
Fig. 4.
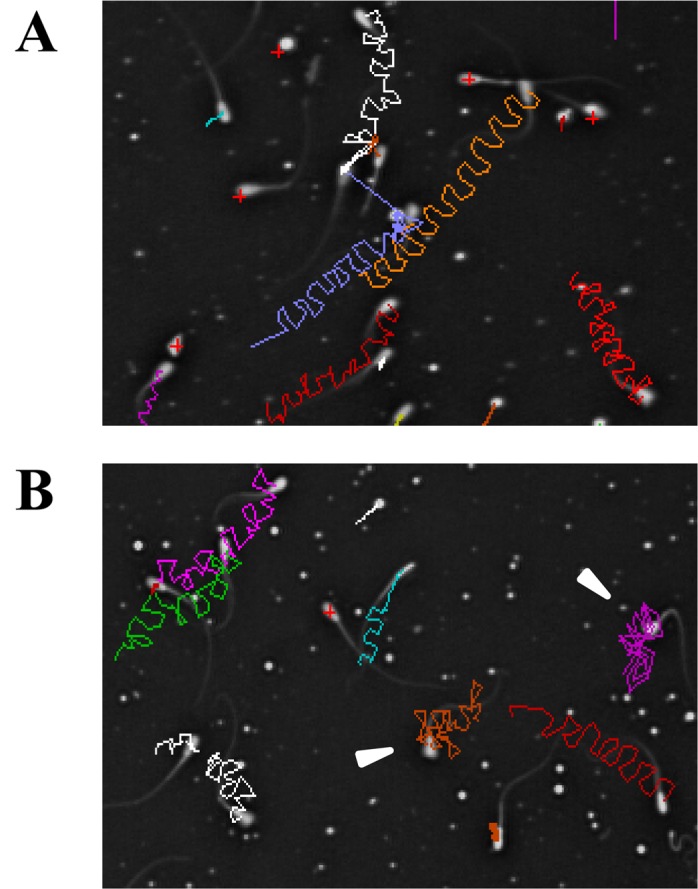
Effect of NT on sperm motility. (A, B) Images showing representative sperm movement 10 min after NT treatment in the control (0 μM) (A) and 10-μM exposure groups (B). Trajectories of individual sperm are shown by colored lines, overlaid on the last frame. Arrowheads indicate hyperactivated sperm.
Table 1. CASA measurements of the effects of neurotensin (NT) on bovine sperm motility.
| Sperm motility parameters | Control | NT | |||
|---|---|---|---|---|---|
| 1 μM | 10 μM | 100 μM | |||
| 0 min | Motile (%) | 52.6 ± 5.0 | 55.7 ± 4.2 | 55.8 ± 3.4 | 57.5 ± 4.2 |
| VSL (μm/sec) | 37.6 ± 5.4 | 43.9 ± 8.1 | 45.4 ± 3.9 | 40.2 ± 7.1 | |
| VCL (μm/sec) | 123.8 ± 15.9 | 140.3 ± 19.2 | 146.2 ± 7.4 | 130.3 ± 13.4 | |
| LIN (%) | 36.0 ± 2.8 | 34.0 ± 1.7 | 32.0 ± 1.3 | 33.0 ± 3.0 | |
| ALH (μm) | 3.3 ± 0.4 | 3.7 ± 0.4 | 4.2 ± 0.1 | 3.7 ± 0.3 | |
| BCF (Hz) | 10.8 ± 0.4 | 10.9 ± 0.4 | 11.1 ± 0.5 | 10.3 ± 0.3 | |
| 10 min | Motile (%) | 52.3 ± 6.0 | 55.4 ± 5.5 | 50.5 ± 4.4 | 55.0 ± 5.4 |
| VSL (μm/sec) | 38.9 ± 7.1 | 43.3 ± 8.8 | 43.9 ± 4.3 | 41.3 ± 7.5 | |
| VCL (μm/sec) | 126.0 ± 17.9 | 142.0 ± 18.2 | 149.0 ± 9.6 | 141.3 ± 19.9 | |
| LIN (%) | 37.2 ± 2.4 | 33.0 ± 1.4 | 31.8 ± 1.2 | 32.4 ± 0.5 | |
| ALH (μm) | 3.4 ± 0.5 | 3.9 ± 0.4 | 4.1 ± 0.2 | 4.0 ± 0.5 | |
| BCF (Hz) | 11.2 ± 0.1 | 11.4 ± 0.5 | 11.1 ± 0.6 | 11.2 ± 0.4 | |
| 30 min | Motile (%) | 47.9 ± 1.2 | 55.5 ± 4.4 | 47.5 ± 4.5 | 50.7 ± 6.9 |
| VSL (μm/sec) | 42.5 ± 10.5 | 45.1 ± 8.8 | 48.8 ± 6.0 | 45.0 ± 8.8 | |
| VCL (μm/sec) | 132.9 ± 28.0 | 143.0 ± 20.6 | 158.2 ± 15.1 | 144.6 ± 19.4 | |
| LIN (%) | 37.2 ± 3.4 | 36.2 ± 2.4 | 32.2 ± 1.1 | 32.2 ± 1.9 | |
| ALH (μm) | 3.7 ± 0.7 | 3.9 ± 0.5 | 4.3 ± 0.3 | 3.8 ± 0.4 | |
| BCF (Hz) | 11.4 ± 0.3 | 11.4 ± 0.6 | 11.7 ± 0.6 | 10.8 ± 0.4 | |
| 60 min | Motile (%) | 51.0 ± 3.2 | 53.8 ± 4.5 | 50.5 ± 5.4 | 46.9 ± 5.2 |
| VSL (μm/sec) | 41.7 ± 10.9 | 43.0 ± 8.5 | 47.5 ± 7.4 | 47.3 ± 7.9 | |
| VCL (μm/sec) | 131.8 ± 28.5 | 136.3 ± 22.6 | 146.1 ± 18.8 | 152.9 ± 21.3 | |
