ABSTRACT
We discovered that Cadherin-11 (CDH11) regulates collagen and elastin synthesis, both affecting the mechanical properties and contractile function of animal tissues. Using a Cdh11-null mouse model, we observed a significant reduction in the mechanical properties [Youngs' modulus and ultimate tensile strength (UTS)] of Cdh11−/− as compared to wild-type (WT) mouse tissues, such as the aorta, bladder and skin. The deterioration of mechanical properties (Youngs' modulus and UTS) was accompanied by reduced collagen and elastin content in Cdh11−/− mouse tissues as well as in cells in culture. Similarly, knocking down CDH11 abolished collagen and elastin synthesis in human cells, and consequently reduced their ability to generate force. Conversely, engagement of CDH11 through homophilic interactions, led to swift activation of the TGF-β and ROCK pathways as evidenced by phosphorylation of downstream effectors. Subsequently, activation of the key transcription factors, MRTF-A (also known as MKL1) and MYOCD led to significant upregulation of collagen and elastin genes. Taken together, our results demonstrate a novel role of adherens junctions in regulating extracellular matrix (ECM) synthesis with implications for many important biological processes, including maintenance of tissue integrity, wound healing and tissue regeneration.
KEY WORDS: Cadherin-11, Extracellular matrix, Tissue regeneration, MRTF-A, Myocardin, Collagen, Elastin, Mechanical properties
Summary: CDH11 regulates collagen and elastin synthesis by both affecting the mechanical properties and contractile function of animal tissues through TGF-β and ROCK pathways.
INTRODUCTION
Cell–cell adhesion through cadherins plays an important role in multiple aspects of cellular behavior including proliferation, differentiation, apoptosis, cell polarity (Niessen and Gumbiner, 2002; Cavallaro and Dejana, 2011), embryonic stem cell self-renewal and differentiation (Li et al., 2012b), tissue morphogenesis and maintenance of tissue integrity (Harris and Tepass, 2010). CDH11 is expressed in osteoblasts and mesenchymal cells as well as in epithelial cells undergoing epithelial–mesenchymal transition (EMT), as for example during progression of cancer cells into a metastatic state (Kimura et al., 1995; Tomita et al., 2000; Zeisberg and Neilson, 2009). Recent work from our laboratory has shown that cell–cell adhesion through CDH11 is crucial for differentiation of mesenchymal stem cells (MSCs) into contractile smooth muscle cells (SMCs) by regulating expression of the key transcription factor SRF. In addition, CDH11 is necessary for development of contractility of smooth-muscle-containing tissues such as artery and bladder (Alimperti et al., 2014).
Extracellular matrix proteins such as collagens and elastin are abundant in all body tissues and play crucial roles in development, tissue remodeling following injury, and maintenance of tissue mechanics and function in homeostasis. As a result, loss of collagen and/or elastin has been implicated in many diseases and is well-documented in aging (Kohl et al., 2011; Wagenseil and Mecham, 2012). For example, fragmented or irregular distribution of collagen and elastin fibrils have been implicated in bladder incontinence as well as cardiovascular disorders such as hypertension, aneurysms and Marfan's syndrome (Goepel and Thomssen, 2006; Li, 2012a; Benke et al., 2013). By contrast, excessive and cumulative deposition of collagen disrupts organ architecture, leading to scar formation and loss of function. Recent studies have implicated CDH11 in lung and skin fibrosis (Schneider et al., 2012; Agarwal, 2014; Wu et al., 2014), myofibroblast migration to site of lung fibrosis (Schneider et al., 2012) and mesenchymal stem cell differentiation into smooth muscle cells (Alimperti et al., 2014). However, it is not clear whether CDH11-mediated adherens junctions regulate ECM production and the signaling mechanisms that might be involved remain unknown.
To this end, we set out to determine the role of CDH11 in ECM production and identify the mechanism of action. Our work was motivated by our initial discovery that smooth-muscle- or myofibroblast-containing tissues such as aorta, bladder and skin from CDH11-null mice (Cdh11−/−) exhibited significantly impaired mechanical strength as well as significantly reduced collagen and elastin content, as compared to WT mice. This is not only a novel but also very surprising result given that Cdh11−/− mice develop normally, are fertile and display no obvious phenotype other than modest osteopenia (Shin et al., 2000; Kawaguchi et al., 2001a,b) and decreased lung and skin fibrosis after lung injury (Schneider et al., 2012; Wu et al., 2014). Using a combination of gene knockdown and gain-of-function approaches, we discovered a direct and novel role of adherens junction molecule CDH11 in extracellular matrix (ECM) production and identified the pathways and transcription factors mediating this action. Taken together, our results implicate cell–cell adhesion as a regulator of ECM production and suggest new ways through which adherens junctions might regulate important biological processes from maintaining tissue integrity to promoting wound healing and tissue regeneration.
RESULTS
Cdh11−/− mouse tissues exhibit diminished mechanical properties such as Youngs' modulus and UTS
Recent work from our laboratory demonstrated that loss of CDH11 impaired the myogenic differentiation capacity of MSCs and diminished the contractile ability of SMCs that were derived from MSCs. In addition, SMC-containing tissues, such as the aorta and bladder, exhibited significantly reduced levels of SMC proteins and diminished contractile ability compared with WT controls (Alimperti et al., 2014). These results prompted us to hypothesize that loss of CDH11 might also affect the mechanical properties of these tissues or organs, possibly by affecting expression of extracellular matrix components.
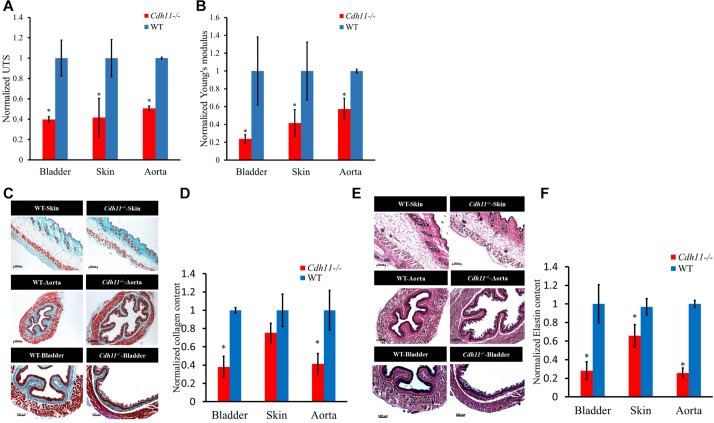
To address this hypothesis, first we examined the mechanical properties of smooth-muscle- and myofibroblast-containing tissues, such as aorta, bladder and skin of 6–8-week-old Cdh11−/− and WT mice. Tissue rings were mounted onto an Instron tensile tester and stretched unilaterally with constant strain until the yielding point. The ultimate tensile stress (UTS) and Young's modulus were normalized to corresponding values of WT tissues (Fig. 1A,B). Surprisingly, we observed a significant reduction in both the UTS and Young's modulus of Cdh11−/− mice tissues (40–60%, n=4, P<0.05), indicating that loss of CDH11 correlated with impaired mechanical strength.
Fig. 1.
Mechanical properties and ECM composition of Cdh11−/− and WT mouse tissues. (A) Ultimate tensile strength (UTS) and (B) Young's moduli of bladder, skin and aorta tissues reported as normalized to corresponding WT tissues (n=4). (C) Histological examination of collagen content in cross-sections of skin, aorta and bladder tissue from Cdh11−/− and WT mice by Masson's trichrome staining. Scale bars: 200 µm. (D) Collagen content as quantified by hydroxyl-proline assay in Cdh11−/− skin, aorta and bladder tissues and reported after normalization to WT (n=4). (E) Histological examination of elastin content in cross-sections of skin, aorta and bladder tissue from Cdh11−/− and WT mice by Verhoeff's elastin staining. Scale bars: 200 µm. (F) Elastin content as quantified by ninhydrin assay in Cdh11−/− skin, aorta and bladder tissues and reported as after normalization to WT (n=4). All quantitative results are mean±s.d. *P<0.05 as compared to WT (unpaired two-tailed Student's t-test).
