Summary
In prokaryotes, members of the High Temperature Requirement A (HtrA) family of serine proteases function in the periplasm to degrade damaged or improperly folded membrane proteins. Borrelia burgdorferi, the agent of Lyme disease, codes for a single HtrA homolog. Two-dimensional electrophoresis analysis of B. burgdorferi B31A3 and a strain that over-expresses HtrA (A3HtrAOE) identified a down-regulated protein in A3HtrAOE with a mass, pI and MALDI-TOF spectrum consistent with outer membrane protein p66. P66 and HtrA from cellular lysates partitioned into detergent-resistant membranes, which contain cholesterol-glycolipid-rich membrane regions known as lipid rafts, suggesting that HtrA and p66 may reside together in lipid rafts also. This agrees with previous work from our laboratory, which showed that HtrA and p66 are constituents of B. burgdorferi outer membrane vesicles. HtrA degraded p66 in vitro and A3HtrAOE expressed reduced levels of p66 in vivo. Fluorescence confocal microscopy revealed that HtrA and p66 co-localize in the membrane. The association of HtrA and p66 establishes that they could interact efficiently and their protease/substrate relationship provides functional relevance to this interaction. A3HtrAOE also showed reduced levels of p66 transcript in comparison to wild-type B31A3, indicating that HtrA-mediated regulation of p66 may occur at multiple levels.
Keywords: Borrelia, HtrA, DegP, p66, protease
Introduction
The membranes of Borrelia burgdorferi, the Lyme disease causative agent, contain free cholesterol and three glycolipids, two of which, cholesteryl 6-O-acyl-β-D-galactopyranoside (ACGal) and cholesteryl-β-D-galactopyranoside (CGal) contain cholesterol. The third glycolipid, mono-α-galactosyl-diacylglycerol (MGalD), contains no cholesterol (Ben-Menachem et al., 2003, Schroder et al., 2003, Stubs et al., 2009). Despite the presence of cholesterol in the membrane, the B. burgdorferi genome does not code for pathways necessary for its biosynthesis (Fraser et al., 1997), thus the medium must be supplemented with it for growth in vitro. In the host, cholesterol is presumably acquired by B. burgdorferi from the surrounding tissue and fluids. Recent evidence from our laboratory describing a novel mechanism, through which cholesterol is acquired by the spirochete via contact with the plasma membrane of cultured eukaryotic cells, supports this idea (Crowley et al., 2013). While the inability to propagate in vitro without cholesterol and the existence of an extraordinary mechanism for acquiring it in vivo bear out its importance for B. burgdorferi viability, the presence of cholesterol in the outer membrane can, at the same time be exploited by host defenses. For example, cholesterol and cholesterol glycolipids in the B. burgdorferi outer membrane are critical for the complement-independent bactericidal mechanism of a monoclonal antibody to outer surface protein (Osp) B, (Coleman et al., 1992, Coleman et al., 1994, LaRocca et al., 2010).
Recently, our laboratory reported that cholesterol glycolipids coalesce into microdomains to form lipid rafts in the outer membrane of B. burgdorferi (LaRocca et al., 2010, LaRocca et al., 2013). Although they are not widely recognized in prokaryotes, lipid rafts are well documented in eukaryotic cells (Brown & London, 2000, London, 2002), where they are discrete clusters of cholesterol and sphingolipids in the plasma membrane, which are more firmly packed and highly ordered than the surrounding phospholipid bilayer (Simons & Ehehalt, 2002). Lipid rafts participate in a number of cellular processes, including endo- and exo-cytosis, membrane vesicle formation, budding, and maintenance of elasticity (Chen & Rand, 1997, Huttner & Zimmerberg, 2001, Nichols, 2003, Salaun et al., 2004), as well as lateral sorting of proteins and receptor clustering (Brown, 1998, Epand, 2008).
In addition to free cholesterol and cholesterol glycolipids, B. burgdorferi lipid rafts contain a discrete set of lipoproteins, including Osps A and B (LaRocca et al., 2010). Another major membrane lipoprotein, OspC, is absent (Toledo et al., 2014). In contrast to B. burgdorferi B31A3, strain B313, which is missing several plasmids and does not express OspA or OspB (Sadziene et al., 1993), exhibits a significantly reduced ability to form ordered lipid rafts. When B313 was transformed with a shuttle plasmid containing OspA, the wild-type B31 phenotype was restored, a strong indication that the impaired lipid raft formation was not due to the loss of genes normally located on the other missing plasmids. Furthermore, transformation with OspC failed to restore the wild-type phenotype despite the use of a range of growth conditions (33°C, 35°C, pH 6.7). This suggests that only lipid raft-associated proteins contribute to the formation of the microdomains (Toledo et al., 2014).
Besides lipoproteins, B. burgdorferi lipid rafts contain other proteinaceous components, one of which is the Escherichia coli Deg homolog, HtrA (BB0104) (Toledo et al., 2014), a putative chaperone and documented serine protease recently characterized independently by several laboratories (Coleman et al., 2013, Kariu et al., 2013, Russell et al., 2013, Russell & Johnson, 2013, Gherardini, 2013). HtrA, a member of the High Temperature Requirement A family of serine proteins, is found in all types of cells. Universal features of this family include the proteolytic domain (Ser-His-Asp catalytic triad) located near the N-terminus and either one or two C-terminal PDZ domains that mediate protein-protein interactions (Clausen et al., 2011, Pallen & Wren, 1997). The E. coli genome codes for three HtrA homologs designated DegP, Deg Q and DegS. DegP, the first of the three to be identified and defined (Swamy et al., 1983) and DegQ, are located in the periplasm where they function alternately as chaperones in a protein folding stress response or as proteases to target and degrade damaged or misfolded proteins (Ehrmann & Clausen, 2004, Raivio, 2005). DegS is a tightly regulated sensory protease, which can identify misfolded proteins in the periplasm and activate signaling mechanisms to trigger a stress response (Alba et al., 2002).
In contrast to that of E. coli, the B. burgdorferi genome codes for only one HtrA homolog (Coleman et al., 2013, Kariu et al., 2013, Russell et al., 2013, Russell & Johnson, 2013) and therefore lacks the redundancy effect of multiple Deg proteins, such as those found in E. coli. This is a likely reason why targeted deletion of HtrA has thus far not been successful, while single Deg deletion mutants are viable in bacteria with additional homologs (Baba et al., 2006). B. burgdorferi HtrA is an immunogenic ~48-kDa protein whose fundamental structural unit is a trimer and is caseinolytic in vitro by virtue of its catalytic serine. HtrA selectively degrades several endogenous proteins, including basic membrane protein D (BmpD/BB0385) and chemotaxis phosphatase CheX (BB0671) (Coleman et al., 2013), as well as virulence factor BB0323, a C-terminal LysM-like domain-containing (Zhang et al., 2009) protein that is processed by HtrA into two discrete N- and C-terminal peptides (Kariu et al., 2013). Additionally, HtrA may promote invasiveness, as it degrades the extracellular matrix components, aggrecan (Russell & Johnson, 2013) and fibronectin, the latter of which results in the generation of pro-inflammatory peptides (Russell et al., 2013). To further investigate the role that HtrA has in protein expression in vivo, we looked for changes in the B. burgdorferi protein expression profile by 2-D gel electrophoresis analysis using a strain engineered to over-express HtrA. We identified the outer membrane integral protein p66 as a proteolytic target for HtrA, both in vitro and in vivo. HtrA and p66 partition into the detergent-resistant membrane fraction (DRM) when treated with Triton X-100, which strongly suggests that they reside together in membrane lipid rafts. Indirect immunofluorescence and confocal microscopy revealed co-localization of HtrA and p66. Membrane co-localization and the likelihood of protein-protein interaction point to a potential regulatory role for HtrA with respect to p66 expression. In addition to the proteolysis of p66, the over-expression of HtrA was shown to have an inhibitory effect on p66 transcript level in A3HtrAOE, suggesting multi-level regulation.
Results
B. burdorferi strain A3HtrAOE over-expresses HtrA
We have previously shown that B. burgdorferi serine protease HtrA selectively degrades outer membrane protein BmpD (BB0385) and chemotaxis phosphatase CheX (BB0671). At the same time, HtrA was shown to be selective in its proteolytic activity, as it did not degrade other proteins such as OspA, OspB, FliL, lpA7P22, or NapA, which immunoprecipitated with HtrA, jointly with BmpD and CheX (Coleman et al., 2013). To further investigate the role(s) of HtrA in B. burgdorferi, and because of an inability to create an HtrA knock out mutant (Coleman et al., 2013), we generated a strain of B31A3 engineered to over-express HtrA through transformation with pKFSS1-derived shuttle plasmid pflaBPhtrA, containing htrA under control of the constitutive flaB promoter (Frank et al., 2003), to form strain A3HtrAOE (Figure S1A, B). Strain A3HtrAOE was analyzed by PCR and verified to contain the full complement of linear and circular plasmids, including virulence plasmids lp25 and lp28-1 (Figure S1C). In addition, A3HtrAOE did not exhibit any growth defects in culture (Figure S1D).
