Abstract
Lizards are amniotes with the remarkable ability to regenerate amputated tails. The early regenerated lizard tail forms a blastema, and the regenerated skeleton consists of a cartilage tube (CT) surrounding the regenerated spinal cord. The proximal, but not distal, CT undergoes hypertrophy and ossifies. We hypothesized that differences in cell sources and signaling account for divergent cartilage development between proximal and distal CT regions. Exogenous spinal cord implants induced ectopic CT formation in lizard (Anolis carolinensis) blastemas. Regenerated spinal cords expressed Shh, and cyclopamine inhibited CT induction. Blastemas containing vertebrae with intact spinal cords formed CTs with proximal hypertrophic regions and distal non-hypertrophic regions, whereas removal of spinal cords resulted in formation of proximal CT areas only. In fate-mapping studies, FITC-labeled vertebra periosteal cells were detected in proximal, but not distal, CT areas. Conversely, FITC-labeled blastema cells were restricted to distal CT regions. Proximal cartilage formation was inhibited by removal of periosteum and could be recapitulated in vitro by periosteal cells treated with Ihh and BMP-2. These findings suggest that proximal CTs are directly derived from vertebra periosteal cells in response to BMP and Ihh signaling, whereas distal CTs form from blastema cells in response to Shh signals from regenerated spinal cords.
KEY WORDS: Lizard, Cartilage, Regeneration, Sonic hedgehog, Indian hedgehog, Bone morphogenetic proteins, Growth plate, Ossification, Calcification, Periosteum, Blastema
Summary: Proximal and distal regenerated lizard tail skeletal regions form independently from different cell sources and in response to different signals.
INTRODUCTION
Lizards are the closest relatives of mammals that exhibit enhanced regenerative abilities of musculoskeletal tissues as adults. As amniotes, lizards follow the same basic blueprints for development, skeletal ossification and wound healing as mammals, yet retain the ability to regenerate amputated tails, a trait shared with urodelian amphibians (Alibardi, 2010). Their distinct evolutionary position intermediate to amphibians and mammals distinguishes lizards from all other regenerative model organisms, and has important and interesting implications in lizard regenerated tissues. Regenerated lizard tails are known as imperfect copies due to several key morphological differences between the regenerated and original tails (Alibardi, 2010; Bellairs and Bryant, 1985; Fisher et al., 2012; Lozito and Tuan, 2015). The most obvious of these differences concerns the regenerated skeleton. Regenerated lizard tail skeletons are almost completely cartilaginous. Vertebrae of the original tail are generated as a single, unsegmented cartilage tube (CT), and the vast majority of the lizard CT resists ossification for the lifetime of the lizard (Lozito and Tuan, 2015). Cartilage is a tissue that most mammals, and humans in particular, have particular difficulty in healing. Lizards, however, represent an organism group that spontaneously generates an abundance of cartilage in response to skeletal injury.
There are generally two mechanisms by which skeletal tissues are regenerated in adult organisms: fracture healing and blastema-based regeneration. In fracture healing, periosteal stem cells activated by the wound environment proliferate and differentiate into cartilage, forming a cartilage callus that bridges the gap between broken bones (Roberts et al., 2015). Callus cartilage development follows many of the same stages as embryonic endochondral ossification and is regulated by a conserved set of pathways. For example, periosteal stem cells proliferate in response to bone morphogenetic protein (BMP) signaling (Wang et al., 2011), and callus chondrocytes regulated by Indian hedgehog (Ihh) signaling undergo hypertrophy and express markers such as alkaline phosphatase (Alk Phos) (Wang et al., 2010). Eventually the cartilage callus ossifies and reforms a periosteum, leading to complete fracture healing.
Regrowth of amputated skeletal elements involves blastema-based regeneration. The term ‘blastema’ refers to a mass of cells that act as progenitors for regenerated tissues. The capacity to form blastemas decreases dramatically with age, and lizards are the only amniotes capable of blastema-based regeneration as adults. Unlike the urodeles, lizards are unable to regenerate amputated limbs, distinguishing lizards as the only adult organisms to combine regenerative (tail) and non-regenerative (limbs) appendages in the same animal (Alibardi, 2010). We have previously characterized development of the regenerated lizard tail skeleton, the CT, which involves blastema-based healing (Lozito and Tuan, 2015). Immediately after tail loss, the tail stump ends in a terminal tail vertebra and involves a severed spinal cord. After 7-10 days, wound epidermis migrates over the tail stump and thickens to form an apical cap. Cells liberated from stump tissues collect under the apical cap and proliferate to form a blastema. The spinal cord regenerates and infiltrates the blastema. The regenerated lizard tail spinal cord is characterized by a prominent ependymal tube. After another week, the CT forms around the regenerated spinal cord and makes contact with the terminal tail vertebra at its proximal end.
We have observed important differences in development and behavior between proximal and distal regions of regenerated lizard tail skeletons (Lozito and Tuan, 2015). Extreme proximal cartilage regions in contact with the original tail vertebrae resemble cartilage calluses formed during mammalian fracture repair. These proximal cartilage regions also exhibit growth plate-like properties and undergo a process similar to endochondral ossification. For example, proximal cartilage chondrocytes undergo hypertrophy, as evidenced by increases in cell size and expression of the hypertrophic chondrocyte markers BMP-6 and Alk Phos. Ossification centers form in between terminal tail vertebrae and proximal cartilage, setting up growth plate-like zones in these regions, which eventually undergo endochondral ossification and are replaced by bone that is continuous with that of original tail vertebrae. This endochondral ossification process is limited to proximal callus-like areas, and does not proceed to distal CT regions. The perichondria of distal CTs calcify, but never develop the growth plate-like structures observed in proximal areas, and distal CTs resist transitioning to bone for the lifetime of the lizards.
This study aims to determine the reasons why proximal, but not distal, regenerated lizard tail skeletons undergo hypertrophy and endochondral ossification. We hypothesize that differences in signaling and cell sources account for divergent development between the proximal and distal regenerated cartilage. Although the topics of cell source and signaling have been addressed in regenerated tissues of other model organisms with modern techniques (Kragl et al., 2009; Rinkevich et al., 2011; Sandoval-Guzman et al., 2014; Stewart and Stankunas, 2012), they have only recently received similar attention in lizards (Bai et al., 2015; Hutchins et al., 2014; Lozito and Tuan, 2015). Furthermore, studies considering regenerated lizard tails performed in the 1960s have remained largely unchallenged and yet are at odds with more recent reports on salamander limb and tail regeneration, particularly relating to cell sources (Cox, 1969; Kragl et al., 2009; Sandoval-Guzman et al., 2014). Here, we distinguish between cells and signals derived directly from the tissues of the original lizard tail versus those derived from the blastema to determine their specific contributions to the regenerated lizard tail skeleton. We conclude that proximal cartilage regions are derived directly from the periosteum of the original tail vertebrae, whereas distal CTs form from blastemal cells in response to signals from the regenerated spinal cord. In fact, our results suggest that proximal cartilage regions of regenerated lizard tail skeletons form independently of blastema-derived, distally located ‘true’ CTs. In recognition of these distinctions and to avoid confusion, hereafter we refer to the proximal regenerated lizard tail skeleton as the cartilage callus (CC) (equivalent to the ‘proximal CT’ term used in the Abstract and in previous publications; Lozito and Tuan, 2015). Similarly, the term CT will be used to describe all regions of regenerated lizard tail skeletons except the proximal CC that eventually ossifies (equivalent to the ‘distal CT’ term used in the Abstract and in previous publications; Lozito and Tuan, 2015). Finally, anterior, medial and posterior are used to designate specific regions within CC and CT regions (i.e. medial CC, anterior CT, etc.).