Cdh11−/− mouse tissues exhibit impaired ECM production
Given that the mechanical properties are derived mostly from extracellular matrix (ECM) we investigated the collagen and elastin content of aorta, bladder and skin from Cdh11−/− and WT mice. Masson's trichrome staining (blue) revealed a reduced amount of collagen in all three tissues of Cdh11−/− as compared to WT mice (Fig. 1C). Interestingly, the collagen layer (blue) in the muscle layer of aorta and bladder of Cdh11−/− animals also appeared thinner (see also Table S1). In addition, quantitative measurements of collagen content using the hydroxyl-proline assay, showed a significant 70% reduction (n=4, P<0.05, unpaired two-tailed Student's t-test) in the Cdh11−/− bladder and aorta tissues (Fig. 1D). Values were internally normalized to WT tissues after the collagen content of each tissue specimen was normalized to its own dry weight. Therefore, although the absolute value of collagen was also lower for Cdh11−/− skin, the dry weight of WT skin of equal surface area was higher, thereby bringing the normalized collagen content closer to that of Cdh11−/− tissues.
Similarly, Verhoeff's staining showed fewer elastin fibers in Cdh11−/− tissues (Fig. 1E, brown-black fibers), as evidenced by lower staining intensity and lack of elastin fiber continuity (see higher magnification images in Fig. S1A). Quantitative measurement of elastin using the colorimetric ninhydrin assay revealed that Cdh11−/− tissues contained 30–60% (P<0.05, n=9, unpaired two-tailed Student's t-test) less fibrous elastin than their WT counterparts (Fig. 1F). Taken together, the reduction in collagen and elastin content is consistent with the reduced mechanical properties exhibited by the Cdh11−/− tissues.
Cdh11−/− cells exhibit reduced expression of collagen and elastin genes
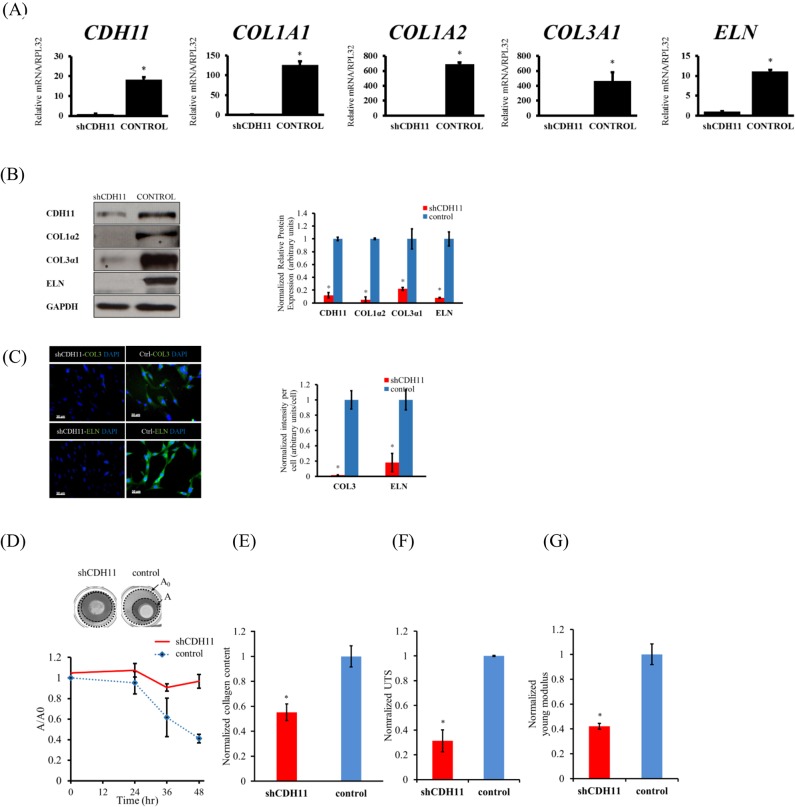
Next, we compared the capacity of Cdh11−/− versus WT cells to synthesize ECM. To this end, dermal fibroblasts and aortic smooth muscle cells (ASMCs) from WT and Cdh11−/− mice were tested for expression levels of ECM genes (Fig. 2A). Interestingly, quantitative real-time PCR (qRT-PCR) revealed that Cdh11−/− ASMCs exhibited significant loss in collagen and elastin mRNA (∼10-fold for Col1a1 and Eln, 20–30-fold for Col1a2 and Col3a1), but the loss was more dramatic for Cdh11−/− dermal fibroblasts (∼450-fold for Col1a1, ∼90-fold for Col1a2, ∼1200-fold for Col3a1 and ∼12-fold for Eln). Similar results were observed at the protein level as evidenced by western blots showing significantly decreased protein levels of COL1α2, COL3α1 and to a lesser extent ELN in Cdh11−/− cells (Fig. 2B). This result was further confirmed by immunohistochemistry for COL1 and ELN (Fig. 2C,D). In agreement, the mRNA levels of several transcription factors that are known to regulate collagen and elastin expression such as Myocd, Mrtfa (also known as Mkl1), Mrtfb (also known as Mkl2) and Srf were significantly reduced in Cdh11−/− as compared to WT mouse dermal fibroblasts (Fig. S1B). By contrast, the level of Fbn1 remained unaffected (Fig. S1C). These results indicate a strong relationship between CDH11 and ECM production.
Fig. 2.
ECM synthesis in Cdh11−/− and WT mouse cells. (A) qRT-PCR for the indicated genes. The relative mRNA levels are reported as the fold change with respect to Cdh11−/− samples (n=4). Dermal fibroblasts (DFs) are shown in top panels (solid) and ASMCs are shown in bottom panels (hatched). (B) Protein levels of COL1α2, ELN and COL3α1 as analyzed by western blotting. Quantification of the band intensity for each protein in Cdh11−/− cells normalized to the corresponding band in WT cells (n=3). (C) Immunostaining for CDH11, COL1 and ELN (green) and counterstained with DAPI (blue). Scale bar: 100 µm. (D) Normalized intensity per cell quantified from n=5 fields of view. (E) Compaction of fibrin hydrogels containing Cdh11−/− or WT cells. Upper panel, picture of hydrogels around a cylindrical mandrel after 48 h. The dotted lines demarcate the inside and outside diameter for each gel. Lower panel, average of the area of each gel as a fraction of the initial gel area (A/A0) plotted over time (h) (n=6). (F) Collagen content of fibrin gel rings as quantified by hydroxyl-proline assay and normalized to WT (n=6). (G) Ultimate tensile strength (UTS) and (H) Young's modulus of fibrin gel rings containing Cdh11−/− or WT cells. The data are normalized to the corresponding WT rings (n=6). All quantitative results are mean±s.d. *P<0.05 as compared to WT (unpaired two-tailed Student's t-test).
Cdh11−/− dermal fibroblasts displayed reduced contractility and ECM production in 3D
The above results prompted us to examine whether loss of Cdh11 might affect the ability of myofibroblasts to generate force. To this end, cells were embedded in fibrin hydrogels (106 cells/ml) and 1 h after polymerization the gels were released from the plate wall and allowed to undergo compaction in the presence of TGF-β1. After 48 h, the area of each gel was measured and normalized to its initial area. As shown in Fig. 2E, Cdh11−/− cells exhibited significantly impaired ability to show compaction as compared to WT cells (Cdh11−/−, 43±6% versus WT, 76±9% of the initial gel area, n=6, P<0.05; mean±s.d.). Furthermore, the initial rate of compaction was ∼2.38% per hour for WT cells but less than half of that at ∼1.01% per hour for Cdh11−/− cells.
We also measured the mechanical properties and collagen content of tissue constructs prepared from Cdh11−/− or WT mouse dermal fibroblasts, as we described in previous publications (Diaz-Chavez et al., 2008; Liang et al., 2013; Koobatian et al., 2016). To this end, we prepared cylindrical tissue equivalents by embedding cells in fibrin hydrogels that were polymerized around cylindrical mandrels and cultured in the presence of TGF-β1 for 2 weeks. At that time, the collagen content was measured using the hydroxyl-proline assay and showed that Cdh11−/− dermal fibroblasts produced only 28.3±0.69% (n=6, P<0.05) of the collagen amount produced by WT cells (Fig. 2F). In addition, both the UTS and Young's modulus of Cdh11−/− tissue constructs were significantly lower at ∼55% (n=6, P<0.05) of tissue constructs containing WT cells (Fig. 2G,H). These results show that, consistent with the in vivo data, Cdh11−/− dermal fibroblasts exhibited significantly impaired capacity to generate force and remodel 3D tissue constructs, which exhibited compromised mechanical properties.