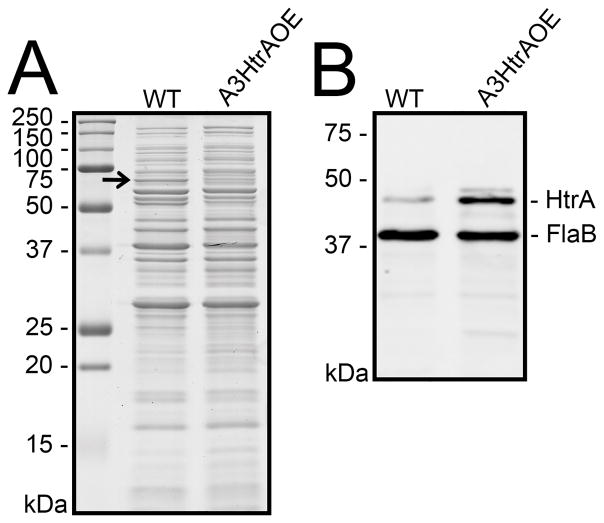
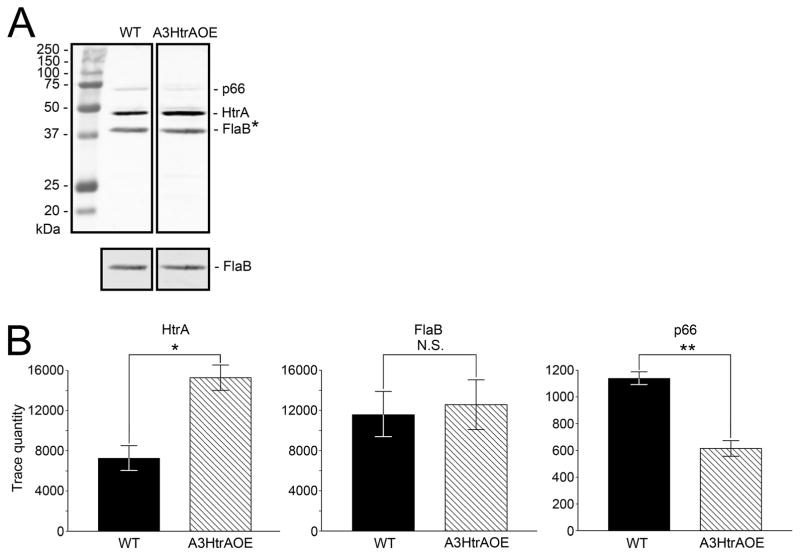
As a putative homolog of E. coli degP (Swamy et al., 1983), over-expression of HtrA could affect the expression levels of any B. burgdorferi proteins recognized as substrates or as chaperone partners. To verify that A3HtrAOE expressed increased levels of HtrA, equivalent numbers of spirochetes from mid-log phase cultures of wild-type B31A3 and A3HtrAOE spirochetes, as demonstrated by the similar levels of constitutively expressed FlaB detected, were analyzed by SDS-PAGE and western blot using rabbit anti-HtrA antibody. No apparent differences in HtrA expression were detected by SDS-PAGE analysis alone. This was not surprising, as it is not heavily expressed by B. burgdorferi during growth in vitro (Coleman et al., 2013) (Figure 1A). A differentially expressed protein of approximately 66-kDa (arrow) was noted at this time (more abundant in the wild type) (Figure 1A). Analysis by western blot, revealed that strain A3HtrAOE reproducibly expressed higher levels of 48-kDa HtrA than wild-type B31A3. Levels of FlaB were similar (Figure 1B).
Figure 1. Borrelia burgdorferi strain A3HtrAOE over-expresses HtrA.
(A) 15% SDS-PAGE of B. burgdorferi wild-type B31A3 and A3HtrAOE (HtrA- over-expressing strain), stained with Coomassie Blue. Arrow, putative p66.
(B) Western blot, using rabbit anti-HtrA antibody, of wild-type B31A3 and A3HtrAOE comparing levels of expressed HtrA. FlaB, the rabbit anti-HtrA cross-reacted with FlaB. Results shown in panels A and B are representative of experiments carried out multiple times with similar results.
2-D electrophoresis analysis reveals reduced levels of a protein suggestive of outer membrane protein p66 in strain A3HtrAOE
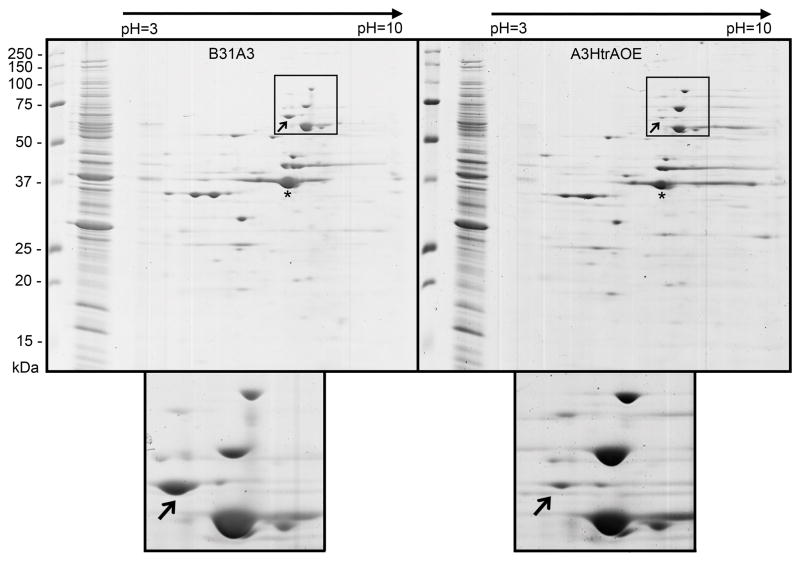
Wild-type B. burgdorferi B31A3 and A3HtrAOE were subsequently analyzed by 2-D electrophoresis (Bjellqvist et al., 1982, O’Farrell, 1975) to identify changes in protein expression that could reflect the presence HtrA substrates (Figure 2). For the first dimension, equivalent amounts of each strain, as determined by enumeration, were subjected to isoelectric focusing (pH 3–10) after which the IPG Strips were overlaid on 12.5% SDS-PAGE gels for the second dimension. One protein spot of approximately 66-kDa was consistently present at higher levels in the wild-type B31A3 than in A3HtrAOE. In addition, the apparent pI of the 66-kDa protein very closely matched that of FlaB (calculated to be 5.31). The electrophoretic characteristics of the unknown band were strongly suggestive of that of B. burgdorferi outer membrane protein p66 (BB0603), the mature form of which (minus the 21 amino acid leader peptide) also has a pI of 5.31 (Figure 2) (Bunikis et al., 1995). The protein was cut out of the B31A3 gel and subjected to mass spectrometry (MALDI-TOF) analysis, which confirmed its identity as p66.
Figure 2.
2-D electrophoretic analysis of Borrelia burgdorferi wild-type B31A3 and HtrA-over-expression strain A3HtrAOE. B. burgdorferi wild-type B31A3 (left panel) and A3HtrAOE (right panel) were subjected to isoelectric focusing (pH 3–10) followed by 12.5% SDS-PAGE and Coomassie Blue staining. The lower boxes are exploded views depicting region of interest delineated by small boxes. Arrows indicate putative p66. Asterisks indicate FlaB. This experiment was repeated four times with comparable results. Representative gels are shown.
B. burgdorferi HtrA and p66 partition into detergent-resistant membranes (DRM) after treatment with Triton X-100
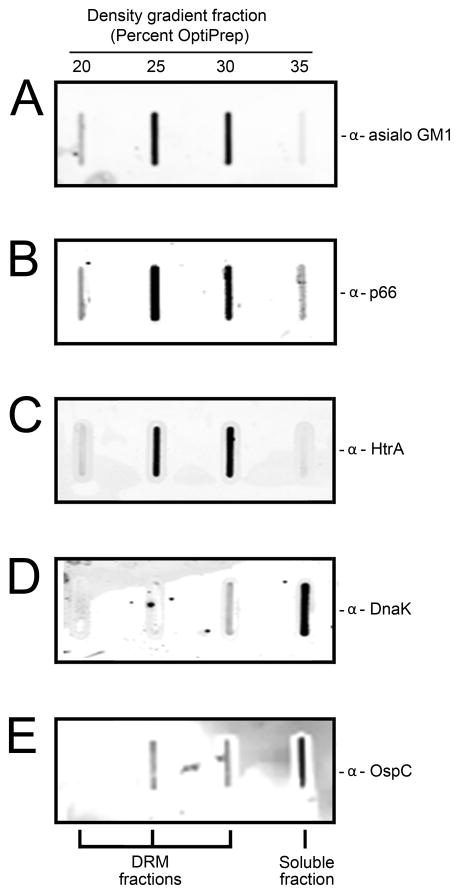
The tendency of membrane cholesterol glycolipids to cluster into distinct microdomains in the bacterial outer membrane is a phenomenon we have shown previously for B. burgdorferi (LaRocca et al., 2010). The presence of these structures is demonstrated after treatment of B. burgdorferi with Triton X-100 at 4°C and fractionation of the lysate on an OptiPrep density step gradient. The cholesterol/glycolipid-containing microdomains are not solubilized by the detergent, and fractionate into DRM structures (Brown & Rose, 1992), which localize mainly to the 20%, 25% and 30% OptiPrep density fractions. The presence of cholesterol-glycolipid in DRM is strongly suggestive that they are organized into lipid rafts. The cholesterol glycolipid/DRM can be detected by analyzing fractions via slot blot using rabbit antibody to asialo GM1, as this antibody cross-reacts with the cholesterol glycolipids of B. burgdorferi (Garcia Monco et al., 1993, Garcia-Monco et al., 1995, LaRocca et al., 2010). Interestingly, B. burgdorferi outer membrane p66 (LaRocca et al., 2010) and serine protease HtrA (Toledo et al., 2014) have also been shown to be present in DRM fractions, though their function in lipid rafts is unknown. In Figure 3A, a slot blot demonstrating cholesterol glycolipid partitioning mainly into the 25% and 30% fractions (DRM) is shown. P66 (Figure 3B) and HtrA (Figure 3C) also partitioned into DRM, evidence for their association with lipid rafts, while the cytosolic chaperone molecule, DnaK and OspC, partitioned into the soluble fraction, as expected (Figures 3D, E). The OspC result is in agreement with our previous findings that OspC is not contained in lipid rafts (Toledo et al., 2014).
Figure 3. Both Borrelia burgdorferi p66 and HtrA partition primarily into detergent resistant membranes (DRM). B. burgdorferi were treated overnight with 1%Triton X-100 at 4°C and the lysate was separated on a discontinuous OptiPrep density gradient. Fractions containing 20%, 25%, 30% and 35% OptiPrep were collected and analyzed by slot blot.
(A) Rabbit anti-α-asialo GM1 purified IgG was used to identify fractions containing DRM.
(B) Rabbit anti-p66 immune serum was used to indicate the presence of p66 protein in DRM.
(C) Rabbit anti-HtrA immune serum was used to indicate the presence of HtrA protein in DRM.
(D) Mouse anti-DnaK was used to indicate the absence of DnaK in DRM and preference for the soluble fraction.
(E) Rabbit anti-OspC was used to indicate the absence of OspC in DRM and preference for the soluble fraction.
