Abstract
RNA-based regulatory mechanisms play important roles in the development and plasticity of neural circuits and neurological disease. Developing axons provide a model well suited to the study of RNA-based regulation, and contain specific subsets of mRNAs that are locally translated and have roles in axon pathfinding. However, the RNA-binding proteins involved in axon pathfinding, and their corresponding mRNA targets, are still largely unknown. Here we find that the RNA-binding protein IMP2 (Igf2bp2) is strikingly enriched in developing axon tracts, including in spinal commissural axons. We used the HITS-CLIP approach to perform a genome-wide identification of RNAs that interact directly with IMP2 in the native context of developing mouse brain. This IMP2 interactome was highly enriched for mRNA targets related to axon guidance. Accordingly, IMP2 knockdown in the developing spinal cord led to strong defects in commissural axon trajectories at the midline intermediate target. These results reveal a highly distinctive axonal enrichment of IMP2, show that it interacts with a network of axon guidance-related mRNAs, and reveal that it is required for normal axon pathfinding during vertebrate development.
KEY WORDS: IMP2, Igf2bp2, RNA interactome, RNA-binding protein, Axon guidance
Highlighted article: The RNA-binding protein IMP2 is enriched in developing axons, interacts with a network of axon guidance-related mRNAs, and is required for normal axon pathfinding in mouse embryos.
INTRODUCTION
During neural development, axons must find their targets by navigating through pathways that may be long and complex (Dickson, 2002). Developing axons are highly specialized structures that express specific and dynamically regulated subsets of proteins. Although axonal proteins can be synthesized in the cell body and transported to the axon, specific subsets of proteins are synthesized locally from mRNAs within the axon. This local protein synthesis allows axons to autonomously regulate their structure and function, and is involved in spatially restricted responses such as growth cone turning, and the changes in axon responsiveness to guidance cues that occur at intermediate targets (Holt and Schuman, 2013).
RNA-binding proteins play key roles in regulating gene expression at the RNA level during normal development (Holt and Schuman, 2013), and many have been linked to human neurological diseases (Castello et al., 2013). However, only a few specific RNA-binding proteins have been studied in the context of neuron and axon development, and more generally the biological functions of most RNA-binding proteins remain little characterized (Hornberg and Holt, 2013). Among the best-studied is IMP1 (also known as Igf2bp1 or ZBP1), which binds to the ‘zipcode’ sequence in the 3′UTR of β-actin mRNA, and regulates β-actin mRNA transport and local translation in fibroblasts, neurons and other cell types (Kiebler and Bassell, 2006; Rodriguez et al., 2008; Gomes et al., 2014). A related member of the IMP family is IMP2 (also known as Igf2bp2), but its function is not well characterized and has only recently begun to emerge. Several studies have now identified roles for IMP2 in adult energy metabolism, obesity and type 2 diabetes (Christiansen et al., 2009; Dai et al., 2015). Additionally, studies have implicated IMP2 in the regulation of myogenesis (Li et al., 2012) and differentiation of neocortical neural precursor cells (Fujii et al., 2013).
Here we find that IMP2 protein distribution in the developing nervous system shows a highly distinctive enrichment in axon tracts. This prompted us to perform a genome-wide identification of RNA targets of IMP2 in the developing mouse nervous system using the HITS-CLIP technique, which allows identification of directly bound RNA targets in native tissues (Licatalosi et al., 2008; Darnell, 2013), and the results revealed an mRNA interactome highly enriched for functions related to axon development. Accordingly, in the well-characterized spinal commissural axon guidance model (Reeber and Kaprielian, 2009; Dickson and Zou, 2010; Nawabi and Castellani, 2011; Preitner et al., 2013; Kaplan et al., 2014), in vivo knockdown of IMP2 led to strong effects on axonal trajectories. Together, these results reveal a characteristic localization and function of IMP2 in axon pathfinding, identify an RNA interactome, and suggest that IMP2 might regulate a network of guidance-related mRNAs within the axon.
RESULTS AND DISCUSSION
IMP2 is enriched in developing axon tracts
We first investigated the expression patterns of the three members of the IMP family in developing mouse spinal cord by immunolabeling with previously characterized antibodies (Hammer et al., 2005). This analysis focused on dorsal spinal commissural neurons (Fig. 1A-C). IMP1 immunolabeling appeared ubiquitous in spinal cord (Fig. 1D,E), consistent with many previous studies showing that IMP1 has widespread roles in neurons and other cell types (Kiebler and Bassell, 2006; Rodriguez et al., 2008; Gomes et al., 2014). IMP3 (Igf2bp3), the family member most closely related to IMP1 in sequence, showed broad labeling comparable to IMP1 (Fig. 1H,I). IMP2 immunolabeling, by contrast, showed a highly distinctive preferential localization over axon tracts, including the ventral commissure and ventral funiculus (Fig. 1F,G) as well as other embryonic axon tracts (Fig. 1F,G; data not shown). The most prominent commissural axon signal was observed in the tracts where contralateral axon segments are bundled, but labeling was also visible in the ipsilateral axon segments that follow less fasciculated trajectories toward the floor plate (Fig. 1F,G). Consistent with these results, a pan-IMP antibody gave essentially a composite of the three distributions, showing some generalized labeling together with strong labeling in commissural axons (data not shown). Given its striking distribution in axon tracts, we focused on IMP2 for further studies.
Fig. 1.
