Abstract
The hemicellulose xylan, which has immunomodulatory effects, has been combined with chitosan to form a composite hydrogel to improve the healing of bone fractures. This thermally responsive and injectable hydrogel, which is liquid at room temperature and gels at physiological temperature, improves the response of animal host tissue compared with similar pure chitosan hydrogels in tissue engineering models. The composite hydrogel was placed in a subcutaneous model where the composite hydrogel is replaced by host tissue within 1 week, much earlier than chitosan hydrogels. A tibia fracture model in mice showed that the composite encourages major remodeling of the fracture callus in less than 4 weeks. A non-union fracture model in rat femurs was used to demonstrate that the composite hydrogel allows bone regeneration and healing of defects that with no treatment are unhealed after 6 weeks. These results suggest that the xylan/chitosan composite hydrogel is a suitable bone graft substitute able to aid in the repair of large bone defects.
Keywords: bone, xylan, hydrogel, hemicellulose, regenerative medicine
Introduction
Natural origin polymers, like polysaccharides, are derived from plants, animals, and bacteria to synthesize improved biomaterials and are widely recognized as biocompatible polymers relevant to tissue engineering.[1,2] Because these polymers are similar in structure to biological macromolecules in humans, the in vivo microenvironment is prepared to recognize and metabolize the degraded components.[1] The bioactivity of polysaccharides in promoting cell growth and differentiation has increased the focus on these under-utilized materials.[3,4] The wide variety of polysaccharide sources, their structural heterogeneity, and their diverse roles in nature reveal a wealth of untapped potential for application to a variety of unmet clinical needs.[5]
Many researchers have opted for synthetic materials, designed to be reproducible and consistent in their chemistry and synthesis.[6,7] However, there are limits to their biocompatibility, and any true bioactivity must be mimicked or incorporated from naturally occurring materials.[8,9] An ideal tissue engineering scaffold would be one that is instead consistent in patient response, compatible with its biological application, and bioactive to encourage healing. While some issues of consistency in demineralized bone products have arisen,[10] naturally occurring polymers have so far had more success in clinical application at meeting all three of these goals than their synthetic counterparts.[11–13] The source for many of these successes has been animal tissue that must be put through careful decellularization and purification steps.[14] Biomaterials from plants, such as cellulose, alginate, and agarose, are more easily processed, but so far have not found as much clinical use in bone tissue engineering as the purified and demineralized bone tissues.[1,15]
Xylan is an important hemicellulose component of plant secondary cell walls. Plants that do not produce xylan have weak stems and cannot bear weight.[16,17] It forms a composite hydrogel with chitosan, creating a scaffold of natural occurring polymers that individually have long histories of biocompatibility.[18,19] Most chitosan gel applications for tissue engineering involve combining chitosan with another bioactive molecule or polymer to achieve successful results.[20] Hemicellulose polymers are greatly underutilized and are normally disposed of as process wastes in biorefinery operations.[21] While studied academically for film properties, their potential for use is largely untapped in other fields of research.[22] Xylan has rarely been used in the context of tissue engineering, although some researchers have begun to use the material in hydrogels for controlled drug delivery.[23–27] The successful application of xylan to fracture healing could reveal a new class of hemicellulose polymers that have not yet been applied in this field.
The field of osteoimmunology has shown that the adaptive immune system has a role in the healing of bone fractures.[28–32] Both xylan and chitosan have been shown to affect the immune system in ways that are now known to be relevant to bone healing. The xylan polymer used in this study has a backbone of xylose sugars with a low substitution ratio of glucuronic acid side chains.[17] Xylans have been shown to modulate the response of T-cells and macrophages, which are relevant to the initial inflammation after bone fracture injuries.[33,34] High numbers of terminally differentiated CD8+ effector memory T-cells in peripheral blood have been shown to be predictive of fracture non-union in human patients.[35] Studies have also revealed that the presence of many pro-inflammatory cytokines produced, or regulated, by T-cells have a negative effect on bone healing, while anti-inflammatory cytokines are necessary to healing.[28–30,35]
These discoveries lead to the hypothesis that a xylan/chitosan composite hydrogel will promote bone repair. Previous attempts at incorporating plant-based polymers into tissue engineering scaffolds have based the application and design on the material properties displayed after the polymers have been extracted from the biomass.[36,37] The approach described here incorporates plant-based polymers based on their observed immunomodulatory effects.