Knockdown of CDH11 in human dermal fibroblasts compromises ECM production
Next, we tested whether the relationship between loss of CDH11 and ECM deposition holds for human cells as well, by knocking down CDH11 in human neonatal foreskin dermal fibroblasts using short hairpin RNA (shRNA)-encoding lentivirus (shCDH11). In agreement with Cdh11−/− mouse cells, the ability of shCDH11 human dermal fibroblasts to synthesize ECM was severely compromised as compared to their WT counterparts. Specifically, shCDH11 cells exhibited considerably lower levels of COL1A1 (∼120-fold), COL3A1 (∼480-fold) and ELN (∼11-fold) compared to WT human dermal fibroblasts (Fig. 3A). The protein levels of CDH11, ELN, COL1α2 and COL3α1 were also reduced dramatically as assessed by western blotting (Fig. 3B) and immunostaining for COL3α1 and ELN (Fig. 3C). Moreover, knocking down of CDH11 significantly reduced the mRNA and protein levels of transcription factors that have been implicated in collagen production such as MYOCD, SRF, and MRTF-A and -B, in agreement with our findings for mouse dermal fibroblasts (Fig. S2A,B).
Fig. 3.
ECM synthesis in shCDH11 and control human dermal fibroblasts. (A) The relative mRNA levels are reported as the fold change with respect to shCDH11 samples (n=4). *P<0.05 as compared to shCDH11 cells. (B) Protein levels of CDH11, ELN, COL1α2, and COL3α1 as analyzed by western blot. GAPDH served as a loading control. A quantification is also shown (n=4). *P<0.05 as compared to control. (C) Immunostaining for COL3 and ELN (green) and counterstained with DAPI (blue). Scale bar: 100 µm. A quantification is also shown (n=4). *P<0.05 as compared to control. (D) Compaction of fibrin hydrogels containing shCDH11 or control human dermal fibroblasts. Upper panel, picture of hydrogels around a cylindrical mandrel after 48 h. The dotted lines demarcate the inside and outside diameter for each gel. Lower panel, average of the area of each gel as a fraction of the initial gel area (A/A0) plotted over time (h) (n=6). (E) Collagen content of fibrin gel rings as quantified by hydroxyl-proline assay and normalized to WT (n=6). *P<0.05 as compared to control. (F) Ultimate tensile strength (UTS) and (G) Young's modulus of fibrin gel rings containing shCDH11 or WT cells. The data are normalized to the corresponding control rings (n=6). *P<0.05 as compared to control. All quantitative results are mean±s.d. and P-values were calculated with an unpaired two-tailed Student's t-test.
In addition, transcriptional activity was measured through the activity of the CArG response element (CArG-RE) or serum response element (SRE), using a triple promoter lentiviral vector that was previously developed in our laboratory (Alimperti et al., 2012). This vector encodes for ZsGreen under the CArG-RE, DsRed2 under the constitutive human PGK promoter and shRNA under a tetracycline regulatable H1 promoter in the viral LTR. Transduced cells are expected to express DsRed constitutively but express ZsGreen only upon CArG-RE activation, which can be quantified by fluorescence microscopy. As shown in Fig. S2C, knocking down CDH11 reduced the CArG-RE activity significantly as compared to control cells (scrambled shRNA), suggesting reduced SRF transcriptional activity.
The contractility of shCDH11 cells was also tested using 3D fibrin gels, as described above. As shown in Fig. 3D, fibrin gel compaction was abolished upon CDH11 knockdown. The collagen content of fibrin hydrogels containing shCDH11 human dermal fibroblasts was reduced by ∼50% as compared to hydrogels containing control cells (Fig. 3E). Finally, reduced collagen production was accompanied by severely compromised mechanical strength as evidenced by a reduction in the UTS (∼70%, n=4, P<0.05) and Young's modulus (∼40%, n=4, P<0.05) of tissue constructs containing shCDH11 human dermal fibroblasts (Fig. 3F,G).
CDH11 engagement induces ECM synthesis through the TGF-β and ROCK pathways
Cell–cell-contact-induced signaling follows engagement of cadherins on the surface of a cell in homotypic interactions with cadherins on the surface of a neighboring cell. However, formation of adherens junctions is also followed by other events such as formation of gap junctions. To isolate the effects of CDH11 signaling from cadherin-dependent juxtacrine signals, we employed a fusion protein of CDH11 with the human Fc region of IgG, herein indicated as CDH11-Fc, which was used to coat the surface of non-tissue culture plates before addition of cells to initiate cadherin engagement (Fig. 4A).
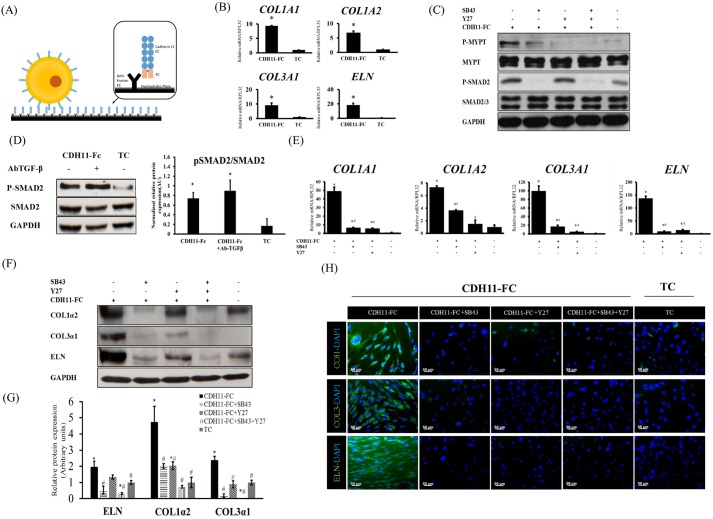
Fig. 4.
CDH11-Fc induced ECM production is mediated through the TGF-β and Rho/ROCK signaling. (A) Schematic of CDH11-Fc coating. (B) The relative mRNA levels are reported as the fold change with respect to TC samples (n=4). *P<0.05 as compared to on tissue culture plates (TC). (C) Activation of ROCK and TGF-β pathways on CDH11-Fc vs tissue culture plates as indicated by MYPT and SMAD-2 phosphorylation (P-MYPT and P-SMAD2, respectively). The cells were plated on CDH11-Fc-coated or tissue culture plates in the presence or absence of SB43 or Y27, chemical inhibitors of ROCK or TGF-β pathways, respectively. After 2 h, the cells were lysed and the levels of phosphorylated MYPT or SMAD2 were measured by western blotting. (D) Protein level of P-SMAD2 upon addition of TGF-β-neutralizing antibody (AbTGF-β). Quantification is shown as relative levels of P-SMAD2 relative to SMAD2. Values reported are normalized to housekeeping gene GAPDH (n=3). *P<0.05 as compared to on tissue culture plates. (E) qRT-PCR for the indicated genes. The relative mRNA levels are reported as the fold change with respect to tissue culture plate samples (n=4). *P<0.05 as compared to on tissue culture plates; #P<0.05 as compared to CDH11-Fc. (F) Protein levels of COL1α2, COL3α1 and ELN as measured by western blotting. GAPDH served as a loading control. (G) Quantification of the band intensity for each protein on CDH11-Fc in the presence or absence of SB43 or Y27 cells normalized to the corresponding band on TC (n=4). *P<0.05 as compared to on tissue culture plates; #P<0.05 as compared to CDH11-Fc. (H) Immunohistochemistry images for COL1, COL3 or ELN (green), counterstained with DAPI (blue). Scale bars: 50 µm. All quantifications results are mean±s.d. and P-values were calculated with an unpaired two-tailed Student's t-test.
Interestingly, after 2 days on CDH11-Fc, the mRNA levels of COL1A1, COL1A2, COL3A1 and ELN were significantly higher [by 5–10-fold for collagen genes and 20-fold for the elastin gene as compared to cells on tissue culture (TC) surface] (Fig. 4B). Increasing the surface concentration of CDH11-Fc led to a step-wise increase in CDH11, as well as of COL1α2 and ELN protein levels (Fig. S2D), supporting the notion that the observed effects were due to increased number of CDH11 contacts. In addition, overexpression of CDH11 using lentiviral delivery (CDH11+ cells) increased expression of COL1α2 and COL3α1 proteins significantly only when cells were plated on CDH11-Fc surface (Fig. S2E), suggesting that engagement of CDH11 in homotypic contacts was necessary for ECM production.