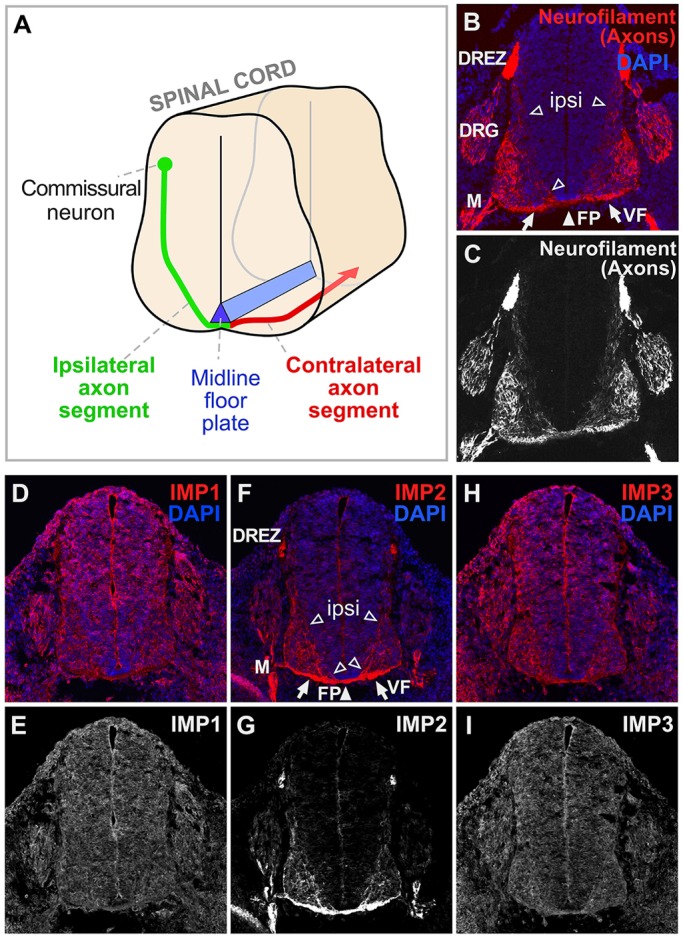
IMP2 is highly enriched in axon tracts. (A) Diagram of axons of dorsal commissural neurons navigating in developing spinal cord. Axon segments on the ipsilateral side (green) orient ventrally and medially from the cell bodies toward the midline floor plate (blue). After crossing the floor plate in the ventral commissure, most axons make a sharp anterior turn, then diverge away from the midline, before growing longitudinally in the ventral and lateral funiculi. (B-I) Transverse sections of E11.5 mouse spinal cord, with DAPI nuclear staining in blue and immunolabeling in red. (B,C) Neurofilament marker for axons. (D,E) Consistent with previous studies, IMP1 labeling was seen broadly in the nervous system as well as in other tissues. (F,G) IMP2 labeling was seen over the ventral funiculus, the ventral commissure, and ipsilateral axon segments oriented towards the floor plate. (H,I) IMP3 labeling, similar to that of IMP1, was seen broadly in the nervous system and other tissues. ipsi, ipsilateral axon segments (open arrowheads); FP, floor plate (white arrowhead); VF, ventral funiculus (arrows); DRG, dorsal root ganglion; DREZ, dorsal root entry zone; M, spinal motor axons.
Identification of the IMP2 RNA interactome in brain by HITS-CLIP
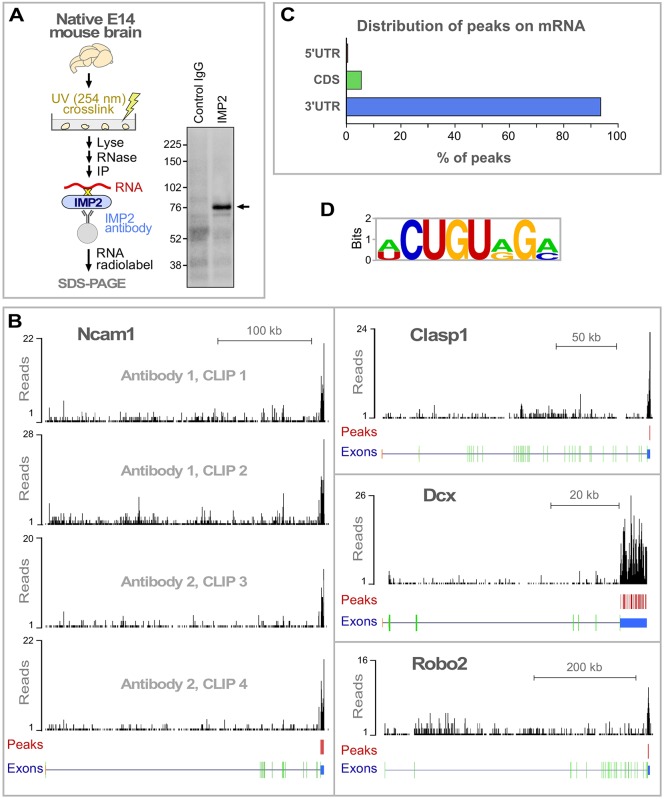
To identify genome-wide RNA targets for IMP2 in brain, we used HITS-CLIP, which combines UV crosslinking and immunoprecipitation (CLIP) with high-throughput sequencing (Fig. 2A) (Licatalosi et al., 2008). Covalent UV crosslinking allows highly stringent washing to remove non-specific interactions, and has a very short range, thereby identifying directly interacting target RNAs. To reduce potential false positives, two different IMP2 antibodies were used, and two independent immunoprecipitation experiments were performed for each, giving a total of four data sets (Fig. S1A), in line with previous HITS-CLIP studies (see Preitner et al., 2014). IMP2 binding peaks were identified by a protocol that gives a low false discovery rate (FDR<0.001; see Fig. S1 and the supplementary Materials and Methods for details), resulting in the identification of 1850 high-confidence binding peaks in mRNAs from 747 genes (Table S1).