The goal of this study is to examine the effects of the hemicellulose xylan on the healing of complex fractures that have a high risk of becoming non-unions without the use of proteins or other growth factors. In the past, a 95% success rate in healing fractures was seen as sufficient. Today, the need to improve on this record is being made evident by the rising costs of treating non-unions.[38] There is also a secondary economic burden that is due to delayed healing: increased time taken off from work for recovery from fractures, increased worker compensation paid, and significant differences between surgeon and patient satisfaction.[39–41] These burdens could all be relieved by preventing or healing non-unions more quickly. While some bone morphogenetic proteins are showing clinical improvement in healing non-unions, they are not reducing overall cost of treatment.[38] Inexpensive hemicellulose polymers that display properties beneficial to bone healing would directly address growing economic and patient satisfaction concerns that result from current treatments. The hypothesis tested in the reported research is that the xylan/chitosan composite hydrogel will aid in healing of complex fractures without the use of growth factors. The influence of xylan on tissue response is demonstrated with in vivo implant studies, and improvement in fracture healing is demonstrated with a non-union in vivo fracture model.
Experimental
Materials
Pharmaceutical grade chitosan (Chitoceutical Chitosan 85/1500) was purchased from Heppe Medical Chitosan (Germany). Xylan from Birchwood, ammonium hydrogen phosphate, and glacial acetic acid were purchased from Sigma-Aldrich (USA). All cell culture supplies were purchased from the University of Virginia core services. All animals were purchased from Charles River and used only in accordance with procedures approved by the Institutional Animal Care and Use Committee at the University of Virginia.
Hydrogel preparation
To prepare the xylan/chitosan composite hydrogel, xylan was dissolved in 5 ml of filtered, deionized water. Acetic acid was then added to create a 0.25% v/v solution. Chitosan was then added to the solution and allowed to dissolve overnight. The ratio of chitosan to xylan was 3:1, and the total polymer mass added to 5-ml water was 0.08 g. This is the highest ratio of xylan that allows a stable hydrogel to form. To initiate gelation, 23 μl of a 4.5 M ammonium hydrogen phosphate solution was added per milliliter of liquid polymer solution. To prepare the pure chitosan hydrogel, similar procedures were followed without the use of xylan in the initial step. Synthesis of this pure chitosan hydrogel system has been previously described.[42] This composite hydrogel retains the previously published thermogelling properties of the pure chitosan hydrogel.
Mouse subcutaneous implant model
Five-week-old, male, CD-1 mice from Charles River were used for testing a subcutaneous implant model to analyze hydrogel degradation and host tissue response. Subcutaneously on the backs of the mice, 0.5 ml of each hydrogel was injected through a 25-gauge needle. Five mice were used with four implants in each mouse: left and right near the front shoulders and left and right rear near the pelvic bone. The implants were left in place for 1 week and then harvested along with some skin where the implants had become attached. The tissue was fixed in glutaraldehyde to crosslink any remaining hydrogel so that it would remain for staining with hematoxylin and eosin along with the host tissue.
Mouse tibia fracture model
A blunt fracture model was used to create tibia fractures in that would form a mineralized callus as part of the healing process.[43] An incision was made in the knee, the patella was dislocated, and a 25-gauge needle was inserted into the medullary canal through the proximal end of the right tibia. The needle was cut to an appropriate length, and the incision was closed. A 300-g weight was dropped from 36 cm onto the center of the tibia using a blunt guillotine. Two groups of 6-week-old, male BALB/c mice from Charles River were included in the study, with five mice in each group: chitosan hydrogel treatment and xylan/chitosan composite hydrogel treatment. The hydrogels were injected into the site of injury approximately 3 hr after creation of the injury. This delay allowed small tears in the skin to close so that the liquid phase of the gels would not drain out. Each injury was injected with 0.5 ml of the gel. Fractures were evaluated at 3, 4, and 6 weeks with X-ray images. A Hewlett-Packard 34805N Faxitron series X-ray system was used at 30 kpV, exposing the Biomax XAR film for 1 min at 21.5 inches from the emitter.
The X-ray images were evaluated using ImageJ (National Institutes of Health, USA) image processing software. A common area of analysis with identical dimensions was chosen for all images, centered on the fracture site. All images were given a common threshold to distinguish the bone, and an algorithm then measured the number of pixels corresponding to bone within the standardized area of analysis, and this number was used to calculate the percentage area of bone in the image. This allowed comparison of callus area in the injured tibias between treatment groups and a comparison with the area of uninjured bone in images of the contralateral tibia. The injury was considered to be further healed as the callus area approached the area of uninjured bone.
Rat femur fracture model
A blunt fracture model was modified to create a femoral fracture in rats that would not heal without intervention. An incision was made in the knee, and a 20-gauge needle was inserted into the medullary canal through the distal end of the left femur and the incision closed. A 432-g weight was then dropped from 30 cm onto the center of the femur using a blunt guillotine. Two groups of 6-week-old, male F344 rats from Charles River, with five rats each group, were included in the study: a non-treated control and treatment with the xylan/chitosan composite hydrogel. In the treatment group, 0.5 ml of the composite hydrogel was injected into the fracture area immediately after creation of the injury. The femurs were X-rayed once per week for 6 weeks to monitor the progress of healing. The previously mentioned X-ray system and settings were used for this study with a 2-min exposure.