RESULTS
Regenerated lizard spinal cord implants induce ectopic CTs
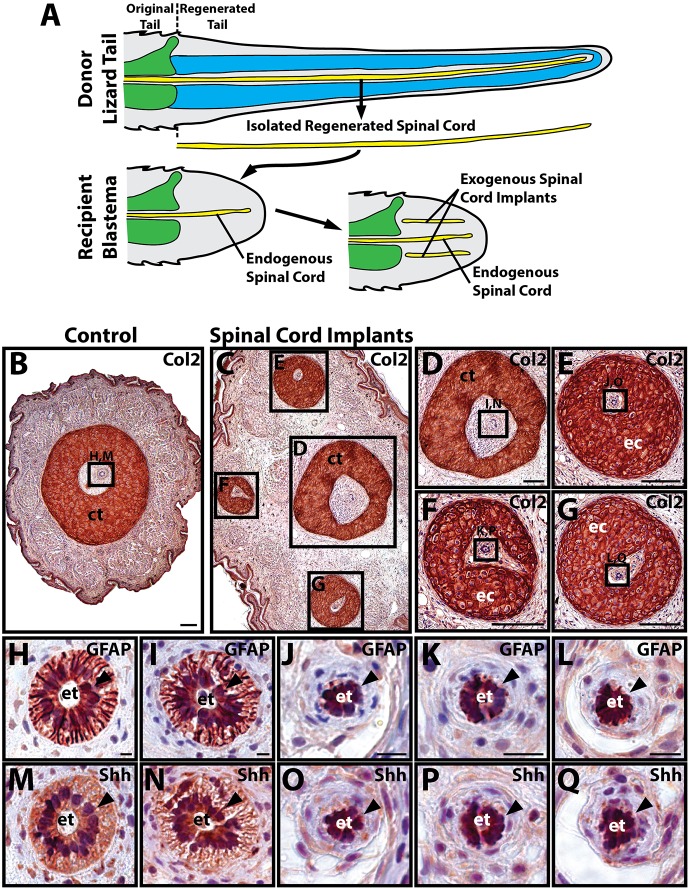
The regenerated spinal cord and ependymal tube have previously been shown to induce cartilage formation in regenerated salamander tails (Holtzer, 1956; Schnapp et al., 2005), and here we tested whether regenerated spinal cords play similar roles in lizard tail regeneration. Spinal cords were isolated from donor regenerated lizard tails (see Fig. S2 for spinal cord isolation procedure), and pieces were implanted into distal regions of recipient blastemas to create tails with both endogenous and exogenous spinal cords, which were analyzed for cartilage development after 14 days (Fig. 1A) (see Fig. S3 for spinal cord implantation procedure). Control tails that did not receive any regenerated spinal cord implants developed single cartilage tubes around endogenous regenerated spinal cords (5/5 tails examined) (Fig. 1B). Experimental tails that received exogenous spinal cord implants formed multiple cartilage tubes (21/24) (Fig. 1C), including single endogenous cartilage tubes around endogenous spinal cords (Fig. 1D) and ectopic cartilage tubes surrounding regenerated spinal cord implants (Fig. 1E-G). Both endogenous regenerated spinal cords (Fig. 1H,I) and exogenous spinal cord implants (Fig. 1J-L) exhibited prominent ependymal tubes that expressed the ependymal cell marker glial fibrillary acidic protein (GFAP) (Fig. 1H-L). Regenerated spinal cord ependymal cells also expressed high levels of the morphogen sonic hedgehog (Shh) (Fig. 1M-Q). These results suggested that lizard CTs are induced by regenerated spinal cords.
Fig. 1.
Lizard regenerated spinal cord implants induce ectopic cartilage formation. (A) Schematic showing experimental set-up for spinal cord implantation studies. Regenerated spinal cords were isolated from donor regenerated lizard tails and implanted into recipient lizard blastemas. Control blastemas contained endogenous spinal cords, whereas experimental blastemas contained both endogenous spinal cords and exogenous spinal cord implants. (B-G) Control blastemas (B) and experimental blastemas with spinal cord implants (C-G) were analyzed by Col2 IHC. Control blastemas (B) formed endogenous CTs around endogenous spinal cords, whereas experimental blastemas formed endogenous CTs around endogenous spinal cords (D) and ectopic CTs around spinal cord implants (E-G). (H-Q) Higher magnification views of endogenous and exogenous regenerated spinal cord regions identified in B,D-G. Endogenous spinal cords (H,I,M,N) and exogenous spinal cord implants (J-L,O-Q) exhibited prominent ependymal tubes (black arrowheads) that expressed GFAP (H-L) and Shh (M-Q). ct, cartilage tube; ec, ectopic cartilage tube; et, ependymal tube. Scale bars: 100 µm (B-G); 12.5 µm (H-Q).
Hedgehog regulates lizard CT induction
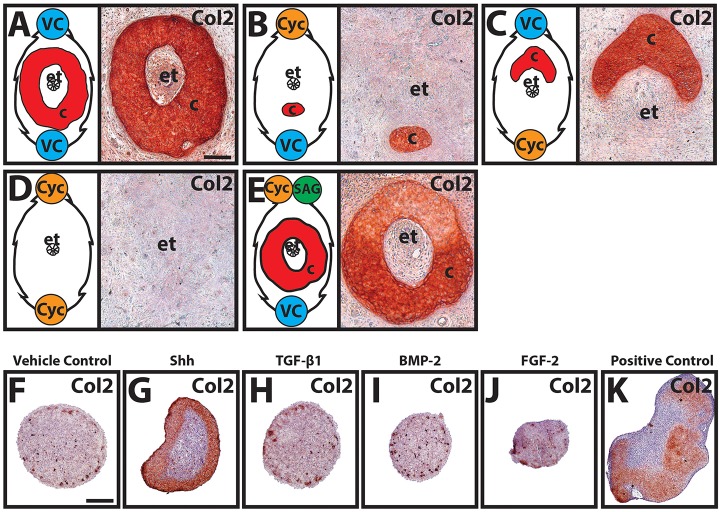
Shh produced by ependymal tubes of regenerated spinal cords was previously shown to be responsible for induction of cartilage skeletons in regenerated salamander tails (Schnapp et al., 2005) and the following set of experiments sought to determine whether Shh plays a similar role in regenerated lizard tail skeletal development. As described above, the ependymal tubes of regenerated lizard tails express high levels of Shh (Fig. 1M-Q), and here we directly tested the role of hedgehog in lizard CT induction. Beads soaked in the hedgehog inhibitor cyclopamine, the hedgehog agonist SAG, and/or vehicle controls were grafted to the dorsal and/or ventral surfaces of distal blastema explants. Explants grafted with vehicle control beads developed typical CTs that completely encircled ependymal tubes (10/10 explants examined) (Fig. 2A). Grafting of cyclopamine beads to the dorsal surfaces of explants restricted cartilage formation to ventral blastema regions (10/10) (Fig. 2B), whereas explants with cyclopamine beads grafted to ventral surfaces formed cartilage that was restricted to dorsal regions (10/10) (Fig. 2C). Cartilage formation was completely inhibited in blastemas that received both dorsal and ventral cyclopamine beads (8/10) (Fig. 2D). Cyclopamine-induced loss of cartilage could be rescued by simultaneously grafting SAG and cyclopamine beads into dorsal blastemas (7/10) (Fig. 2E). In summary, cyclopamine inhibited chondrogenesis in surrounding areas and restricted cartilage formation to opposite sides of ependymal tubes, which expressed high levels of Shh. Taken together, these results suggested that the lizard CT forms from blastema cells in response to Shh signals from the ependymal tube of the regenerated spinal cord.
Fig. 2.
Manipulation of lizard CT patterning with cyclopamine and SAG. (A-E) Beads soaked in cyclopamine (Cyc), SAG, and/or vehicle control (VC) were grafted to the dorsal (top) and/or ventral (bottom) surfaces of lizard blastemas, explant-cultured for 14 days, and analyzed by Col2 IHC. Line drawings on the left of each panel illustrate relative positions of implanted beads and effects on cartilage formation. Cyclopamine beads inhibited cartilage formation in neighboring areas, and cartilage induction was rescued by co-treatment with SAG. (F-J) Lizard blastema cells pellet-cultured and treated with 10 ng/ml Shh, TGF-β1, BMP-2, FGF-2 or vehicle control for 21 days were immunostained for Col2. (K) Lizard embryonic limb bud cell pellets served as positive controls for chondrogenesis. c, cartilage; et, ependymal tube. Scale bars: 100 µm.
The effects of Shh and other growth factors with known effects on mammalian chondrogenesis were also directly tested on lizard tail blastema cells in vitro. Cells isolated from blastemas were pellet cultured and treated with 10 ng/ml Shh, TGF-β1, BMP-2 or FGF-2 for 21 days (Fig. 2F-K). Only Shh-treated pellets developed significant cartilage areas, which were located along the periphery of the blastema cell pellets (Fig. 2G). These results supported the specificity of hedgehog signaling in inducing blastema cell chondrogenesis.
Proximal regenerated lizard tail skeletons are induced by vertebrae, whereas distal regions are induced by ependymal tubes
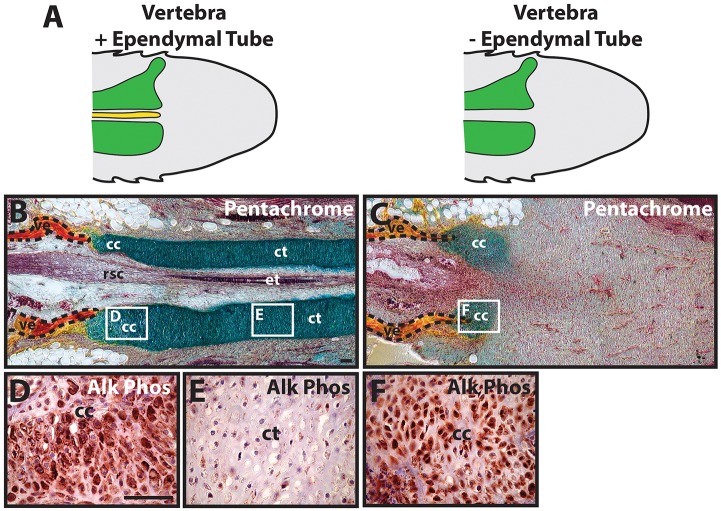
We next examined the effects of ependymal tube removal with experiments testing the individual activities of vertebrae and ependymal tubes in proximal and distal cartilage formation. Two groups of blastema explants (Fig. 3A), one with vertebrae and ependymal tubes intact, and one in which ependymal tubes were removed (see Fig. S4 for ependymal tube removal procedure), were assayed for cartilage development. Tails with both vertebrae and ependymal tubes developed skeletons with both proximal CCs and distal CTs (5/5 tails examined) (Fig. 3B), whereas tails containing vertebrae without ependymal tubes developed proximal CC areas only (5/5) (Fig. 3C). CTs did not form in samples in which regenerated spinal cord tissue other than ependymal tube remained following surgical manipulations, validating the dependency of CT formation on intact ependymal tubes. In skeletons with both CC and CT regions, proximal CCs underwent hypertrophy and expressed Alk Phos (5/5) (Fig. 3D), whereas distal CTs did not undergo hypertrophy or express Alk Phos (5/5) (Fig. 3E). Proximal CCs formed after spinal cord removal also exhibited hypertrophic morphologies and Alk Phos expression (5/5) (Fig. 3F). These results reinforced the conclusion that lizard CTs are induced by signals originating from the regenerated spinal cord ependymal tube, and introduced the idea that proximal CCs are induced and influenced by signals from the vertebra.