Fig. 2.
HITS-CLIP identification of RNAs that interact with IMP2. (A) IMP2 HITS-CLIP. After UV-crosslinking protein-RNA complexes in native E14 mouse brain tissue, radiolabeled RNAs were co-immunoprecipitated with IMP2. CLIP results are shown on the right. Arrow marks the major band with increased intensity in the experimental lane, at the size expected for IMP2-RNA complexes, which was taken for high-throughput sequencing. Molecular mass markers in kDa. (B) Distribution of IMP2 HITS-CLIP sequence signals on representative target genes. Red vertical bars indicate high-confidence IMP2 binding peaks (see Fig. S1A and the supplementary Materials and Methods for details on peak identification). Structure of each gene is illustrated beneath: 5′UTR, orange; protein-coding, green; 3′UTR, blue. (C) Distribution of IMP2 binding peaks on target mRNAs. CDS, coding sequence. (D) A consensus sequence motif identified within the IMP2 binding regions using MEME software (see also Fig. S2).
Fig. 2B shows examples of peak distribution for representative target mRNAs encoding proteins with known functions in axon development: Ncam1, Clasp1, Dcx and Robo2. Over 90% of the identified binding peaks were located in mRNA 3′UTRs (Fig. 2C), as is typical of many other regulatory RNA-binding proteins (Holt and Schuman, 2013). A consensus sequence was identified for IMP2 interaction (Fig. 2D, Fig. S2). This relatively short 7-8 nt sequence was found in only a fraction of targets (Fig. 2D, Fig. S2). These features are comparable to motifs associated with many other RNA-binding proteins (Anko and Neugebauer, 2012), and suggest that the motif is unlikely to be an obligate IMP2 binding consensus and might instead be associated with RNA secondary structure or the binding of accessory factors. Previously, IMP2 was used among a panel of several RNA-binding proteins to validate that the PAR-CLIP technique can detect protein-RNA interactions, resulting in identification of a shorter and highly degenerate 3-4 nt motif (Hafner et al., 2010); that a different motif was observed could reflect the use of overexpressed recombinant IMP2 in HEK 293 cells (Bell et al., 2013). The HITS-CLIP approach used here examined RNAs associated with endogenously expressed IMP2 in the native context of developing brain. We next used bioinformatic tools to assess the functions of the identified targets.
Global functional analysis of brain IMP2 target mRNAs
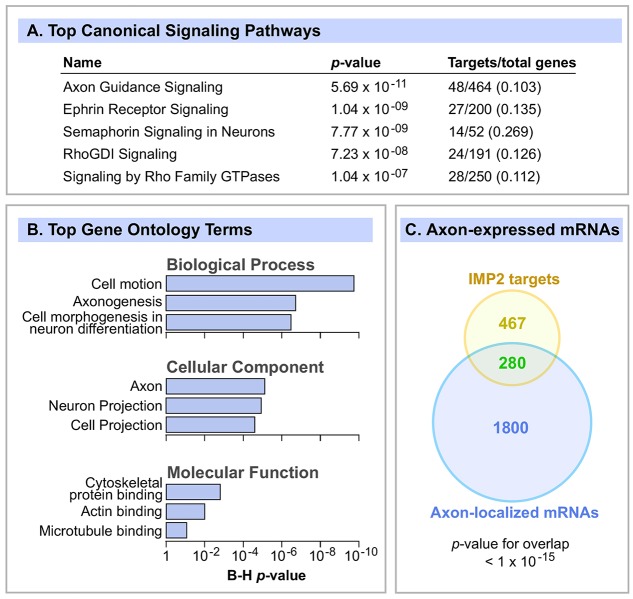
First, the Ingenuity Pathway Analysis (IPA) package was used to detect enriched signaling pathways. The top canonical signaling category was axon guidance signaling, with strong enrichment and a highly significant P-value (Fig. 3A). The next four categories were related to specific axon guidance cues, namely the ephrins and semaphorins, followed by Rho GTPase signaling, which also plays important roles in cell migration and axon guidance. We also performed a Gene Ontology (GO) analysis using the DAVID bioinformatics platform and, very consistent with the IPA analysis, the most enriched functions were related to cell migration, axon and neuron morphogenesis, and cytoskeleton organization (Fig. 3B). Although the use of brain as a source clearly selects for brain-related functions, the analysis used the E14 brain transcriptome as background, and considering that many neurodevelopmental processes are occurring in the brain at that stage, the enrichment of functions related to axon migration was robust and highly significant.
Fig. 3.
IMP2 target mRNAs are highly enriched for functions related to axon development. (A) IMP2 target mRNAs were analyzed by IPA to identify enriched canonical signaling pathways, of which the top five are shown. Right-hand column shows target genes as a fraction of the total number of genes in the IPA category. (B) The top GO terms enriched among IMP2 target mRNAs, identified by the DAVID bioinformatic tools. P-values were adjusted for multiple comparisons by the Benjamini-Hochberg (B-H) method. (C) Venn diagram comparing IMP2 target mRNAs with a catalog of axon-localized mRNAs in embryonic mouse DRG axons (Gumy et al., 2011). Statistics used Fisher's exact test.