| LIN (%) | 35.2 ± 3.6 | 35.2 ± 1.1 | 34.8 ± 2.9 | 32.0 ± 2.2 | |
| ALH (μm) | 3.4 ± 0.6 | 3.7 ± 0.5 | 3.8 ± 0.4 | 4.2 ± 0.5 | |
| BCF (Hz) | 11.4 ± 0.5 | 11.3 ± 0.2 | 11.3 ± 0.2 | 11.3 ± 0.4 | |
Data are shown as the means ± SE. VSL, straight line velocity; VCL, curvilinear velocity, LIN, linearity; ALH, amplitude of lateral head displacement; BCF, beat cross frequency.
Discussion
In this study, we revealed that the expression of NTR1 is restricted to the neck region of bovine sperm (Fig. 1). Consistently with a recent report [16], we also detected the NT precursor protein in bovine oviduct, where sperm hyperactivation and fertilization occur. Moreover, we also found expression of the NT precursor in the uterus, where sperm capacitation occurs. Importantly, the expression pattern of the NT precursor includes an extended area of the female reproductive tract, and NTR1 localization in the sperm neck region is conserved between cattle and mouse [15]. In mice, it was reported that NT can act as a promoter of sperm capacitation and the acrosome reaction [15]. Thus, we hypothesized that NT could promote bovine sperm functional changes in mouse. In this study, we conducted three experiments to investigate the effects of NT on bovine sperm functions that were essential for normal fertilization.
First, we showed that NT facilitates protein tyrosine phosphorylation in bovine sperm in a dose-dependent manner, suggesting that NT induces capacitation of bovine sperm (Fig. 2). Such a dose-dependent increase of broadly distributed protein tyrosine phosphorylation during sperm capacitation has also been reported in cattle and buffalo, by using other capacitation inducers [20, 21, 24, 25]. It has been accepted that cAMP elevation and subsequent PKA activation is necessary for protein tyrosine phosphorylation in sperm [26]. Interestingly, several reports showed NT regulated cAMP levels in other types of cells [27,28,29]. Thus, we argue that NT induces cAMP production via its receptor(s) in bovine sperm.