Statistical methods
One-way analysis of variance, which collapses to simple t-tests when only two groups are compared, was used to determine statistical significance of differences between independent population means. Paired sample t-tests were used to determine statistical significance of differences between time points within the same experimental group.
Results
Hydrogel preparation
Chitosan was made water soluble by dissolution in a weak acid, such as acetic acid, that gives a proton to the insoluble NH2 group. In this case, the chitosan hydrogel was formed by adding an amino salt, ammonium hydrogen phosphate, which scavenges the added proton, causing an irreversible phase change and formation of the gel. This phase change has previously been shown to be temperature sensitive.[42] The addition of xylan changed the amount of salt that is required for the phase change, but did not otherwise affect the process. The xylan freely associated with the chitosan in solution and was brought out of the solution along with the chitosan as the phase change took place. The association between chitosan and xylan is assumed to be a complex between the amino groups of chitosan and the glucuronic acid side chains of xylan.[44] Figure 1 describes the basic structure of the chitosan and xylan polymers.
Figure 1.
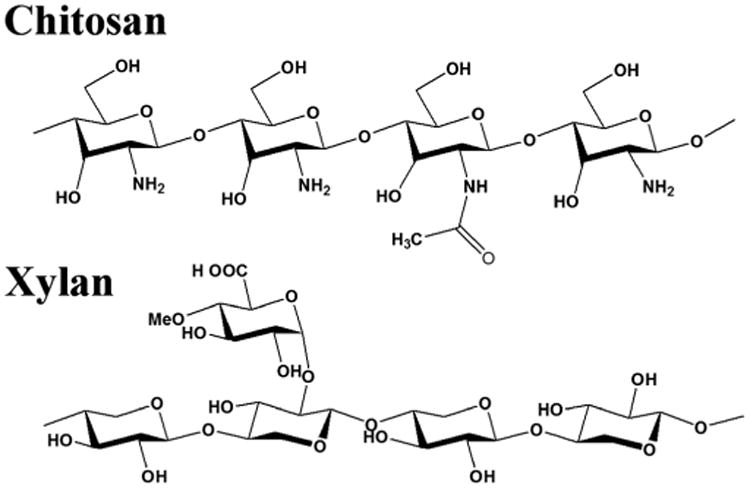
The structures of chitosan, β-(1→4)-2-amino-2-deoxy-D-glycopyranose, with incomplete deacetylation, such as the 85% deacetylated chitosan used in these experiments, and Xylan, β-(1→4)-D-xylopyranose, with a D-glucuronic acid side chain as found in xylans from hardwoods.
In vivo implant model
The composite hydrogel was injected subcutaneously in mice to analyze host response to the gel and in situ gel degradation. After 1 week, the pure chitosan hydrogel was still visible in large portions that had allowed little cell penetration (Fig. 2). At the same time point, the composite hydrogel implants were no longer present and were completely replaced with vascularized tissue in distinct nodules in place of the injected polymer. No significant lymphocytic infiltration or foreign-body granulation was seen.
Figure 2.
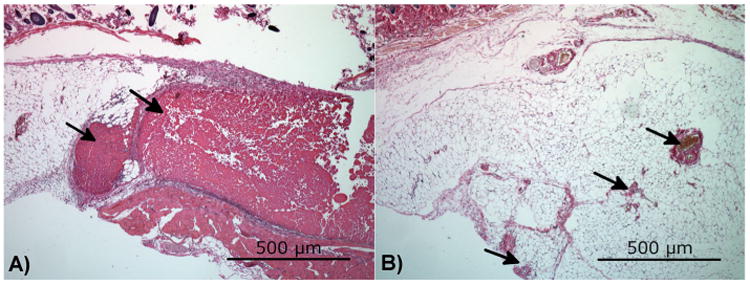
A pure chitosan hydrogel (A) and a xylan/chitosan composite hydrogel (B) were injected subcutaneously in the backs of mice. After 1 week, large portions of the pure chitosan implant remained, while the xylan composite had been completely replaced with vascularized tissue. Tissue was stained with hematoxylin and eosin. The arrows in panel (A) point to the remaining chitosan hydrogel. The arrows in panel (B) point to blood vessels. This figure is available in color online at wileyonlinelibrary.com/journal/pat.
Mouse tibia fracture model
Fractures of the tibia were created in mice, and the size of the fracture callus was compared by measuring the area of the bone in X-rays of the fracture site. Each data point is given in the figures along with the averages so that the inherent variation in healing and normal bone size from one animal to another is not hidden. At 3 weeks, there was no significant difference between treatment with the xylan/chitosan composite and a pure chitosan hydrogel (Fig. 3). At 4 weeks, the group treated with the xylan composite showed a significantly smaller callus size, approaching the size of uninjured bone (Figs 4 and S1). The difference between each treatment group and the uninjured, contralateral control at 3 weeks were statistically significant, revealing no advantage from either treatment at this time point (p ≤ 0.015). The difference in xylan composite treatment group from Week 3 to Week 4 revealed a significant change in callus size that occurred between these time points (p= 0.017). However, there was no statistical difference in the pure chitosan group from Week 3 to Week 4 (p=0.15). The difference between xylan treatment and the uninjured, contralateral bone at 4 weeks was only marginally significant (p=0.051), and a significant difference was seen between xylan and chitosan treatments at 4 weeks (p=0.018), which revealed an improvement in healing with the xylan composite compared with the pure chitosan treatment group. At 6 weeks (Fig. S2), the lack of a statistical difference between the xylan/chitosan composite group and the uninjured bone was even more pronounced (p = 0.084).