Since our localization experiments revealed enrichment of IMP2 in axon tracts (Fig. 1), we compared IMP2 targets with known axonally localized mRNAs. Strong overlap was observed between a list of axonally localized mRNAs (Gumy et al., 2011) and IMP2 target mRNAs (37% of IMP2 targets; P<10−15; Fig. 3C). This result seems very consistent with a model in which IMP2 regulates mRNAs locally within the axon.
In addition to biological functions, we were also interested in disease associations. IPA Disease and Disorder Analysis showed that neurological disease was the most significantly enriched category, including enrichment of multiple neurological disease subcategories (Fig. S3). Moreover, 112 of the IMP2 target mRNAs correspond to genes responsible for human Mendelian diseases (Table S2). The significant association with Alzheimer's disease is especially intriguing, given that IMP2 is also associated with type 2 diabetes (Scott et al., 2007; Zeggini et al., 2007; Saxena et al., 2007; Lyssenko et al., 2008): type 2 diabetes is a risk factor for Alzheimer's disease (Haan, 2006; Li et al., 2007) and pathological processes such as insulin resistance underlie both conditions (Li et al., 2007; Kim and Feldman, 2012). This suggests the intriguing possibility that IMP2 might provide a molecular link between type 2 diabetes and Alzheimer's disease by regulating targets such as insulin receptor mRNA or other mRNAs in its interactome that are related to both diabetes and neurodegeneration.
IMP2 is required for normal commissural axon trajectories in vivo
Since our studies of the IMP2 expression pattern and mRNA interactome strongly pointed to a role in axon development, we next performed experiments on commissural axon guidance in vivo. Commissural axon trajectories are described in Fig. 1A. In terms of molecular guidance mechanisms, initial growth toward the midline is mediated by floor plate attractants. Since the floor plate is an intermediate target, after crossing the axons must undergo a drastic ‘midline switch' in responsiveness to cues, losing their responsiveness to midline attractants and becoming sensitive instead to midline repellents including Slits. In addition to ensuring that axons do not recross, this switch also renders the axons responsive to cues that guide their post-crossing trajectories, including anteroposterior guidance gradients, and divergence away from the midline due to Slit-mediated repulsion.
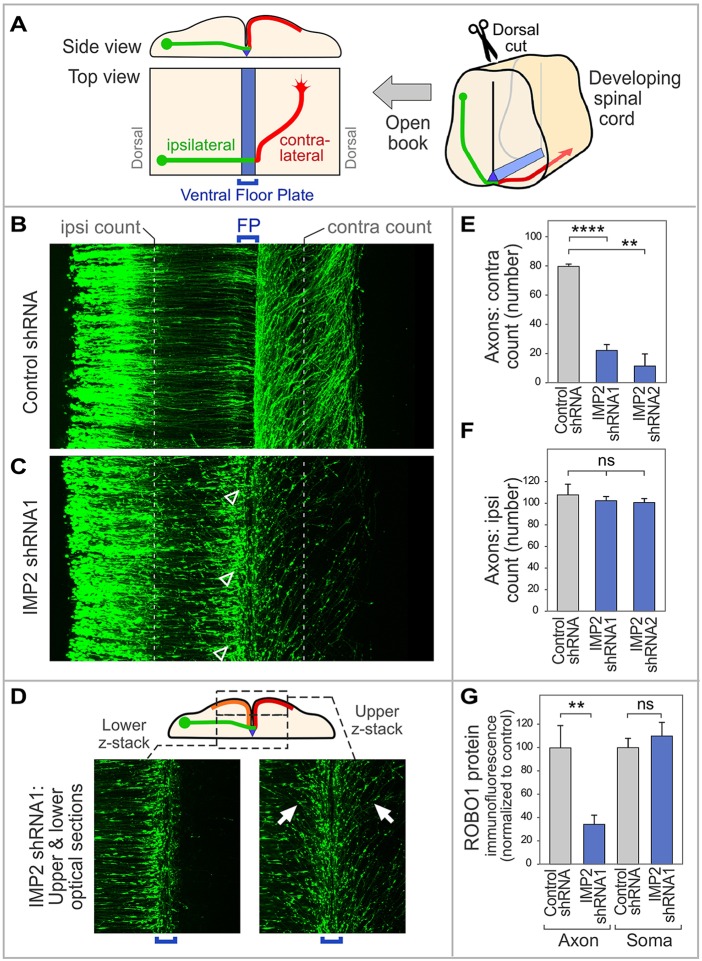
Spinal cords of chick embryos in ovo were electroporated 1-2 days before commissural axons reach the floor plate, unilaterally with plasmids encoding IMP2-specific shRNAs or control shRNA, along with GFP under control of the Math1 (Atoh1) promoter to trace commissural axons (Helms and Johnson, 1998; Lumpkin et al., 2003; Reeber et al., 2008). Commissural axon trajectories were then visualized 64 h later (E5.5, HH stage 24-25) in spinal cord open-book preparations. In embryos treated with control shRNA at this stage, most axons have crossed the midline (Fig. 4B,E). By contrast, in embryos subjected to IMP2 shRNAs, a much lower proportion of axons had crossed to the contralateral side, and instead many growth cones were observed at or near the ipsilateral side of the floor plate, where they had the typical morphology of stalled growth cones (Fig. 4C, arrowheads; see also Fig. 4E,F and left panel of 4D). Similar phenotypes were observed using two different shRNAs that knock down IMP2 (Fig. 4E,F, Fig. S4A). Interestingly, in the IMP2 RNAi embryos, for those axons that progressed beyond the initial encounter with the floor plate, approximately half turned contralaterally and half turned ipsilaterally (Fig. 4D, right panel, orange and red trajectories in the diagram), in contrast to the classical contralateral trajectory (red trajectory in Fig. 4A). These aberrant ipsilateral trajectories approximately mirrored the contralateral trajectories, turning anteriorly and away from the midline (Fig. 4D, right panel and diagram).