Second, our analysis showed that sperm acrosomal loss was significantly increased in a dose-dependent manner by NT (Fig. 3). Since the acrosomal disappearance in frozen and thawed sperm can be caused by artificial cryo-injury [30], we cannot exclude the possibility that the NT treatment enhances the acrosomal damage of cryopreserved and thawed sperm. However, considering the evidence for NT presence in the bovine oviduct and the previous findings that NT facilitates the acrosome reaction of unfrozen mouse sperm [15], the involvement of the NT signaling in the acrosome reaction of capacitated sperm is likely to occur in cattle. The acrosome reaction is an exocytotic process triggered by elevation of Ca2+ levels [31, 32]. Our data showed that NT-signaling is received at the sperm neck region. How does the acceptance of NT-signaling at the sperm neck region induce acrosome reaction in the sperm head region? Our recent paper suggested that NT induces the elevation of Ca2+ levels and Ca2+ mobilization from the neck region to the head region in mouse sperm [15]. Considering this report, we propose that the elevation in Ca2+ levels and the calcium mobilization triggered by NT-signaling induce the acrosome reaction in bull sperm.
Finally, here we revealed that the average of the sperm motilities is not significantly affected by NT, while a small proportion of sperm exhibit hyperactivated motility (Table 1 and Fig. 4). Thus, our results indicate that NT does not have major effects on sperm motility.
Taken together, our results suggest that the major role of NT is the facilitation of both the tyrosine phosphorylation and acrosome reaction, rather than hyperactivation of bovine sperm motility. It was reported that different signaling pathways regulate capacitation and hyperactivation in bovine sperm, and therefore, hyperactivation can occur independently of capacitation [33, 34]. NT signaling may primarily regulate signaling pathways upstream of capacitation, rather than pathways that regulate hyperactivation in bovine sperm. Further studies are needed to clarify the working molecular mechanism for NT signaling in bovine sperm.
The results of enhancing tyrosine phosphorylation and acrosome reaction are consistent with our previous report in mouse [15]. However, there are various anatomical and physiological differences between both species, such as length of oviduct and time required for fertilization from ejaculation. Thus, further elucidation of the physiology of NT secretion in females will help the understanding of these inter-species differences.
In Japan and other countries, low conception rate after artificial insemination caused by unknown mechanism has become a serious problem in bovine reproduction. This study provides a plausible hypothesis that NT levels in the female uterus affect the sperm capacitation in bovine artificial insemination. In this regard, it will be interesting to investigate epidemiologically the correlation between past conception rates and NT levels of individual female cows. If the positive correlation between low NT levels and low conception rate is clarified, exogenous NT administration to the female reproductive tract at the time of sperm injection may be useful to rescue female function and enhance sperm capacitation. This study firstly highlights the potential usefulness of NT-mediated signaling to control bovine sperm functions.
Acknowledgments
The authors want to thank the Miyagi livestock experimental station for giving the bovine spermatozoa. This study was supported by the Meat Inspection Office, Sendai, Japan.
