Abstract
Background
This study aimed to investigate the expression of DJ-1 in cervical carcinoma and its effects on cell viability and apoptosis.
Material/Methods
Cervical carcinoma cell line Hela and 85 tissue samples, including 45 primary tumor biopsies, 30 para-carcinoma tissues, and 10 normal cervical tissues samples were used in this study. The expressions of DJ-1 in cervical carcinoma tissue, para-carcinoma tissue, and normal tissue samples were investigated by immunohistochemistry. DJ-1 expression in Hela cells was also investigated by quantitative reverse transcription-polymerase chain reaction (RT-PCR) and Western blot. DJ-1 was interfered and transfected with siRNA, then cell viability and apoptosis were assayed by MTT and flow cytometry, respectively. Additionally, the expressions of phosphatase and tensin homolog (PTEN), AKT, and phospho-AKT (P-AKT) were detected.
Results
Immunohistochemistry results showed that DJ-1 was highly expressed in cervical carcinoma tissues. In Hela cells, the expression of DJ-1 was significantly higher than that in normal controls (P<0.05). When cells were treated with DJ-1 siRNA, the cell viability decreased significantly (P<0.05), and the percentage of apoptosis cells increased significantly (P<0.05). In addition, the expressions of PTEN and AKT were significantly higher in the DJ-1 siRNA treatment group than those in the control group (P<0.05). The expression of p-AKT was significantly lower in the DJ-1 siRNA treatment group than in the control group and the DJ-1 over-expression group (P<0.05).
Conclusions
The aberrant up-regulation of DJ-1 expression might be an important step in the pathogenesis of cervical carcinoma.
MeSH Keywords: Apoptosis, Cell Survival, PTEN Phosphohydrolase, RNA Interference, Uterine Cervical Neoplasms
Background
Cervical carcinoma is one of the most common malignancies of the female reproductive tract and is responsible for about 200 000 deaths per year among women worldwide [1,2], with more than 80% of the mortality occurring in developing countries. Furthermore, Chinese women have the highest age-standardized incidence rate of 23.2 per 100 000 population [3]. Infection with some types of human papillomavirus (HPV) is the greatest risk factor for cervical cancer, and appears to be involved in the development of more than 90% of cases [4]. This disease has a poor prognosis and its 5-year survival rate for patients at stage IV is only 3–13% [5]. The progression of cervical carcinoma from low-grade to high-grade is closely associated with cell cycle regulation, apoptosis, and DNA repair [6]. Therefore, understanding the molecular mechanisms underlying cervical carcinoma progression is very important.
DJ-1, a 189 amino acid protein, was initially identified as a putative oncogene that can strongly transform NIH3T3 cells in combination with H-Ras or c-Myc. It is ubiquitously expressed in various human tissues [7]. DJ-1 is overexpressed in many cancers [8–10] and is implicated in tumor progression [11,12]. Importantly, DJ-1 has been suggested to have an effect on cell survival by regulating cellular signaling cascades, such as phosphatase and tensin homolog (PTEN)/phosphatidylinositol 3-kinase (PI3K)/AKT [13]. Interestingly, the PTEN/PI3K pathway plays a critical role in tumorigenesis, including cervical carcinoma [14,15]. However, the role and possible mechanism of DJ-1 in cervical carcinoma remain unclear.
In the present study, the expression of DJ-1 in cervical carcinoma tissue and cell line was analyzed. In addition, the expression of DJ-1 was also analyzed after cervical carcinoma cells were transfected with siRNA. We detected cell viability and apoptosis, followed by analysis of the expression of PTEN, AKT, and phospho-AKT (P-AKT). We aimed to investigate the role of DJ-1 in cervical carcinoma and to further explore its possible mechanism.
Material and Methods
We obtained all appropriate approval from the Institutional Review Board of Ankang Hospital and we performed the study in accordance with ethics standards.
Cell line and tissue specimens
Cervical carcinoma cell line Hela was used in the study, which was purchased from the Chinese Academy of Sciences Shanghai Institutes for Biological Sciences Cell resource center (Shanghai, China). The cells were cultured in RPMI1640 culture medium (Hyclone, Logan, Utah, USA) supplemented with 10% fetal bovine serum (FBS; Gibco, Shanghai, China) in a humidified atmosphere at 37°C and 5% CO2, and passaged using 0.25% trypsin for digestion.
