Abstract
Objective
The Timed Up and Go (TUG), one of the most widely used tests of mobility, has been validated and associated with adverse outcomes in the community, acute care and nursing home setting. It is composed of several distinct subtasks, however, the temporal relationship when transitioning between subtasks has not been well-studied. We tested the hypothesis that longer transition durations between the final turn to the sitting subtasks are associated with worse motor and cognitive performance in older adults.
Methods
1,055 participants (80.33±7.57 yrs, 76.96% female) performed the TUG while wearing a 3-D inertial sensor on their lower back. We employed a series of linear regressions to examine the association of the duration between the turn and sitting subtasks with clinical characteristics including motor and cognitive functions.
Results
Subjects employed two different strategies when they transitioned from turning to sitting: (1) Distinct Transition strategy (DTS): 816 participants (77.34%) first completed the turn before starting to sit. The average duration between these distinct subtasks (D-interval) was 715±980msec. (2) Overlapping Transition strategy (OTS): 239 participants (22.65%) started to sit before completing the turn. The average overlap duration between these tasks (O-interval) was 237±269msec. Participants who employed the DTS were slightly younger than those who employed the OTS (p≤0.013). Higher D-intervals and O-intervals were associated with worse TUG performance (p≤0.02), with poorer motor and cognitive function, i.e. worse parkinsonian gait (p≤0.001), lower level of perceptual speed (p≤0.03), and with worse mobility disability (p≤0.001). A longer D-interval was associated with worse gait speed and bradykinesia (p≤0.001), while a longer O-interval was associated with increased rigidity (p=0.004).
Conclusion
Older adults apparently employ two different strategies when transitioning from turning to sitting. The instrumented TUG can characterize additional gait and balance aspects that cannot be derived from traditional TUG assessments. These new measures offer novel targets for intervention to decrease the burden of late-life gait impairment.
Keywords: aging, disability, cognition, inertial sensors, accelerometers, turn-to-sit, Timed Up and Go
INTRODUCTION
The Timed Up and Go (TUG) is a widely used measure of mobility in older adults. Since its introduction in 1991, it has been cited more than 6000 times. It has been validated and associated with adverse outcomes in the community, acute care and nursing home setting. The TUG requires subjects to integrate several movement subtasks together for its successful completion. When it was first developed, a stopwatch was used to measure the duration of the TUG. The total time to complete the TUG is associated with poorer motor function and has been shown to predict a variety of adverse health outcomes associated with aging1–3.
The successful performance of a specified motor task like the TUG depends on the integration and timing of the several subtasks necessary to meet the overall task demands. Our group and others have employed an instrumented TUG1, 2, 4–10 and have shown that the TUG can be decomposed into several different subtasks. Objective measures extracted from these recordings show preferential impairment and prolongation of distinct subtasks underlying a completed TUG that may account in part for the heterogeneity of gait and balance impairment in older adults. The total TUG duration is composed of the duration of the individual subtasks plus the duration of the transitions between each of its subcomponents. Prior TUG studies have quantified the duration of the individual subtasks1, 2, 4–10, but have not investigated the timing between the individual TUG subtasks. The inter-relationship between the duration during the transition between TUG subcomponents, gait and balance capacity, and disability is unknown.
To fill this gap and provide a more complete characterization of a completed TUG, we examined data from 1,055 older adults without dementia who wore a whole body sensor to record TUG performance during annual gait testing in the community-setting. These analyses focused on the duration for the final turn to sit since the beginning and end times of both subtasks are easily identified and this transition may be associated with increased risk of falls11. Since a prolonged total TUG duration is associated with poorer function and increased disability, we tested the hypothesis that a longer duration between its subtasks (the interval between the final turn to the sitting subtask) is associated with poorer motor and cognitive performance and more disability in older adults.
METHODS
Subjects
Participants were from two ongoing community-based cohort studies of aging. The Memory and Aging Project (MAP) and the Minority Aging Research Study (MARS)12, 13. Since both studies employ common data collection and operational methods, we combined their data for these analyses. These studies have rolling admission and the whole body worn sensor (see below) was added to both studies in 2011. For these analyses, we included all cases that had completed TUG testing with the whole body sensor and did not have clinical dementia or Parkinson’s disease. At the time of these analyses, 1159 participants had undergone TUG testing with the sensor at least once. We excluded 100 cases with clinical dementia and 4 participants diagnosed with Parkinson disease, leaving 1055 cases for these analyses. The study was conducted in accordance with the latest version of the Declaration of Helsinki and was approved by the institutional review board of Rush University Medical Center.
Instrumented TUG Testing
Participants performed the TUG while wearing a body-fixed sensor as previously described1, 2, 9. A portable small, light-weight body-fixed sensor (DynaPort MiniMod Hybrid Module, McRoberts B.V., The Hague, The Netherlands) was worn on a neoprene belt placed on the lower back at the level of anterior iliac crest. The sensor weighs 74 gm and its dimensions are 87 × 45 × 14 mm. The device includes a triaxial accelerometer (sensor range and resolution are: ±2g and ± 1mg respectively) and a triaxial gyroscope (sensor range and resolution are: ± 100 deg/s and ± 0.0069 deg/s, respectively).