References
- 1.Dochi O, Kabeya S, Koyama H. Factors affecting reproductive performance in high milk-producing Holstein cows. J Reprod Dev 2010; 56(Suppl): S61–S65. [DOI] [PubMed] [Google Scholar]
- 2.Barbat A, Le Mézec P, Ducrocq V, Mattalia S, Fritz S, Boichard D, Ponsart C, Humblot P. Female fertility in French dairy breeds: current situation and strategies for improvement. J Reprod Dev 2010; 56(Suppl): S15–S21. [DOI] [PubMed] [Google Scholar]
- 3.Kishida K, Sakase M, Minami K, Arai MM, Syoji R, Kohama N, Akiyama T, Oka A, Harayama H, Fukushima M. Effects of acrosomal conditions of frozen-thawed spermatozoa on the results of artificial insemination in Japanese Black cattle. J Reprod Dev 2015; 61: 519–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghersevich S, Massa E, Zumoffen C. Oviductal secretion and gamete interaction. Reproduction 2015; 149: R1–R14. [DOI] [PubMed] [Google Scholar]
- 5.Yanagimachi R, Chang MC. Fertilization of hamster eggs in vitro. Nature 1963; 200: 281–282. [DOI] [PubMed] [Google Scholar]
- 6.Puente MA, Tartaglione CM, Ritta MN. Bull sperm acrosome reaction induced by gamma-aminobutyric acid (GABA) is mediated by GABAergic receptors type A. Anim Reprod Sci 2011; 127: 31–37. [DOI] [PubMed] [Google Scholar]
- 7.Ramírez AR, Castro MA, Angulo C, Ramió L, Rivera MM, Torres M, Rigau T, Rodríguez-Gil JE, Concha II. The presence and function of dopamine type 2 receptors in boar sperm: a possible role for dopamine in viability, capacitation, and modulation of sperm motility. Biol Reprod 2009; 80: 753–761. [DOI] [PubMed] [Google Scholar]
- 8.Meizel S, Turner KO. Serotonin or its agonist 5-methoxytryptamine can stimulate hamster sperm acrosome reactions in a more direct manner than catecholamines. J Exp Zool 1983; 226: 171–174. [DOI] [PubMed] [Google Scholar]
- 9.Carraway R, Leeman SE. The isolation of a new hypotensive peptide, neurotensin, from bovine hypothalami. J Biol Chem 1973; 248: 6854–6861. [PubMed] [Google Scholar]
- 10.Thibault D, Albert PR, Pineyro G, Trudeau LE. Neurotensin triggers dopamine D2 receptor desensitization through a protein kinase C and beta-arrestin1-dependent mechanism. J Biol Chem 2011; 286: 9174–9184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Binder EB, Kinkead B, Owens MJ, Nemeroff CB. Neurotensin and dopamine interactions. Pharmacol Rev 2001; 53: 453–486. [PubMed] [Google Scholar]
- 12.Zhao D, Pothoulakis C. Effects of NT on gastrointestinal motility and secretion, and role in intestinal inflammation. Peptides 2006; 27: 2434–2444. [DOI] [PubMed] [Google Scholar]
- 13.Ramez M, Bagot M, Nikolova M, Boumsell L, Vita N, Chalon P, Caput D, Ferrara P, Bensussan A. Functional characterization of neurotensin receptors in human cutaneous T cell lymphoma malignant lymphocytes. J Invest Dermatol 2001; 117: 687–693. [DOI] [PubMed] [Google Scholar]
- 14.Pelaprat D. Interactions between neurotensin receptors and G proteins. Peptides 2006; 27: 2476–2487. [DOI] [PubMed] [Google Scholar]
- 15.Hiradate Y, Inoue H, Kobayashi N, Shirakata Y, Suzuki Y, Gotoh A, Roh SG, Uchida T, Katoh K, Yoshida M, Sato E, Tanemura K. Neurotensin enhances sperm capacitation and acrosome reaction in mice. Biol Reprod 2014; 91: 53. [DOI] [PubMed] [Google Scholar]