A total of 45 primary tumor biopsies and 30 para-carcinoma tissues samples from cervical carcinoma patients were obtained from Ankang Hospital. All tissues were verified by pathologists under a microscope. These tissue samples were isolated, immediately frozen in liquid nitrogen, and stored at −80°C. Additionally, 10 normal cervical tissues samples from healthy volunteers were included as controls. All patients and volunteers gave their informed consent.
Immunohistochemistry
Immunohistochemical staining was performed for normal tissues, para-carcinoma tissues, and carcinoma tissues (n=3 per group). All of them were stained using a Benchmark automatic immunostaining device (Ventana Medical System, Tucson, AZ). Subsequently, the slide sections were incubated with polyclonal antibody against DJ-1 (1:50, FL-189; Santa Cruz Biotechnology, CA, USA), biotinylated anti-rabbit immunoglobulins, peroxidase-labeled streptavidin (LSAB kit; Dako, Carpentaria, CA), and 3,3−-diaminobenzidine (Sigma, Germany). The slides were counterstained using Harris hematoxylin. Immunohistochemical staining was evaluated based on the location, percentage, and intensity of positively stained cells. DJ-1 was scored positive when more than 10% of nuclear staining was observed in the tumor cells. All of the immunohistochemically stained slides were reviewed by 3 experienced cervical pathologists for improved accuracy.
siRNA interference and transfection
DJ-1-specific siRNAs were designed as described before [16]. The sequences of sense and antisense nucleotides were: 5−-AATGGAGGTCATTACACCTACCCTGTCTC-3− and 5−-AAGTAGGTGTAATGACCTCCACCTGTCTC-3−. A total of 5×105 cells were plated into 35 mm plates. We transfected 30 nM siRNA into Hela cells using Lipofectamine 2000 reagent (Invitrogen, CA, USA) following the manufacturer’s protocol. siRNA validation was carried out at 24 h by checking the expression of DJ-1 via RT-PCR and Western blot.
Cell transfections were conducted using Lipofectamine 2000 reagent (Invitrogen, CA, USA) following the manufacturer’s protocol. Stable DJ-1 transfectants were generated under G418 (Gibco, Paisley, UK) selection as described before.
Quantitative reverse transcription-polymerase chain reaction (RT-PCR)
Total mRNA was isolated from cells using Trizol Reagent. The RNA was reverse transcribed into complementary DNA (cDNA) using reverse transcriptase (iScript™ cDNA Synthesis Kit; Bio-Rad Laboratories). The expression levels of mRNAs were measured by SYBR green-based quantitative RT-PCR (SYBR Green Master mix; Thermo Scientific, Waltham, MA, USA). Primer sequences were GAPDH: forward ACCACAGTCCATGCCATCAC, reverse TCCACCACCCTGTTGCTGTA; DJ-1: forward GGAGACGGTCATCCCTGTAGAT, reverse GCTACACTGTACTGGGTCTTTTCCA. Relative mRNA amounts and expression ratios were calculated with 2−ΔΔCT method [17].
Western blot
Hela cells were lysed in RIPA. The total protein concentration was determined by Protein Quantitative Reagent Kit-BCA method (Sigma, Germany). Protein samples were separated on a 10–12% sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE) and then blotted onto polyvinylidene difluoride membranes (Bio-Rad Laboratories, Hercules, CA). After that, the membranes were blocked in PBST (0.1% triton in 19 PBS) and probed with primary antibodies overnight at 4ºC. The primary antibodies included anti-DJ-1 antibody (1:1000), anti-PTEN antibody (1:1000), anti-AKT antibody (1:1000), and anti-pAKT antibody (1:1000). Then, membranes were incubated with appropriate horseradish peroxidase-conjugated secondary antibodies (1:1000). All antibodies were purchased from Santa Cruz Biotechnology, Santa Cruz, CA. The protein bands were then washed and developed by enhanced chemiluminescence.
Cell viability assay
Cell concentration of Hela in logarithmic growth phase was adjusted to 5×104/mL, and cultured in a 96-well plate with 200 μL per well for 24 h, 48 h, 72 h, and 96 h. Each group had 4 repeats. Then, 20 μL fresh medium with 0.5 mg/mL methyl thiazolyl tetrazolium (MTT) was added to each well 4 h before termination. After 4-h incubation, 200 μL dimethyl sulfoxide (DMSO) was added to each well. The optical density (OD) was measured at 492 nm. The experiment was repeated 3 times.