Six acceleration and angular velocity signals are recorded continuously during TUG testing. Signals include 3 acceleration axes: vertical acceleration (V), medio-lateral acceleration (ML), anterior-posterior acceleration (AP), and 3 angular velocity axes: yaw- the rotation around the vertical axis, pitch- the rotation around the medio-lateral axis, and roll- the rotation around the anterior-posterior axis. The six signals were saved on a Secure Digital (SD) card at a sample frequency of 100 Hz. After testing was completed, the data was transferred to a personal computer for further analysis (using Matlab, the Mathworks software).
TUG Subtasks Measures
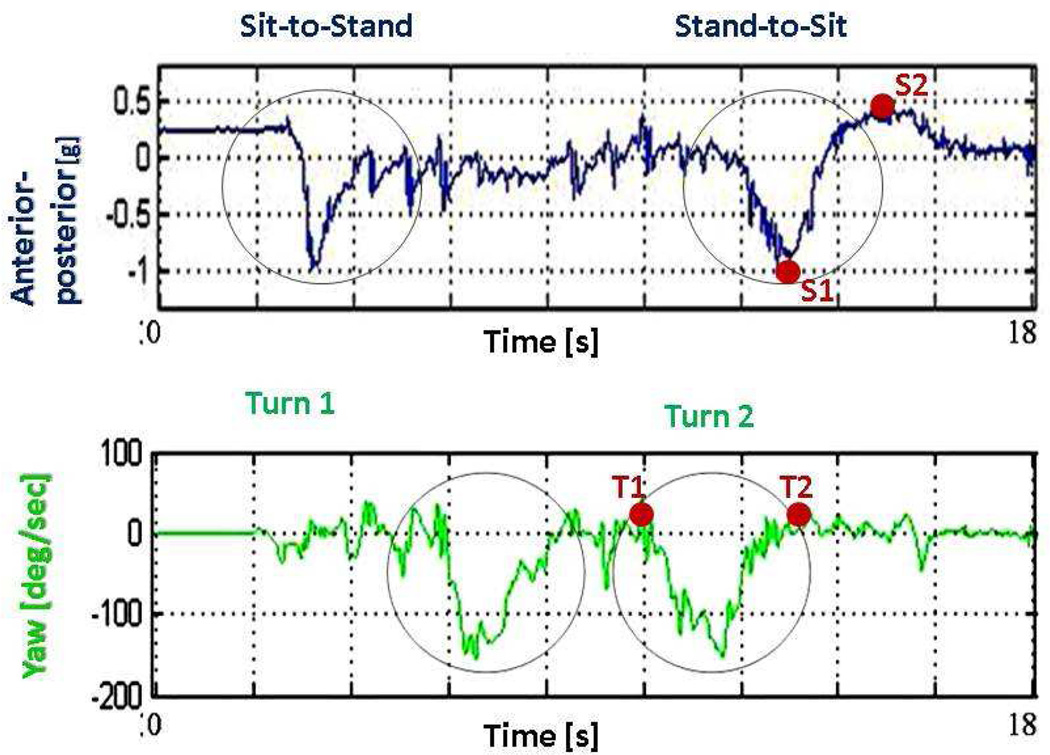
Participants performed the TUG twice. The present analysis was performed on the 2nd trial. Previously published algorithms1, 2, 9 were used to derive measures of the TUG subtasks: sit to stand, walking, turning, and stand to-sit. Our analyses focused on the final two subtasks. We measured the duration of the entire turn to sit interval, i.e. from the beginning of the final turn to the end of the final sit subtasks. As illustrated in Figure 1, we also measured the interval between the two subtasks, i.e. the duration between the end of the turn (determined by the yaw axis4, 9) and the beginning of the stand to sit subtask (determined by the AP axis1, 2, 9).
Figure 1.
Start and end times of the second turn of the Timed Up and Go, as derived from the yaw signal (points T1 and T2, respectively) and start and end times of the stand-to-sit subtask, as derived from the anterior-posterior signal (points S1 and S2, respectively). The interval S1 to S2 represents the "active transition" part of this transfer, i.e., the leaning backward of the trunk when sitting back on the chair which includes the lowering of the center of mass backward and the free fall into the chair. The interval before S1 represents the "preparation part", i.e., the leaning forward of the trunk when preparing to sit back on the chair, and is more challenging to determine. We therefore focus on the "active transition" part.
Assessment of Other Covariates
Demographic Variables
Age was based on date of birth and date of TUG; sex, years of education and race were obtained at baseline interview.
Motor Assessments
Several instruments were employed to measure motor function. Parkinsonian symptoms: Trained nurses assessed 26-items from the motor section of the Unified Parkinson’s Disease Rating Scale (UPDRS). Four previously established scores for parkinsonism, i.e., gait disturbance, bradykinesia, rigidity and tremor, were derived from these 26 items14. Other Motor Performances: We also assessed gait speed, which was based on time to complete a walking task. Mobility Disability: Rosow-Breslau Scale was used as a self-report measure of mobility disability.
Cognitive Assessment and Clinical Diagnoses
Subjects underwent a uniform structured clinical evaluation, as described elsewere13, 15–17. Summary scores for five cognitive abilities were derived from 19 cognitive tests: episodic memory, semantic memory, working memory, perceptual speed and visual spatial15, 18. Higher scores reflect better performance.