Figure 3.
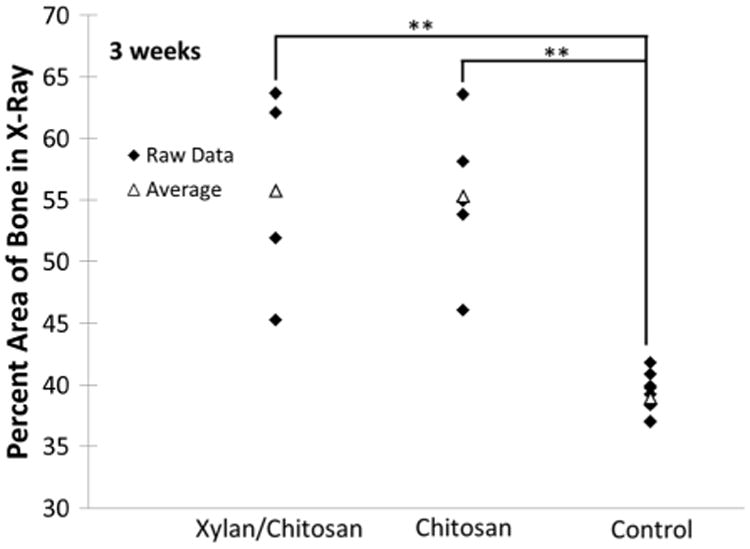
Callus area in X-ray images of tibia fractures was measured and compared with the area of normal bone at the same site on the opposite leg of the mice. Three weeks after injury, no difference can be seen between treatment with the xylan/chitosan composite and a pure chitosan hydrogel. The control is the uninjured, contralateral bone. Each treatment group had five animals. The symbol “**” denotes a p-value ≤0.01.
Figure 4.
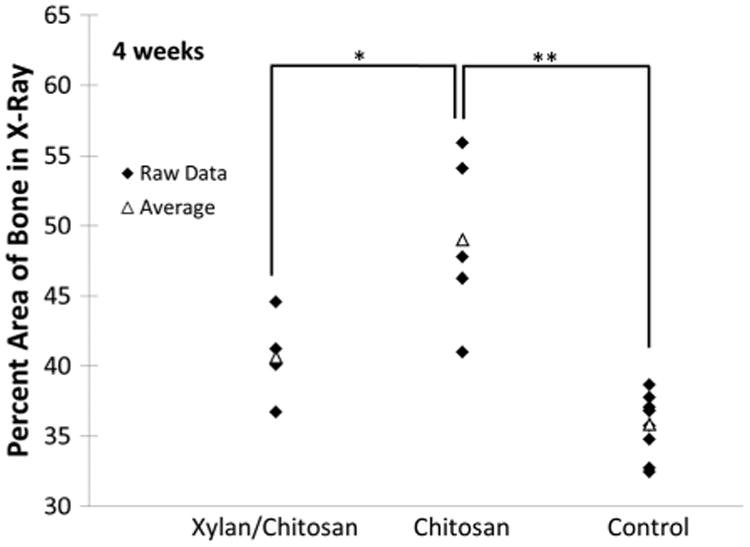
Callus area in X-ray images of tibia fractures was measured and compared with the area of normal bone at the same site on the opposite leg of the mice. Four weeks after injury, the decreased bone area of the xylan/chitosan composite group reveals the desired remodeling of hard callus, indicating faster healing compared with the group treated with pure chitosan hydrogel. The control is the uninjured, contralateral bone. Each treatment group had five animals. The symbol “*” denotes a p-value ≤0.05, and the symbol “**” denotes a p-value ≤0.01.
Rat femur non-union fracture model
A rat femur fracture model was chosen to demonstrate how the xylan/chitosan hydrogel affects healing in a bone injury that does not heal without treatment. Two of the untreated control animals died within 2 weeks of the injury. All of the animals treated with the xylan/chitosan composite remained otherwise healthy, and none were removed from the experiment before the 6-week end point. The injury model resulted in displaced, comminuted fractures in the femurs of all animals. The untreated fractures in the control group did not heal and remained as a non-union even after 6 weeks (Fig. 5). However, when the injury was treated with the xylan/chitosan composite hydrogel, the fracture showed complete bridging of the bone through the site of injury.