B. burgdorferi HtrA degrades p66 in vitro
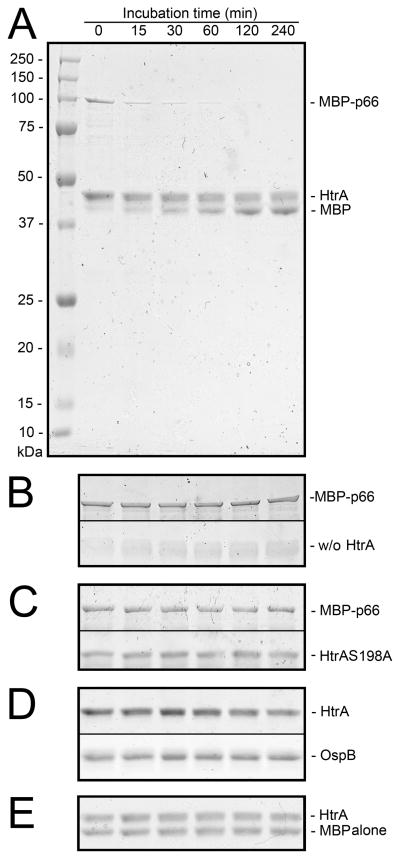
The co-localization of HtrA and p66 in the lipid rafts of B. burgdorferi provides an environment in which they could interact efficiently. To determine if HtrA could recognize p66 as a substrate, we carried out in vitro digestion experiments in which recombinant p66 (200 μg ml−1) and recombinant HtrA (100 μg ml−1) were co-incubated at 37°C. P66 was in the form of a maltose binding protein (MBP) fusion protein (MBP-p66) to maintain its solubility (Coburn et al., 1999). Aliquots (10 μl) were removed over a range of time points (0 – 240 min) and processed for SDS-PAGE under non-reducing conditions and without heating. As shown in Figure 4A, MBP-p66, which appeared as a single ~100-kDa band at T0, was quickly and efficiently degraded by HtrA in as little as 15 min. Over time, increasing amounts of full-length (42-kDa) MBP appeared near the 42-kDa area of the gel, suggesting that it was being released and is by itself not a substrate. The 42-kDa band was verified separately to be MBP by western blot (not shown). The presence of HtrA in the mixture was indicated by the 48-kDa band immediately above MBP. When HtrA was omitted from the digestion mixture, MBP-p66 remained intact (Figure 4B). Catalytic site mutant HtrAS198A (Coleman et al., 2013) was not active against MBP-p66 (Figure 4C). We previously reported that HtrA does not degrade OspB (Coleman et al., 2013). B. burgdorferi OspB, employed as an additional negative control in this study, was not affected by HtrA (Figure 4D). Purified MBP alone was verified to not be an HtrA substrate (Figure 4E). Taken together, the data from this experiment demonstrates that p66 is extremely sensitive to catalysis by HtrA, while its fusion partner, MBP, is not targeted.
Figure 4. HtrA degrades Borrelia burgdorferi p66 in vitro. For 5B–5D, discontinuous areas of the gel are shown in separate panels. Gels shown are representative of experiments carried out separately a minimum of two times each.
(A) Coomassie Blue-stained 12% SDS-PAGE gel showing proteolysis of 100-kDa fusion protein MBP-p66 by HtrA. HtrA (48-kDa) and released MBP (42-kDa) are also shown.
(B) Coomassie Blue-stained 12% SDS-PAGE gel showing that MBP-p66 (upper panel) is not degraded in the absence of HtrA (lower panel).
(C) Coomassie Blue-stained 12% SDS-PAGE gel showing that MBP-p66 (upper panel) is not degraded by catalytically inactive B. burgdorferi mutant HtrAS198A (48-kDA) (lower panel).
(D) Coomassie Blue-stained 12% SDS-PAGE gel showing that HtrA (upper panel) does not degrade recombinant OspB (34-kDa) (lower panel).
(E) Coomassie Blue-stained 12% SDS-PAGE gel showing that HtrA (upper band) does not degrade MBP alone (lower band).
Expression of B. burgdorferi p66 is impaired in A3HtrAOE
The 2-D electrophoretic results from Figure 2 indicated that HtrA might have a role in regulation of p66 in vivo. To determine whether the proteolytic activity of HtrA toward p66 has relevance in vivo and to expand on the 2-D electrophoretic evidence, B31A3 and A3HtrAOE were cultured to mid-log phase and the expression of HtrA, p66 and FlaB in both strains were compared by western blot. Equivalent amounts of wild-type and A3HtrAOE spirochetes were separated by SDS-PAGE, transferred to nitrocellulose and the membrane was probed with antibodies to p66, HtrA and FlaB. The relative levels of constitutively expressed FlaB in each strain were indistinguishable, verifying that equivalent amounts of spirochetes were loaded for SDS-PAGE (Figure 5A, upper panels). The 41-kDa FlaB band shown in the upper panel of Figure 5A (asterisk) is the result of cross-reactivity of the rabbit anti-HtrA antibody with FlaB, as determined by western blot (not shown). The FlaB level was additionally verified by use of mouse monoclonal antibody CB1 (Coleman & Benach, 1989) (Figure 5A, lower panels). The expression of p66 in A3HtrAOE, as detected by rabbit antibody, however, was impaired in comparison to the wild-type B31A3 (Figure 5A, upper panels). At the same time, the level of HtrA was increased in A3HtrAOE, as expected. To quantify the data, western blot images from both experiments were analyzed by Quantity One Software (Bio Rad). Vertical scans for each band were made in three different places (left side, middle and right side). Analysis of the combined data (a total of 6 readings each for FlaB, HtrA and p66, encompassing both experiments) revealed that the HtrA level in A3HtrAOE showed a 111% increase over the wild-type level (Figure 5B left, P = 0.005). This was expected, due to the additive effect of plasmid-encoded htrA. There was no significant difference in the FlaB levels of the strains (P = 0.7731) (Figure 5B center), which confirmed that the gels were loaded with equivalent numbers of spirochetes. Most importantly, the p66 level in strain A3HtrAOE was reduced by 46% compared to the wild-type level (P < 0.0001) (Figure 5B right).
Figure 5. In vivo expression of Borrelia burgdorferi p66 is reduced in HtrA over-expression strain A3HtrAOE.
(A) Western blot of wild-type and A3HtrAOE B. burgdorferi whole cell lysates in which p66, HtrA and FlaB were detected by rabbit anti-p66, rabbit anti-HtrA and mouse anti-FlaB, respectively. This experiment was done twice with similar results. A representative blot is shown. Asterisk, upper panels, rabbit anti-HtrA cross-reacted with B. burgdorferi FlaB. Lower panels, FlaB as detected by mouse monoclonal antibody CB1 (Coleman & Benach, 1989).
(B) The western blot image data was analyzed by Quantity One Quantitation software (Bio Rad) to graphically show the results presented in panel A. Figure shows the combined data from two independent experiments. Bars represent the mean of six measurements for each gel band. Error bars represent the standard error of the mean. Statistical significance was determined by two-tailed t-test. P values: *HtrA, P=0.0005; **p66, P<0.0001; FlaB, P=0.773.
In control western blots, A3HtrAOE grown without streptomycin exhibited the same p66 deficit phenotype as spirochetes grown with the antibiotic. B31A3 spirochetes transformed with shuttle plasmid pKFSS1 containing PflaB alone were similar to wild-type B31A3 (Figure S2A).
HtrA and p66 colocalize in the B. burgdorferi membrane
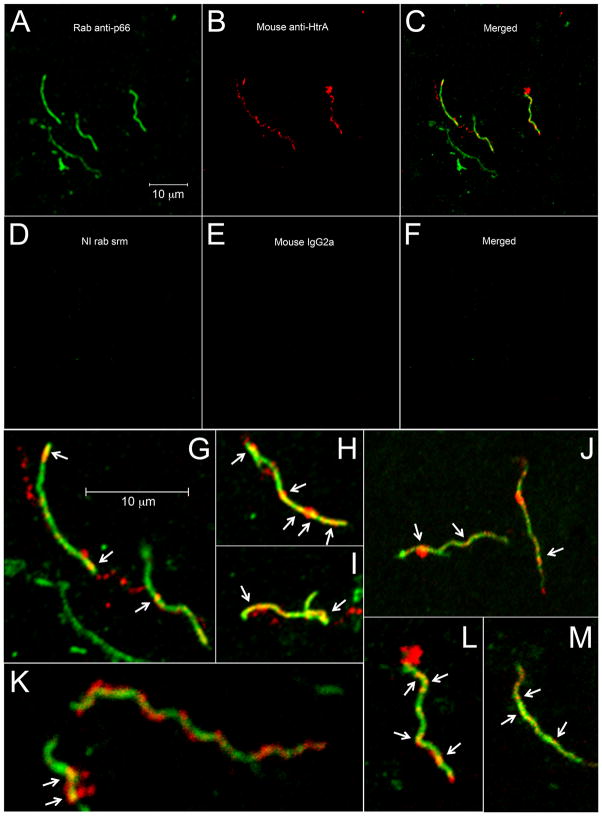
Confocal microscopy was used to further confirm that p66 and HtrA interact in the spirochete membrane. B. burgdorferi on glass slides were probed simultaneously with rabbit antibody to p66 and a mouse monoclonal antibody to HtrA. Both antibody probes were confirmed to be mono-specific for their intended targets (Figure S2B). Both p66 (green) and HtrA (red) fluoresced brightly when observed separately (Figure 6A and 6B). When the images were merged, numerous areas of discontinuous yellow signal representing co-localization of p66 and HtrA were visible (Figure 6C and 6G). Other merged images exemplifying co-localization are also shown (Figure 6H–6M). Control spirochetes stained with non-immune rabbit serum (green), mouse IgG2a (red) were uniformly non-reactive (Figure 6D–6F). Interestingly, the mouse monoclonal antibody to HtrA labeled spirochete structures resembling membrane blebs (Figure 6B). This bleb-like morphology could also be seen in all of the merged images (Figure 6G–6M) and in those labeled with rabbit anti-HtrA (not shown). This is consistent with what we have seen previously in that HtrA is found in membrane vesicles (Toledo et al., 2012).
Figure 6. HtrA and p66 colocalize in the Borrelia burgdorferi membrane. B. burgdorferi strain B31A3 spirochetes on printed well slides were probed with rabbit serum specific for p66 and mouse monoclonal antibody 1A3:1E2, specific for HtrA, followed by goat anti-rabbit Alexa Fluor 488-labeled (green) and goat anti-mouse Alexa Fluor 594-labeled (red) secondary antibodies, respectively.
(A) Green image detecting p66. Size bar applies to panels A–F.
(B) Red image detecting HtrA.
(C) Merged image of A and B showing co-localization (yellow) of p66 and HtrA.
(D) Negative control primary antibody [non-immune (NI) rabbit serum].
(E) Negative control primary antibody (mouse IgG2a).
(F) Merged image of and E.