Statistical Analysis
We first examined the inter-relationship between the turn-to-sit duration (i.e. from the beginning of the turn (T1) to the end of the sitting subtask (S2)) (see Figure 1) and the other TUG measures derived from the whole body sensor using linear regression (enter method) with the duration of the turn-to-sit and the squared duration of turn-to-sit as predictors, adjusted for age and sex. Then we examined the clinical correlates between the interval between the two subtasks (i.e. the interval between the end of the final turn (T2) to the start of the sitting subtask (S1)) (see Figure 1) and between motor and cognitive performance measures using linear regressions (enter method, with the interval between the two subtasks as an output), adjusted for age, sex, height, weight, race and years of education). Beta values and 95% confidence intervals (CI) are reported for the regression analyses. All data was assessed for normality using the Komogorov-Smirnov test. Corrections for multiple comparisons were made using the Hochberg-Benjamini method19. Because of the lack of normality, Mann Whitney tests were used for between group comparisons, while group values are reported as mean ± standard deviation. Statistical analyses were carried out using SPSS version 19.
RESULTS
Transitioning between the Final Turn and the Stand to Sit TUG Subtask
The clinical characteristics of 1,055 participants with completed the instrumented TUG are included in Table 1. During our initial evaluation of the TUG data, we noted that both a linear term for turn to sit duration (B=0.42; CI: 0.012–0.025; p<0.001) and a squared term (B=0.29; CI: 0.00001–0.00002; p<0.001) were associated with the overall TUG duration. The presence of the significant squared term in the regression analyses suggested that the turn to sit duration was a non-linear association and led us to investigate more closely the interval between the two subtasks.
Table 1.
Clinical characteristics
| Demographic measures | Entire cohort | Distinct (DTS) |
Overlap (OTS) |
p-value DTS vs OTS |
|---|---|---|---|---|
| # of subjects (N) | 1055 | 816 | 239 | |
| Age (yrs) | 80.33±7.57 | 79.77±7.71 | 82.24±6.75 | <0.001 * |
| Gender (% women) | 76.96% | 77.30% | 75.70% | 0.606 |
| Race (%minorities) | 29.10% | 31.86% | 20.10% | 0.01 * |
| Height (m) | 1.63±0.09 | 1.63±0.09 | 1.63±0.09 | 0.737 |
| Weight (kg) | 74.81±17.46 | 75.32±17.63 | 73.06±16.80 | 0.056 |
| Years of education (yrs) | 15.08±3.13 | 15.13±3.19 | 14.93±2.90 | 0.483 |
| Cognitive measures | ||||
| Episodic memory score | 0.25±0.70 | 0.25±0.71 | 0.22±0.68 | 0.417 |
| Semantic memory score | 0.14±0.69 | 0.14±0.68 | 0.16±0.70 | 0.825 |
| Working memory score | 0.06±0.74 | 0.06±0.74 | 0.06±0.73 | 0.906 |
| Perceptual speed score | 0.11±0.75 | 0.11±0.76 | 0.09±0.71 | 0.499 |
| Visual-spatial score | 0.16±0.77 | 0.18±0.79 | 0.12±0.71 | 0.125 |
| Parkinsonian scores | ||||
| Bradykinesia score | 6.76±9.88 | 6.93±10.17 | 6.16±8.77 | 0.793 |
| Rigidity score | 0.33±2.39 | 0.33±2.42 | 0.34±2.30 | 0.749 |
| Tremor score | 1.20±3.53 | 1.13±3.37 | 1.42±4.05 | 0.255 |
| Gait score | 10.97±12.39 | 10.88±12.46 | 11.27±12.19 | 0.569 |
| Mobility Disability Rosow-Breslau Scale | 0.73±0.95 | 0.74±0.95 | 0.71±0.95 | 0.655 |
| Gait speed (m/s) | 0.52±0.17 | 0.52±0.17 | 0.50±0.17 | 0.228 |
Measures which were significantly different between groups.
Most participants (77.34%, N=816) completed the final turn before they began to sit down. Thus, we were able to measure a variable time interval in between these two distinct subtask performances. However, not all participants completed the final turn before they initiated sitting. Because the sensor recordings included tri-axial recordings, we were able to document that a minority of cases (22.65%, N=239) began to sit before completing the final turn, i.e. they showed an overlap between these two subtasks.
We refer to the first strategy as Distinct Transition strategy (DTS) and to the second strategy as Overlapping Transition strategy (OTS). Figure 2 depicts the body-fixed sensor signals obtained during the two strategies. Among subjects who used the DTS, the average duration from the end of the turn to the initiation of the sitting (D-interval) was 715.08±980.32 msec. Among those subjects who used the OTS, the average duration of the overlap from the initiation of the sitting to the end of the turn subtask (O-interval) was 237.15±269.87 msec. While participants who employed the DTS were about 2 years younger, on average, than those who employed OTS, no other clinical differences were observed in these two groups (Table 1). Participants who employed the DTS had a longer overall TUG duration (DTS: 13.85±6.09 sec; OTS: 12.83±5.32; p=0.008) and a lower turn duration (DTS: 2.01±0.61 sec; OTS: 2.32±0.68; p<0.001).