Given that the TGF-β and ROCK pathways are well known for regulating ECM synthesis, the contribution of these pathways to CDH11-mediated signaling was investigated with the aid of the CDH11-Fc surface. Surprisingly, plating cells on CDH11-Fc induced phosphorylation of SMAD2 and MYPT within 2 h, suggesting that engagement of CDH11 was sufficient to activate the TGF-β and ROCK pathways (Fig. 4C). Indeed, blocking TGF-β by using a neutralizing antibody had no effect on the level of phosphorylated (p-)SMAD2, indicating that the phosphorylation of SMAD2 was induced by the CDH11-Fc surface, independent of TGF-β-ligand–receptor interactions (Fig. 4D; Fig. S3A). By contrast, engagement of N-cadherin (CDH2) on CDH2-Fc surface led to much less phosphorylation of MYPT and its effect on SMAD2 was negligible as compared to that of CDH11-Fc (Fig. S3B), suggesting that CDH11 is the dominant cadherin activating these two pathways.
In addition, blocking the TGF-β1 pathway by use of SB43152 (SB43) or the ROCK pathway by use of Y27632 (Y27) reduced significantly the CDH11-Fc-mediated increase of COL1A1, COL1A2, COL3A1 and ELN mRNA (Fig. 4E). Similarly, both inhibitors decreased COL1α2, COL3α1 and ELN proteins as shown by western blotting (Fig. 4F,G) and immunostaining (Fig. 4H), although the TGF-β1 pathway seemed to have a greater effect, especially on ELN expression. Interestingly, blocking the ROCK pathway with Y27 had little or no effect on the phosphorylation of SMAD2, whereas blocking the TGF-β pathway with SB43 reduced p-MYPT levels as well (Fig. 4C). Similarly, the RhoA inhibitor, C3 eliminated p-MYPT but had no effect on p-SMAD2 (Fig. S3C). These results indicate partial activation of the ROCK pathway by TGF-β1, which could also explain the greater decline of ECM synthesis upon treatment with SB43.
CDH11-induced collagen and elastin synthesis is mediated by MRTF and MYOCD
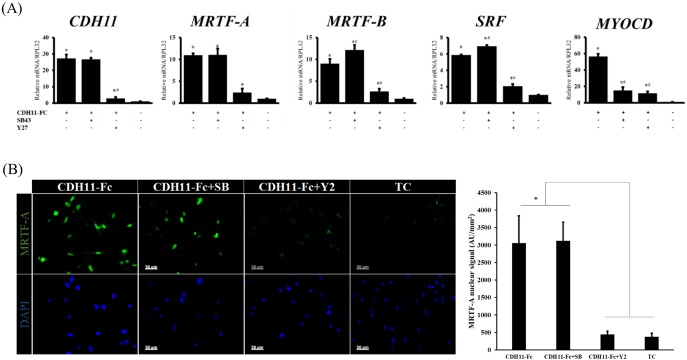
To further investigate the targets of the CDH11 signaling, several key transcription factors that are known to mediate collagen and elastin transcription, such as SRF, MYOCD and MRTF-A and -B were assessed by qRT-PCR (Fig. 5A). Interestingly, cell adhesion on the CDH11-Fc surface significantly increased the mRNA levels of CDH11 itself (∼25-fold, n=3, P<0.05), as well as SRF (∼6-fold, n=3, P<0.01), MRTF-A (11-fold, n=3, P<0.05), MRTF-B (9-fold, n=3, P<0.05) and especially MYOCD (50-fold, n=3, P<0.05). This increase was mediated through the ROCK pathway, as the mRNA levels of CDH11 and all transcription factors were significantly reduced by Y27 (SRF, MRTF-A and -B were reduced by 3–5-fold, n=3, P<0.05). By contrast, blocking the TGF-β1 pathway had no effect, except on MYOCD, which decreased significantly by ∼5-fold (n=3, P<0.05). In addition, blocking RhoA with C3 also eliminated the CDH11-mediated increase in gene expression of MRTF-A and -B, MYOCD and SRF (Fig. S3D), implicating the canonical Rho–ROCK pathway in MRTF-A and -B transcription. Notably, adhesion to CDH11-Fc significantly increased the protein level of MRTF-A in the cell nucleus, which was blocked by Y27 but was unaffected by SB43 (Fig. 5B). Conversely, knocking down CDH11 with shRNA reduced expression and completely blocked MRTF-A nuclear localization (Fig. S4A).
Fig. 5.
CDH11-Fc engagement induces expression of several transcription factors. (A) Cells were plated on CDH11-Fc or tissue culture plates (TC) in the presence or absence of SB43 or Y27 and the next day (16 h later) they were lysed and the levels of indicated genes were measured by qRT-PCR. The relative mRNA levels are reported as the fold change with respect to tissue culture plate samples (n=4). *P<0.05 as compared to tissue culture plate; #P<0.05 as compared to CDH11-Fc. (B) Immunostaining for MRTF-A (green) on CDH11-Fc in the presence or absence of SB43 or Y27; cells were counterstained with DAPI. Cells on tissue culture plates were used as a control. Scale bars: 50 µm. A quantification of the relative nuclear signal of MRTF-A shown in right panel (n=5 fields of view). *P<0.05 as compared to tissue culture plates. All quantifications results are mean±s.d. and P-values were calculated with an unpaired two-tailed Student's t-test.
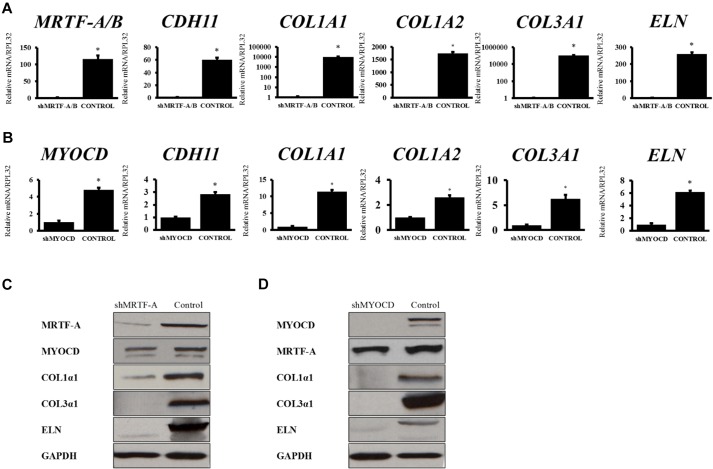
Loss of MRTF-A, MRTF-B or MYOCD abolishes CDH11-Fc-mediated collagen and elastin production
To determine which transcription factor(s) mediated CDH11-Fc-induced collagen and elastin production, we employed shRNA-encoding lentivirus to knockdown MYOCD, MRTF-A and -B or SRF (shMYOCD, shMRTF-A/B and shSRF, respectively). Knocking down MRTF-A and -B, or MYOCD drastically reduced the mRNA levels of COL1A1, COL3A1 and ELN (Fig. 6A,B) as well as the protein levels of COL1A2, COL3A1 and ELN (Fig. 6C,D), indicating that both transcription factors were required for transciptional activation of collagen and elastin genes. By contrast, knocking down SRF affected only CDH11-Fc mediated ELN production but had no significant effect on COL1α2 or COL3α1 (Fig. S4B).
Fig. 6.
CDH11-induced ECM synthesis is mediated by MRTF-A and MYOCD. qRT-PCR data for CDH11, MRTF-A, MYOCD, COL1A1, COL1A2, COL3A1 and ELN for (A) shMRTF-A and (B) shMYOCD human dermal fibroblasts. The relative mRNA levels are reported as the fold change with respect to tissue culture plate samples (n=4). *P<0.05 as compared to the indicated knockdown sample (unpaired two-tailed Student's t-test). Protein levels of MYOCD, MRTF-A, ELN, COL1α2, COL3α1 as analyzed by western blot in (C) shMRTF-A or (D) shMYOCD human dermal fibroblasts and control dermal fibroblasts. All quantitative results are mean±s.d.
Interestingly, CDH11 levels were reduced dramatically by loss of MRTF-A and -B (∼50-fold, n=3, P<0.03) and to a much lesser extent by loss of MYOCD (∼3-fold, n=3, P<0.03) (Fig. 6A,B). In order to determine whether loss of MRTF-A or MYOCD affected ECM synthesis directly or indirectly by decreasing CDH11, we measured the protein levels of COL1A2, COL3A1 and ELN in shMRTF-A/B or shMYOCD cells overexpressing CDH11. As shown in Fig. S4C, lack of MRTF-A or MYOCD impaired collagen and elastin production, even in the presence of CDH11, suggesting that both transcription factors are necessary for CDH11-induced ECM synthesis.