Fig. 3.
Proximal and distal regenerated lizard tail skeletal regions form independently. (A) Lizard tail blastemas, with or without endogenous ependymal tubes, were studied for differences in cartilage development. (B,C) The effects of ependymal tube removal on cartilage formation was analyzed by pentachrome staining. Tails with both vertebrae and ependymal tubes developed both proximal CCs and distal CTs (B), whereas tails lacking ependymal tubes developed CC areas only (C). (D-F) Higher magnification views of CC (D,F) and CT (E) areas analyzed by Alk Phos IHC. Vertebrae boundaries are traced in dashed lines. cc, cartilage callus; ct, cartilage tube; et, ependymal tube; rsc, regenerated spinal cord; ve, vertebra. Scale bar: 100 µm.
Proximal regenerated lizard tail cartilage hypertrophy is regulated by signals originating from original tail vertebrae
Numerous lines of evidence supported the link between CC hypertrophy and original tail vertebrae. Results from spinal cord implant studies indicated that, in the absence of vertebrae, proximal cartilage did not undergo hypertrophy (Fig. S8). Pro-hypertrophy effects were specific for vertebral tissue. Lizard tails re-amputated in regenerated CT portions did not develop hypertrophic cartilage regions (Fig. S9). Similarly, non-vertebral original tail stump tissues did not induce hypertrophy in CCs (Fig. S10). Finally, we performed experiments specifying lizard tail vertebrae, and the periosteum in particular, as sources of pro-hypertrophy signals (Fig. S11). Indeed, lizard periosteal cells induce hypertrophic markers in CC, but not CT, cells (Fig. S12). Taken together, these results demonstrated that signals responsible for inducing hypertrophy in proximal regenerated lizard tail cartilage originate from original tail vertebrae, and the following group of studies aimed to identify the specific signals involved.
Hedgehog signaling regulates proximal cartilage induction and hypertrophy
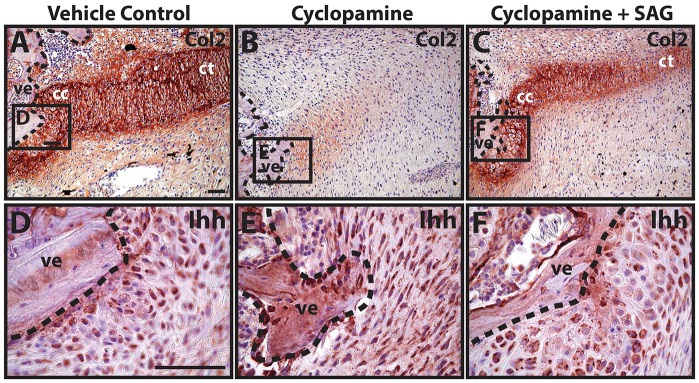
Western blot analysis of growth factors expressed by the proximal CC identified Ihh, BMP-2 and BMP-6 as the most abundant/detectable (Fig. S13) and spatially distinct (Fig. S14). The next set of experiments investigated the specific roles of each of these molecules in proximal cartilage development. The first group of experiments focused on hedgehog signaling via Ihh. Ihh expression begins around 12 days post-amputation (DPA) and increases in the CC over the first 21 days of regenerated lizard tail cartilage formation (Fig. S15). During this time-frame, Ihh expression is restricted to the proximal CC rather than the distal CT (Fig. S15). To test the role of Ihh and hedgehog signaling in early CC formation, nine DPA blastema explants were treated with vehicle control or cyclopamine for 7 days, during the inductive phase of proximal cartilage tube development. Whereas vehicle control samples developed proximal CCs of normal appearance (7/7 explants examined) (Fig. 4A), treatment with cyclopamine inhibited proximal cartilage formation (9/9) (Fig. 4B). Cyclopamine-induced loss of cartilage formation could be rescued by co-treatment with SAG (7/9) (Fig. 4C). To validate the hypothesis that cyclopamine and SAG were acting through hedgehog inhibition rather than modulation of hedgehog expression, the same samples were analyzed by Ihh immunostaining (Fig. 4D-F). Neither cyclopamine nor SAG treatment significantly affected Ihh expression levels, suggesting that the observed effects on cartilage formation were, in fact, signaling effects of drug treatments. Thus, these results suggested that proximal cartilage formation is induced by Ihh signaling.
Fig. 4.
Cyclopamine inhibits proximal cartilage induction. (A-C) Lizard tail blastema explants (9 DPA) were treated with vehicle control (A), cyclopamine (B) or cyclopamine and SAG (C) and analyzed by Col2 immunostaining to assess the role of hedgehog signaling in proximal CC formation. (D-F) Higher magnification views of regions identified in A-C analyzed by Ihh immunostaining. Dashed lines trace vertebral boundaries. cc, cartilage callus; ct, cartilage tube; ve, vertebra. Scale bars: 50 µm.
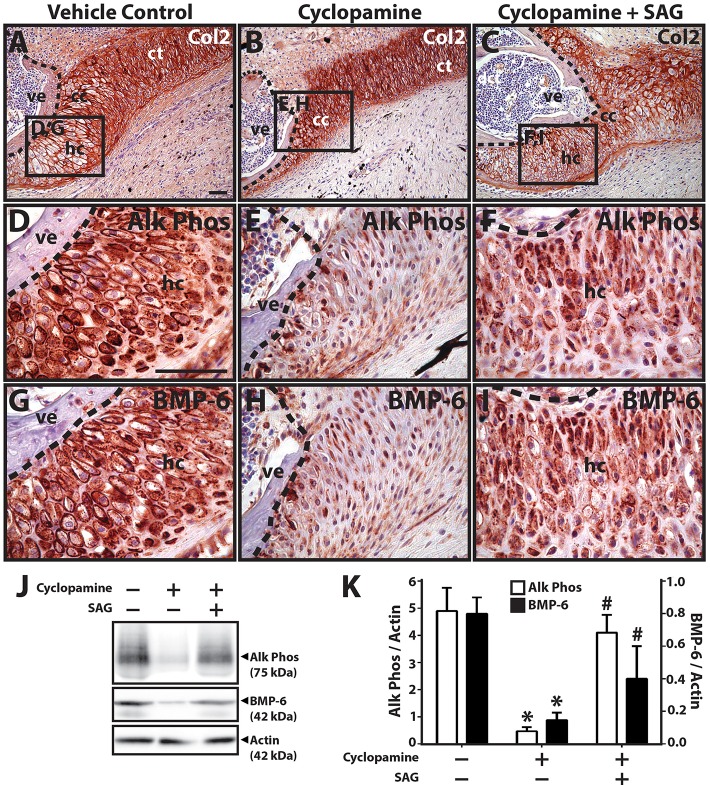
The next set of experiments involved treating blastema explants with cyclopamine beginning after CC formation (14 DPA) to focus on the role of hedgehog in later stages of cartilage development. Proximal CCs of explants treated with vehicle control underwent hypertrophy (9/9 explants examined) (Fig. 5A), as indicated by cell morphology (Fig. 5A,D) and Alk Phos expression (Fig. 5D), whereas cyclopamine treatment inhibited hypertrophy (9/9) (Fig. 5B,E) and Alk Phos expression (9/9) (Fig. 5E). Co-treatment with SAG partially rescued cartilage hypertrophic morphology (8/9) (Fig. 5C,F) and Alk Phos expression (7/9) (Fig. 5F), although the hypertrophic regions appeared disorganized (Fig. 5C) compared with controls. Samples were also analyzed by BMP-6 immunostaining (Fig. 5G-I) to gain a better understanding of the interplay between hedgehog and BMP signaling. Hypertrophic chondrocyte regions expressed BMP-6 under control conditions (Fig. 5G). Cyclopamine treatment significantly inhibited BMP-6 expression in these regions (9/9) (Fig. 5H), and co-treatment with SAG partially rescued BMP-6 expression levels (9/9) (Fig. 5I). Alk Phos and BMP-6 immunostaining results were validated by western blotting (Fig. 5J,K). Taken together, these results suggested that hedgehog signaling regulated proximal CC hypertrophy and acted to induce BMP-6 expression in hypertrophic cartilage regions.