Fig. 4.
IMP2 knockdown causes defects in commissural axon pathfinding in vivo. (A) Diagram of spinal cord open-book preparation. The developing spinal cord is cut open along the dorsal midline, then mounted ventricular face downwards. A typical commissural axon ipsilateral segment (green), contralateral segment (red) and the floor plate (blue) are shown. (B-F) IMP2 or control shRNAs were introduced by in ovo electroporation into one side of the chick embryo spinal cord, with a Math1-GFP construct to trace commissural axons. Spinal cord was dissected 64 h later and imaged as an open-book. (B) Commissural axons in a confocal z-stack of open-book, viewed from the top. Electroporated cell bodies are visible on the left, and axons grow toward the right. (C) After IMP2 knockdown, in contrast to the control many growth cones are seen to have stalled at or near the ipsilateral side of the floor plate, showing an enlarged fusiform morphology typical of stalled growth cones (arrowheads). Correspondingly, a reduced number of axons was seen on the contralateral side, where they appeared to follow roughly normal trajectories without obvious signs of degeneration. (D) The lower confocal z-stack shows axon segments approaching the floor plate (green in diagram). The upper z-stack shows axon trajectories diverging away from the midline on both sides (contralateral, red in diagram; and ipsilateral, orange in diagram; examples of the approximately mirror image trajectories are arrowed). (E,F) Quantitation of ipsilateral and contralateral axon numbers, counted at the positions indicated by the white dashed lines in B and C. IMP2 knockdown did not significantly affect the number of axons emerging from the dorsal commissural neuron cell bodies and growing toward the floor plate, but strongly reduced the percentage of axons on the contralateral side. Each replicate was a separate embryo. (G) Effect of IMP2 RNAi on Robo1 expression in spinal commissural neurons. IMP2 or control shRNAs were introduced by in ovo electroporation into chick embryo spinal cord, with a Math1-GFP construct to identify transfected neurons; 64 h later, commissural neurons were dissociated and cultured for analysis of Robo1 immunofluorescence in axons and cell bodies. Immunolabeling was performed on permeabilized cells to detect both intracellular and cell surface Robo1. IMP2 shRNA reduced Robo1 expression in the axon, but not in the soma (see also Fig. S4B). Each replicate in this experiment was a different neuron (n=16 for control and n=28 for IMP2 shRNA), and the experiment was repeated three times to ensure reproducibility. Comparisons used Student's unpaired two-tailed t-test. Error bars show s.e.m. **P<0.01, ****P<0.0001; ns, not significant.
The observation that IMP2 RNAi leads to frequent stalling of commissural axons at the midline is intriguing because this phenotype is strikingly similar to the loss-of-function phenotype of one of the IMP2 targets, Robo1, in both mouse and chick embryos (Long et al., 2004; Philipp et al., 2012). Robo1 is a receptor for the midline repellent Slit, and this receptor is upregulated on commissural axons upon reaching the midline, and enables commissural axons to escape the midline. To test whether IMP2 might be involved in the expression of Robo1, we performed IMP2 RNAi in ovo, and then cultured commissural neurons for analysis of Robo1 protein levels in axons and cell bodies. The results showed that IMP2 knockdown reduced Robo1 immunolabeling in axons but not in the cell body (Fig. 4G), providing functional evidence that axonal Robo1 expression is downstream of IMP2.
Our observations that IMP2 labeling is strongly enriched in axon tracts, that it binds mRNAs highly enriched for functions in axon guidance, and that knockdown leads to strong defects in axon trajectories in vivo, provide consistent evidence for a role of IMP2 in axon development. The enrichment of a large number of axon guidance-related mRNA targets in the IMP2 interactome suggests that it might coordinately regulate a broad RNA program for axon development. This could entail binding a large number of mRNAs within individual neurons or, alternatively, a smaller subset of mRNAs in multiple diverse neuron types. The enrichment of IMP2 in axon tracts is particularly interesting; indeed, we do not know of any other mammalian RNA-binding protein that has been described to show such a distinctive enrichment in developing axon tracts, and our data suggest a model whereby IMP2 regulates mRNAs locally within the axon.