Apoptosis assay
The cells were seeded at a density of 2×105 per 35-mm culture dish and cell apoptosis was assayed by annexin V and proprium iodide (PI) staining (BD PharMingen, CA, USA) according to the manufacturer’s instructions. Cells were analyzed with a FACS Calibur flow cytometer (BD, CA, USA). The percentage of total apoptotic events was defined as the sum of the cells in the early stage (annexin V-positive/PI-negative) and late stage (annexin V-positive/PI-positive) of apoptosis, as previously described [18].
Statistical analysis
The results of multiple experiments are presented as mean ±SD. Statistical analyses were performed using SPSS 19.0 statistical software (SPSS Inc., Chicago, IL, USA). All collected data were first tested for the normal distribution using the one-sample K-S test. Measurement data were tested by one-way analysis of variance (ANOVA). Enumeration data were analyzed by chi-square test or rank-sum test. P<0.05 was considered statistically significant.
Results
Immunohistochemistry results
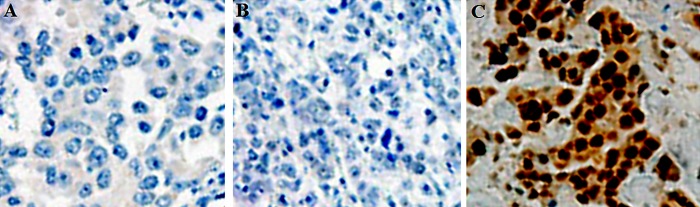
Representative photomicrographs of histologic findings and DJ-1 immunohistochemistry for normal, para-carcinoma, and cervical carcinoma tissues are shown in Figure 1. As indicated by the brown particles, DJ-1 was highly expressed in cervical carcinoma tissues. Compared to the cervical carcinoma tissues, para-carcinoma and normal cervical tissues showed a much lower DJ-1 expression (P<0.05).
Figure 1.
Immunohistochemical images for the expression of DJ-1 in normal cervical tissue, para-carcinoma tissue, and primary cervical carcinoma tissue. (A) Normal cervical tissue; (B) Para-carcinoma tissues; (C) Primary cervical carcinoma tissues (200×).
Expression of DJ-1 in Hela cell
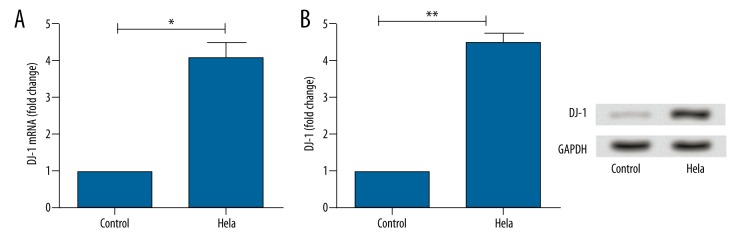
The expression of CDC42 mRNA was assayed by RT-PCR in Hela cells and normal cervical cells with GAPDH as an internal reference (Figure 2A). The expression of CDC42 protein was assayed by Western blot (Figure 2B). As shown in the figures, the expressions of DJ-1 mRNA and protein in Hela cells were significantly higher than that in normal controls (mRNA: P<0.05; protein: P<0.01).
Figure 2.
Histograms for the expression of DJ-1 in Hela cells and normal cervical cells assayed by RT-PCR (A) and Western blot (B). Y-axis represented the fold change of DJ-1 mRNA (A) or protein (B). Error bars indicated means ±SD (P value was determined by ANOVA; ** P<0.01, *** P<0.001).
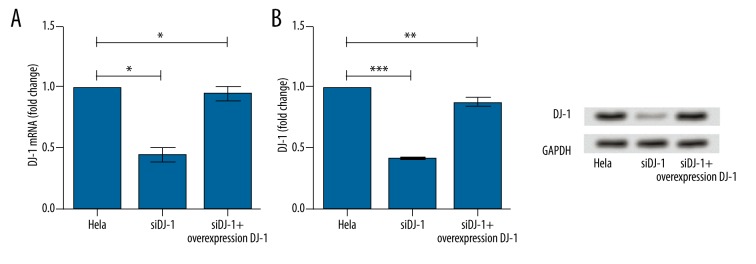
To determine the efficiency of siRNA in Hela cells, the mRNA and protein levels of DJ-1 gene were analyzed. Compared with controls, the expressions of DJ-1 decreased significantly when DJ-1 was interfered with DJ-1 siRNA (mRNA: P<0.05; protein: P<0.001). However, when DJ-1 was transfected, the expressions of DJ-1 increased significantly (mRNA: P<0.05; protein: P<0.01) (Figure 3).