Figure 5.
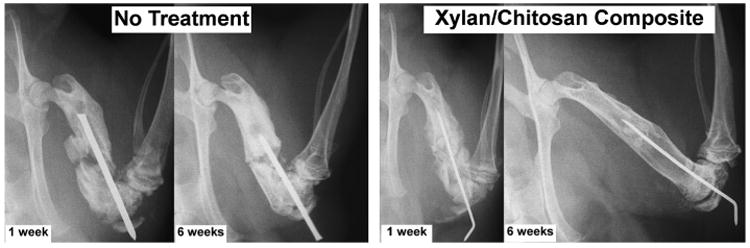
X-ray images at 1 and 6 weeks after injury and treatment. The displaced comminuted fractures did not heal without treatment. However, when the xylan/chitosan composite hydrogel is injected into the injury site, the bone is completely healed by 6 weeks.
Discussion
A composite hydrogel containing a polymer ratio of 1:3 xylan to chitosan was observed to improve the healing of tibia fractures and displaced comminuted femur fractures caused by blunt force trauma. This was accomplished without the use of any exogenous therapeutic proteins or growth factors. Previous investigations that combined xylan with chitosan in a hydrogel were not able to create a material with suitable properties for tissue engineering because it readily dissolved when exposed to excess water, nor was it tested in any in vitro or in vivo models.[44,45] The material investigated in this study has improved on those previous attempts by synthesizing a composite hydrogel that does not immediately dissolve with excess water and has thermogelling properties that ease the method of delivery. Chitosan has been extensively studied for decades. It promotes cell attachment, retains growth factors, has bacteriostatic properties, and is easily made into gels and scaffolds.[18,46] The exact mechanism by which xylan contributed to the improved healing shown here is unknown. However, the effect of xylan on the inflammatory response provides a starting point for investigation.
The main cytokines identified that inhibit bone healing are IFN-γ and TNF-α. Slight inhibition of bone repair is also seen with IL-6 and IL-1.[28–30,35] Regulatory T-cells, or Treg cells, can inhibit T-cell activation and decrease concentrations of IFN-γ and TNF-α.[29] Suppressing production of these cytokines rescues compromised bone healing.[29,35] An increase in IL-10, an anti-inflammatory cytokine produced by Treg cells, and IL-4, another Th2 cytokine, have been connected with improving bone healing and osteoblast differentiation.[29,30] Xylan polymers have been shown to decrease TNF-α, IL-6, and IL-1 in mouse models.[33] They can also enhance the Th2, Th17, and Treg populations of T-cells important for bone regeneration, affecting both the innate and acquired immune response.[34,47] The cytokine known to benefit fracture healing, IL-10, is increased by treatment with arabinoxylans.[33] Chitosan can also inhibit the over-production of TNF-α and increase concentrations of IL-10.[48
The combination of xylan and chitosan delivered in this hydrogel could be affecting healing either by inhibiting the subset of T-cells and macrophages and their production of pro-inflammatory cytokines that are known to delay bone healing or by enhancing T-cells beneficial to repair and increase production of IL-10.[28,30]
Chitosan hydrogels have several properties that provide a beneficial foundation on which to build for healing fractures at risk of non-union. Chitosan has antibacterial properties, in situ gelling can immobilize chipped pieces of bone in the fracture space, and injectable gels conform to the complex geometry of fractures. Each of these characteristics directly address the cause of many non-unions.[49] However, as the in vivo implant experiment demonstrated, the chitosan hydrogel also can act as a barrier to tissue penetration into the injury area, which would further delay any healing. The addition of xylan to this hydrogel preserved of chitosan's benefits while improving its healing potential.
The ability of the composite hydrogel to encourage in growth of tissue was demonstrated in subcutaneous models where the pure chitosan hydrogel acted as a barrier to cell migration. In the subcutaneous injection model, the chitosan was seen in histological staining, and the hydrogel was acellular with an encapsulating layer of cells at the edges. The enzymatic degradation products of chitosan, chitooligosaccharides, have been shown to inhibit cell migration in angiogenesis models.[50] The presence of xylan in the hydrogel appeared to overcome this barrier characteristic. The basis of cell-based therapies for healing bone is the hypothesis that not enough therapeutic cells from the host are able tomigrate into critical sized defects to affect healing. The xylan/chitosan composite showed that it is able to encourage migration of host cells into the area of injection. This subcutaneous model resulted in formation of vascularized nodules in host tissue at the site of injection, demonstrating that the xylan not only overcomes the barrier nature of the chitosan hydrogel but also encourages host tissue to replace the biomaterial.