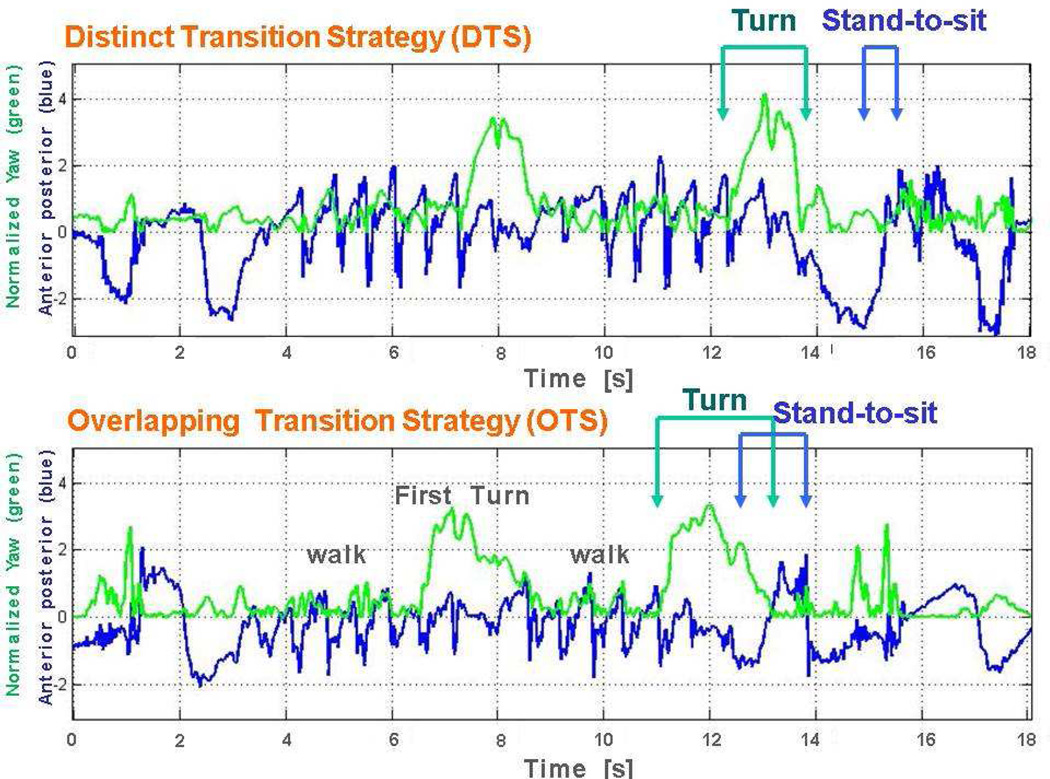
Figure 2.
The two turn-to-sit strategies employed by older adults in the current study are illustrated here: 1. Distinct Transition Strategy (DTS) and 2. Overlapping Transition Strategy (OTS). The start and end times of the turn and the stand-to-sit were derived from the normalized yaw (green) and Anterior-posterior (AP) (blue) axes, respectively. The upper figure shows the TUG yaw and AP signal of a subject with DTS. The turn is completed (green) before the initiation of the stand-to-sit movement (blue). The lower figure shows the TUG yaw and AP signal of a subject with OTS. The turn is completed (green) after the initiation of the stand-to-sit movement (blue).
Association of the Transition Strategy Duration with the other TUG Measures
A higher D-interval and a higher O-interval were both significantly associated with a longer time to complete the entire TUG (Spearman’s correlation coefficient ρ=0.15–0.54, p≤0.02), with longer durations of the TUG subtasks (walk, transitions, and turn-to-sit), and with lower pitch and yaw ranges during the transitions and turns (Spearman’s correlation coefficient ρ=0.09–0.47, p≤0.012).
Clinical Correlates of Distinct Transition Strategy (DTS) Duration (D-interval)
Subject characteristics
As summarized in Table 2a, with age, sex, height, weight, race and education as covariates and D-interval as the dependent variable, a longer D-interval was related to an older age (p<0.001), higher weight (p=0.003), more years of education (p=0.03), a and a higher probability to be white (p<0.001).
Table 2.