(G) Expanded view of merged image from panel C. Arrows indicate areas of co-localization. Size bar applies to panels G–M.
(H–M) Additional merged confocal images indicating co-localization of p66 and HtrA. Arrows indicate areas of co-localization.
HtrA cleaves p66 at multiple sites
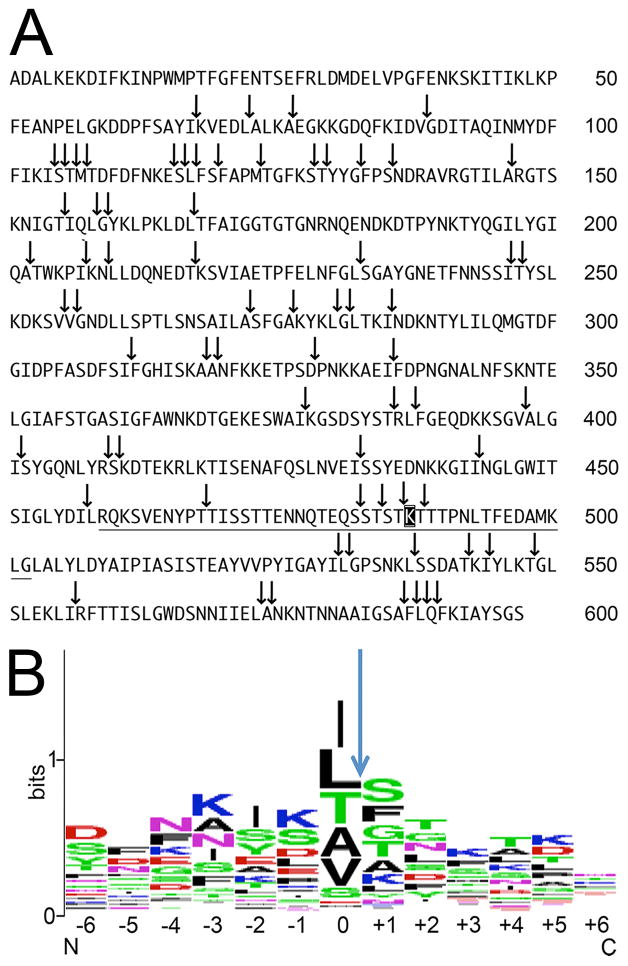
To map the cleavage sites in p66 for HtrA, both enzyme and MBP-p66 were incubated for 30 min at 37°C, followed by mass spectrometry. Numerous proteolytic sites were identified (Figure 7A and Table 1) and amino acid residue preference was determined. A clear preference for the hydrophobic amino acids isoleucine and leucine was noted, followed by threonine, alanine, valine and serine. P66 has a surface exposed loop structure containing a trypsin-sensitive lysine residue (Bunikis et al., 1998, Kenedy et al., 2014). The surface-exposed loop sequence was cleaved at 5 different positions, including on either side of trypsin-sensitive residue K487. Four undigested peptides of the p66 molecule were also noted: residues 1–67, 168–202, 336–377 and 489–530 with calculated molecular weights of 7726, 3807, 4507 and 4583, respectively (Figure 7A). All of the peptides generated by the digestion were analyzed using weblogo (weblogo.berkley.edu) to generate a summary of residue preference (Figure 7B).
Figure 7. MS analysis of HtrA cleavage sites in p66.
(A) HtrA cleavage sites in p66 at 30 min (arrows). Underlined sequence, p66 amino acid residues 459 to 502, represents the putative surface-exposed loop at C-terminus. The trypsin-sensitive lysine K487 within the loop is shown on a black background.
(B) Amino acid residue preference was determined and revealed a clear preference for the hydrophobic amino acids isoleucine and leucine, followed by threonine, alanine, valine and serine. All of the peptides generated by the digestion were analyzed using Weblogo (weblogo.berkley.edu).
Table 1.
Analysis of p66 cut sites by preference.
| Amino acid residue | a Times |
|---|---|
| I | 94 |
| L | 88 |
| T | 64 |
| A | 47 |
| V | 41 |
| S | 26 |
| E | 11 |
| G | 5 |
| F | 3 |
| M | 2 |
| N | 2 |
| D | 2 |
| Q | 2 |
| R | 1 |
The number of times the enzyme cutting event was detected.
Transcript for p66 in B. burgdorferi A3HtrAOE is reduced
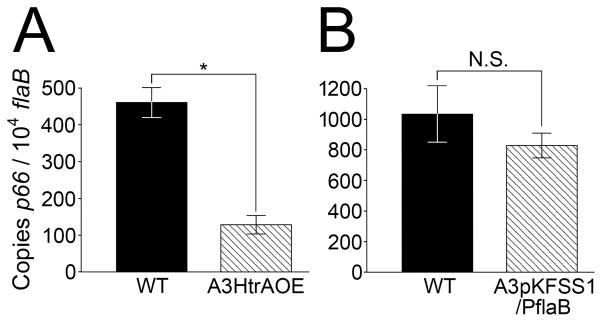
We have provided evidence that HtrA degrades recombinant p66 in vitro. We have also shown by several different methodologies that an increase in cellular HtrA is associated with a decrease in p66 protein in the HtrA-over-expressing A3HtrAOE strain. To investigate whether over-expression of HtrA has an effect on p66 transcription, the levels of p66 transcript in both wild-type and HtrA-over-expressing strains were assessed by quantitative real-time RT-PCR (qRT-PCR). Total RNA from equivalent amounts (5X107 cells) of B. burgdorferi wild-type B31A3 and A3HtrAOE were extracted and reversed transcribed to cDNA. cDNA from B31A3 spirochetes transformed with pKFSS1 containing the flaB promoter sequence but not htrA (A3flaB) was also prepared. The cDNA was analyzed by qRT-PCR analysis to determine the levels of p66 transcript during growth at 33°C. The level of p66 transcript in A3HtrAOE was significantly reduced (p≤0.0001) in comparison to wild-type spirochetes (Figure 8A). The level of control A3flaB was not significantly different from the wild-type (Figure 8B). qRT-PCR of samples containing mock-reverse transcripted cDNA showed minimal product, indicating that DNA contamination in the RNA samples, if any, was low (Table S1). This result, in combination with the in vitro and in vivo degradation of p66 by HtrA, suggests that HtrA may be affecting the expression of p66 at both the transcriptional and protein levels.
Figure 8. Quantitative real-time RT-PCR analysis of p66 transcript levels in Borrelia burgdorferi grown at 33°C. Each figure is one representative of two independent experiments in which the results were consistent. Bars represent the mean of quadruplicate samples. Error bars represent the standard deviation of the means from one representative experiment. Statistical significance was determined by two-tailed t-test. P values: *, P<0.0001; N.S. not statistically significant (P=0.1349).
(A) Comparison of p66 transcript level in B31A3 wild-type and A3HtrAOE.
(B) Comparison of p66 transcript level in B31A3 wild-type and A3pKFSSIPflab (B31A3 transformed with shuttle plasmid pKFSS1 containing the sequence for the flab promoter).
Discussion
B. burgdorferi HtrA is a caseinolytic serine protease (Coleman et al., 2013, Russell & Johnson, 2013, Kariu et al., 2013) and predicted chaperone that likely carries out many of the cellular quality control functions attributed to its E. coli Deg homologs. In some gram-negative bacteria, including Salmonella (Baumler et al., 1994), Brucella (Elzer et al., 1996), Yersinia (Li et al., 1996) and Helicobacter (Hoy et al., 2010), HtrA is also a virulence factor. The same may hold true for HtrA, as it regulates endogenous protein BB0323 (Kariu et al., 2013), which plays a role in B. burgdorferi outer membrane organization (Stewart et al., 2004) and is essential for infectivity (Zhang et al., 2009). Additionally, HtrA degrades structural elements of the extracellular matrix (Russell et al., 2013, Russell & Johnson, 2013).
B. burgdorferi endogenous proteins CheX and BmpD have also been identified as potential HtrA-binding partners and substrates, indicative of a possible role for HtrA in regulation of motility and outer membrane protein fate. Several other proteins, among them OspA and OspB, bound HtrA but were not degraded by it (Coleman et al., 2013). The proteolytic selectivity of binding partners by HtrA may reflect its role in the periplasm where it could degrade some proteins while binding to and stabilizing others in its role as a chaperone. Immunoprecipitation using whole cell lysates, however, must be interpreted with caution due to the potential for inappropriate mixing of proteins normally found in separate cellular compartments. We therefore attempted to further identify potential HtrA binding/substrate partners by genetic means. Ideally, the most direct approach to investigate protein function in bacteria is to knock out the gene of interest, however, repeated attempts at targeted deletion of HtrA in our laboratory were not successful, and none have been reported in the literature. As an alternative, we generated a strain of B. burgdorferi engineered to over-express HtrA (A3HtrAOE), with the goal of identifying endogenous substrate candidates in vivo. Visualization of proteins separated by 2-D electrophoresis analysis of wild-type spirochetes and strain A3HtrAOE led to the identification of p66 as a specific substrate that is quickly and efficiently degraded in vitro by HtrA.
P66, a chromosomally encoded 66-kDa integral membrane protein of B. burgdorferi, has homologs in the related Lyme borreliosis (LB)-causing Borrelia species B. afzelii and B. garinii in Europe and other LB-causing Borrelia species from Europe and South America (Bunikis et al., 1998, Bunikis et al., 1995, Madden, 2002), as well as a number of relapsing fever Borrelia worldwide (Barcena-Uribarri et al., 2010, Madden, 2002). The outer membrane organization of p66 is oligomeric (Barcena-Uribarri et al., 2013, Kenedy et al., 2014), where individual monomers form outer membrane β-barrel structures (Kenedy et al., 2014). Predicted β-sheet regions in p66 suggestive of β–barrel structures have also been described for relapsing fever Borrelia (Barcena-Uribarri et al., 2010). Taken together, these findings are likely related to the strong evidence that it forms pore structures in the lipid bilayer (Pinne et al., 2007, Skare et al., 1997), which may be oligomers of approximately eight channels each (Barcena-Uribarri et al., 2013). In addition, p66 acts as an adhesin for β3-chain integrins of eukaryotic cells, which may facilitate the invasion and colonization of host tissues and organs (Coburn et al., 1999, Coburn & Cugini, 2003, Defoe & Coburn, 2001, Ristow et al., 2015). Finally, p66 has an affinity for B. burgdorferi Osps in the spirochete membrane, particularly OspA (Bunikis & Barbour, 1999, Kenedy et al., 2014, Bunikis et al., 2001), and OspB (LaRocca et al., 2009, Yang et al., 2011), the consequences of which may be a hindrance of access by proteases and antibodies and a limit to its usefulness as a vaccine.