DISCUSSION
We have previously shown that in high-density MSC cultures, CDH11-mediated intercellular adhesion activated ROCK leading to mesenchymal stem cell differentiation into smooth muscle cells (Alimperti et al., 2014). In this study, we presented in vivo and in vitro experimental evidence that CDH11 is necessary for extracellular matrix production contributing to the mechanical properties of tissues. We discovered that smooth-muscle-containing tissues of Cdh11−/− mice exhibited significantly reduced mechanical strength, which correlated with significant reduction in collagen and elastin content. Indeed, expression of Col1a1, Col1a2, Col3a1 and Eln at the mRNA as well as the protein level were dramatically reduced both in mouse Cdh11−/− fibroblasts and human shCDH11 fibroblasts. Similarly, mRNAs of key transcription factors that are known to regulate collagen and elastin were reduced so as to be effectively not present. In addition, the force generation ability of cells lacking CDH11 was dramatically reduced as shown by their ability to compact fibrin hydrogels. These data implicate intercellular adhesion as a new and crucial regulator of ECM production with significant consequences for tissue biomechanics.
Using a CDH11-Fc-decorated surface, we identified the signaling pathways mediating CDH11-induced ECM production. Examination of early signaling dynamics revealed activation of both the ROCK and TGF-β pathways shorty after cell attachment to the surface (within 2 h or less), as evidenced by phosphorylation of MYPT and SMAD2, respectively. Interestingly, blocking TGF-β1 with a function-blocking antibody had no effect on CDH11-mediated SMAD2 phosphorylation, suggesting that activation of the TGF-β pathway was the direct effect of CDH11 engagement, rather than through paracrine action of de novo transcribed soluble TGF-β1. This result suggests that engagement of CDH11 might activate the TGF-β receptor or phosphorylate SMAD2 or SMAD3 through a currently unidentified pathway. Interestingly, CDH2 engagement activated ROCK to a much lesser extent and failed to phosphorylate SMAD2, indicating a unique role of CDH11 in activating the TGF-β1 pathway.
In addition, activation of the TGF-β pathway by CDH11 had a direct effect on ROCK activation, as inhibition of SMAD2 or SMAD3 phosphorylation also reduced the phosphorylation of MYPT. The converse was not true, as inhibition of ROCK or RhoA had no effect on CDH11-induced phosphorylation of SMAD2 or SMAD3. The crosstalk between the two pathways has been previously reported especially in the context of the role of TGF-β in ECM production (Itoh et al., 2007; Zhu et al., 2013) but our study demonstrates that such crosstalk also exists in the context of CDH11-mediated intercellular adhesion. Currently, the nature of the effector(s) mediating this inter-connection remains unknown.
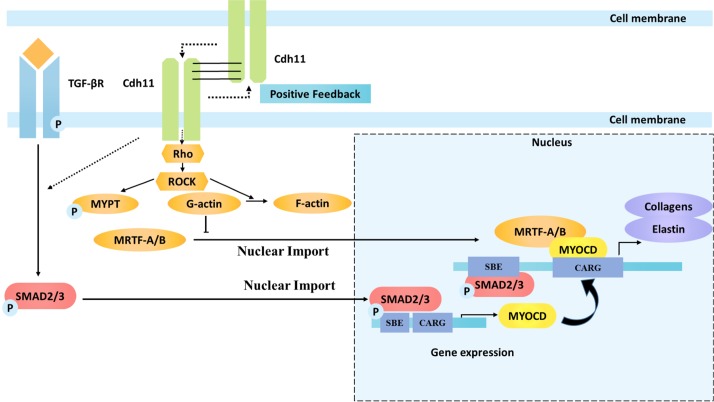
Furthermore, loss of CDH11 either in Cdh11−/− mouse dermal fibroblasts or in shCDH11 human dermal fibroblasts abolished the expression of transcription factors known to affect ECM synthesis (Ueyama et al., 2003; Medjkane et al., 2009; Small et al., 2010; Leitner et al., 2011; Velasquez et al., 2013; Johnson et al., 2014; Parreno et al., 2014), such as MYOCD, MRTF-A, SRF and to a lesser extent MRTF-B. In agreement, knocking down either MYOCD or MRTF-A and -B decreased CDH11-mediated expression of collagen and elastin, but the effects of MRTF-A and -B were significantly stronger. Although past studies have only recognized MRTF and MYOCD as co-activators of SRF, some recent studies have shown that they can act as transcription factors independently (Tang et al., 2008; Asparuhova et al., 2011; Luchsinger et al., 2011; Smith et al., 2012; Kitchen et al., 2013). MRTF-A has been shown to control Col1A2 promoter activity as well as expression of other extracellular matrix molecules (Asparuhova et al., 2011; Luchsinger et al., 2011). MYOCD has also been implicated in studies showing CArG-RE-independent regulation of ECM (Tang et al., 2008; Kitchen et al., 2013). Interestingly, engagement of CDH11 on surface-immobilized CDH11-Fc increased the mRNA levels of all these transcription factors significantly. In agreement, the CArG-RE activity was blocked almost completely when CDH11 was knocked down by shRNA. CDH-11-mediated MRTF-A and -B expression was dependent solely on ROCK, whereas MYOCD required both ROCK and TGF-β1 activation. Given that expression of the ECM genes COL1A1, COL1A2, COL3A1 and ELN depended on both pathways, we conclude that TGF-β is required to promote expression of MYOCD, whereas ROCK activation is necessary for expression of MRTF-A and -B (see Fig. 7). Interestingly, activation of the Rho–ROCK pathway by CDH11 engagement led to increased MRTF-A and -B, MYOCD and SRF gene expression, ultimately leading to high levels of ECM synthesis.
Fig. 7.
Schematic showing proposed mechanism of ECM synthesis following CDH11 engagement. CDH11 engagement activates TGF-β and ROCK pathways as evidenced by phosphorylation of MYPT and Smad2. Activation of Rho–ROCK leads to actin polymerization and nuclear localization of MRTF-A. Phosphorylation of Smad2–Smad3 leads to increased levels of MYOCD. These two transcription factors, perhaps with the help of other co-factors such as SRF, bind to the SBE or CARG response elements leading to transcription of collagen and elastin as well as CDH11, indicating the presence of a positive-feedback loop. The dotted lines denote that the pathway is not fully defined.
Our results might have implications for restoration of tissue function, wound healing and regenerative medicine. Recent studies implicated CDH11 in lung and skin fibrosis following chemical injury, leading to impaired wound healing and loss of function (Schneider et al., 2012; Wu et al., 2014). Our results provide mechanistic insight into this process and suggest, for the first time, that CDH11 might be contributing to collagen deposition following injury, by directly activating both the TGF-β and ROCK pathways, with subsequent activation of the transcription factors necessary for ECM production. Interestingly, CDH2 engagement did not have the same effects, suggesting that CDH11 might be a distinct target for preventing fibrosis and promoting tissue regeneration. By contrast, a number of diseases are attributed to a loss or impaired state of the ECM, for example, stress urinary incontinence or improper bladder function, which is related to irregular distribution of elastin fibers (Goepel and Thomssen, 2006) and reduced collagen type I and III (Li et al., 2012a), and Marfan's syndrome, which is caused by a mutation in the fibrillin gene leading to lack of elastin fibers and reduced tissue levels of TGF-β in the aorta, with subsequent increase in stiffness (Benke et al., 2013). However, loss of CDH11 had no effect on the mRNA expression of the fibrillin gene, as shown in Cdh11−/− mouse dermal fibroblasts. In addition, age, hypertension and cardiovascular disease are associated with significantly reduced collagen and elastin content in large arteries and skin, among other tissues (Kohl et al., 2011; Wagenseil and Mecham, 2012). In this context, strategies that promote CDH11 engagement might be a viable strategy to restore the levels of ECM, thereby preventing deterioration of the mechanical properties and the proper physiological function of these tissues.