For bone fracture healing, the host tissue must be able to bridge the injury, allow osteogenic differentiation, and allow mineralization of the deposited extracellular matrix, which is largely collagen.[51] If an acellular scaffold is used to aid in this process, it must allow migration of host cells into the scaffold to encourage this process to take place.[8] The xylan/chitosan composite hydrogel used here demonstrated that it is capable of meeting these demands in models of fracture injuries. The tibia fracture modeled common fractures that form a hard callus as part of the repair process. The mineralized callus in the group treated with the xylan/chitosan composite hydrogel was remodeled to normal bone earlier than the group treated with the pure chitosan hydrogel. Between 3 and 4 weeks, a significant improvement was seen in the injuries treated with the composite. There was a statistically significant difference between the measurements of callus area seen at Week 3 compared with that seen at Week 4. This change was only seen in the group treated with the composite. At Week 3, there was no difference in callus area between treated groups, but a difference was clearly seen at Week 4. This could imply that the xylan component of the composite accelerates normal callus formation and the remodeling process, which the literature on xylan's immunomodulatory properties may suggest.
The femur fracture demonstrated that the xylan/chitosan composite hydrogel can modify the healing response of a severe bone fracture that results in a delayed healing or non-union. This model imitates blast and crush injuries seen in automobile accidents and war injuries. The femur fracture remained as a non-union after 6 weeks without treatment, but when treated with the composite hydrogel, the bone was allowed to heal. This showed that the composite is able to help the normal healing process in severe injuries without adding additional growth factors or therapeutic cells that are often seen as necessary in regenerative medicine.[52] This also suggests that the composite hydrogel could be active in the repair process by one of the suggested mechanisms. The subcutaneous implant model suggests that the composite hydrogel is replaced by tissue early in the repair process, so it does not provide long-term stability to the injury that would replace or aid fixation of the bone provided by the internal pin. This behavior allows the composite hydrogel to easily complement current clinical methods, without disrupting the fixation techniques that surgeons already trust.
If xylan's mechanism of action does affect the inflammatory response of macrophages, which stimulate deposition of connective tissue,[53] the impact the study could have a much broader impact with application to a wide range of injuries and diseases that cause collagen fibers to be laid down in a disorganized or overstimulated manner, such as fibrosis.[54,55] The development of this newly discovered composite hydrogel closely aligns with discoveries in osteoimmunology, connecting the immunomodulatory effects of xylan and chitosan to known regulators of the fracture healing process. Targeting the activity of T-cells and their related cytokines at the site of a bone injury, especially in patients susceptible to nonunions, could have a substantial impact on the treatment of bone injuries. The relative simplicity of processing plant-based polymers with known biocompatibility and activity that directly addresses the causes for impaired or delayed bone healing calls for a significant shift in how commercial tissue engineering scaffolds are designed and developed.
Conclusion
These data together point to an injectable biomaterial synthesized from naturally occurring polymers that aid in the healing of bone fractures. The injectable nature of the material ensures easy application of the hydrogel, and the invasion of tissue into the delivery site addresses the main issue that characterizes delayed healing and non-unions. The data show that the incorporation of the second polymer, xylan, has helped to overcome limitations of using a pure chitosan hydrogels in this application.
Supplementary Material
Acknowledgments