The immune response to p66 in patients indicates that it is expressed by B. burgdorferi during infection (Bunikis et al., 1996, Coleman & Benach, 1987, Ntchobo et al., 2001, Ornstein et al., 2002). P66 is also expressed by B. burgdorferi in the arthropod vector, Ixodes scapularis, although in a feeding stage-dependent manner. In the unfed tick, the expression pattern of p66 is such that very little, if any protein (Cugini et al., 2003) or transcript (Medrano et al., 2010) can be detected. However, as the tick begins to take a blood meal, p66 becomes detectable and expression increases until about 7 days post repletion. At 16 days post repletion, p66 expression is once again greatly reduced (Medrano et al., 2010). Manipulation of growth conditions in vitro does not explain this variability, as neither pH nor temperature have a major influence on p66 expression (Cugini et al., 2003). This is interesting because pH and temperature do have an effect on the expression of other membrane proteins, such as OspA/B and OspC (Schwan et al., 1995, Yang et al., 2000), and likely reflects that they are under the control of distinct regulatory pathways, of which there are several that are relevant to p66 (Samuels, 2011): Targeted disruption of RNA helicase HrpA (BB0827), results in the down regulation of expression of p66, along with varied effects on other B. burgdorferi genes (Salman-Dilgimen et al., 2011, Salman-Dilgimen et al., 2013). In addition, the DNA binding protein Hbb (BB0232) (Kobryn et al., 2000, Mouw & Rice, 2007), interacts with the p66 promoter, possibly acting to repress gene product expression (Medrano et al., 2010). Both of these pathways may contribute to regulation of p66, and the Hbb model could account for the quenching of p66 transcription in the tick after repletion; however, the mechanism for the spirochete to divest itself of accumulated p66 could involve the action of one or more proteases. HtrA has been shown to be present in the B. burgdorferi outer membrane (Russell & Johnson, 2013), where contiguity with p66 could enable it to carry out such a proteolytic role. In this study, IFA and confocal microscopic analysis revealed that HtrA and p66 colocalize in the outer membrane. However, the distribution of the merged labeling was not uniform, as the distribution of HtrA was more clustered than p66, which was more dispersed. This result is in agreement with the proteome analysis of he lipid rafts (Toledo et al., 2015b) in which HtrA was found enriched 116-fold in lipid rafts whereas p66 was enriched only 1.67-fold. Therefore the distribution of both proteins in the membrane of B. burgdorferi is expected to be different, as the IFA indicated. HtrA should have well-defined clusters that correspond to lipid raft domains whereas p66 will have a more heterogeneous distribution in the membrane. In addition, the uneven distribution may be related in part to the efficacy of the protease toward p66 as demonstrated in vitro. That is, the observed co-localization is may be a transient event due to the rapid turnover of p66. Lastly, the results could indicate solely that p66 is dispersed throughout the cell whereas HtrA forms aggregates, however we do not believe this is the case, as the close relationship between the two proteins, as evidenced by the DRM partitioning, enzymatic proteolysis, and through HtrA-overexpression is supportive of true co-localization.
In addition to the membrane, HtrA, as a homolog of E. coli Deg proteins, is likely to function also as a chaperone-protease in the periplasmic space. In such a context, HtrA could be a positive or negative determinant in whether p66 is exported to the outer membrane, either by chaperoning it or degrading it as it is formed. Therefore, the proteolytic effects of HtrA on p66 expression could occur over several regulatory levels.
In addition to investigating the possibility and implications of direct protein-protein interactions between HtrA and p66, we also sought to determine if HtrA could be affecting p66 RNA transcription in any way. If HtrA were exclusively binding and degrading p66 protein in the membrane, one would expect the relative levels of p66 transcript from B31A3 and A3HtrAOE to be similar, evidence that HtrA acts post-translationally. To determine if this was the case, we used qRT-PCR to quantify the amount of p66 transcript found in both wild-type B31A3 and A3HtrAOE. The over-expression of HtrA in A3HtrAOE was surprisingly associated with a statistically signiificant reduction of p66 RNA transcript in comparison to that of wild-type B31A3. Thus, the possibility exists that in addition to acting directly on p66 protein to affect p66 expression, HtrA may be exerting an effect on p66 transcript levels, perhaps by proteolytic regulation of an unknown transcriptional activator for p66. In this fashion, B. burgdorferi Hbb, previously described as a transcriptional regulator for p66 (Medrano et al., 2010), would be an interesting candidate for further study. There is also a possibility that over-expression of HtrA could lead to protein changes in the periplasm, creating a non-specific stress signal, thereby altering the transcriptional process.
P66 is required for B. burgdorferi to infect mice (Ristow et al., 2012). Consequently, in the mouse model, a logical expectation would be that the A3HtrAOE would be attenuated due to the under-expression of p66. However, based on organ culture and qPCR, there were no discernible differences in infectivity between A3HtrAOE and wild-type A3. All mice cultured positive for heart, bladder and skin, and organs in the two groups did not show any statistically significant differences in spirochete burden (data not shown). One possible explanation for the lack of attenuation for A3HtrAOE is that although the detectable amount of p66 is less in the over-expressing strain, it is still there as demonstrated by western blot. The diminished level of p66 could have been enough to offset any loss due to HtrA.
The importance of the regulation of p66 by HtrA may lie in its pore-forming capability. A major function of outer membrane porins in bacteria is to allow for the intake of nutrients and other substances vital for the cell as well as the efflux of toxins. The enzymatic activity of HtrA may serve to isolate the organism from the environment by inhibiting the formation of p66 channels in the outer membrane. As an example, during a period of unfavorable conditions, such as extremes of temperature, pH, or a nutrient-poor environment likely to be found in an unfed tick, the HtrA-mediated loss of p66 could constitute a survival mechanism to allow the organism to persist.
Experimental Procedures
Proteins and antibodies
Recombinant full-length p66 (BB0603) protein fused to maltose binding protein (MBP-p66) was used as the target substrate for most degradation experiments was the gift of Dr. Jenifer Coburn (the inherent insolubility of p66 necessitated its use in experiments as an MBP fusion protein) (Coburn et al., 1999, Bunikis et al., 1998). Purified MBP (Novus Biologicals, Littleton, CO) and recombinant B. burgdorferi OspB (Coleman et al., 2013, Katona et al., 2000) and were used as control substrates. The cloning, expression and purification of recombinant wild-type HtrA and active site mutant, HtrAS198A, have been described previously (Coleman et al., 2013). Polyclonal rabbit anti-p66 antibodies were the gifts of Dr. Jenifer Coburn and Dr. Utpal Pal, University of Maryland, College Park, MD. Polyclonal rabbit anti-HtrA antibody has been described previously (Coleman et al., 2013). Mouse monoclonal antibody to HtrA was the gift of Dr. Barbara Johnson, Centers for Disease Control, Fort Collins CO. Mouse monoclonal antibody to MBP was purchased from Thermo Fisher Scientific, Pittsburgh, PA. Mouse anti-FlaB (Coleman & Benach, 1989) and DnaK (Coleman & Benach, 1992) monoclonal antibodies have been described previously. Polyclonal rabbit antibody to asialo GM1 was purchased from Abcam, Cambridge, MA. Secondary antibodies used for western blots were goat ant-rabbit IgG IR700 and goat anti-mouse IgG IR800 (Rockland Immunochemicals, Gilbertsville, PA). Secondary antibodies used for confocal fluorescence microscopy were goat anti-mouse IgG Alexa Fluor 594 and Goat anti-rabbit IgG Alexa Fluor 488 (Molecular Probes, Life Technologies, Grand Island, NY).
Triton X-100 treatment and OptiPrep density gradient separation
The procedure for identification of detergent-resistant membranes (DRM) has been previously described (LaRocca et al., 2010, Toledo et al., 2014). Briefly, B31 spirochetes were treated with 1% Triton X-100 for 16 hours at 4°C. The lysate was separated on a discontinuous OptiPrep density gradient and the fractions were analyzed for α-asialo-GM1, p66 and HtrA by slot blot using rabbit polyclonal antibodies to ganglioside asialo GM1, p66 and HtrA, and mouse anti-DnaK. Secondary antibodies used were goat ant-rabbit IgG IR700 and goat anti-mouse IgG IR800 (Rockland Immunochemicals).
Generation of HtrA-over-expressing strain A3HtrAOE
A B. burgdorferi strain that over-expressed HtrA under the control of the FlaB promoter was created to assess its effect on spirochete protein levels. To accomplish this, DNA coding for the constitutive FlaB promoter (PflaB) (351 bp) (Bono et al., 2000) was amplified from B. burgdorferi B31A3 template by PCR using the primers PFlaBF and PFlaBR (Table S2) and the purified product was ligated into the KpnI and XbaI restriction sites of previously digested shuttle plasmid pKFSS1, which contains the streptomycin-resistance cassette aadA, (Frank et al., 2003) to form plasmid pflaBP. E. coli Dh5α was transformed with purified pflaBP. Streptomycin-resistant colonies were screened by PCR and by agarose gel electrophoresis for size. Subsequently, the 1425 bp htrA (BB0104), including the leader sequence (Coleman et al., 2013), was PCR-amplified from B. burgdorferi template using primers BB0104F and BB0104R (Table S2) and ligated into the XbaI and PstI restriction sites of pre-digested plasmid pflaBP, immediately downstream of PflaB to form plasmid pflaBPhtrA. Streptomycin-resistant colonies were screened as described above. All constructs were verified by sequencing. Purified plasmid pflaBPhtrA (12–15 μg) was introduced into electrocompetent B. burgdorferi strain B31A3 by electroporation. The B. burgdorferi were then diluted in 15 ml of BSK II medium and allowed to recover overnight at 33°C. The culture was then expanded to 42 ml in BSK II containing streptomycin (30 μg ml−1) and incubated at 33°C in 96-well plates (225 μl/well). Positive cultures as evidenced by a change in the medium color from red to yellow were expanded in BSK II/streptomycin and screened by PCR using primers PFlaBF and BB0104-9R. A single clone (A3HtrAOE) was identified, expanded and sequenced (Table S2). This clone was used for subsequent experiments.