Interestingly, engagement of CDH11 increased the expression of CDH11 itself, thereby suggesting the presence of a positive-feedback loop, which required the presence of MRTF-A- and -B but not MYOCD, as shown by knockdown experiments. Overexpression of CDH11 did not increase ECM production until the cells were plated either at high density or on surface-immobilized CDH11-Fc, suggesting that CDH11 engagement in homophilic interactions was necessary for the activation of signaling pathways leading to expression of CDH11, collagen type I and III and elastin. Interestingly, formation of adherens junctions through cadherins has been shown to generate mechanical forces between adjacent cells (Tzima et al., 2005; Liu et al., 2007b), ultimately regulating basic cellular functions such as proliferation, differentiation and migration. Given that engagement of CDH11 induced ECM production, our findings implicate intercellular adhesion force as a new and crucial regulator of ECM production, ultimately affecting the mechanical properties of tissues and consequently biological processes such as tissue development, regeneration and wound healing. Our findings reveal new functions of CDH11 with significant repercussions for the function of vital tissues including the vasculature. It would be interesting to determine whether these observations can be extended to other smooth-muscle-containing tissues or organs such as the lung, stomach and intestine, as well as to other muscle types such as skeletal or cardiac muscle.
MATERIALS AND METHODS
Animals and tissue collection
Cdh11−/− mice and wild-type B6:129 F1 intercross mice were bred and maintained as previously described (Schneider et al., 2012), and were housed at Baylor College of Medicine with the approval of the Baylor College of Medicine Institutional Animal Care and Use Committee. Mice were killed at 6–8 weeks of age in a standard CO2 chamber, and tissues, namely, aorta, bladder and skin (from the back) were immediately harvested. Fresh tissue was reserved for mechanical testing after extensive washes in PBS. Tissues used for histological analyses were fixed in 10% neutral buffered formalin.
Mechanical testing of tissues
Tissues obtained from mice were cut into rings (for aorta and bladder) or strips (for skin tissue) and diameters or thicknesses were measured for calculation of contact area. These were subjected to mechanical testing using a uniaxial Instron tensile tester (Model 3343, 50N load cell, Instron Corporation, Norwood, MA) as previously described (Row et al., 2015). Ring samples or strips were mounted onto tester grips directly (for strips of skin) or by using stainless steel hooks (for aorta and bladder tissue rings). Samples were displaced in the vertical axis at constant speed (18 mm/min crosshead speed) until failure. Young's moduli were obtained from the linear portion of the stress–strain curve, exported from the software (Bluehill 3, Instron Corporation, Norwood, MA) and the UTS was calculated as the breaking force per unit area of tissue and expressed in MPa.
Colorimetric quantification of collagen and elastin in tissues
A ninhydrin assay was used for evaluation of elastin content and a hydroxyl-proline assay was used for the quantification of collagen as described previously (Koobatian et al., 2016). Briefly, tissues used for quantification of elastin and collagen were lyophilized to obtain the dry weight, which served as a normalization factor. These were then boiled at 95°C for 45 min in 0.1 M NaOH to yield cross-linked elastin as an insoluble residue. The pellet and supernatant were separated by centrifugation and the supernatant was reserved for collagen quantification. The pellet was then dried again for acid hydrolysis, using 6 M HCl, overnight at 105°C. Lyophilized hydrolyzed protein was resuspended in water and samples at the desired dilution and standards (Alpha elastin, EPC) were loaded into 96-well plates. Ninhydrin reagent (Sigma) was then added and the plates were incubated for 40 min at 65°C. Plates were then read at 550 nm using a plate reader (Synergy 4, BioTek, Winooski, VT).
The reserved supernantant was also subjected to acid hydrolysis after lyophilization. The protocol for the hydroxyl-proline assay is as described elsewhere (Liang et al., 2013).
Histological examination of tissues
Pressure-fixed samples were dehydrated in a series of graded ethanol solutions and xylene substitutes and then embedded in paraffin as reported before (Geer et al., 2002; Swartz et al., 2005). For histological evaluation, 5-μm paraffin-embedded tissue sections were stained with Masson's trichrome or Verhoeff's elastin using a Masson trichrome staining kit or Elastic stain kit (Chromaview, Richard-Allen scientific, Kalamazoo, MI), following the manufacturer's directions.
Cell isolation and culture
Human neonatal foreskin fibroblasts and mice dermal fibroblasts were isolated from tissues as reported elsewhere (Bajaj et al., 2001). Mice aortic smooth muscle cells (ASMCs) were isolated from mice aorta sections as described previously (Swartz et al., 2005). Human dermal fibroblasts were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with antibiotic-antimycotic cocktail and 10% MSC-qualified fetal bovine serum, supplemented with 2 ng/ml basic fibroblast growth factor (bFGF, all components from Life Technologies, Burlington, ON, Canada). Passages between 2 and 12 were used in all experiments. Mice dermal fibroblasts and ASMCs were cultured in DMEM supplemented with 10% MSC-qualified fetal bovine serum, penicillin-streptomycin-glutamine and β-mercaptoethanol (Life Technologies), non-essential amino acids and essential amino acids (Hyclone Labs, GE Healthcare, Logan, UT), supplemented with 2 ng/ml bFGF. These cells were used between passage 2 and 6.
Knockdown of genes by RNA interference
shLVDP, as characterized by our group in a previous study (Alimperti et al., 2012) was utilized and modified by cloning in the oligonucleotide sequences presented in Table S2, using ClaI and MluI restriction enzyme sites in the 3′ LTR of the vector. Resulting plasmids were used along with psPAX2 and PMD2.G in a standard calcium phosphate transfection method using HEK 293T to make lentivirus as described elsewhere (Alimperti et al., 2012). Lentiviral transduction of human dermal fibroblasts was carried out with 8 µg/ml polybrene, and infected cells were immediately used for experimental analyses.
Overexpression of CDH11
The CDH11-encoding region was PCR-amplified from pCMV-sport6-OBCAD (Open Biosystems) using PCR primers (5′-AACAAACCGGTTTGGGCCCCTCGAGGGATA-3′ sense and 5′-ACAAGCTAGCGGTCCGGAATTCCCGGGATA-3′ antisense) and ligated into pSIN-EF2-Sox2-puro (Addgene) using restriction enzyme BamHI. The resulting vector was named pSIN-EF2-OBCAD-puro, which was used for lentiviral delivery after packaging in HEK 293T cell line as described above.
CDH11-Fc- and CDH2-Fc-coated surface
Non-tissue culture plates were utilized owing to their hydrophobicity, and coated with goat anti-human IgG (FCγ-specific, Jackson ImmunoResearch, West Grove, PA) in attachment buffer overnight at 4°C as described previously (Lira et al., 2008). 50 ng/cm2 of recombinant human CDH11-Fc chimera (R&D systems, Minneapolis, MN) was then added in binding buffer for 1 h at 37°C (Lira et al., 2008). CDH11-Fc consisted of the five extracellular domains of CDH11 linked to the constant heavy chain domains CH2 and CH3 of FCγ through the hinge region, which existed in solution as a homodimer with disulfide bridges between the monomeric subunits (see Fig. 4A). A similar method was followed for CDH2-Fc-coated plates (R&D systems, Minneapolis, MN). When cells were plated on this surface, homophillic interactions occurred between cell surface cadherins and exposed homodimers of CDH11-Fc. An optimized cell density of 7000 cells/cm2 was used in order to avoid cell–cell interactions and single out the effect of CDH11 contacts between the cells and the CDH11-Fc surface.
Fibrin gel compaction
Fibrin gels were formed around a mandrel as previously described (Swartz et al., 2005; Liu et al., 2007a). Following polymerization at 37°C for 1 h, gels were released from the wall and images of wells were obtained at indicated times. Experimental triplicates or quadruplicates with each group were used to measure area of fibrin gel as a fraction of total area (A/A0) of a standard 24-well plate (A0=2 cm2). These gels were further cultured in vessel medium (Liang et al., 2013) in the same plates for 14 days, to allow for collagen deposition and remodeling.
qRT-PCR
Total mRNA was extracted from cell monolayers by using an RNeasy Mini Kit (Qiagen, Balencia, CA). Single-strand cDNA was reverse transcribed and synthesized from purified 1.0 µg mRNA using the QuantiTect Reverse Transcription Kit (Qiagen, Balencia, CA). Real-time PCR analysis was performed in CFX96 real-time system (Bio-Rad, Hercules, CA) with a mixture of iQ SYBR Green Supermix (Bio-Rad, Hercules, CA), primers (Table S3) and cDNA prepared by following manufacturer's instructions. Gene expression levels were quantified and analyzed using the ΔCT method, and are reported as normalized to the housekeeping gene (RPL32 for human samples and Gapdh for mice).