Regarding a mechanistic explanation for the observed effect of IMP2 knockdown on commissural axon development, one model is that IMP2 could be required for the expression of a general program of mRNAs needed for axon growth and guidance. However, after IMP2 knockdown, axons initially grew toward the floor plate without obvious abnormalities, and then showed highly specific phenotypes near the midline, including both stalling and mirror trajectories. These observations suggest a more specific model whereby IMP2 participates in the midline switch of axon behavior. In this latter scenario, IMP2 might first be involved in the transport of mRNAs into the axon, and then the regulation of IMP2 function by a presumptive midline signal could initiate a program of mRNA translation in post-crossing axon segments. Indeed, multiple proteins, including Robo receptors, are known to be upregulated in post-crossing axon segments, and translational control mechanisms within the axon can provide a mechanism for this regulation (Brittis et al., 2002; Colak et al., 2013; Preitner et al., 2013). Also potentially consistent with this model is the identification, among the IMP2 targets, of Robo1, a receptor for the midline repellent Slit, which produces a loss-of-function phenotype of stalling at the midline (Brose et al., 1999; Long et al., 2004; Jaworski et al., 2010; Philipp et al., 2012) comparable to the strong stalling phenotype seen here in the IMP2 loss-of-function analysis. These observations also fit with the prominent expression of both IMP2 and Robo1 in commissural axons, as well as our finding that axonal Robo1 expression is reduced by IMP2 loss-of-function. Together, these findings lead to a model in which IMP2 regulation of Robo1 contributes to the midline switch. IMP2 regulation of other targets might also contribute, since our CLIP list includes mRNAs for additional proteins involved in axon growth and guidance, including some known to have midline guidance phenotypes. Moreover, RNA-based mechanisms of the type studied here (Brittis et al., 2002; Colak et al., 2013; Preitner et al., 2013) are likely to act in concert with switch mechanisms at other molecular levels (Nawabi and Castellani, 2011; Neuhaus-Follini and Bashaw, 2015; Alther et al., 2016) to accomplish this complex biological process.
In connection with a possible role of IMP2 in the midline switch, it is intriguing that IMP1 phosphorylation upregulates translation of its target β-actin mRNA (Rodriguez et al., 2008), and it seems conceivable that IMP2 might undergo a similar form of regulation by signals at the midline. Further studies would be required to investigate specific molecular mechanisms that might lead to the regulated action of IMP2 in axons. Also, although other genes that cause strong commissural axon crossing phenotypes do not necessarily produce obvious behavioral defects in mice, it would be interesting to determine whether Imp2 gene knockout mice (Dai et al., 2015) have behavioral phenotypes, as this has not yet been characterized.
In conclusion, it has been appreciated for well over a decade that RNA-based regulation plays important and unique roles in axon development, but little is known about the RNA-binding proteins involved, or their RNA targets. By genome-wide identification of target mRNAs for IMP2 in a native physiological context, namely the developing brain, we find strong enrichment for targets involved in axon growth and guidance. Taken together with the striking localization of IMP2 in developing axon tracts, and the strong functional effects of IMP2 loss-of-function on axon trajectories in vivo, these results identify novel roles of IMP2 in axon development, and now open the door to further studies into the mechanistic basis of RNA-based processes in the axon.
MATERIALS AND METHODS
Immunolabeling
Immunolocalization of IMP1-3 in mouse E11.5 spinal cord sections was performed as described in the supplementary Materials and Methods. All animal experiments were performed in compliance with relevant ethical regulations, and were approved by the IACUC at Harvard Medical School.
HITS-CLIP
HITS-CLIP, RNA-seq and associated bioinformatic analysis (including peak identification, motif searching, GO, IPA) were performed as previously described (Preitner et al., 2014), except as noted in the supplementary Materials and Methods.
In ovo electroporation
In ovo electroporation in chicken embryos was performed essentially as described (Reeber et al., 2008). Fertilized eggs (Charles River Laboratories) were incubated 38°C for 64 h. A DNA mix containing shRNA and EGFP plasmid with Trypan Blue dye was introduced with a glass micropipette into the spinal cord lumen of HH stage 17 embryos and unilaterally electroporated. Windowed eggs were further incubated at 37°C for 64 h, then mounted as open-books and imaged by confocal microscopy. Details of the shRNA constructs and electroporation procedures are provided in the supplementary Materials and Methods.
Acknowledgements
We thank Jane Johnson for sharing the Math1 plasmid; Jiangwen Zhang, Christian Daly and the Bauer Center for Genomic Research for support with deep sequencing; and Jennifer Waters and the Nikon Imaging Center at Harvard Medical School for imaging equipment and expertise.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
N.P. designed the study and participated in all experiments. J.Q. participated in the RNAi experiments and performed the bioinformatic analysis. X.L. participated in the RNAi experiments, made the Math1-GFP construct and participated in methods development for the in vivo experiments. F.C.N. provided IMP antibodies and consulted on the project. J.G.F. supervised the study, and designed and analyzed it with the other authors. J.Q., N.P. and J.G.F. wrote the manuscript.
Funding
This work was supported by grants from the National Institutes of Health [R01 NS069913, R37 HD029417, R01 EY011559] and a fellowship from the Charles A. King Trust (to N.P.). Deposited in PMC for release after 12 months.
Data availability
HITS-CLIP data have been deposited at NCBI Gene Expression Omnibus and are accessible through GEO Series accession number GSE83822 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE83822).