Fig. 5.
Cyclopamine inhibits proximal cartilage hypertrophy. (A-C) Lizard tail blastema explants (14 DPA) were treated with vehicle control (A), cyclopamine (B) or cyclopamine and SAG (C) and analyzed by Col2 immunostaining to assess the role of hedgehog signaling in proximal CT hypertrophy. (D-I) Higher magnification views of regions identified in A-C analyzed by Alk Phos (D-F) and BMP-6 (G-I) expression. Dashed lines trace vertebral boundaries. (J) Western blot analysis of Alk Phos and BMP-6 expression in CC explants treated with vehicle control, cyclopamine and/or SAG. Actin blots were included as loading controls. (K) Densitometric quantification of Alk Phos and BMP-6 western blot results normalized to actin loading controls (n=3). *P<0.05 compared with vehicle controls; #P<0.05 compared with 0 ng/ml SAG condition; Student's t-test. Data are mean±s.d. cc, cartilage callus; ct, cartilage tube; hc, hypertrophic chondrocytes; ve, vertebra. Scale bars: 50 µm.
Interestingly, treatment with Ihh-soaked beads did not significantly induce exogenous regions of proximal or distal cartilage (Fig. S16). Similarly, none of the SAG treatments described above induced cartilage in blastema areas that would otherwise form other tissues, such as muscle. These results suggest that cartilage formation within the regenerated lizard tail is not only dependent upon hedgehog signaling, but is also influenced by the responsiveness of the cell population, an idea that is further explored in the experiments concerning cartilage cell origins described below.
BMP signaling regulates proximal cartilage proliferation and hypertrophy
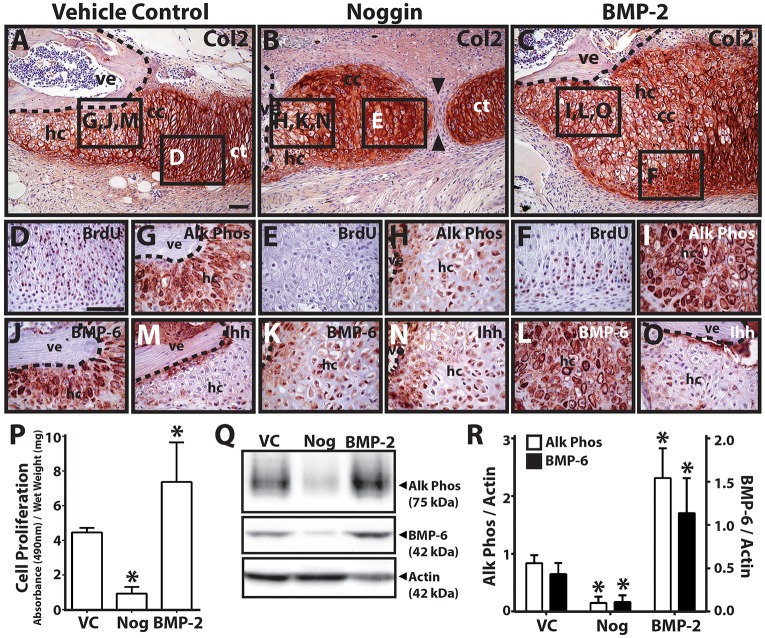
The final set of drug/growth treatment experiments tested the effects of BMP inhibition and stimulation on proximal CC development. Explants (9 DPA) treated with vehicle control exhibited normal, fully developed CCs (9/9 explants examined) (Fig. 6A). Treatment with the BMP antagonist noggin resulted in gaps between CCs and distal CTs (8/9) (Fig. 6B), and treatment with exogenous BMP-2 caused significant enlargement of proximal CCs (9/9) (Fig. 6C). These results suggested an effect on proliferation, so we assayed for proliferation with bromodeoxyuridine (BrdU) immunostaining (Fig. 6D-F). Inhibition of BMP signaling by treatment with noggin resulted in a reduction of proliferative cells in proximal CC areas (9/9) (Fig. 6E), suggesting that BMP regulated proliferation of this region. BrdU-positive proliferating chondrocytes were smaller than non-proliferating cells (Fig. 6D,F), and proximal CCs of noggin-treated samples were almost entirely made up of larger, rather than smaller, cells (Fig. 6E). The reduced numbers of these smaller, proliferating chondrocytes in non-union areas of noggin-treated samples suggested that noggin treatment caused reductions in proliferating chondrocyte populations.
Fig. 6.
BMP inhibition and stimulation modulates proximal cartilage proliferation and hypertrophy. (A-C) Lizard tail blastema explants (9 DPA) were treated with vehicle control (A), noggin (B) or BMP-2 (C) and analyzed by Col2 immunostaining to assess the role of hedgehog signaling in proximal CT hypertrophy. Noggin treatment resulted in non-unions between proximal and distal CT regions (black arrowheads). (D-O) Higher magnification views of regions identified in A-C analyzed by BrdU (proliferating cells; D-F), Alk Phos (G-I), BMP-6 (J-L) and (M-O) Ihh immunostaining. Dashed lines trace vertebral boundaries. (P) Quantification of cell proliferation in CC explants treated with vehicle control (VC), noggin (Nog) or BMP-2 (n=3). *P<0.05 compared with vehicle control; Student's t-test. (Q) Western blot analysis of Alk Phos and BMP-6 expression in CC explants treated with VC, noggin or BMP-2. Actin blots were included as loading controls. (R) Densitometric quantification of Alk Phos and BMP-6 western blot results normalized to actin loading controls (n=3). *P<0.05 compared with vehicle controls; Student's t-test. Data are mean±s.d. cc, cartilage callus; ct, cartilage tube; hc, hypertrophic chondrocytes; ve, vertebra. Scale bars: 50 µm.
We also noticed differences in hypertrophic region sizes among the three conditions (Fig. 6A-C), and staining for Alk Phos expression (Fig. 6G-I) confirmed that noggin treatment inhibited hypertrophy (9/9) (Fig. 6H) and BMP-2 treatment enhanced hypertrophy (9/9) (Fig. 6I) compared with vehicle controls (Fig. 6G). These results suggested that BMP signaling also regulated proximal CC hypertrophy. The same samples were also analyzed for BMP-6 (Fig. 6J-L) and Ihh (Fig. 6M-O) expression. Noggin treatment decreased BMP-6 expression levels (9/9) (Fig. 6K), and BMP-2 treatment enhanced BMP-6 expression (9/9) (Fig. 6L). Neither noggin (Fig. 6N) nor BMP-2 (Fig. 6O) treatment significantly affected Ihh expression levels, although noggin treatment resulted in disorganized Ihh expression less closely associated with vertebrae (Fig. 6N) compared with control and BMP-2 conditions (Fig. 6M,O). Observations on the effects of noggin and BMP-2 on CC cell proliferation were quantified with cell proliferation assays (Fig. 6P), and Alk Phos and BMP-6 immunostaining results were verified by western blots (Fig. 6Q,R). Taken together, these results suggested that BMP signaling regulates both primal CC proliferation and hypertrophy. Furthermore, as hedgehog inhibition reduced BMP expression, and BMP inhibition reduced BMP but not Ihh expression, these results support the hypothesis that BMP regulates its own expression and acts downstream of Ihh in proximal CC development.
Proximal regenerated lizard tail skeletons are directly derived from vertebrae periosteum, whereas more distal regions are derived from blastema cells
Having shown that the proximal and distal regenerated lizard tail skeletal regions were regulated by signals originating from different tissues, we wanted to determine whether the cell sources of the two regions also differed. These experiments were also motivated by considering wound healing in lizard skeletal tissues outside of the tail. Although lizards can regenerate amputated tails, they are unable to regrow lost limbs, so limb and tail injuries were compared to identify healing responses common to both tissues and, thus, independent of blastema-based regeneration. Amputated lizard limbs and toes do not form blastemas (0/10 limbs/toes examined), but significant CCs do form around the damaged bone (10/10) (Fig. S17A) that express Col2 (10/10) (Fig. S17B) and Alk Phos (10/10) (Fig. S17C), similar to the proximal CCs of regenerated tail skeletons. These observations suggested the possibility that proximal cartilage originates from the bones of original tails and forms independently of blastema-derived regenerated cartilage.