Western blotting
Protein lysates were isolated from pre-incubated cell monolayers in standard lysis buffer at the indicated times. The lysates were subjected to western blot analysis as described previously (Alimperti et al., 2014). Primary antibodies (Table S4) were incubated overnight at 4°C followed by washes and incubation with secondary antibody at room temperature for 1 h. Visualized bands were developed with LumiGLO reagent (Cell Signaling Technologies, Beverly, MA) according to the manufacturer's protocol. Protein content was analyzed by densitometric analysis using Image J and results are reported as normalized to GAPDH.
Immunohistochemistry
Cell monolayers, at indicated times, were fixed with 4% paraformaldehyde for 15 min at room temperature. Following permeabilization with 0.1% Triton X-100 in PBS and incubation in blocking buffer (5% goat serum in PBS) at room temperature for 1 h, primary antibodies were added in dilutions as mentioned (Table S5). After incubation with primary antibodies overnight at 4°C, secondary antibody (1:200 in PBS, Alexa-Fluor-488-conjugated goat anti-rabbit IgG, Life Technologies, Burlington, ON, Canada) was added for 1 h at room temperature. Phalloidin–Alexa-Fluor-594 (Life Technologies) was used to stain for F-actin as per the manufacturer's instructions. Cell nuclei were counterstained with Hoechst 33342 (10 mg/ml; 1:200 dilution; 5 min at room temperature; EMD Millipore Laboratory Chemicals, Billerica, MA). Fluorescence microscopy images were acquired using the Zeiss Axiovision observer Z1 (LSM 510; Zeiss, Oberkochen, Germany) equipped with a digital camera (ORCA-ER C4742-80; Hamamatsu, Bridgewater, NJ) and images were analyzed using the ImageJ software.
Statistical analysis
Values are mean±s.d. Significant differences between animal groups were determined by Student's t-test (unpaired, two-tailed) or ANOVA with post-hoc analysis using Student–Newman–Keuls multiple comparison test or Spearman correlations analysis.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
S.R. and Y.L. contributed equally as first authors. They participated in primary data collection, statistical analyses, intellectual contribution and preparation and editing of the manuscript. S.A. contributed intellectually and in primary data collection for mice tissues as well as preparation of plasmids. S.K.A. maintained and bred the mice at Baylor College of Medicine, contributed towards mice tissue and data collection and cell isolation and participated in editing the manuscript. S.T.A. is the corresponding author and contributed intellectually to the entire study and specifically to experimental design, data interpretation and writing of the manuscript. He is also responsible for management of resources and planning of the study. S.K.A. and S.T.A. contributed to grant acquisition and management. All authors agree to the authorship as listed in the manuscript.
Funding
This study was supported by the National Institutes of Health (NIH) [grant number R01 AR062056-05 to S.K.A.]; and the National Science Foundation (NSF) [grant number CBET 1403086 to S.T.A.]. Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://jcs.biologists.org/lookup/doi/10.1242/jcs.183772.supplemental
References
- Agarwal S. K. (2014). Integrins and cadherins as therapeutic targets in fibrosis. Front. Pharmacol. 5, 131 10.3389/fphar.2014.00131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alimperti S., Lei P., Tian J. and Andreadis S. T. (2012). A novel lentivirus for quantitative assessment of gene knockdown in stem cell differentiation. Gene Ther. 19, 1123-1132. 10.1038/gt.2011.208 [DOI] [PubMed] [Google Scholar]
- Alimperti S., You H., George T., Agarwal S. K. and Andreadis S. T. (2014). Cadherin-11 regulates both mesenchymal stem cell differentiation into smooth muscle cells and the development of contractile function in vivo. J. Cell Sci. 127, 2627-2638. 10.1242/jcs.134833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asparuhova M. B., Ferralli J., Chiquet M. and Chiquet-Ehrismann R. (2011). The transcriptional regulator megakaryoblastic leukemia-1 mediates serum response factor-independent activation of tenascin-C transcription by mechanical stress. FASEB J. 25, 3477-3488. 10.1096/fj.11-187310 [DOI] [PubMed] [Google Scholar]
- Bajaj B., Lei P. and Andreadis S. T. (2001). High efficiencies of gene transfer with immobilized recombinant retrovirus: kinetics and optimization. Biotechnol. Prog. 17, 587-596. 10.1021/bp010039n [DOI] [PubMed] [Google Scholar]
- Benke K., Ágg B., Szilveszter B., Tarr F., Nagy Z. B., Pólos M., Daróczi L., Merkely B. and Szabolcs Z. (2013). The role of transforming growth factor-beta in Marfan syndrome. Cardiol. J. 20, 227-234. 10.5603/CJ.2013.0066 [DOI] [PubMed] [Google Scholar]
- Cavallaro U. and Dejana E. (2011). Adhesion molecule signalling: not always a sticky business. Nat. Rev. Mol. Cell Biol. 12, 189-197. 10.1038/nrm3068 [DOI] [PubMed] [Google Scholar]
- Diaz-Chavez J., Hernandez-Pando R., Lambert P. F. and Gariglio P. (2008). Down-regulation of transforming growth factor-beta type II receptor (TGF-betaRII) protein and mRNA expression in cervical cancer. Mol. Cancer 7, 3 10.1186/1476-4598-7-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geer D. J., Swartz D. D. and Andreadis S. T. (2002). Fibrin promotes migration in a three-dimensional in vitro model of wound regeneration. Tissue Eng. 8, 787-798. 10.1089/10763270260424141 [DOI] [PubMed] [Google Scholar]
- Goepel C. and Thomssen C. (2006). Changes in the extracellular matrix in periurethral tissue of women with stress urinary incontinence. Acta Histochem. 108, 441-445. 10.1016/j.acthis.2006.07.001 [DOI] [PubMed] [Google Scholar]
- Harris T. J. C. and Tepass U. (2010). Adherens junctions: from molecules to morphogenesis. Nat. Rev. Mol. Cell Biol. 11, 502-514. 10.1038/nrm2927 [DOI] [PubMed] [Google Scholar]
- Itoh Y., Kimoto K., Imaizumi M. and Nakatsuka K. (2007). Inhibition of RhoA/Rho-kinase pathway suppresses the expression of type I collagen induced by TGF-beta2 in human retinal pigment epithelial cells. Exp. Eye Res. 84, 464-472. 10.1016/j.exer.2006.10.017 [DOI] [PubMed] [Google Scholar]
- Johnson L. A., Rodansky E. S., Haak A. J., Larsen S. D., Neubig R. R. and Higgins P. D. R. (2014). Novel Rho/MRTF/SRF inhibitors block matrix-stiffness and TGF-beta–induced fibrogenesis in human colonic myofibroblasts. Inflamm. Bowel Dis. 20, 154-165. 10.1097/01.MIB.0000437615.98881.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi J., Azuma Y., Hoshi K., Kii I., Takeshita S., Ohta T., Ozawa H., Takeichi M., Chisaka O. and Kudo A. (2001a). Targeted disruption of cadherin-11 leads to a reduction in bone density in calvaria and long bone metaphyses. J. Bone Miner. Res. 16, 1265-1271. 10.1359/jbmr.2001.16.7.1265 [DOI] [PubMed] [Google Scholar]
- Kawaguchi J., Kii I., Sugiyama Y., Takeshita S. and Kudo A. (2001b). The transition of cadherin expression in osteoblast differentiation from mesenchymal cells: consistent expression of cadherin-11 in osteoblast lineage. J. Bone Miner. Res. 16, 260-269. 10.1359/jbmr.2001.16.2.260 [DOI] [PubMed] [Google Scholar]
- Kimura Y., Matsunami H., Inoue T., Shimamura K., Uchida N., Ueno T., Miyazaki T. and Takeichi M. (1995). Cadherin-11 expressed in association with mesenchymal morphogenesis in the head, somite, and limb bud of early mouse embryos. Dev. Biol. 169, 347-358. 10.1006/dbio.1995.1149 [DOI] [PubMed] [Google Scholar]
- Kitchen C. M., Cowan S. L., Long X. and Miano J. M. (2013). Expression and promoter analysis of a highly restricted integrin alpha gene in vascular smooth muscle. Gene 513, 82-89. 10.1016/j.gene.2012.10.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl E., Steinbauer J., Landthaler M. and Szeimies R.-M. (2011). Skin ageing. J. Eur. Acad. Dermatol. Venereol. 25, 873-884. 10.1111/j.1468-3083.2010.03963.x [DOI] [PubMed] [Google Scholar]