2-D electrophoresis
For the first dimension, isoelectric focusing was carried out by use a PROTEAN IEF Cell (Bio Rad, Hercules, CA) according to methods outlined in the IEF Cell manual. Briefly, B. burgdorferi WT and HtrA-over-expression strain A3HtrAOE were grown in 50 ml of BSK II medium at 33°C. Mid-log phase cells (3.2 × 1010) were harvested by centrifugation (7,000 × g, 20 min, 4°C) and washed three times (1.2 ml per wash) with Dulbecco’s Phosphate Buffered Saline (DPBS). The pellets were resuspended in 800 μl of a rehydration buffer consisting of 8M urea, 15 mM DTT, 1% CHAPS, 0.2% BioLytes 3/10 (Bio Rad) and 0.001% bromophenol blue, and sonicated on ice for 1 minute at a setting of 4 (Microson ultrasonic cell disruptor, Farmingdale, NY). Lysates were examined by darkfield to ensure complete disruption. Lysate samples of 250 μl were used to passively rehydrate 7 cm ReadyStrip IPG Strips, pH 3/10 (Bio Rad) for 16 hours at 20°C, followed by a voltage ramping step (2 hours) to 4000V. The final isoelectric focusing step was carried out for 2.5 hours at 4000V. Following the run, the strips were equilibrated for 10 min in Equilibration buffer I, consisting of 6M urea, 20% glycerol, 2% SDS, 375 mM-Tris HCl and 130 mM DTT and Equilibration buffer II consisting of 6M urea, 20% glycerol, 2% SDS, 375 mM Tris-HCl and 135 mM iodoacetamide. For the second dimension, the proteins were further separated by SDS-PAGE, using 10 cm gels of 12.5% acrylamide and stained with Coomassie Brilliant blue.
In vivo analysis of p66 levels for WT and HtrA-over-expresssion strain A3HtrAOE
B. burgdorferi strains B31A3 and A3HtrAOE were harvested from BSK II medium and washed two times with DPBS. A total of 2 × 107 cells from each were separated by SDS-PAGE and analyzed by western blot using rabbit polyclonal anti-p66, rabbit anti-HtrA and mouse monoclonal anti-FlaB. Secondary antibodies used were goat ant-rabbit IgG IR700 and goat anti-mouse IgG IR800 (Rockland Immunochemicals). Digital images of western blot bands were analyzed using a Bio Rad GD/Chemi Documentation System and Quantity One Quantitation Software (Bio Rad) to determine optical densities. Each band was quantitated three times (left end, middle, right end) and the combined data from two independent experiments was tested for statistical significance by unpaired t test (Graph Pad, La Jolla, CA).
Quantitative real-time reverse transcription PCR (qRT-PCR)
Wild-type and A3HtrAOE spirochetes were grown to mid log phase at 33°C and adjusted to a density of 5 × 107/ml. Four replicates of 5 × 107 spirochetes for each strain (1 ml) were centrifuged at 7,000 × and total RNA was extracted using TRIzol reagent (Ambion-Life Technologies, Grand Island, NY) according to the manufacturer’s instructions. In an identical manner, total RNA was extracted from 5 × 107 B31A3 transformed with pKFSS1 containing pFlaB without htrA inserted. To remove any contaminating DNA from the RNA extracts, the samples were treated with DNase using the DNA-free Kit (Ambion-Life Technologies). The total RNA was quantified using a NanoDrop ND-1000 spectrophotometer (NanoDrop Products, Wilmington, DE). Equivalent amounts (200 ng) of DNase-treated RNA were reverse transcribed to cDNA with the Transcriptor First Strand cDNA Synthesis Kit using the supplied Random Hexamer Primer (Roche Applied Science, Indianapolis IN). Mock reverse transcriptions without reverse transcriptase were also carried out to control for contaminating DNA. Absolute qRT-PCR assays were carried out using the 7500 Real Time PCR System. Each 25 μl reaction contained 1X Power SYBR Green PCR Master Mix (Applied Biosystems-Life Technologies) and 2 μM of primers for either flaB (BB0147/47/472F and BB0147/34/342R) or p66 (p66qPCRF and p66qPCRR) (Table S2), which produced amplicons of 150 bp and 175 bp, respectively, in addition to 2.5 μl of cDNA template diluted to 1:100 or PCR-amplified standard curve DNA for flaB (1.64 μl) or p66 (2 μl). Standard curves for flaB and p66, prepared by PCR using the above primers, were done in duplicate for each assay and ranged from 101 to 107 copies. Unknown samples were carried out in triplicate. Water (in place of cDNA) template controls were also included for each run. Instrument parameters were: 1 cycle at 95°C for 15 min; 40 cycles of 95°C for 15 sec, 55°C for 30 sec, and 72°C for 1 min.
Quantitative real-time PCR (qPCR) for spirochete burdens in mouse tissues
This procedure was done as described previously (Toledo et al., 2015a). Briefly, total DNA was isolated on day 21 post-infection from mouse bladder, heart and ear tissues, using the DNEasy kit (Qiagen) and further purified using SpinSmart PCR Purification columns (Denville Scientific, Metuchen, NJ). All samples were diluted to a concentration of 10 ng μl−1. Quantitative PCR was carried out on a 7500 Real-Time PCR System (Applied Biosystems) using oligonucleotide primers for FlaB and nidogen (2 μm) (Table S2) (Morrison et al., 1999). In addition, each 25 μl reaction included 50 ng of template DNA, 300 nM of each primer, 250 ng μl−1 BSA, and 1X Power SYBR Green PCR Master Mix (Applied Biosystems). Instrument parameters were 1 cycle at 95°C for 15 min, followed by 40 cycles at 95°C for 15 s, 55°C (nidogen) or 60°C (flaB) for 30 s, and 72°C for 1 min. All reactions were carried out in duplicate and repeated three times in separate plates for a total of six replicates of gene copy numbers. The mean quantity of B. burgdorferi flaB was normalized using the mean number of mouse nidogen copies.
Proteolysis of MBP-p66 by HtrA
In vitro proteolysis of MBP-p66 by HtrA was carried out as previously described (Coleman et al., 2013) for other substrates. Briefly, MBP-p66 (200 μg ml−1) and HtrA (100 μg ml−1) in DPBS (80 μl) were incubated at 37°C. A fraction (10 μl) of the mixture was collected at various time points and Protease inhibitor (Complete Mini, EDTA-Free Protease Inhibitor Cocktail, Roche Diagnostics, Mannheim Germany) was added to stop proteolysis. Samples were subjected to 12% SDS-PAGE (Bio Rad Mini PROTEAN Precast Gels) followed by Coomassie Brilliant blue staining. Protein bands were visualized using a Bio Rad GS scanner/densitometer.
Indirect immunofluorescence and confocal microscopy
B. burgdorferi B31A3 were harvested from the BSK II medium, centrifuged for 20 min. at 4°C and 5,000X g and washed three times in Dulbecco’s phosphate buffered saline supplemented with 5 mM MgCl2 (DPBS-Mg). The cells were resuspended in DPBS-Mg and applied to printed slide wells, followed by air-drying and fixation for 10 min. in ice-cold acetone. The spirochetes were incubated with 20 μl of primary antibodies (rabbit anti-p66 and mouse anti-HtrA together) at 25°C for 45 min. The slides were placed in a glass staining tray, containing DPBS with gentle stirring for 10 min. This was followed by incubation with 20 μl of goat anti-rabbit IgG Alexa Fluor 488 (green) and goat anti-mouse IgG Alexa Fluor 594 (red) (Molecular Probes, Grand Island, NY) and washing as described above. Coverslips were applied with Slow-Fade anti-fade reagent (Molecular Probes) in glycerol/PBS. Confocal microscopy was carried out with a Zeiss LSM 510 META NLO Two-Photon Laser Scanning Confocal Microscope System at the Stony Brook University Central Microscopy Imaging Center.
Mass Spectrometry
All mass spectrometric analysis was carried out at the Proteomics Center at Stony Brook University. For identification of p66 from 2-D gels, the spot containing putative p66 was cut out and digested with trypsin, followed by MALDI-TOF analysis. For analysis of HtrA cleavage sites for p66, in vitro proteolysis was carried out as described above with the exceptions that incubation time was fixed at 30 min, and all proteolysis was stopped with the addition of 0.1M acetic acid. A trypsin digestion of p66 was included as a positive control. The digest sample (20 μl) was run on an Orbitrap LC/MS/MS and the MS/MS spectra were analyzed against the HtrA and p66 amino acid sequences.
Statistics
The mouse infection data was tested for statistical significance by the non-parametric Mann-Whitney test. The mass spectrometry and western blot densitometry data were tested for statistical significance by two-tailed t-test.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health (NIH) grant AI-027044 to J.L.B. We thank Dr. Jenifer Coburn, Division of Infectious Diseases, Department of Medicine, Medical College of Wisconsin, Milwaukee, WI 53226, USA for the generous gift of recombinant MBP-p66 and rabbit anti-p66 antiserum, Dr. Utpal Pal, Dept. of Veterinary Medicine, University of Maryland, College Park, MD, for rabbit anti-p66 antiserum, and Drs. Barbara Johnson and Theresa Russell, Centers for Disease Control and Prevention, Fort Collins, CO, for mouse anti-HtrA monoclonal antibodies. We also thank Dr. Javier Monzon for guidance with qPCR and Indra Jayatilaka for excellent technical assistance. Recombinant HtrA and HtrAS198A expression and purification was done at the NBC Protein Expression Core, New York State Department of Health, Wadsworth Center, Albany, NY. Mass spectrometry was carried out at the Proteomics Center, Stony Brook University (NIH/NCRR 1 S10 RR023680-1). Confocal microscopy was done at the Central Microscopy Imaging Center, Stony Brook University.
Footnotes
The authors have no conflict of interest to declare.