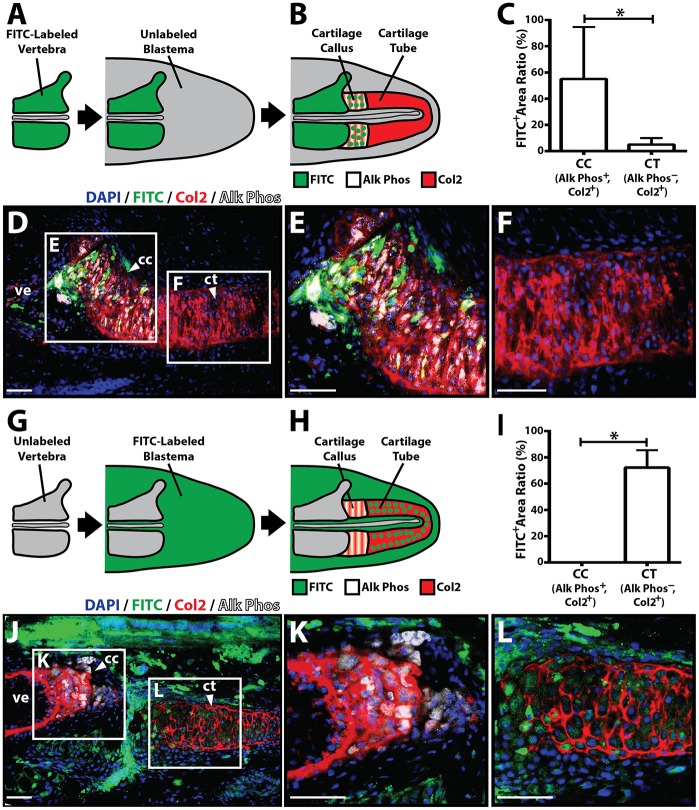
To test this hypothesis directly, vertebral cell fate-mapping experiments were performed. Lizard tail vertebral tissue was treated with carboxyfluorescein diacetate-succinimidyl ester (CFDA-SE), which irreversibly labels cells with FITC, and implanted into unlabeled blastema explants (Fig. 7A). After 2 weeks of culture, explants were assayed for co-expression of FITC, Col2 and Alk Phos to identify Alk Phos+ cartilage regions directly derived from vertebral tissue (Fig. 7B). A significant majority of FITC-positive cells were detected in proximal Alk Phos+ CCs rather than distal Alk Phos− CTs (n=5 explants) (Fig. 7C-F), suggesting that proximal cartilage is directly derived from vertebrae. Penetration of the labeling reagent was found to be restricted to the periosteum of CDFA-treated vertebral tissue (Fig. S18A,B), suggesting that the periosteum is the specific tissue source of the proximal CC. Lizard tail vertebra periosteum could be efficiently removed (Fig. S18C,D), and periosteal cells were isolated from periosteal tissue and cultured in vitro (Fig. S18E). Isolated periosteal cells expressed the stem/progenitor cell markers CD166, CD144 and CD90 (Fig. S19). Interestingly, isolated CC, but not CT, cells also expressed these markers, strengthening the finding that the lizard tail CC is derived from periosteal cells (Fig. S19A). Periosteal cells alone expressed high levels of Ihh (Fig. S19A,E), indicating these cells, rather than CC cells, as the original source for Ihh in the regenerating lizard tail. Having also shown that lizard periosteal cells induce Ihh in CC cells during co-culture (Fig. S12), these results also suggest that CC Ihh is induced by periosteal cells. Finally, we tested the dependency of CC formation on vertebra periosteum (Fig. S20), finding that removal of periostium resulted in loss of CC formation (Fig. S20B,C), and that isolated periosteal cells formed cartilage in vitro (Fig. S20D-F). Furthermore, periosteal cells proliferated and migrated in response to BMP stimulation (Fig. S21). Taken together, these results suggested that proximal CCs are derived from periosteal cells.
Fig. 7.
Vertebral and blastema cells fate map to proximal and distal regenerate lizard tail skeletal regions, respectively. (A,B) Experimental scheme used to fate map vertebral-derived cartilage regions. (A) FITC-labeled vertebrae were implanted into unlabeled blastema explants. (B) After 2 weeks, during which time CCs/CTs formed, samples were co-immunostained for Alk Phos and Col2 to determine whether FITC-labeled cells traced to Alk Phos+ Col2+ cartilage callus (CC) regions or Alk Phos− Col2+ cartilage tube (CT) regions. (C) Areas of FITC+, Alk Phos+ and Col2+ regions were quantified using ImageJ and compared between Alk Phos+ Col2+ CC regions and Alk Phos− Col2+ CT regions (n=5). *P<0.05; Student's t-test. (D) Representative unlabeled blastema sample implanted with FITC-labeled vertebra following 2 weeks of explant culture and analyzed for FITC, Col2 and Alk Phos expression. (E,F) Higher magnification views of CC (E) and CT (F) regions identified in D. (G,H) Experimental scheme used to fate map blastema-derived cartilage regions. (G) Unlabeled vertebrae were implanted into FITC-labeled blastema explants. (H) Resultant CCs/CTs were analyzed for Col2 and Alk Phos expression to identify cartilage regions derived from blastemal cells. (I) Quantification of FITC+ areas within Alk Phos+ Col2+ CC regions and Alk Phos− Col2+ CT regions (n=5). *P<0.05; Student's t-test. (J) Representative cultured FITC-labeled blastema sample implanted with unlabeled vertebra and analyzed for FITC, Col2 and Alk Phos expression. (K,L) Higher magnification views of CC (K) and CT (L) regions identified in J. Data are mean±s.d. cc, cartilage callus; ct, cartilage tube; ve, vertebra. Scale bars: 25 µm.
To validate these results, the reverse experiment, in which unlabeled vertebrae were implanted into FITC-labeled blastemas (Fig. 7G), was also performed. The same type of analysis as described above was conducted, but here regions that expressed both FITC and Col2 represented cartilage derived from blastema cells (Fig. 7H). In these experiments, FITC was only detected in Alk Phos− CT areas, not in proximal Alk Phos+ CCs (n=5) (Fig. 7I-L), confirming that CTs, but not proximal cartilage, are derived from blastemas.
Proximal and distal regenerated lizard tail skeletons form independently from distinct cell sources in response to different signals
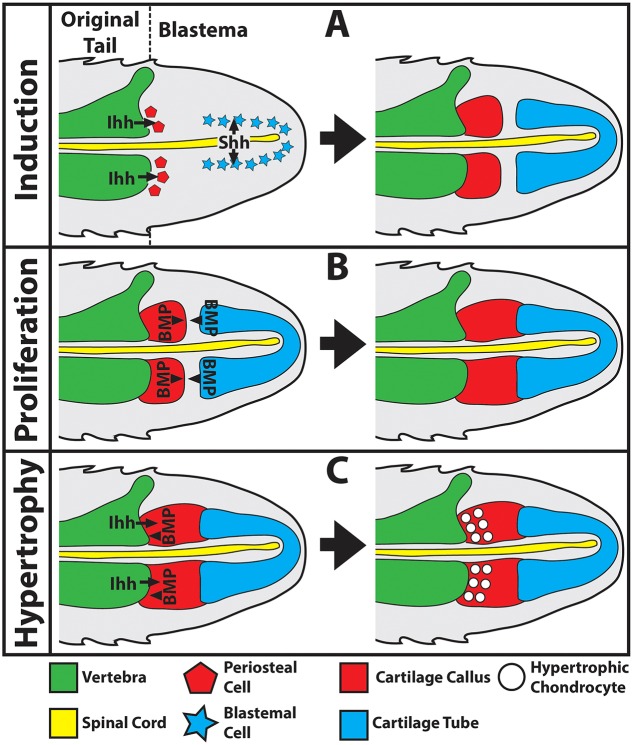
Our current understanding of early regenerated lizard tail skeletal development as supported by the results presented in this report are presented in Fig. 8. Lizard skeletal regeneration is divided into three main phases: (1) induction (Fig. 8A); (2) proliferation (Fig. 8B); and (3) hypertrophy (Fig. 8C). During the induction phase, proximal CCs form from vertebra periosteal cells in response to Ihh signaling, whereas the distal CT forms from blastemal cells in response to Shh signals from the ependymal tube of the regenerated spinal cord (Fig. 8A). Next, proximal CCs proliferate in response to BMP signaling (Fig. 8B). BMP-6 is produced by the cartilage callus itself, and BMP-2 produced by anterior ends of CTs activates cartilage callus chemotaxis to close gaps between proximal CC and distal CTs, effectively linking original and regenerated tail skeletons. Finally, proximal CCs undergo hypertrophy in response to both Ihh and BMP signals linked to original tail vertebrae (Fig. 8C). Eventually, hypertrophic CC chondrocytes complete endochondral ossification and are replaced by bone.
Fig. 8.
Summary of cells and signals regulating regenerated lizard tail skeletal development. (A-C) Schematics of sagittal sections of a regenerated lizard tail to highlight cell sources and signals regulating regenerated cartilage induction (A), proliferation (B) and hypertrophy (C). (A) The proximal cartilage callus forms from periosteal cells derived from original tail vertebrae in response to Ihh signaling, whereas the cartilage tube forms from blastemal cells in response to Shh signals from the regenerated spinal cord ependymal tube. (B) The proximal cartilage callus proliferates and expands towards the cartilage tube in response to BMP signaling. (C) The proximal cartilage callus undergoes hypertrophy in response to both Ihh and BMP vertebral signals.