All work was performed in the Orthopaedic Research Center and Department of Biomedical Engineering at the University of Virginia.
Funding for this research was provided by the DOD Hypothesis Development Award (W81XWH-10-1-0886) and the R. Clifton Brooks Medical Research Fund.
Footnotes
Supporting Information: Supporting information may be found in the online version of this paper.
References
- 1.Mano JF, Silva GA, Azevedo HS, Malafaya PB, Sousa RA, Silva SS, Boesel LF, Oliveira JM, Santos TC, Marques AP, Neves NM, Reis RL. J R Soc Interface. 2007;4:999–1030. doi: 10.1098/rsif.2007.0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shelke NB, James R, Laurencin CT, Kumbar SG. Polym Adv Technol. 2014;25:448–460. [Google Scholar]
- 3.Matyash M, Despang F, Mandal R, Fiore D, Gelinsky M, Ikonomidou C. Tissue Eng Part A. 2012;18:55–66. doi: 10.1089/ten.TEA.2011.0097. [DOI] [PubMed] [Google Scholar]
- 4.Kokkonen H, Verhoef R, Kauppinen K, Muhonen V, Jorgensen B, Damager I, Schols HA, Morra M, Ulvskov P, Tuukkanen J. J Biomed Mater Res A. 2012;100A:111–119. doi: 10.1002/jbm.a.33240. [DOI] [PubMed] [Google Scholar]
- 5.Barbucci R, Consumi M, Lamponi S, Leone G. Macromol Symp. 2003;204:37–58. [Google Scholar]
- 6.Hoffman A. Adv Drug Deliv Rev. 2002;54:3–12. doi: 10.1016/s0169-409x(01)00239-3. [DOI] [PubMed] [Google Scholar]
- 7.Gunatillake PA, Adhikari R. Eur Cell Mater. 2003;5:1–16. doi: 10.22203/ecm.v005a01. [DOI] [PubMed] [Google Scholar]
- 8.Jabbari E. Curr Opin Biotechnol. 2011;22:655–660. doi: 10.1016/j.copbio.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meyers S, Khoo X, Huang X, Walsh E, Grinstaff M, Kenan D. Biomaterials. 2009;30:277–286. doi: 10.1016/j.biomaterials.2008.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee KI, Lee KS, Kim IH, Jang JW, Kim DH, Kim HY, Moon SH, Chun HJ. Tissue Eng Regen Med. 2009;6:170–178. [Google Scholar]
- 11.Balakrishnan B, Banerjee R. Chem Rev. 2011;111:4453–4474. doi: 10.1021/cr100123h. [DOI] [PubMed] [Google Scholar]
- 12.Derwin KA, Badylak SF, Steinmann SP, Iannotti JP. J Shoulder Elbow Surg. 2010;19:467–476. doi: 10.1016/j.jse.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 13.Badylak SF. Biomaterials. 2007;28:3587–3593. doi: 10.1016/j.biomaterials.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 14.Keane TJ, Londono R, Turner NJ, Badylak SF. Biomaterials. 2012;33:1771–1781. doi: 10.1016/j.biomaterials.2011.10.054. [DOI] [PubMed] [Google Scholar]
- 15.Reddy N, Yang Y. Trends Biotechnol. 2011;29:490–498. doi: 10.1016/j.tibtech.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Scheller HV, Ulvskov P. In: Annu Rev Plant Biol. Merchant S, Briggs W, Ort D, editors. Vol. 61. Annual Reviews; Palo Alto: 2010. pp. 263–289. [DOI] [PubMed] [Google Scholar]
- 17.Ebringerova A, Hromadkova Z, Heinze T. Polysacch 1 Struct Charact Use. 2005;186:1–67. [Google Scholar]
- 18.Muzzarelli RAA, Muzzarelli C. Adv Polym Sci. 2005;186:151–209. [Google Scholar]
- 19.Samuelsen AB, Rieder A, Grimmer S, Michaelsen TE, Knutsen SH. Int J Mol Sci. 2011;12:570–587. doi: 10.3390/ijms12010570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Martino A, Sittinger M, Risbud MV. Biomaterials. 2005;26:5983–5990. doi: 10.1016/j.biomaterials.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 21.Edlund U, Albertsson A. J Bioact Compat Polym. 2008;23:171–186. [Google Scholar]
- 22.Edlund U, Svensson M, Albertsson AC. Eur Polym J. 2012;48:372–383. [Google Scholar]
- 23.Krishnan R, Rajeswari R, Venugopal J, Sundarrajan S, Sridhar R, Shayanti M, Ramakrishna S. J Mater Sci-Mater Med. 2012;23:1511–1519. doi: 10.1007/s10856-012-4630-6. [DOI] [PubMed] [Google Scholar]
- 24.Venugopal J, Rajeswari R, Shayanti M, Sridhar R, Sundarrajan S, Balamurugan R, Ramakrishna S. Mater Sci Eng C-Mater Biol Appl. 2013;33:1325–1331. doi: 10.1016/j.msec.2012.12.032. [DOI] [PubMed] [Google Scholar]
- 25.Guan Y, Zhang B, Bian J, Peng F, Sun RC. Cellulose. 2014;21:1709–1721. [Google Scholar]
- 26.Kuzmenko V, Hagg D, Toriz G, Gatenholm P. Carbohydr Polym. 2014;102:862–868. doi: 10.1016/j.carbpol.2013.10.077. [DOI] [PubMed] [Google Scholar]
- 27.Pahimanolis N, Sorvari A, Luong ND, Seppala J. Carbohydr Polym. 2014;102:637–644. doi: 10.1016/j.carbpol.2013.11.058. [DOI] [PubMed] [Google Scholar]