- Koobatian M. T., Row S., Smith R. J. Jr., Koenigsknecht C., Andreadis S. T. and Swartz D. D. (2016). Successful endothelialization and remodeling of a cell-free small-diameter arterial graft in a large animal model. Biomaterials 76, 344-358. 10.1016/j.biomaterials.2015.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitner L., Shaposhnikov D., Mengel A., Descot A., Julien S., Hoffmann R. and Posern G. (2011). MAL/MRTF-A controls migration of non-invasive cells by upregulation of cytoskeleton-associated proteins. J. Cell Sci. 124, 4318-4331. 10.1242/jcs.092791 [DOI] [PubMed] [Google Scholar]
- Li G.-Y., Cui W.-S., Zhou F., Gao Z.-Z., Xin H., Liu T., Li W.-R., Gong Y.-Q., Bai G.-Y., Guo Y.-L. et al. (2012a). Pathology of urethral fibromuscular system related to parturition-induced stress urinary incontinence and TGF-beta1/Smad pathway. Mol. Cell. Biochem. 364, 329-335. 10.1007/s11010-012-1234-x [DOI] [PubMed] [Google Scholar]
- Li L., Bennett S. A. and Wang L. (2012b). Role of E-cadherin and other cell adhesion molecules in survival and differentiation of human pluripotent stem cells. Cell. Adh. Migr. 6, 222-233. 10.4161/cam.19583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang M.-S., Koobatian M., Lei P., Swartz D. D. and Andreadis S. T. (2013). Differential and synergistic effects of mechanical stimulation and growth factor presentation on vascular wall function. Biomaterials 34, 7281-7291. 10.1016/j.biomaterials.2013.05.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lira C. B. B., Chu K., Lee Y.-C., Hu M. C.-T. and Lin S.-H. (2008). Expression of the extracellular domain of OB-cadherin as an Fc fusion protein using bicistronic retroviral expression vector. Protein Expr. Purif. 61, 220-226. 10.1016/j.pep.2008.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J. Y., Swartz D. D., Peng H. F., Gugino S. F., Russell J. A. and Andreadis S. T. (2007a). Functional tissue-engineered blood vessels from bone marrow progenitor cells. Cardiovasc. Res. 75, 618-628. 10.1016/j.cardiores.2007.04.018 [DOI] [PubMed] [Google Scholar]
- Liu W. F., Nelson C. M., Tan J. L. and Chen C. S. (2007b). Cadherins, RhoA, and Rac1 are differentially required for stretch-mediated proliferation in endothelial versus smooth muscle cells. Circ. Res. 101, e44-e52. 10.1161/CIRCRESAHA.107.158329 [DOI] [PubMed] [Google Scholar]
- Luchsinger L. L., Patenaude C. A., Smith B. D. and Layne M. D. (2011). Myocardin-related transcription factor-A complexes activate type I collagen expression in lung fibroblasts. J. Biol. Chem. 286, 44116-44125. 10.1074/jbc.M111.276931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medjkane S., Perez-Sanchez C., Gaggioli C., Sahai E. and Treisman R. (2009). Myocardin-related transcription factors and SRF are required for cytoskeletal dynamics and experimental metastasis. Nat. Cell Biol. 11, 257-268. 10.1038/ncb1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niessen C. M. and Gumbiner B. M. (2002). Cadherin-mediated cell sorting not determined by binding or adhesion specificity. J. Cell Biol. 156, 389-400. 10.1083/jcb.200108040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parreno J., Raju S., Niaki M. N., Andrejevic K., Jiang A., Delve E. and Kandel R. (2014). Expression of type I collagen and tenascin C is regulated by actin polymerization through MRTF in dedifferentiated chondrocytes. FEBS Lett. 588, 3677-3684. 10.1016/j.febslet.2014.08.012 [DOI] [PubMed] [Google Scholar]
- Row S., Peng H., Schlaich E. M., Koenigsknecht C., Andreadis S. T. and Swartz D. D. (2015). Arterial grafts exhibiting unprecedented cellular infiltration and remodeling in vivo: the role of cells in the vascular wall. Biomaterials 50, 115-126. 10.1016/j.biomaterials.2015.01.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider D. J., Wu M., Le T. T., Cho S.-H., Brenner M. B., Blackburn M. R. and Agarwal S. K. (2012). Cadherin-11 contributes to pulmonary fibrosis: potential role in TGF-beta production and epithelial to mesenchymal transition. FASEB J. 26, 503-512. 10.1096/fj.11-186098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin C. S., Lecanda F., Sheikh S., Weitzmann L., Cheng S.-L. and Civitelli R. (2000). Relative abundance of different cadherins defines differentiation of mesenchymal precursors into osteogenic, myogenic, or adipogenic pathways. J. Cell Biochem. 78, 566-577. [DOI] [PubMed] [Google Scholar]
- Small E. M., Thatcher J. E., Sutherland L. B., Kinoshita H., Gerard R. D., Richardson J. A., DiMaio J. M., Sadek H., Kuwahara K. and Olson E. N. (2010). Myocardin-related transcription factor-a controls myofibroblast activation and fibrosis in response to myocardial infarction. Circ. Res. 107, 294-304. 10.1161/CIRCRESAHA.110.223172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E. C., Thon J. N., Devine M. T., Lin S., Schulz V. P., Guo Y., Massaro S. A., Halene S., Gallagher P., Italiano J. E. Jr.. et al. (2012). MKL1 and MKL2 play redundant and crucial roles in megakaryocyte maturation and platelet formation. Blood 120, 2317-2329. 10.1182/blood-2012-04-420828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz D. D., Russell J. A. and Andreadis S. T. (2005). Engineering of fibrin-based functional and implantable small-diameter blood vessels. Am. J. Physiol. Heart Circ. Physiol. 288, H1451-H1460. 10.1152/ajpheart.00479.2004 [DOI] [PubMed] [Google Scholar]
- Tang R.-H., Zheng X.-L., Callis T. E., Stansfield W. E., He J., Baldwin A. S., Wang D.-Z. and Selzman C. H. (2008). Myocardin inhibits cellular proliferation by inhibiting NF-kappaB(p65)-dependent cell cycle progression. Proc. Natl. Acad. Sci. USA 105, 3362-3367. 10.1073/pnas.0705842105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita K., van Bokhoven A., van Leenders G. J., Ruijter E. T., Jansen C. F., Bussemakers M. J. and Schalken J. A. (2000). Cadherin switching in human prostate cancer progression. Cancer Res. 60, 3650-3654. [PubMed] [Google Scholar]
- Tzima E., Irani-Tehrani M., Kiosses W. B., Dejana E., Schultz D. A., Engelhardt B., Cao G., DeLisser H. and Schwartz M. A. (2005). A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 437, 426-431. 10.1038/nature03952 [DOI] [PubMed] [Google Scholar]
- Ueyama T., Kasahara H., Ishiwata T., Nie Q. and Izumo S. (2003). Myocardin expression is regulated by Nkx2.5, and its function is required for cardiomyogenesis. Mol. Cell. Biol. 23, 9222-9232. 10.1128/MCB.23.24.9222-9232.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasquez L. S., Sutherland L. B., Liu Z., Grinnell F., Kamm K. E., Schneider J. W., Olson E. N. and Small E. M. (2013). Activation of MRTF-A-dependent gene expression with a small molecule promotes myofibroblast differentiation and wound healing. Proc. Natl. Acad. Sci. USA 110, 16850-16855. 10.1073/pnas.1316764110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenseil J. E. and Mecham R. P. (2012). Elastin in large artery stiffness and hypertension. J. Cardiovasc. Transl. Res. 5, 264-273. 10.1007/s12265-012-9349-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M., Pedroza M., Lafyatis R., George A.-T., Mayes M. D., Assassi S., Tan F. K., Brenner M. B. and Agarwal S. K. (2014). Identification of cadherin 11 as a mediator of dermal fibrosis and possible role in systemic sclerosis. Arthritis Rheumatol. 66, 1010-1021. 10.1002/art.38275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisberg M. and Neilson E. G. (2009). Biomarkers for epithelial-mesenchymal transitions. J. Clin. Invest. 119, 1429-1437. 10.1172/JCI36183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Nguyen D., Ouyang H., Zhang X. H., Chen X. M. and Zhang K. (2013). Inhibition of RhoA/Rho-kinase pathway suppresses the expression of extracellular matrix induced by CTGF or TGF-beta in ARPE-19. Int. J. Ophthalmol. 6, 8-14. 10.3980/j.issn.2222-3959.2013.01.02 [DOI] [PMC free article] [PubMed] [Google Scholar]