Comparisons of lizard (Anolis carolinensis) and salamander (Ambystoma mexicanum) tail regeneration
Several key experiments described in this manuscript were repeated with salamanders to identify similarities and differences between lizard and salamander tail regeneration. First, we sought to determine whether salamander tail regeneration involves the formation of a CC similar to lizards. As described above, the distinction between CC and CT components of regenerated lizard tail skeletons is facilitated by the fact that lizard CC, but not lizard CT, undergoes endochondral ossification. We could not use this technique to determine whether regenerated salamander skeletons included CCs because no portions of regenerated salamander skeletons ossify. So, to focus on vertebrae-derived cartilage in regenerated salamander tails, we took advantage of the dorsoventral patterning exhibited by regenerated salamander skeletons. Blastema-based salamander tail skeletal regeneration is restricted to ventral regenerated salamander tails (Fig. S22A). Thus, to identify vertebral-derived salamander CCs, we focused on cartilage formed in dorsal proximal regenerated salamander tails (Fig. S22B). Dorsal salamander cartilage regions were associated with original tail vertebrae and resembled CC regions of regenerated lizard tails. Identification of dorsal salamander CCs was further validated with ependymal tube removal experiments, similar to those carried out for lizards (Fig. 3). As in lizards, removal of salamander tail ependymal tubes resulted in loss of blastema-derived cartilage (Fig. S22C), and also led to expansion of dorsal salamander CC regions (Fig. S22D).
Although both lizards and salamanders developed CCs, only lizard CCs underwent endochondral ossification. In lizards, proximal CCs associated with original tail vertebrae (Fig. S23A) were converted to bone (Fig. S23A,B). Lizard CC chondrocytes in contact with the terminal vertebra underwent hypertrophy as they enlarged and expressed Alk Phos (Fig. S23C). Periosteum/perichondrium formed around regions of hypertrophic chondrocytes and expressed Ihh (Fig. S23D), and ossification centers formed in between terminal tail vertebrae and CCs (Fig. S23C,D). Thus, lizard CCs developed growth plate-like organization as they underwent endochondral ossification. This entire process was absent in regenerated salamander tails. Salamander CCs contacted original tail vertebrae similar to lizard CCs (Fig. S23E), but salamander CCs did not undergo endochondral ossification (Fig. S23F), and salamander CC chondrocytes did not enter hypertrophy. There was no evidence of growth plate-like structures in salamander CCs, which also lacked Alk Phos (Fig. S23G) and Ihh (Fig. S23H) expression. We believe that these interesting differences are functionally related to differences in development between lizard and salamander tail skeletons. Original lizard tails develop and grow through endochondral ossification (Fig. S24A), whereas the centrum of the salamander vertebrae remains cartilaginous without transitioning to bone during all life stages (Fig. S24B). Thus, we believe that endochondral ossification processes that occur in lizard, but not salamander, CCs reflect embryonic developmental mechanisms.
Important differences between regenerated lizard and salamander skeletons in terms of dorsoventral patterning were also observed. These differences are obvious when 4-week-old regenerated lizard and salamander tails are viewed in cross-section (Fig. S25A,B). Lizard CTs surround ependymal tubes (Fig. S25A), whereas salamander CRs are ventral to ependymal tubes (Fig. S25B). This lack of dorsoventral patterning in lizard versus salamander tails is mirrored in ependymal tubes of regenerated spinal cords. Lizard ependymal tubes express Shh along their entire circumference and do not express the roof plate/neural crest marker Pax7 (Fig. S25C), whereas salamander ependymal tubes exhibit distinct Shh+ floor plate and Pax7+ roof plate domains (Fig. S25D). Salamander ependymal tube Shh and Pax7 expression patterns closely resemble those of the embryonic lizard neural tube (Fig. S25E); we hypothesize that this reflects the embryonic nature of the salamander nervous system and plays a role in the divergent dorsoventral patterns of salamander versus lizard regenerated tail skeletons.
DISCUSSION
Unique in the animal kingdom, the lizard CT is an adult skeletal organ that only exists in regenerated lizard tails. This study finds that regenerated lizard tails also generate a CC proximal to the CT, and that these two distinct cartilage regions differ in cell sources and regulatory signals. These conclusions were made possible by virtue of two singular properties of regenerated lizard tail skeletons. First, unlike regenerated salamander tail cartilage, which transitions into segmented vertebral columns, the vast majority of the lizard CT persists unchanged for the lifetime of the lizard/regenerate. Thus, the lizard regenerate lacks segmentation and proximodistally stratified structures other than what is provided by original tail stump tissues at extreme proximal tail regions. Second, we took advantage of the fact that lizard vertebra-derived cartilage (described here as the CC) undergoes hypertrophy, whereas blastema-derived cartilage (which makes up the CT) does not. The combination of these unique characteristics distinguished the regenerated lizard tail skeleton as an ideal model for investigating contributions of stump versus blastema cells and signals to regenerated skeletal tissues. Indeed, the most important finding reported here concerns the differences in cell sources between the proximal and distal regions of regenerated lizard tail skeletons. Ever since the first description of the blastema in 1911 (Fritsch, 1911), the cellular origin of regenerated tissues has been one of the primary questions in regenerative sciences. Studies that have focused on this area have essentially supported one of two hypotheses on blastema/regenerated tissue formation (Stocum, 2002). In the first hypothesis, blastemas form from de-differentiated stumps cells that then re-differentiate to form regenerated tissues. In the second hypothesis, reserve stem/progenitor cells that reside within original tail stump tissues differentiate directly into cell lineages that reconstitute regenerates. The various studies that have been performed, the majority of which focused on muscle regeneration in amputated amphibian limbs, have yielded conflicting conclusions (Echeverri et al., 2001; Hay, 1959; Kragl et al., 2009; Kumar et al., 2004; Lo et al., 1993; Morrison et al., 2006, 2010; Stocum, 2002). Adding to the confusion are recent reports that suggest cellular origins of regenerated tissues vary between species (Sandoval-Guzman et al., 2014). Among regenerative amphibians, newts regenerate muscle in regrown limbs via the first hypotheses involving cell de-differentiation, whereas axolotl salamanders regrow muscle from stem cell populations within stump tissues according to the second hypothesis (Sandoval-Guzman et al., 2014).
Our study shows that, in the case of lizard tail skeletal regeneration, both mechanisms are at play, and that cell sources contributing to regeneration differ by location within the regrown tail. Specifically, proximal regenerated lizard tail skeletons are derived from chondrogenic periosteal stem/progenitor cells of the original tail vertebrae, whereas distal skeletons form from multipotent blastema cells. Here, we made the distinction between cells derived directly from vertebrae and cells derived directly from blastemas. That is not to say populations of blastema cells are not derived from vertebrae. However, our studies have shown that, whatever their initial origins, blastema cells (as defined here) are distinct from progenitor cells found within stump tissues, at least as they relate to skeletal regeneration.
To our knowledge, this study reports the first direct evidence of distinctions in cell origin between proximal and distal skeletal structures and lays the groundwork for investigating whether similar trends exist in other examples of regeneration. For example, salamanders also develop proximal callus-like cartilage regions in their regenerated tail skeletons (Fig. S22). However, unlike lizards, salamander CCs do not undergo hypertrophy and endochondral ossification (Fig. S23). We posit that differences in cartilage development between lizard versus salamander regenerated tail skeletons reflect the evolutionary position of lizards relative to salamanders. Salamander skeletons are evolutionarily primitive compared with those of lizards and exhibit several embryonic characteristics. For example, salamander skeletons are largely cartilaginous and retain notochord throughout adulthood, whereas lizard skeletons are fully formed and ossified. We can imagine that development of the original and regenerated skeletons are related, and that lizard regenerated proximal cartilage ossifies because the original lizard skeleton ossifies, whereas the inability of salamander proximal cartilage to ossify is linked to the absence of endochondral ossification in the original salamander tail. Furthermore, the realities of joining cartilaginous and ossified skeletons may present additional mechanical and structural challenges to the regenerated lizard tail, and the proximal lizard CC may also function as enhanced attachment sites for binding the regenerated and original tail skeletons. These hypotheses are supported by work presented here showing that cartilage regenerated from amputated lizard CTs does not ossify. Future work will be aimed at determining why lizard, but not salamander, proximal cartilage undergoes hypertrophy and endochondral ossification by comparing salamander and lizard periosteal cells and signals.
Differences between the salamander and lizard are not limited to the proximal CC, and this study highlights important differences between distal skeletal elements regenerated by the two animals, particularly as they relate to dorsoventral patterning. Regenerated lizard tail skeletons lack dorsoventral patterning compared with those of salamander. Lizard CTs surround ependymal tubes, whereas salamander tails contain cartilage rods (CRs) ventral to ependymal tubes. Our study complements earlier work with salamanders (Schnapp et al., 2005), and we can now say that both lizard CTs and salamander CRs are induced by Shh signals derived from ependymal tubes of regenerated spinal cords. However, comparisons of spinal cord Shh expression between the two animals indicated that salamander, but not lizard, ependymal tubes are dorsoventrally patterned. Salamander Shh expression is confined to the ventral ependymal tube ‘floor plate’, whereas lizard Shh is expressed by the entire circumference. The dorsal salamander ependymal tube has also been shown to express neural crest/roof plate markers, such as Pax7 and MSX1, which we have found to be absent in lizard ependymal tubes. Thus, in terms of neural tube patterning, lizard ependymal tubes are entirely made up of cartilage-inductive Shh+ ‘floor plate’. From this, it is reasonable to conclude that regenerated lizard tail skeletons lack patterning because regenerated lizard tail ependymal tubes lack dorsoventral patterning. Again, this may reflect differences in evolutionary position between lizards and salamanders; neotenic salamanders, the primitive spinal cords of which retain neural tube dorsoventral patterning even as adults, regenerate similarly patterning spinal cords. By contrast, adult lizard spinal cords lose patterning during development and are unable to recreate embryonic patterning during regeneration.