References
- Alba BM, Leeds JA, Onufryk C, Lu CZ, Gross CA. DegS and YaeL participate sequentially in the cleavage of RseA to activate the sigma(E)-dependent extracytoplasmic stress response. Genes Dev. 2002;16:2156–2168. doi: 10.1101/gad.1008902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, et al. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol. 2006;2 doi: 10.1038/msb4100050. 2006 0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcena-Uribarri I, Thein M, Maier E, Bonde M, Bergstrom S, Benz R. Use of nonelectrolytes reveals the channel size and oligomeric constitution of the Borrelia burgdorferi P66 porin. PLoS One. 2013;8:e78272. doi: 10.1371/journal.pone.0078272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcena-Uribarri I, Thein M, Sacher A, Bunikis I, Bonde M, Bergstrom S, Benz R. P66 porins are present in both Lyme disease and relapsing fever spirochetes: a comparison of the biophysical properties of P66 porins from six Borrelia species. Biochim Biophys Acta. 2010;1798:1197–1203. doi: 10.1016/j.bbamem.2010.02.011. [DOI] [PubMed] [Google Scholar]
- Baumler AJ, Kusters JG, Stojiljkovic I, Heffron F. Salmonella typhimurium loci involved in survival within macrophages. Infect Immun. 1994;62:1623–1630. doi: 10.1128/iai.62.5.1623-1630.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Menachem G, Kubler-Kielb J, Coxon B, Yergey A, Schneerson R. A newly discovered cholesteryl galactoside from Borrelia burgdorferi. Proc Natl Acad Sci U S A. 2003;100:7913–7918. doi: 10.1073/pnas.1232451100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjellqvist B, Ek K, Righetti PG, Gianazza E, Gorg A, Westermeier R, Postel W. Isoelectric focusing in immobilized pH gradients: principle, methodology and some applications. J Biochem Biophys Methods. 1982;6:317–339. doi: 10.1016/0165-022x(82)90013-6. [DOI] [PubMed] [Google Scholar]
- Bono JL, Elias AF, Kupko JJ, 3rd, Stevenson B, Tilly K, Rosa P. Efficient targeted mutagenesis in Borrelia burgdorferi. J Bacteriol. 2000;182:2445–2452. doi: 10.1128/jb.182.9.2445-2452.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DA, London E. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J Biol Chem. 2000;275:17221–17224. doi: 10.1074/jbc.R000005200. [DOI] [PubMed] [Google Scholar]
- Brown DA, Rose JK. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- Brown RE. Sphingolipid organization in biomembranes: what physical studies of model membranes reveal. J Cell Sci. 1998;111( Pt 1):1–9. doi: 10.1242/jcs.111.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunikis J, Barbour AG. Access of antibody or trypsin to an integral outer membrane protein (P66) of Borrelia burgdorferi is hindered by Osp lipoproteins. Infect Immun. 1999;67:2874–2883. doi: 10.1128/iai.67.6.2874-2883.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunikis J, Luke CJ, Bunikiene E, Bergstrom S, Barbour AG. A surface-exposed region of a novel outer membrane protein (P66) of Borrelia spp. is variable in size and sequence. J Bacteriol. 1998;180:1618–1623. doi: 10.1128/jb.180.7.1618-1623.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunikis J, Mirian H, Bunikiene E, Barbour AG. Non-heritable change of a spirochaete’s phenotype by decoration of the cell surface with exogenous lipoproteins. Mol Microbiol. 2001;40:387–396. doi: 10.1046/j.1365-2958.2001.02382.x. [DOI] [PubMed] [Google Scholar]
- Bunikis J, Noppa L, Bergstrom S. Molecular analysis of a 66-kDa protein associated with the outer membrane of Lyme disease Borrelia. FEMS Microbiol Lett. 1995;131:139–145. doi: 10.1111/j.1574-6968.1995.tb07768.x. [DOI] [PubMed] [Google Scholar]
- Bunikis J, Noppa L, Ostberg Y, Barbour AG, Bergstrom S. Surface exposure and species specificity of an immunoreactive domain of a 66-kilodalton outer membrane protein (P66) of the Borrelia spp. that cause Lyme disease. Infect Immun. 1996;64:5111–5116. doi: 10.1128/iai.64.12.5111-5116.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Rand RP. The influence of cholesterol on phospholipid membrane curvature and bending elasticity. Biophys J. 1997;73:267–276. doi: 10.1016/S0006-3495(97)78067-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen T, Kaiser M, Huber R, Ehrmann M. HTRA proteases: regulated proteolysis in protein quality control. Nat Rev Mol Cell Biol. 2011;12:152–162. doi: 10.1038/nrm3065. [DOI] [PubMed] [Google Scholar]
- Coburn J, Chege W, Magoun L, Bodary SC, Leong JM. Characterization of a candidate Borrelia burgdorferi beta3-chain integrin ligand identified using a phage display library. Mol Microbiol. 1999;34:926–940. doi: 10.1046/j.1365-2958.1999.01654.x. [DOI] [PubMed] [Google Scholar]
- Coburn J, Cugini C. Targeted mutation of the outer membrane protein P66 disrupts attachment of the Lyme disease agent, Borrelia burgdorferi, to integrin alphavbeta3. Proc Natl Acad Sci U S A. 2003;100:7301–7306. doi: 10.1073/pnas.1131117100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman JL, Benach JL. Isolation of antigenic components from the Lyme disease spirochete: their role in early diagnosis. J Infect Dis. 1987;155:756–765. doi: 10.1093/infdis/155.4.756. [DOI] [PubMed] [Google Scholar]
- Coleman JL, Benach JL. Identification and characterization of an endoflagellar antigen of Borrelia burgdorferi. J Clin Invest. 1989;84:322–330. doi: 10.1172/JCI114157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman JL, Benach JL. Characterization of antigenic determinants of Borrelia burgdorferi shared by other bacteria. J Infect Dis. 1992;165:658–666. doi: 10.1093/infdis/165.4.658. [DOI] [PubMed] [Google Scholar]
- Coleman JL, Crowley JT, Toledo AM, Benach JL. The HtrA protease of Borrelia burgdorferi degrades outer membrane protein BmpD and chemotaxis phosphatase CheX. Mol Microbiol. 2013;88:619–633. doi: 10.1111/mmi.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman JL, Rogers RC, Benach JL. Selection of an escape variant of Borrelia burgdorferi by use of bactericidal monoclonal antibodies to OspB. Infect Immun. 1992;60:3098–3104. doi: 10.1128/iai.60.8.3098-3104.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman JL, Rogers RC, Rosa PA, Benach JL. Variations in the ospB gene of Borrelia burgdorferi result in differences in monoclonal antibody reactivity and in production of escape variants. Infect Immun. 1994;62:303–307. doi: 10.1128/iai.62.1.303-307.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley JT, Toledo AM, LaRocca TJ, Coleman JL, London E, Benach JL. Lipid exchange between Borrelia burgdorferi and host cells. PLoS Pathog. 2013;9:e1003109. doi: 10.1371/journal.ppat.1003109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cugini C, Medrano M, Schwan TG, Coburn J. Regulation of expression of the Borrelia burgdorferi beta(3)-chain integrin ligand, P66, in ticks and in culture. Infect Immun. 2003;71:1001–1007. doi: 10.1128/IAI.71.2.1001-1007.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defoe G, Coburn J. Delineation of Borrelia burgdorferi p66 sequences required for integrin alpha(IIb)beta(3) recognition. Infect Immun. 2001;69:3455–3459. doi: 10.1128/IAI.69.5.3455-3459.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrmann M, Clausen T. Proteolysis as a regulatory mechanism. Annu Rev Genet. 2004;38:709–724. doi: 10.1146/annurev.genet.38.072902.093416. [DOI] [PubMed] [Google Scholar]
- Elzer PH, Phillips RW, Robertson GT, Roop RM., 2nd The HtrA stress response protease contributes to resistance of Brucella abortus to killing by murine phagocytes. Infect Immun. 1996;64:4838–4841. doi: 10.1128/iai.64.11.4838-4841.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epand RM. Proteins and cholesterol-rich domains. Biochim Biophys Acta. 2008;1778:1576–1582. doi: 10.1016/j.bbamem.2008.03.016. [DOI] [PubMed] [Google Scholar]
- Frank KL, Bundle SF, Kresge ME, Eggers CH, Samuels DS. aadA confers streptomycin resistance in Borrelia burgdorferi. J Bacteriol. 2003;185:6723–6727. doi: 10.1128/JB.185.22.6723-6727.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser CM, Casjens S, Huang WM, Sutton GG, Clayton R, Lathigra R, et al. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 1997;390:580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- Garcia Monco JC, Wheeler CM, Benach JL, Furie RA, Lukehart SA, Stanek G, Steere AC. Reactivity of neuroborreliosis patients (Lyme disease) to cardiolipin and gangliosides. J Neurol Sci. 1993;117:206–214. doi: 10.1016/0022-510x(93)90175-x. [DOI] [PubMed] [Google Scholar]
- Garcia-Monco JC, Seidman RJ, Benach JL. Experimental immunization with Borrelia burgdorferi induces development of antibodies to gangliosides. Infect Immun. 1995;63:4130–4137. doi: 10.1128/iai.63.10.4130-4137.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gherardini FC. Borrelia burgdorferi HtrA may promote dissemination and irritation. Mol Microbiol. 2013;90:209–213. doi: 10.1111/mmi.12390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoy B, Lower M, Weydig C, Carra G, Tegtmeyer N, Geppert T, et al. Helicobacter pylori HtrA is a new secreted virulence factor that cleaves E-cadherin to disrupt intercellular adhesion. EMBO Rep. 2010;11:798–804. doi: 10.1038/embor.2010.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttner WB, Zimmerberg J. Implications of lipid microdomains for membrane curvature, budding and fission. Curr Opin Cell Biol. 2001;13:478–484. doi: 10.1016/s0955-0674(00)00239-8. [DOI] [PubMed] [Google Scholar]
- Kariu T, Yang X, Marks CB, Zhang X, Pal U. Proteolysis of BB0323 results in two polypeptides that impact physiologic and infectious phenotypes in Borrelia burgdorferi. Mol Microbiol. 2013;88:510–522. doi: 10.1111/mmi.12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona LI, Ayalew S, Coleman JL, Benach JL. A bactericidal monoclonal antibody elicits a change in its antigen, OspB of Borrelia burgdorferi, that can be detected by limited proteolysis. J Immunol. 2000;164:1425–1431. doi: 10.4049/jimmunol.164.3.1425. [DOI] [PubMed] [Google Scholar]