Associations between D-interval or O-interval and clinical measures – adjusted linear regression models*
|
Subgroup 1: DTS: (n=816) (dependent variable: D-interval [msec] |
Subgroup 1: OMS: (n=239) (dependent variable: O-interval [msec]) |
|
|---|---|---|
| (A) Demographic measuresa | ||
| Age | B=0.24 (CI:−33.00– −15.95) p<0.001 | B=0.124 (CI: −1.65–11.43) p=0.142 |
| Sex | B=−0.07 (CI: −49.2– 334.06) p=0.145 | B=0.06 (CI: −93.25–176.82) p=0.542 |
| Race | B=−0.22(CI: 4.77–10.31) p<0.001 | B=0.14 (CI: −11.55–207.75) p=0.079 |
| Education | B=0.088 (CI: −41.64—1.74) p=0.03 | B=0.03 (CI: −10.88–16.58) p=0.683 |
| Height | B=0.139 (CI: −10.76 – −2.26) p=719 | B=−0.13 (CI: −1035–271) p=0.250 |
| Weight | B=0.13 (CI:−10.76– −2.26) p=0.003 | B=0.21 (CI: 0.40–6.53) p=0.027 |
| (B) Parkinsonian scoresb | ||
| Bradykinesia score | B=0.18 (CI:−20.3– −7.3) p<0.001 | B=0.16 (CI:−0.05–6.8) p=0.053 |
| Rigidity score | B=−0.02 (CI:−20.3– 34.2) p=0.617 | B=0.23 (CI:5.8–30.7) p=0.004 |
| Tremor score | B=0.05 (CI:−30.9– 7.6) p=0.235 | B=−0.04 (CI:−9.8– 5.4) p=0.57 |
| Gait score | B=0.45 (CI:−35.6– −23.7) p<0.001 | B=0.34 (CI:2.8–8.5) p<0.001 |
| Global parkinsonian score | B=0.35 (CI:−66.4– −39.4) p<0.001 | B=0.29 (CI:5.3–18.7) p<0.001 |
| (C) Cognitive Abilitiesb | ||
| Episodic memory score | B=−0.04 (CI:−41.0– 146.9) p=0.269 | B=−0.06 (CI:−89.8– 38.2) p=0.428 |
| Semantic memory score | B=−0.122 (CI:−41.2–228.6) p=0.005 | B=0.01 (CI:−62.2– 74.8) p=0.856 |
| Working memory score | B=−0.06 (CI:−17.8–146.6) p=0.125 | B=−0.08 (CI:−88.5– 28.5) p=0.313 |
| Perceptual speed score | B=−0.16 (CI:−80.03–253.77) p<0.001 | B=−0.17 (CI:−128.5– −5.4) p=0.033 |
| Visual-spatial score | B=−0.07 (CI:−5.6–160.8) p=0.068 | B=−0.10(CI:−100.7– 23.1) p=0.217 |
| Global Cognitive score | B=−0.12 (CI:−47.6– 287.5) p=0.006 | B=−0.13 (CI:−155.9– 21.3) p=0.136 |
Adjusted Multivariate model
Adjusted Univariate model
Entries are the B values, 95% confidence intervals and the associated p-value. The multivariate and univariate models were adjusted for age, sex, race, height, weight, and years of education. The linear regression was performed using the enter method.
Motor performance
As summarized in Table 2B, in the adjusted regression models, a longer D-interval was related to a higher (worse) parkinsonian scores, specifically bradykinesia (p<0.001) and parkinsonian gait (p<0.001). Gait Speed: In the adjusted regression model, a higher D-interval was correlated with lower gait speed (B=0.36; CI: 1325.9– 2381.8; p<0.001). Mobility disability: In the adjusted regression model, a higher D-interval was correlated with higher (worse) Rosow-Breslau Scale of disability (B=−0.31; CI:−328.5– −196.1; p<0.001)).
Cognition
As summarized in Table 2c, in the adjusted regression models, a longer D-interval was related to a lower (worse) semantic memory (p=0.005) and to a lower perceptual speed score (p<0.001).
Clinical Correlates Overlapping Transition Strategy (OTS) Duration (O-interval)
Subject Characteristics
As summarized in Table 2a, in the regression model that included age, sex, height, weight, race and education as covariates with O-interval as the dependent variable, a longer O-interval was related to higher weight (p=0.027).
Motor performance
As summarized in Table 2b, in the adjusted regression models, a longer O-interval was related to a higher (worse) parkinsonian scores, specifically rigidity (p=0.004) and parkinsonian gait (p<0.001). Gait Speed: No significant relation was found between the O-interval and gait speed (p=0.446). Mobility disability: In the adjusted regression model, a higher O-interval was correlated with a higher (worse) Rosow-Breslau Scale of disability (B=0.25; CI: 28.1–112.2; p=0.001).
Cognition
As summarized in Table 2c, in the adjusted regression models, a longer O-interval was related to a lower (worse) perceptual speed (p=0.033).
DISCUSSION
Recent work has shown that the instrumented TUG can provide a more detailed description of the complexity of the TUG performance that cannot be assessed through simple observation or with a standard stopwatch1, 2, 4, 8–10. While prior studies using the instrumented TUG have shown that the TUG is composed of several subtasks1, 2, 9, the present study focused on the timing of the transition between the two final subtasks employed to complete the TUG. Here, we show that it is not sufficient to assess only the duration of the turn-to-sit; it is essential to evaluate the nature of the interval between the turning and the sitting subtask in order to obtain a more complete picture of the performance of these subtasks. We found that almost 80% of individuals completed their final turn before they initiated sitting. In these individuals, there was no temporal overlap between the two subtasks. A longer intervening interval between the two subtasks was associated with poorer motor and cognitive outcomes. A small, but significant minority of older participants used a different strategy when turning to sit; they began to sit before they completed the final turn. In this group, a longer overlap between these two subtasks was associated with worse motor and cognitive functions.
It is not yet clear what drives a participant to choose one strategy over the other. Still, once a strategy is chosen, the present findings indicate that the profile is unique. In other words, different factors are apparently related to the degradation of each strategy's performance. Older adults may exhibit slower performance of the movement sequence20, leading to a higher separation between subtasks when using the DTS strategy. Alternatively, they might have less control of the movement sequence, less flexibility, and impaired segmental coordination21–25 that could lead to failure to properly separate between the turn and sit tasks and eventually result in a higher overlap between subtasks when using the OTS strategy. Consistent with these possibilities, although the participants in the two strategy groups were similar on average, worse DTS (i.e., longer D-interval) performance was associated with bradykinesia and lower gait speed, while a worse OTS performance (i.e., longer O-interval) was associated with rigidity.