The above discussion has focused on differences between salamander and lizard tail regeneration, but this study has also highlighted important similarities between lizard and salamander regenerated skeletons. For example, neither salamander nor lizard regenerated skeletons ossify. The perichondria of both salamander CRs and lizard CTs calcify, but both regenerated skeletons are decidedly cartilaginous. Thus, we must consider the possibility that, as a rule, blastema-derived axial skeletons simply do not ossify, and perhaps the regenerated lizard tail CT should be considered as a primitive skeleton regenerated by an amniote. Future work will be aimed at exploring this intriguing possibility.
The result that distal, but not proximal, regenerated lizard tail cartilage is derived from blastema cells may be controversial because it has long been thought that lizard tails do not form true blastemas. This view is based on reports from the 1960s published by P. G. Cox highlighting differences between the early regenerated lizard tail and the classical blastema described from amphibian limb regeneration (Cox, 1969). Our findings reported here have shown that these observations are actually due to differences in limb versus tail regeneration rather than differences between salamanders and lizards, and have little bearing on blastema classification. For example, according to Cox, salamander limb blastema cell proliferation is apically associated with the wound epidermis, whereas lizard cell proliferation centers more proximally (Cox, 1969). With the results from this report, this difference can now be explained by the divergent locations of Shh-producing organization centers in limb versus tail regeneration. Since the report by Cox, numerous studies have demonstrated apical salamander regenerating limb wound epidermis as the source of Shh signals responsible for organizing blastema cell proliferation and differentiation (Imokawa and Yoshizato, 1997; Roy and Gardiner, 2002; Torok et al., 1999). In this article, we show that in the regenerating lizard tail it is the spinal cord ependymal tube, not the wound epidermis, that is the source of Shh signals. This mirrors what has been described for ependymal tube-derived Shh signals regulating differentiation and proliferation in regenerated salamander tail blastemas. Thus, differences in lizard versus salamander blastema descriptions observed in previous studies are more a reflection of the differences in Shh signaling sources, and the true distinction to be made is between limb and tail blastemas rather than excluding early lizard tail regenerates from classification as blastemas.
Earlier studies have also refrained from classifying early regenerated lizard tails as true blastemas due to lack of evidence indicating tail stump cell de-differentiation, which, at the time, was held as a key trait of blastema cell origination. More recent experiments have demonstrated that even the classical blastema cells described for axolotl salamander limbs are derived from resident stem cells rather than de-differentiated pluripotent cells (Sandoval-Guzman et al., 2014), casting the appropriateness of some traditional requirements of blastema classification into doubt. In addition, earlier lizard studies assumed that all regenerated lizard tail tissues, including cartilage, were formed either directly from stump skeletal cells or from undifferentiated reserve stem cells. In light of the results presented here indicating two distinct cell sources for regenerated cartilage, we believe that these earlier reports made assumptions for the whole of the lizard CT based on observations concerning proximal, but not distal, cartilage regions. For example, the results presented by Cox (1969) match our observations of regenerated lizard tail proximal CC regions, which are derived directly from chondrogenic periosteal cells of the original tail vertebrae. However, our work with distal cartilage, particularly experiments concerning spinal cord implants, show that the CT originates from a separate, blastemal cell population capable of differentiating into either cartilage or muscle, and perhaps other lineages. In this regard, our study also challenges the prevailing theory of blastema cell plasticity during regeneration. Work done with salamander limbs has unequivocally shown that, under normal circumstances, blastema cells retain lineage-restricted differentiation capabilities during regeneration (Kragl et al., 2009). For example, blastema cells derived from original limb muscle give rise to regenerated muscle tissue. However, these salamander studies did not test whether muscle-derived blastemal cells could differentiate into cartilage cells in artificially manipulated regeneration circumstances. Our experiments demonstrating ectopic CT induction by spinal cord implants showed that lizard blastema cells exhibit some degree of plasticity or transdifferentiation, at least as they pertain to cartilage and muscle differentiation. In these experiments, spinal cord implants induced chondrogenesis in blastema cells that would otherwise have differentiated into muscle. Having identified Shh as the factor responsible for inducing chondrogenesis in lizard blastemal cells, it is tempting to speculate that, in the absence of Shh stimulation, lizard blastema cells default towards the muscle lineage. Future work will be aimed at testing this hypothesis and at defining the differentiation capacities of lizard blastema cell lineages.
In conclusion, this study demonstrates that proximal and distal regenerated lizard tail skeletal regions form independently from different cell sources and in response to different signals. The proximal skeleton is directly derived from periosteum from the original tail vertebrae and resembles a CC formed during fracture repair, whereas the distal CT is derived from the blastema similar to the salamander regenerated tail. Therefore, we propose referring to the proximal cartilage region as the proximal CC, rather than CT, to reflect its independence from the true, blastema-derived CT. In challenging several long-held assumptions, particularly related to blastema formation and cell sources of regenerated lizard tail cartilage, this study also provides a much needed update on the mechanisms and cell populations regulating lizard skeletal regeneration. In doing so, this report places lizard tail regeneration in better context with modern observations on mammalian fracture healing and amphibian limb/tail regeneration, highlighting common themes as well as important distinctions that result in very different regenerative outcomes. In the end, the findings suggest that lizards, evolutionarily intermediate to amphibians and mammals, employ an amalgam of lower and higher vertebrate wound healing responses during tail skeletal regeneration, hinting at an evolutionary role in regenerative outcomes.
MATERIALS AND METHODS
Lizard tail blastema surgical manipulations and explant culture
Lizard (Anolis carolinensis) tail blastemas (Fig. S1) were subjected to surgical procedures prior to embedding in Col1 gels. All surgical manipulations were performed under observation with an Olympus SZX16 dissecting microscope. Tissue to be manipulated was soaked in HBSS supplemented with Antibiotic-Antimycotic (Life Technologies). See supplementary Materials and Methods for specific details on surgical manipulations, including CT and regenerated spinal cord isolation (Fig. S2), spinal cord transplantation (Fig. S3), ependymal tube removal (Fig. S4) and CT relocation (Fig. S5), as well as descriptions of lizard tail blastema explant culture.
Lizard cell culture isolation and culture
Cells were isolated from lizard tail blastemas, periosteum, CCs and CTs, as well as from chicken embryonic limb buds, and cultured in vitro. See supplementary Materials and Methods for specifics on cell isolations and culture conditions.
Drug and growth factor treatments
Lizard tail blastema explants were treated with LBEM supplemented with 600 nM cyclopamine (LC Laboratories), 40 nM SAG [N-methyl-N′-(3-pyridinylbenzyl)-N′-(3-chlorobenzo[b]thiophene-2-carbonyl)-1,4-diaminocyclohexane] (Stem RD), 75 ng/ml noggin, (Peprotech), 100 ng/ml BMP-2 (Peprotech) or vehicle control (PBS or ethanol). In experiments involving beads, AG1-X2 resin beads (Bio-Rad) were soaked in either 20 mg/ml cyclopamine, 2.5 mg/ml SAG or vehicle control (ethanol) overnight at 4°C, washed three times in HBSS, and implanted into the dorsal and/or ventral surfaces of lizard tail blastemas. Comparatively hard resin beads easily slipped under soft blastema epidermis. Explants were cultured as described in the supplementary Materials and Methods. SAG and cyclopamine concentrations used in studies involving SAG treatments to rescue hedgehog signaling inhibition by cyclopamine were determined with titration experiments carried out for both soluble and bead-delivered drugs (Fig. S6).
Histology and immunohistochemistry (IHC)
Lizard tissue samples were analyzed by histology (Movat's pentachrome stain) and IHC exactly as previously described (Lozito and Tuan, 2015). Pentachrome stains cartilage green and bone red/orange, making it the ideal stain for analyzing skeletal regeneration. See Table S1 for IHC antibody specifics and Fig. S7 for validation of collagen type X (Col10) antibodies. All histology/IHC images of sagittal sections are presented dorsal towards the top, ventral bottom, distal right and proximal left.0
Fluorescent labeling of lizard tail tissues
Lizard vertebrae and blastemal tissue were isolated from original and regenerated lizard tails, respectively, washed with HBSS, and incubated in 10 µM carboxyfluorescein diacetate-succinimidyl ester (CFDA-SE; Life Technologies) in HBSS at 37°C for 15 min (Sinha et al., 1994). Labeled lizard tissues were then incubated for another 30 min in label-free LBEM at 37°C to ensure the complete modification of the probe and then washed in HBSS.