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.128348.supplemental
References
- Alther T. A., Domanitskaya E. and Stoeckli E. T. (2016). Calsyntenin 1-mediated trafficking of axon guidance receptors regulates the switch in axonal responsiveness at a choice point. Development 143, 994-1004. 10.1242/dev.127449 [DOI] [PubMed] [Google Scholar]
- Änkö M.-L. and Neugebauer K. M. (2012). RNA-protein interactions in vivo: global gets specific. Trends Biochem. Sci. 37, 255-262. 10.1016/j.tibs.2012.02.005 [DOI] [PubMed] [Google Scholar]
- Bell J. L., Wächter K., Mühleck B., Pazaitis N., Köhn M., Lederer M. and Hüttelmaier S. (2013). Insulin-like growth factor 2 mRNA-binding proteins (IGF2BPs): post-transcriptional drivers of cancer progression? Cell. Mol. Life Sci. 70, 2657-2675. 10.1007/s00018-012-1186-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittis P. A., Lu Q. and Flanagan J. G. (2002). Axonal protein synthesis provides a mechanism for localized regulation at an intermediate target. Cell 110, 223-235. 10.1016/S0092-8674(02)00813-9 [DOI] [PubMed] [Google Scholar]
- Brose K., Bland K. S., Wang K. H., Arnott D., Henzel W., Goodman C. S., Tessier-Lavigne M. and Kidd T. (1999). Slit proteins bind robo receptors and have an evolutionarily conserved role in repulsive axon guidance. Cell 96, 795-806. 10.1016/S0092-8674(00)80590-5 [DOI] [PubMed] [Google Scholar]
- Castello A., Fischer B., Hentze M. and Preiss T. (2013). RNA-binding proteins in Mendelian disease. Trends Genet. 29, 318-327. 10.1016/j.tig.2013.01.004 [DOI] [PubMed] [Google Scholar]
- Christiansen J., Kolte A. M., Hansen T. V. O. and Nielsen F. C. (2009). IGF2 mRNA-binding protein 2: biological function and putative role in type 2 diabetes. J. Mol. Endocrinol. 43, 187-195. 10.1677/JME-09-0016 [DOI] [PubMed] [Google Scholar]
- Colak D., Ji S. J., Porse B. T. and Jaffrey S. R. (2013). Regulation of axon guidance by nonsense-mediated mRNA decay. Cell 153, 1252-1265. 10.1016/j.cell.2013.04.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai N., Zhao L., Wrighting D., Krämer D., Majithia A., Wang Y., Cracan V., Borges-Rivera D., Mootha V. K., Nahrendorf M. et al. (2015). IGF2BP2/IMP2-Deficient mice resist obesity through enhanced translation of Ucp1 mRNA and Other mRNAs encoding mitochondrial proteins. Cell Metab. 21, 609-621. 10.1016/j.cmet.2015.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell R. B. (2013). RNA protein interaction in neurons. Annu. Rev. Neurosci. 36, 243-270. 10.1146/annurev-neuro-062912-114322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson B. J. (2002). Molecular mechanisms of axon guidance. Science 298, 1959-1964. 10.1126/science.1072165 [DOI] [PubMed] [Google Scholar]
- Dickson B. J. and Zou Y. (2010). Navigating intermediate targets: the nervous system midline. Cold Spring Harb. Perspect. Biol. 2, a002055 10.1101/cshperspect.a002055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii Y., Kishi Y. and Gotoh Y. (2013). IMP2 regulates differentiation potentials of mouse neocortical neural precursor cells. Genes Cells 18, 79-89. 10.1111/gtc.12024 [DOI] [PubMed] [Google Scholar]
- Gomes C., Merianda T. T., Lee S. J., Yoo S. and Twiss J. L. (2014). Molecular determinants of the axonal mRNA transcriptome. Dev. Neurobiol. 74, 218-232. 10.1002/dneu.22123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumy L. F., Yeo G. S., Tung Y.-C. L., Zivraj K. H., Willis D., Coppola G., Lam B. Y. H., Twiss J. L., Holt C. E. and Fawcett J. W. (2011). Transcriptome analysis of embryonic and adult sensory axons reveals changes in mRNA repertoire localization. RNA 17, 85-98. 10.1261/rna.2386111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haan M. N. (2006). Therapy Insight: type 2 diabetes mellitus and the risk of late-onset Alzheimer's disease. Nat. Clin. Pract. Neurol. 2, 159-166. 10.1038/ncpneuro0124 [DOI] [PubMed] [Google Scholar]
- Hafner M., Landthaler M., Burger L., Khorshid M., Hausser J., Berninger P., Rothballer A., Ascano M., Jungkamp A.-C., Munschauer M. et al. (2010). Transcriptome-wide identification of RNA-binding protein and microRNA target sites by PAR-CLIP. Cell 141, 129-141. 10.1016/j.cell.2010.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer N. A., Hansen T. V. O., Byskov A. G., Rajpert-De Meyts E., Grondahl M. L., Bredkjaer H. E., Wewer U. M., Christiansen J. and Nielsen F. C. (2005). Expression of IGF-II mRNA-binding proteins (IMPs) in gonads and testicular cancer. Reproduction 130, 203-212. 10.1530/rep.1.00664 [DOI] [PubMed] [Google Scholar]
- Helms A. and Johnson J. (1998). Progenitors of dorsal commissural interneurons are defined by MATH1 expression. Development 125, 919-928. [DOI] [PubMed] [Google Scholar]
- Holt C. E. and Schuman E. M. (2013). The central dogma decentralized: new perspectives on RNA function and local translation in neurons. Neuron 80, 648-657. 10.1016/j.neuron.2013.10.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hörnberg H. and Holt C. (2013). RNA-binding proteins and translational regulation in axons and growth cones. Front. Neurosci. 7, 81 10.3389/fnins.2013.00081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski A., Long H. and Tessier-Lavigne M. (2010). Collaborative and specialized functions of Robo1 and Robo2 in spinal commissural axon guidance. J. Neurosci. 30, 9445-9453. 10.1523/JNEUROSCI.6290-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan A., Kent C. B., Charron F. and Fournier A. E. (2014). Switching responses: spatial and temporal regulators of axon guidance. Mol. Neurobiol. 49, 1077-1086. 10.1007/s12035-013-8582-8 [DOI] [PubMed] [Google Scholar]