Hase and Stein26 previously showed that there are two types of turns during walking: step turns and spin turns. The step strategy involves the inner foot stepping towards the turning direction while the outer foot stands in place. The spin strategy involves the outer foot swinging towards the step direction while the inner foot is at stance. The step strategy apparently is more stable27 and has a lower biomechanical cost27, 28, while spin turns impose a greater challenge to the locomotor system and perhaps are related to greater chance of tripping27, 29. Another study30 showed that that healthy older adults preferred spin turns when walking either slower or faster than their natural walking speed. This indicates that spin turns are perhaps not the natural preference of healthy older adults. Based on these previous findings26, 30, it might be suggested that the more common DTS strategy derives from the step turn strategy which contributes to higher stability. In contrast, the OTS strategy may derive from a spin strategy that requires greater postural control and is not typically performed at a person's natural walking speed. This might explain why most of our cohort preferred the DTS over the OTS strategy. People who use the OTS were slightly older and tended to weigh less and may therefore be prone to using a "less stable" strategy.
Nonetheless, choosing one strategy over the other does not necessarily mean that one strategy is worse than the other. The duration of the transition apparently plays a critical role. Although the DTS seems to be the more stable and the more common choice among older adults, it may, at times, be considered as a “lower functioning” strategy, for example, in cases where gait deterioration causes the participant to walk too slowly. In some instances, an overlap strategy may be considered as a "higher functioning" strategy. For example, motor learning using co-contractions can provide more movement control and smoother movement31, 32. However, in other cases, overlapping movements may be considered as a poorer strategy, i.e. when they occur in an inaccurate timing or magnitude, causing inefficient movements, impaired segmental coordination and less control (resulting for example in a "free fall" into the chair). Co-contractions can also be a sign of central nervous system abnormalities, dystonia, stroke33, 34 and unsuccessful ageing35.
Limitations and future work
As technology advances the miniaturization of devices ensures that instrumented TUG testing can be done unobtrusively without increased burden. In fact, most current devices have the recording capacity which allows for post hoc analyses long after the testing has been completed. While the custom algorithms employed in the current study have been previously validated, in their current form implementing these algorithms requires special training and experience. Investigators and commercial entities are actively pursuing the development of automated data analysis packages that will eventually enable results in real time. Widespread use of instrumented TUG will eventually provide clinicians with a more comprehensive characterization of gait and balance in older adults. This offers the potential for the targeting of mor sensitive and specific outcome metrics in the efforts to monitor and improve impaired gait and balance in our rapidly growing aging population. While the current study examined a large number of community-dwelling older adults, the associations with clinical performances identified were cross sectional. Longitudinal studies are needed to determine the predictive validity of these measures and to determine whether they provide associations which are independent of more traditional TUG metrics.
CONCLUSIONS
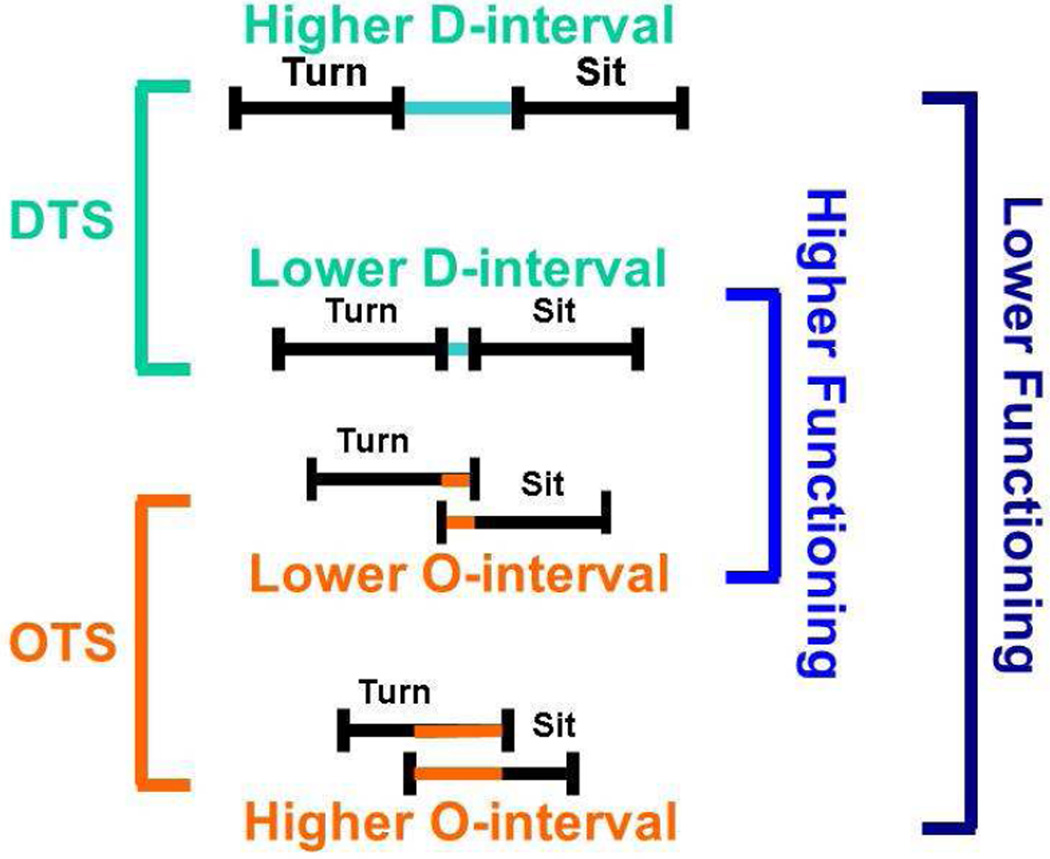
In summary, longer separation between movement subtasks and more prolonged overlap between the turn and stand-to-sit subtasks are both related to poorer motor and cognitive function and more disability. It seems that slower gait and higher bradykinesia is related to worse DTS performance while higher rigidity is related to worse OTS performance. Figure 2 and 3 depict this idea more clearly. Turn-to-sit analysis using a body-worn sensor in the community-setting provides a tool for identifying two different turn-to-sit strategies used by older persons, as well as assessing the performance in each strategy. Perhaps these measures can be utilized as possible additional markers of risk for developing cognitive and motor impairments. The use of unobtrusive body worn sensory in the community setting provides both clinicians and investigators with the means of decomposing the components of complex movements and the ability to investigate different strategies for linking together the individual components. It can also provide clinicians with novel targets for interventions to decrease the burden of impaired gait and balance in older adults. Further studies of older adults with motor disorders associated with peripheral nerve and muscle disorders or central motor disorders such as Parkinson’s disease and stroke are needed to further explicate the neurobiology of these strategies and the clinical basis for the difference in how movements are performed.