- 28.Colburn NT, Zaal KJM, Wang F, Tuan RS. Arthritis Rheum. 2009;60:1694–1703. doi: 10.1002/art.24520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Y, Wang L, Kikuiri T, Akiyama K, Chen C, Xu X, Yang R, Chen W, Wang S, Shi S. Nat Med. 2011;17:1594–1601. doi: 10.1038/nm.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dighe AS, Yang S, Madhu V, Balian G, Cui Q. J Orthop Res. 2013;31:227–234. doi: 10.1002/jor.22212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Onuora S. Nat Rev Rheumatol. 2015;11:257. doi: 10.1038/nrrheum.2015.51. [DOI] [PubMed] [Google Scholar]
- 32.Takayanagi H. Nat Rev Rheumatol. 2015;11:74–75. doi: 10.1038/nrrheum.2014.219. [DOI] [PubMed] [Google Scholar]
- 33.Fang HY, Chen YK, Chen HH, Lin SY, Fang YT. Food Chem. 2012;134:836–840. doi: 10.1016/j.foodchem.2012.02.190. [DOI] [PubMed] [Google Scholar]
- 34.Zhou S, Liu X, Guo Y, Wang Q, Peng D, Cao L. Carbohydr Polym. 2010;81:784–789. [Google Scholar]
- 35.Reinke S, Geissler S, Taylor WR, Schmidt-Bleek K, Juelke K, Schwachmeyer V, Dahne M, Hartwig T, Akyüz L, Meisel C, Unterwalder N, Singh NB, Reinke P, Haas NP, Volk HD, Duda GN. Sci Transl Med. 2013;5:177ra36. doi: 10.1126/scitranslmed.3004754. [DOI] [PubMed] [Google Scholar]
- 36.Lenaghan SC, Serpersu K, Xia L, He W, Zhang M. Bioinspir Biomim. 2011;6:046009. doi: 10.1088/1748-3182/6/4/046009. [DOI] [PubMed] [Google Scholar]
- 37.Kumbar SG, Toti US, Deng M, James R, Laurencin CT, Aravamudhan A, Harmon M, Ramos DM. Biomed Mater. 2011;6:065005. doi: 10.1088/1748-6041/6/6/065005. [DOI] [PubMed] [Google Scholar]
- 38.Dahabreh Z, Calori GM, Kanakaris NK, Nikolaou VS, Giannoudis PV. Int Orthop. 2009;33:1407–1414. doi: 10.1007/s00264-008-0709-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heckman JD, Sarasohn-Kahn J. Bull Hosp Jt Dis N Y N. 1997;56:63–72. [PubMed] [Google Scholar]
- 40.St John TA, Vaccaro AR, Sah AP, Schaefer M, Berta SC, Albert T, Hilibrand A. Am J Orthop. 2003;32:18–23. [PubMed] [Google Scholar]
- 41.Harris IA, Dao ATT, Young JM, Solomon MJ, Jalaludin BB. Inj-Int J Care Inj. 2009;40:377–384. doi: 10.1016/j.injury.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 42.Nair LS, Starnes T, Ko JWK, Laurencin CT. Biomacromolecules. 2007;8:3779–3785. doi: 10.1021/bm7006967. [DOI] [PubMed] [Google Scholar]
- 43.Schindeler A, Morse A, Harry L, Godfrey C, Mikulec K, McDonald M, Gasser JA, Little DG. J Orthop Res. 2008;26:1053–1060. doi: 10.1002/jor.20628. [DOI] [PubMed] [Google Scholar]
- 44.Gabrielii I, Gatenholm P. J Appl Polym Sci. 1998;69:1661–1667. [Google Scholar]
- 45.Gabrielii I, Gatenholm P, Glasser W, Jain R, Kenne L. Carbohydr Polym. 2000;43:367–374. [Google Scholar]
- 46.Muzzarelli RAA, Greco F, Busilacchi A, Sollazzo V, Gigante A. Carbohydr Polym. 2012;89:723–739. doi: 10.1016/j.carbpol.2012.04.057. [DOI] [PubMed] [Google Scholar]
- 47.Akhtar M, Tariq AF, Awais MM, Iqbal Z, Muhammad F, Shahid M, Hiszczynska-Sawicka E. Carbohydr Polym. 2012;90:333–339. doi: 10.1016/j.carbpol.2012.05.048. [DOI] [PubMed] [Google Scholar]
- 48.Oliveira MI, Santos SG, Oliveira MJ, Torres AL, Barbosa MA. Eur Cell Mater. 2012;24:136–153. doi: 10.22203/ecm.v024a10. [DOI] [PubMed] [Google Scholar]
- 49.Bishop JA, Palanca AA, Bellino MJ, Lowenberg DW. J Am Acad Orthop Surg. 2012;20:273–282. doi: 10.5435/JAAOS-20-05-273. [DOI] [PubMed] [Google Scholar]
- 50.Wu H, Aam BB, Wang W, Norberg AL, Sorlie M, Eijsink VGH, Du Y. Carbohydr Polym. 2012;89:511–518. doi: 10.1016/j.carbpol.2012.03.037. [DOI] [PubMed] [Google Scholar]
- 51.Del Fattore A, Teti A, Rucci N. Front Biosci. 2012;4:2302–2321. doi: 10.2741/543. [DOI] [PubMed] [Google Scholar]
- 52.Varghese S, Elisseeff JH. Adv Polym Sci. 2006;203:95–144. [Google Scholar]
- 53.King SN, Hanson SE, Chen X, Kim J, Hematti P, Thibeault SL. J Biomed Mater Res A. 2014;102:890–902. doi: 10.1002/jbm.a.34746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jones NF, Ahn HC, Eo S. Plast Reconstr Surg. 2012;129:683–692. doi: 10.1097/PRS.0b013e3182402c37. [DOI] [PubMed] [Google Scholar]
- 55.de la Motte CA. Am J Physiol-Gastrointest Liver Physiol. 2011;301:G945–G949. doi: 10.1152/ajpgi.00063.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