- Kiebler M. A. and Bassell G. J. (2006). Neuronal RNA granules: movers and makers. Neuron 51, 685-690. 10.1016/j.neuron.2006.08.021 [DOI] [PubMed] [Google Scholar]
- Kim B. and Feldman E. L. (2012). Insulin resistance in the nervous system. Trends Endocrinol. Metab. 23, 133-141. 10.1016/j.tem.2011.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L. and Holscher C. (2007). Common pathological processes in Alzheimer disease and type 2 diabetes: a review. Brain Res. Rev. 56, 384-402. 10.1016/j.brainresrev.2007.09.001 [DOI] [PubMed] [Google Scholar]
- Li Z., Gilbert J. A., Zhang Y., Zhang M., Qiu Q., Ramanujan K., Shavlakadze T., Eash J. K., Scaramozza A., Goddeeris M. M. et al. (2012). An HMGA2-IGF2BP2 axis regulates myoblast proliferation and myogenesis. Dev. Cell 23, 1176-1188. 10.1016/j.devcel.2012.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licatalosi D. D., Mele A., Fak J. J., Ule J., Kayikci M., Chi S. W., Clark T. A., Schweitzer A. C., Blume J. E., Wang X. et al. (2008). HITS-CLIP yields genome-wide insights into brain alternative RNA processing. Nature 456, 464-469. 10.1038/nature07488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long H., Sabatier C., Ma L., Plump A., Yuan W., Ornitz D. M., Tamada A., Murakami F., Goodman C. S. and Tessier-Lavigne M. (2004). Conserved roles for Slit and Robo proteins in midline commissural axon guidance. Neuron 42, 213-223. 10.1016/S0896-6273(04)00179-5 [DOI] [PubMed] [Google Scholar]
- Lumpkin E. A., Collisson T., Parab P., Omer-Abdalla A., Haeberle H., Chen P., Doetzlhofer A., White P., Groves A., Segil N. et al. (2003). Math1-driven GFP expression in the developing nervous system of transgenic mice. Gene Expr. Patterns 3, 389-395. 10.1016/S1567-133X(03)00089-9 [DOI] [PubMed] [Google Scholar]
- Lyssenko V., Jonsson A., Almgren P., Pulizzi N., Isomaa B., Tuomi T., Berglund G., Altshuler D., Nilsson P. and Groop L. (2008). Clinical risk factors, DNA variants, and the development of type 2 diabetes. N. Engl. J. Med. 359, 2220-2232. 10.1056/NEJMoa0801869 [DOI] [PubMed] [Google Scholar]
- Nawabi H. and Castellani V. (2011). Axonal commissures in the central nervous system: how to cross the midline? Cell. Mol. Life Sci. 68, 2539-2553. 10.1007/s00018-011-0691-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus-Follini A. and Bashaw G. J. (2015). Crossing the embryonic midline: molecular mechanisms regulating axon responsiveness at an intermediate target. Wiley Interdiscip. Rev. Dev. Biol. 4, 377-389. 10.1002/wdev.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipp M., Niederkofler V., Debrunner M., Alther T., Kunz B. and Stoeckli E. T. (2012). RabGDI controls axonal midline crossing by regulating Robo1 surface expression. Neural Dev. 7, 36 10.1186/1749-8104-7-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preitner N., Quan J. and Flanagan J. G. (2013). This message will self-destruct: NMD regulates axon guidance. Cell 153, 1185-1187. 10.1016/j.cell.2013.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preitner N., Quan J., Nowakowski D. W., Hancock M. L., Shi J., Tcherkezian J., Young-Pearse T. L. and Flanagan J. G. (2014). APC is an RNA-binding protein, and its interactome provides a link to neural development and microtubule assembly. Cell 158, 368-382. 10.1016/j.cell.2014.05.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeber S. L. and Kaprielian Z. (2009). Leaving the midline: how Robo receptors regulate the guidance of post-crossing spinal commissural axons. Cell Adh. Migr. 3, 300-304. 10.4161/cam.3.3.9156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeber S. L., Sakai N., Nakada Y., Dumas J., Dobrenis K., Johnson J. E. and Kaprielian Z. (2008). Manipulating Robo expression in vivo perturbs commissural axon pathfinding in the chick spinal cord. J. Neurosci. 28, 8698-8708. 10.1523/JNEUROSCI.1479-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez A. J., Czaplinski K., Condeelis J. S. and Singer R. H. (2008). Mechanisms and cellular roles of local protein synthesis in mammalian cells. Curr. Opin. Cell Biol. 20, 144-149. 10.1016/j.ceb.2008.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena R., Voight B., Lyssenko V., Burtt N. l., de Bakker P., Chen H., Roix J., Kathiresan S., Hirschhorn J., Daly M. et al. (2007). Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science 316, 1331-1336. 10.1126/science.114235 [DOI] [PubMed] [Google Scholar]
- Scott L., Mohlke K., Bonnycastle L., Willer C., Li Y., Duren W., Erdos M., Stringham H., Chines P., Jackson A. et al. (2007). A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science 316, 1341-1345. 10.1126/science.1142382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeggini E., Weedon M., Lindgren C., Frayling T., Elliott K., Lango H., Timpson N., Perry J., Rayner N., Freathy R. et al. (2007). Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science 316, 1336-1341. 10.1126/science.1142364 [DOI] [PMC free article] [PubMed] [Google Scholar]