Figure 3.
A graphical representation which juxtaposes both transition strategies associated with lower and higher levels of clinical function.
Acknowledgments
The authors wish to thank the staff of the RADC and participants of the Memory and Aging Project and the Minority and Aging Research Study for clinical supervision and data collection, and the Tel Aviv Sourasky Medical Center Movement Disorders unit for helpful input.
FUNDING
This work was supported in part by NIH grants R01AG17917, R01AG022018, RO1NS078009 and R01AG47976.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No conflicts of interest.
Reference List
- 1.Weiss A, Herman T, Plotnik M, et al. Can an accelerometer enhance the utility of the Timed Up & Go Test when evaluating patients with Parkinson's disease? Med Eng Phys. 2010;32:119–125. doi: 10.1016/j.medengphy.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 2.Weiss A, Herman T, Plotnik M, et al. An instrumented timed up and go: the added value of an accelerometer for identifying fall risk in idiopathic fallers. Physiol Meas. 2011;32:2003–2018. doi: 10.1088/0967-3334/32/12/009. [DOI] [PubMed] [Google Scholar]
- 3.Podsiadlo D, Richardson S. The timed "Up & Go :"a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 4.Herman T, Weiss A, Brozgol M, et al. Identifying axial and cognitive correlates in patients with Parkinson's disease motor subtype using the instrumented Timed Up and Go. Exp Brain Res. 2013 doi: 10.1007/s00221-013-3778-8. [DOI] [PubMed] [Google Scholar]
- 5.Mancini M, Priest KC, Nutt JG, Horak FB. Quantifying freezing of gait in Parkinson's disease during the instrumented timed up and go test. Conf Proc IEEE Eng Med Biol Soc. 2012;2012:1198–1201. doi: 10.1109/EMBC.2012.6346151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palmerini L, Mellone S, Rocchi L, Chiari L. Dimensionality reduction for the quantitative evaluation of a smartphone-based Timed Up and Go test. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:7179–7182. doi: 10.1109/IEMBS.2011.6091814. [DOI] [PubMed] [Google Scholar]
- 7.Palmerini L, Mellone S, Avanzolini G, et al. Quantification of motor impairment in Parkinson's disease using an instrumented timed up and go test. IEEE Trans Neural Syst Rehabil Eng. 2013;21:664–673. doi: 10.1109/TNSRE.2012.2236577. [DOI] [PubMed] [Google Scholar]
- 8.Salarian A, Horak FB, Zampieri C, et al. iTUG, a sensitive and reliable measure of mobility. IEEE Trans Neural Syst Rehabil Eng. 2010;18:303–310. doi: 10.1109/TNSRE.2010.2047606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiss A, Mirelman A, Buchman AS, et al. Using a body-fixed sensor to identify subclinical gait difficulties in older adults with IADL disability: maximizing the output of the timed up and go. PLoS One. 2013;8:e68885. doi: 10.1371/journal.pone.0068885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zampieri C, Salarian A, Carlson-Kuhta P, et al. The instrumented timed up and go test: potential outcome measure for disease modifying therapies in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2010;81:171–176. doi: 10.1136/jnnp.2009.173740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aziz O, Park EJ, Mori G, Robinovitch SN. Distinguishing the causes of falls in humans using an array of wearable tri-axial accelerometers. Gait Posture. 2014;39:506–512. doi: 10.1016/j.gaitpost.2013.08.034. [DOI] [PubMed] [Google Scholar]
- 12.Barnes LL, Shah RC, Aggarwal NT, et al. The Minority Aging Research Study: ongoing efforts to obtain brain donation in African Americans without dementia. Curr Alzheimer Res. 2012;9:734–745. doi: 10.2174/156720512801322627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bennett DA, Schneider JA, Buchman AS, et al. Overview and findings from the rush Memory and Aging Project. Curr Alzheimer Res. 2012;9:646–663. doi: 10.2174/156720512801322663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buchman AS, Shulman JM, Nag S, et al. Nigral pathology and parkinsonian signs in elders without Parkinson disease. Ann Neurol. 2012;71:258–266. doi: 10.1002/ana.22588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bennett DA, Schneider JA, Buchman AS, et al. The Rush Memory and Aging Project: study design and baseline characteristics of the study cohort. Neuroepidemiology. 2005;25:163–175. doi: 10.1159/000087446. [DOI] [PubMed] [Google Scholar]