- Kenedy MR, Luthra A, Anand A, Dunn JP, Radolf JD, Akins DR. Structural modeling and physicochemical characterization provide evidence that P66 forms a beta-barrel in the Borrelia burgdorferi outer membrane. J Bacteriol. 2014;196:859–872. doi: 10.1128/JB.01236-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobryn K, Naigamwalla DZ, Chaconas G. Site-specific DNA binding and bending by the Borrelia burgdorferi Hbb protein. Mol Microbiol. 2000;37:145–155. doi: 10.1046/j.1365-2958.2000.01981.x. [DOI] [PubMed] [Google Scholar]
- LaRocca TJ, Crowley JT, Cusack BJ, Pathak P, Benach J, London E, et al. Cholesterol lipids of Borrelia burgdorferi form lipid rafts and are required for the bactericidal activity of a complement-independent antibody. Cell Host Microbe. 2010;8:331–342. doi: 10.1016/j.chom.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRocca TJ, Holthausen DJ, Hsieh C, Renken C, Mannella CA, Benach JL. The bactericidal effect of a complement-independent antibody is osmolytic and specific to Borrelia. Proc Natl Acad Sci U S A. 2009;106:10752–10757. doi: 10.1073/pnas.0901858106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRocca TJ, Pathak P, Chiantia S, Toledo A, Silvius JR, Benach JL, London E. Proving lipid rafts exist: membrane domains in the prokaryote Borrelia burgdorferi have the same properties as eukaryotic lipid rafts. PLoS Pathog. 2013;9:e1003353. doi: 10.1371/journal.ppat.1003353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SR, Dorrell N, Everest PH, Dougan G, Wren BW. Construction and characterization of a Yersinia enterocolitica O:8 high-temperature requirement (htrA) isogenic mutant. Infect Immun. 1996;64:2088–2094. doi: 10.1128/iai.64.6.2088-2094.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London E. Insights into lipid raft structure and formation from experiments in model membranes. Curr Opin Struct Biol. 2002;12:480–486. doi: 10.1016/s0959-440x(02)00351-2. [DOI] [PubMed] [Google Scholar]
- Madden T. The BLAST Sequence Analysis Tool. In: McEntyre J, Ostell J, editors. The NCBI Handbook [Internet] Bethesda, MD: National Center for Biotechnology Information (US); 2002. [Google Scholar]
- Medrano MS, Policastro PF, Schwan TG, Coburn J. Interaction of Borrelia burgdorferi Hbb with the p66 promoter. Nucleic Acids Res. 2010;38:414–427. doi: 10.1093/nar/gkp1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison TB, Ma Y, Weis JH, Weis JJ. Rapid and sensitive quantification of Borrelia burgdorferi-infected mouse tissues by continuous fluorescent monitoring of PCR. J Clin Microbiol. 1999;37:987–992. doi: 10.1128/jcm.37.4.987-992.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouw KW, Rice PA. Shaping the Borrelia burgdorferi genome: crystal structure and binding properties of the DNA-bending protein Hbb. Mol Microbiol. 2007;63:1319–1330. doi: 10.1111/j.1365-2958.2007.05586.x. [DOI] [PubMed] [Google Scholar]
- Nichols B. Caveosomes and endocytosis of lipid rafts. J Cell Sci. 2003;116:4707–4714. doi: 10.1242/jcs.00840. [DOI] [PubMed] [Google Scholar]
- Ntchobo H, Rothermel H, Chege W, Steere AC, Coburn J. Recognition of multiple antibody epitopes throughout Borrelia burgdorferi p66, a candidate adhesin, in patients with early or late manifestations of Lyme disease. Infect Immun. 2001;69:1953–1956. doi: 10.1128/IAI.69.3.1953-1956.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Farrell PH. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975;250:4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Ornstein K, Ostberg Y, Bunikis J, Noppa L, Berglund J, Norrby R, Bergstrom S. Differential immune response to the variable surface loop antigen of P66 of Borrelia burgdorferi sensu lato species in geographically diverse populations of lyme borreliosis patients. Clin Diagn Lab Immunol. 2002;9:1382–1384. doi: 10.1128/CDLI.9.6.1382-1384.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallen MJ, Wren BW. The HtrA family of serine proteases. Mol Microbiol. 1997;26:209–221. doi: 10.1046/j.1365-2958.1997.5601928.x. [DOI] [PubMed] [Google Scholar]
- Pinne M, Thein M, Denker K, Benz R, Coburn J, Bergstrom S. Elimination of channel-forming activity by insertional inactivation of the p66 gene in Borrelia burgdorferi. FEMS Microbiol Lett. 2007;266:241–249. doi: 10.1111/j.1574-6968.2006.00529.x. [DOI] [PubMed] [Google Scholar]
- Raivio TL. Envelope stress responses and Gram-negative bacterial pathogenesis. Mol Microbiol. 2005;56:1119–1128. doi: 10.1111/j.1365-2958.2005.04625.x. [DOI] [PubMed] [Google Scholar]
- Ristow LC, Bonde M, Lin YP, Sato H, Curtis M, Wesley E, et al. Integrin binding by Borrelia burgdorferi P66 facilitates dissemination but is not required for infectivity. Cell Microbiol. 2015 doi: 10.1111/cmi.12418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristow LC, Miller HE, Padmore LJ, Chettri R, Salzman N, Caimano MJ, et al. The beta(3)-integrin ligand of Borrelia burgdorferi is critical for infection of mice but not ticks. Mol Microbiol. 2012;85:1105–1118. doi: 10.1111/j.1365-2958.2012.08160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell TM, Delorey MJ, Johnson BJ. Borrelia burgdorferi BbHtrA degrades host ECM proteins and stimulates release of inflammatory cytokines in vitro. Mol Microbiol. 2013;90:241–251. doi: 10.1111/mmi.12377. [DOI] [PubMed] [Google Scholar]
- Russell TM, Johnson BJ. Lyme disease spirochaetes possess an aggrecan-binding protease with aggrecanase activity. Mol Microbiol. 2013;90:228–240. doi: 10.1111/mmi.12276. [DOI] [PubMed] [Google Scholar]
- Sadziene A, Wilske B, Ferdows MS, Barbour AG. The cryptic ospC gene of Borrelia burgdorferi B31 is located on a circular plasmid. Infect Immun. 1993;61:2192–2195. doi: 10.1128/iai.61.5.2192-2195.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salaun C, James DJ, Chamberlain LH. Lipid rafts and the regulation of exocytosis. Traffic. 2004;5:255–264. doi: 10.1111/j.1600-0854.2004.0162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salman-Dilgimen A, Hardy PO, Dresser AR, Chaconas G. HrpA, a DEAH-box RNA helicase, is involved in global gene regulation in the Lyme disease spirochete. PLoS One. 2011;6:e22168. doi: 10.1371/journal.pone.0022168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salman-Dilgimen A, Hardy PO, Radolf JD, Caimano MJ, Chaconas G. HrpA, an RNA helicase involved in RNA processing, is required for mouse infectivity and tick transmission of the Lyme disease spirochete. PLoS Pathog. 2013;9:e1003841. doi: 10.1371/journal.ppat.1003841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels DS. Gene regulation in Borrelia burgdorferi. Annu Rev Microbiol. 2011;65:479–499. doi: 10.1146/annurev.micro.112408.134040. [DOI] [PubMed] [Google Scholar]
- Schroder NW, Schombel U, Heine H, Gobel UB, Zahringer U, Schumann RR. Acylated cholesteryl galactoside as a novel immunogenic motif in Borrelia burgdorferi sensu stricto. J Biol Chem. 2003;278:33645–33653. doi: 10.1074/jbc.M305799200. [DOI] [PubMed] [Google Scholar]
- Schwan TG, Piesman J, Golde WT, Dolan MC, Rosa PA. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc Natl Acad Sci U S A. 1995;92:2909–2913. doi: 10.1073/pnas.92.7.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K, Ehehalt R. Cholesterol, lipid rafts, and disease. J Clin Invest. 2002;110:597–603. doi: 10.1172/JCI16390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skare JT, Mirzabekov TA, Shang ES, Blanco DR, Erdjument-Bromage H, Bunikis J, et al. The Oms66 (p66) protein is a Borrelia burgdorferi porin. Infect Immun. 1997;65:3654–3661. doi: 10.1128/iai.65.9.3654-3661.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart PE, Hoff J, Fischer E, Krum JG, Rosa PA. Genome-wide transposon mutagenesis of Borrelia burgdorferi for identification of phenotypic mutants. Appl Environ Microbiol. 2004;70:5973–5979. doi: 10.1128/AEM.70.10.5973-5979.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubs G, Fingerle V, Wilske B, Gobel UB, Zahringer U, Schumann RR, Schroder NW. Acylated cholesteryl galactosides are specific antigens of Borrelia causing lyme disease and frequently induce antibodies in late stages of disease. J Biol Chem. 2009;284:13326–13334. doi: 10.1074/jbc.M809575200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swamy KH, Chung CH, Goldberg AL. Isolation and characterization of protease do from Escherichia coli, a large serine protease containing multiple subunits. Arch Biochem Biophys. 1983;224:543–554. doi: 10.1016/0003-9861(83)90242-4. [DOI] [PubMed] [Google Scholar]
- Toledo A, Coleman JL, Kuhlow CJ, Crowley JT, Benach JL. The enolase of Borrelia burgdorferi is a plasminogen receptor released in outer membrane vesicles. Infect Immun. 2012;80:359–368. doi: 10.1128/IAI.05836-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo A, Crowley JT, Coleman JL, Larocca TJ, Chiantia S, London E, Benach JL. Selective Association of Outer Surface Lipoproteins with the Lipid Rafts of Borrelia burgdorferi. MBio. 2014;5 doi: 10.1128/mBio.00899-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo A, Monzon JD, Coleman JL, Garcia-Monco JC, Benach JL. Hypercholesterolemia and ApoE deficiency result in severe infection with Lyme disease and relapsing-fever Borrelia. Proc Natl Acad Sci U S A. 2015a doi: 10.1073/pnas.1502561112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo A, Perez A, Coleman JL, Benach JL. The lipid raft proteome of Borrelia burgdorferi. Proteomics. 2015b doi: 10.1002/pmic.201500093. [DOI] [PubMed] [Google Scholar]
- Yang X, Goldberg MS, Popova TG, Schoeler GB, Wikel SK, Hagman KE, Norgard MV. Interdependence of environmental factors influencing reciprocal patterns of gene expression in virulent Borrelia burgdorferi. Mol Microbiol. 2000;37:1470–1479. doi: 10.1046/j.1365-2958.2000.02104.x. [DOI] [PubMed] [Google Scholar]
- Yang X, Promnares K, Qin J, He M, Shroder DY, Kariu T, et al. Characterization of multiprotein complexes of the Borrelia burgdorferi outer membrane vesicles. J Proteome Res. 2011;10:4556–4566. doi: 10.1021/pr200395b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Yang X, Kumar M, Pal U. BB0323 function is essential for Borrelia burgdorferi virulence and persistence through tick-rodent transmission cycle. J Infect Dis. 2009;200:1318–1330. doi: 10.1086/605846. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.