- 16.Cerny KL, Garrett E, Walton AJ, Anderson LH, Bridges PJ. A transcriptomal analysis of bovine oviductal epithelial cells collected during the follicular phase versus the luteal phase of the estrous cycle. Reprod Biol Endocrinol 2015; 13: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parrish JJ, Susko-Parrish JL, Uguz C, First NL. Differences in the role of cyclic adenosine 3′,5′-monophosphate during capacitation of bovine sperm by heparin or oviduct fluid. Biol Reprod 1994; 51: 1099–1108. [DOI] [PubMed] [Google Scholar]
- 18.Parrish JJ, Susko-Parrish J, Winer MA, First NL. Capacitation of bovine sperm by heparin. Biol Reprod 1988; 38: 1171–1180. [DOI] [PubMed] [Google Scholar]
- 19.Cross NL, Watson SK. Assessing acrosomal status of bovine sperm using fluoresceinated lectins. Theriogenology 1994; 42: 89–98. [DOI] [PubMed] [Google Scholar]
- 20.Galantino-Homer HL, Visconti PE, Kopf GS. Regulation of protein tyrosine phosphorylation during bovine sperm capacitation by a cyclic adenosine 3’5′-monophosphate-dependent pathway. Biol Reprod 1997; 56: 707–719. [DOI] [PubMed] [Google Scholar]
- 21.Visconti PE, Kopf GS. Regulation of protein phosphorylation during sperm capacitation. Biol Reprod 1998; 59: 1–6. [DOI] [PubMed] [Google Scholar]
- 22.Suárez SS, Osman RA. Initiation of hyperactivated flagellar bending in mouse sperm within the female reproductive tract. Biol Reprod 1987; 36: 1191–1198. [DOI] [PubMed] [Google Scholar]
- 23.Katz DF, Vanagimachi R. Movement characteristics of hamster spermatozoa within the oviduct. Biol Reprod 1980; 22: 759–764. [DOI] [PubMed] [Google Scholar]
- 24.Roy SC, Atreja SK. Tyrosine phosphorylation of a 38-kDa capacitation-associated buffalo (Bubalus bubalis) sperm protein is induced by L-arginine and regulated through a cAMP/PKA-independent pathway. Int J Androl 2008; 31: 12–24. [DOI] [PubMed] [Google Scholar]
- 25.Roy SC, Atreja SK. Capacitation-associated protein tyrosine phosphorylation starts early in buffalo (Bubalus bubalis) spermatozoa as compared to cattle. Anim Reprod Sci 2009; 110: 319–325. [DOI] [PubMed] [Google Scholar]
- 26.Breitbart H. Signaling pathways in sperm capacitation and acrosome reaction. Cell Mol Biol (Noisy-le-grand) 2003; 49: 321–327. [PubMed] [Google Scholar]
- 27.Carraway RE, Mitra SP. Neurotensin enhances agonist-induced cAMP accumulation in PC3 cells via Ca2+ -dependent adenylyl cyclase(s). Mol Cell Endocrinol 1998; 144: 47–57. [DOI] [PubMed] [Google Scholar]
- 28.Shi WX, Bunney BS. Actions of neurotensin: a review of the electrophysiological studies. Ann N Y Acad Sci 1992; 668: 129–145. [DOI] [PubMed] [Google Scholar]
- 29.Firth SI, Boelen MK, Morgan IG. Enkephalin, neurotensin and somatostatin increase cAMP levels in the chicken retina. Aust N Z J Ophthalmol 1998; 26(Suppl 1): S65–S67. [DOI] [PubMed] [Google Scholar]
- 30.Holt WV. Basic aspects of frozen storage of semen. Anim Reprod Sci 2000; 62: 3–22. [DOI] [PubMed] [Google Scholar]
- 31.Talbot P, Summers RG, Hylander BL, Keough EM, Franklin LE. The role of calcium in the acrosome reaction: an analysis using ionophore A23187. J Exp Zool 1976; 198: 383–392. [DOI] [PubMed] [Google Scholar]
- 32.Breitbart H. Intracellular calcium regulation in sperm capacitation and acrosomal reaction. Mol Cell Endocrinol 2002; 187: 139–144. [DOI] [PubMed] [Google Scholar]
- 33.Suarez SS, Ho HC. Hyperactivated motility in sperm. Reprod Domest Anim 2003; 38: 119–124. [DOI] [PubMed] [Google Scholar]
- 34.Marquez B, Suarez SS. Different signaling pathways in bovine sperm regulate capacitation and hyperactivation. Biol Reprod 2004; 70: 1626–1633. [DOI] [PubMed] [Google Scholar]