Figure 3.
Histograms for the expression of DJ-1 in Hela cells (control group), DJ-1 siRNA treatment group, and DJ-1 overexpression group assayed by RT-PCR (A) and Western blot (B). Y-axis represented the fold change of DJ-1 mRNA (A) or protein (B). Error bars indicate means ±SD (P value was determined by ANOVA; * P<0.05, ** P<0.01, *** p<0.001).
Cell viability
As shown in Figure 4, the cell viability increased in a time-dependent manner in control and DJ-1 overexpression groups. However, cell viability decreased significantly over time with DJ-1 siRNA treatment (P<0.05).
Figure 4.
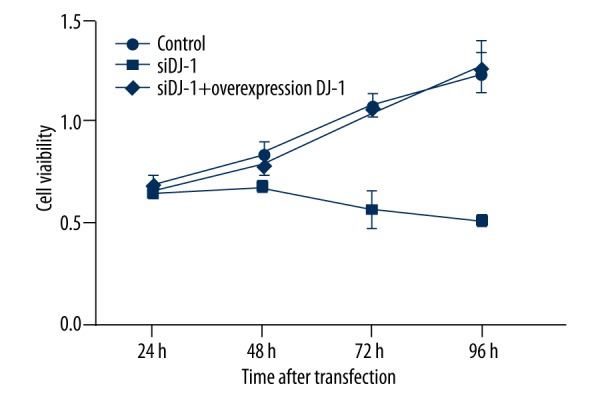
Line chart of cell viability assayed by MTT. Cell viability of Hela cells decreased significantly upon DJ-1 siRNA treatment. Error bars indicated means ± SD (P value was determined by ANOVA; * P<0.05).
Apoptosis
The percentages of apoptosis cells in DJ-1 siRNA treatment, DJ-1 overexpression, and control groups are shown in Figure 5. The percentage of apoptosis cells in the DJ-1 silence group was significantly higher than that in control and DJ-1 over-expression groups (P<0.01).
Figure 5.
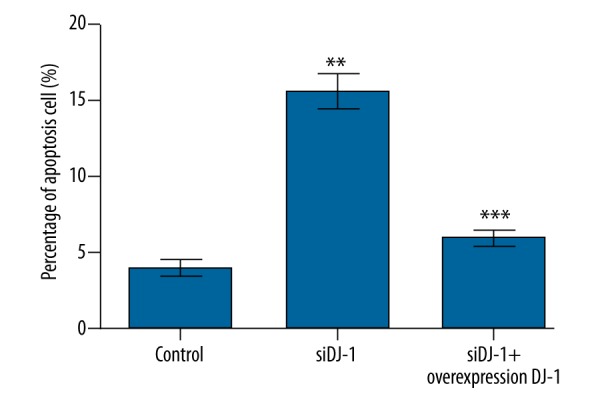
Histograms for the apoptosis rate of Hela cells assayed by flow cytometer. DJ-1 siRNA treatment enhances the apoptosis of Hela cells. Y-axis represents the percentages of apoptosis cells (%). Error bars indicate means ±SD (P value was determined by ANOVA; ** P<0.01, *** p<0.001).
Expression of PTEN, AKT and p-AKT
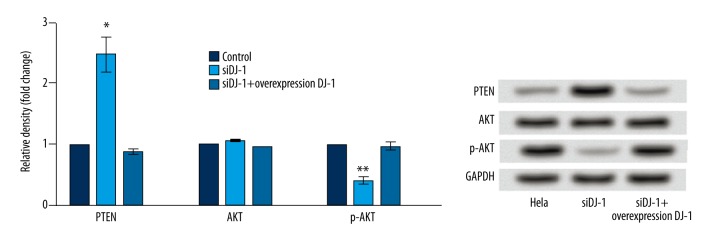
The expressions of PTEN was significantly higher in the DJ-1 siRNA treatment group than in the control group (P<0.05). When DJ-1 was overexpressed, the expression of PTEN significantly decreased (P<0.05). For the expression of AKT, no significant difference was found among the 3 groups. p-AKT expression was significantly lower in the DJ-1 siRNA treatment group than in the control and DJ-1 over-expression groups (P<0.01) (Figure 6).
Figure 6.