- 16.Bennett DA, Launer LJ. Longitudinal epidemiologic clinical-pathologic studies of aging and Alzheimer's disease. Curr Alzheimer Res. 2012;9:617–620. doi: 10.2174/156720512801322645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buchman AS, Wilson RS, Yu L, et al. Total daily activity declines more rapidly with increasing age in older adults. Arch Gerontol Geriatr. 2014;58:74–79. doi: 10.1016/j.archger.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buchman AS, Boyle PA, Yu L, et al. Total daily physical activity and the risk of AD and cognitive decline in older adults. Neurology. 2012;78:1323–1329. doi: 10.1212/WNL.0b013e3182535d35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benjamini Y. HY Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. 1995;57:125–133. [Google Scholar]
- 20.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gabriel DA, Kamen G, Frost G. Neural adaptations to resistive exercise: mechanisms and recommendations for training practices. Sports Med. 2006;36:133–149. doi: 10.2165/00007256-200636020-00004. [DOI] [PubMed] [Google Scholar]
- 22.Kamen G, Knight CA. Training-related adaptations in motor unit discharge rate in young and older adults. J Gerontol A Biol Sci Med Sci. 2004;59:1334–1338. doi: 10.1093/gerona/59.12.1334. [DOI] [PubMed] [Google Scholar]
- 23.Macaluso A, Nimmo MA, Foster JE, et al. Contractile muscle volume and agonist-antagonist coactivation account for differences in torque between young and older women. Muscle Nerve. 2858–863:25. doi: 10.1002/mus.10113. 002. [DOI] [PubMed] [Google Scholar]
- 24.Macaluso A, De VG. Muscle strength, power and adaptations to resistance training in older people. Eur J Appl Physiol. 2004;91:450–472. doi: 10.1007/s00421-003-0991-3. [DOI] [PubMed] [Google Scholar]
- 25.Ochi A, Yokoyama S, Abe T, et al. Differences in muscle activation patterns during step recovery in elderly women with and without a history of falls. Aging Clin Exp Res. 2014;26:213–220. doi: 10.1007/s40520-013-0152-4. [DOI] [PubMed] [Google Scholar]
- 26.Hase K, Stein RB. Turning strategies during human walking. J Neurophysiol. 1999;81:2914–2922. doi: 10.1152/jn.1999.81.6.2914. [DOI] [PubMed] [Google Scholar]
- 27.Taylor MJ, Dabnichki P, Strike SC. A three-dimensional biomechanical comparison between turning strategies during the stance phase of walking. Hum Mov Sci. 2005;24:558–573. doi: 10.1016/j.humov.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 28.Patla AE, Prentice SD, Robinson C, Neufeld J. Visual control of locomotion: strategies for changing direction and for going over obstacles. J Exp Psychol Hum Percept Perform. 1991;17:603–634. doi: 10.1037//0096-1523.17.3.603. [DOI] [PubMed] [Google Scholar]
- 29.Xu D, Chow JW, Wang YT. Effects of turn angle and pivot foot on lower extremity kinetics during walk and turn actions. J Appl Biomech. 2006;22:74–79. doi: 10.1123/jab.22.1.74. [DOI] [PubMed] [Google Scholar]
- 30.Akram SB, Frank JS, Chenouri S. Turning behavior in healthy older adults: Is there a preference for step versus spin turns? Gait Posture. 2010;31:23–26. doi: 10.1016/j.gaitpost.2009.08.238. [DOI] [PubMed] [Google Scholar]
- 31.Di NF, Mengarelli A, Maranesi E, et al. Assessment of the ankle muscle co-contraction during normal gait: a surface electromyography study. J Electromyogr Kinesiol. 2015;25:347–354. doi: 10.1016/j.jelekin.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 32.Arias P, Espinosa N, Robles-Garcia V, et al. Antagonist muscle co-activation during straight walking and its relation to kinematics: insight from young, elderly and Parkinson's disease. Brain Res. 2012;1455:124–131. doi: 10.1016/j.brainres.2012.03.033. [DOI] [PubMed] [Google Scholar]
- 33.Rosa MC, Marques A, Demain S, Metcalf CD. Lower limb co-contraction during walking in subjects with stroke: A systematic review. J Electromyogr Kinesiol. 2014;24:1–10. doi: 10.1016/j.jelekin.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 34.Khanmohammadi R, Talebian S, Hadian MR, et al. Characteristic muscle activity patterns during gait initiation in the healthy younger and older adults. Gait Posture. 2016;43:148–153. doi: 10.1016/j.gaitpost.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 35.Lebiedowska MK, Gaebler-Spira D, Burns RS, Fisk JR. Biomechanic characteristics of patients with spastic and dystonic hypertonia in cerebral palsy. Arch Phys Med Rehabil. 2004;85:875–880. doi: 10.1016/j.apmr.2003.06.032. [DOI] [PubMed] [Google Scholar]