Micro-computed tomography (microCT)
Samples were immersed in 70% ethanol and scanned with a vivaCT 40 (Scanco Medical, Switzerland) (resolution, 19 µm; energy, 70 kVp; current, 114 µA).
5-Bromo-2'-deoxyuridine (BrdU) labeling
Lizard blastema explants were treated with 1 µM BrdU (Life Technologies) for 16 hours in culture prior to collection. Explants were then processed for histology, and BrdU+ cells were detected by IHC using anti-BrdU primary antibody (Abcam, ab6326; 1:200).
Migration and proliferation assays
The effects of lizard tissues and/or exogenous growth factors on isolated lizard cell migration and proliferation were measured using the xCELLigence System. See supplementary Materials and Methods for descriptions of the xCELLigence System and experiment specifics. Cell proliferation in lizard cartilage explant cultures were measured with the CellTiter 96 AQueous One Solution Cell Proliferation Assay (MTS) according to the manufacturer's instructions.
Statistics
Results from experimental observations, such as those involving surgical manipulations, are indicated as ratios between numbers of samples yielding positive results and total sample numbers (e.g. 4/5). Cell migration, proliferation, and western blot densitometry measurements for three experimental replicates are expressed as the mean±s.d., and significant differences between control and experimental conditions were determined by two-tailed Student's t-tests.
Acknowledgements
We acknowledge helpful comments on the manuscript by Aaron Sun.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
T.P.L. designed the research study, performed the experiments, analyzed the data and wrote the paper. R.S.T. acted as mentor to T.P.L., aided in developing the experimental approach and interpreting the results, and revised and edited the paper.
Funding
This project was supported by the Pennsylvania Department of Health [SAP 4100050913]; and the National Institutes of Health [R01 GM115444]. Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.129585.supplemental
References
- Alibardi L. (2010). Morphological and cellular aspects of tail and limb regeneration in lizards. A model system with implications for tissue regeneration in mammals. Adv. Anat. Embryol. Cell Biol. 207, iii, v-x, 1-49. 10.1007/978-3-642-03733-7_1 [DOI] [PubMed] [Google Scholar]
- Bai X., Wang Y., Man L., Zhang Q., Sun C., Hu W., Liu Y., Liu M., Gu X. and Wang Y. (2015). CD59 mediates cartilage patterning during spontaneous tail regeneration. Sci. Rep. 5, 12798 10.1038/srep12798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellairs A. D. and Bryant S. V. (1985). Autotomy and regeneration in reptiles. In The Biology of The Reptilia, Vol. 15 (ed. Billet F. and Gans C.), pp. 303-410. New York: John Wiley & Sons, Inc. [Google Scholar]
- Cox P. G. (1969). Some aspects of tail regeneration in the lizard, Anolis carolinensis .I. A description based on histology and autoradiography. J. Exp. Zool. 171, 127-149. 10.1002/jez.1401710202 [DOI] [Google Scholar]
- Echeverri K., Clarke J. D. W. and Tanaka E. M. (2001). In vivo imaging indicates muscle fiber dedifferentiation is a major contributor to the regenerating tail blastema. Dev. Biol. 236, 151-164. 10.1006/dbio.2001.0312 [DOI] [PubMed] [Google Scholar]
- Fisher R. E., Geiger L. A., Stroik L. K., Hutchins E. D., George R. M., Denardo D. F., Kusumi K., Rawls J. A. and Wilson-Rawls J. (2012). A histological comparison of the original and regenerated tail in the green anole, Anolis carolinensis. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 295, 1609-1619. 10.1002/ar.22537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch C. (1911). Experimentelle studien uber regenerations-vorgange des gleidmassenskeletts der amphibien. Zool. Jqhrb Abt. Allg. Zool. Physiol. 30, 377-472. [Google Scholar]
- Hay E. D. (1959). Electron microscopic observations of muscle dedifferentiation in regenerating amblystoma limbs. Dev. Biol. 1, 555-585. 10.1016/0012-1606(59)90018-1 [DOI] [Google Scholar]
- Holtzer S. (1956). The inductive activity of the spinal cord in urodele tail regeneration. J. Morphol. 99, 1-39. 10.1002/jmor.1050990102 [DOI] [Google Scholar]
- Hutchins E. D., Markov G. J., Eckalbar W. L., George R. M., King J. M., Tokuyama M. A., Geiger L. A., Emmert N., Ammar M. J., Allen A. N. et al. (2014). Transcriptomic analysis of tail regeneration in the lizard anolis carolinensis reveals activation of conserved vertebrate developmental and repair mechanisms. PLoS ONE 9, e105004 10.1371/journal.pone.0105004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imokawa Y. and Yoshizato K. (1997). Expression of Sonic hedgehog gene in regenerating newt limb blastemas recapitulates that in developing limb buds. Proc. Natl. Acad. Sci. USA 94, 9159-9164. 10.1073/pnas.94.17.9159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kragl M., Knapp D., Nacu E., Khattak S., Maden M., Epperlein H. H. and Tanaka E. M. (2009). Cells keep a memory of their tissue origin during axolotl limb regeneration. Nature 460, 60-65. 10.1038/nature08152 [DOI] [PubMed] [Google Scholar]
- Kumar A., Velloso C. P., Imokawa Y. and Brockes J. P. (2004). The regenerative plasticity of isolated urodele myofibers and its dependence on Msx1. PLoS Biol. 2, e218 10.1371/journal.pbio.0020218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo D. C., Allen F. and Brockes J. P. (1993). Reversal of muscle differentiation during urodele limb regeneration. Proc. Natl. Acad. Sci. USA 90, 7230-7234. 10.1073/pnas.90.15.7230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozito T. P. and Tuan R. S. (2015). Lizard tail regeneration: regulation of two distinct cartilage regions by Indian hedgehog. Dev. Biol. 399, 249-262. 10.1016/j.ydbio.2014.12.036 [DOI] [PubMed] [Google Scholar]
- Morrison J. I., Lööf S., He P. P. and Simon A. (2006). Salamander limb regeneration involves the activation of a multipotent skeletal muscle satellite cell population. J. Cell Biol. 172, 433-440. 10.1083/jcb.200509011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison J. I., Borg P. and Simon A. (2010). Plasticity and recovery of skeletal muscle satellite cells during limb regeneration. FASEB J. 24, 750-756. 10.1096/fj.09-134825 [DOI] [PubMed] [Google Scholar]
- Rinkevich Y., Lindau P., Ueno H., Longaker M. T. and Weissman I. L. (2011). Germ-layer and lineage-restricted stem/progenitors regenerate the mouse digit tip. Nature 476, 409-413. 10.1038/nature10346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts S. J., van Gastel N., Carmeliet G. and Luyten F. P. (2015). Uncovering the periosteum for skeletal regeneration: the stem cell that lies beneath. Bone 70, 10-18. 10.1016/j.bone.2014.08.007 [DOI] [PubMed] [Google Scholar]
- Roy S. and Gardiner D. M. (2002). Cyclopamine induces digit loss in regenerating axolotl limbs. J. Exp. Zool. 293, 186-190. 10.1002/jez.10110 [DOI] [PubMed] [Google Scholar]
- Sandoval-Guzmán T., Wang H., Khattak S., Schuez M., Roensch K., Nacu E., Tazaki A., Joven A., Tanaka E. M. and Simon A. (2014). Fundamental differences in dedifferentiation and stem cell recruitment during skeletal muscle regeneration in two salamander species. Cell Stem Cell 14, 174-187. 10.1016/j.stem.2013.11.007 [DOI] [PubMed] [Google Scholar]
- Schnapp E., Kragl M., Rubin L. and Tanaka E. M. (2005). Hedgehog signaling controls dorsoventral patterning, blastema cell proliferation and cartilage induction during axolotl tail regeneration. Development 132, 3243-3253. 10.1242/dev.01906 [DOI] [PubMed] [Google Scholar]
- Stewart S. and Stankunas K. (2012). Limited dedifferentiation provides replacement tissue during zebrafish fin regeneration. Dev. Biol. 365, 339-349. 10.1016/j.ydbio.2012.02.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocum D. L. (2002). Regenerative biology and medicine. J. Musculoskelet. Neuronal Interact. 2, 270-273. [PubMed] [Google Scholar]
- Torok M. A., Gardiner D. M., Izpisua-Belmonte J.-C. and Bryant S. V. (1999). Sonic hedgehog (shh) expression in developing and regenerating axolotl limbs. J. Exp. Zool. 284, 197-206. [DOI] [PubMed] [Google Scholar]
- Wang Q., Huang C., Zeng F., Xue M. and Zhang X. (2010). Activation of the Hh pathway in periosteum-derived mesenchymal stem cells induces bone formation in vivo: implication for postnatal bone repair. Am. J. Pathol. 177, 3100-3111. 10.2353/ajpath.2010.100060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Huang C., Xue M. and Zhang X. (2011). Expression of endogenous BMP-2 in periosteal progenitor cells is essential for bone healing. Bone 48, 524-532. 10.1016/j.bone.2010.10.178 [DOI] [PMC free article] [PubMed] [Google Scholar]