Histograms for the expressions of PTEN, AKT and p-AKT assayed by Western blot. Y-axis represents the fold change of relative density. Error bars indicate means ±SD (P value was determined by ANOVA; * P<0.05, ** P<0.01).
Discussion
In recent years, the molecular mechanisms for cancer cell invasion and metastasis have been widely explored [19]. In the present study, we analyzed the expression of DJ-1 in cervical carcinoma tissues and cells, and found that DJ-1 showed a much higher expression level in cervical carcinoma tissues and cells than in normal cervical tissues and cells. Interestingly, after siRNA interference, the expressions of DJ-1 mRNA and protein in Hela cells were reduced significantly, but the expressions of DJ-1 mRNA and protein increased significantly after transfection. Our findings suggested that there might be a significant correlation between DJ-1 overexpression and the occurrence of cervical carcinoma.
DJ-1 is frequently up-regulated in many cancer types, such as prostate cancer, breast cancer, and pancreatic cancer [11,20,21]. Importantly, Arnouk et al. [10] suggested that DJ-1 is one of molecular markers of cervical carcinoma and shows higher expression in cervical carcinoma than in normal cervical tissues. In the present study, the results of immunohistochemistry showed that DJ-1 expression was significantly increased in cervical carcinoma tissues compared with para-cancerous tissues and normal cervical tissue, which was in accordance with the findings of Arnouk et al.
Sustaining proliferative signaling, evading growth suppressors, and resisting cell death are the hallmarks of tumors [22]. In this study, DJ-1 siRNA treatment led to impaired cell viability and increased percentage of apoptosis cells compared to those of the control group. Further investigation found that overexpression of DJ-1 increased cell viability and reduced the percentage of apoptosis cells compared to those of the control group and DJ-1 siRNA-treated group. Therefore, DJ-1 may contributed to the development of cervical carcinoma by a possible pathway of enhancing cell viability and inhibiting apoptosis.
To elucidate the molecular mechanisms underlying the DJ-1 dependent cell viability and apoptosis, the expressions of PTEN, AKT, and p-AKT were assayed. Studies have shown that PTEN is negatively regulated by DJ-1, and overexpression of DJ-1 can provide a mechanism for down-regulation of the PTEN gene [23–25]. Many human cancers overexpress DJ-1 mRNA during cancer progression, and escape from PTEN-induced cell death [26]. PTEN is one of the most frequently mutated tumor suppressor genes in human cancer [27] and can prevent cells from growing and dividing too rapidly [28]. Growing evidence shows that PTEN plays a critical role in pathogenesis of some tumors [29,30]. Song et al. [31] suggested that mutations of PTEN that inactivate its enzymatic activity induce increased cell proliferation, reduced cell death, and tumor development. Additionally, PTEN antagonizes PI3K, which can active AKT by a variety of growth factors [32]. AKT is a serine/threonine protein kinase, which is ubiquitously expressed in normal tissues and mediates growth factor-associated cell survival, and cell proliferation [33]. Once activated, p-AKT is a powerful promoter of cell survival because it can inactivate various components of the apoptotic cascade [34]. Importantly, Hossein et al. [35] indicated that lack of DJ-1 led to a reduction in AKT phosphorylation, which is in agreement with our study.
Conclusions
In conclusion, our results suggested that the aberrant up-regulation of DJ-1 expression might be an important step in the pathogenesis of cervical carcinoma. DJ-1 could serve as a potential molecular target for the treatment of cervical carcinoma.
Footnotes
Conflict of interests
None.
Source of support: Departmental sources
References
- 1.Ma D, Cheng Y, Zhang Y, et al. Expression of CDC42 in cervical squamous cell carcinoma and its correlation with clinicopathologic characteristics. Chin J Cancer Res. 2013;25:656–61. doi: 10.3978/j.issn.1000-9604.2013.11.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sankaranarayanan R, Ferlay J. Worldwide burden of gynaecological cancer: The size of the problem. Best Prac Res Clin Obstet Gynaecol. 2006;20:207–25. doi: 10.1016/j.bpobgyn.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Lim GCC, Rampal S, Yahaya H. Cancer incidence in peninsular Malaysia, 2003–2005: The third report of the National Cancer Registry, Malaysia. National Cancer Registry; 2008. [Google Scholar]
- 4.Hong WK, Hait WN. Holland Frei cancer medicine eight. PMPH-USA; 2010. [Google Scholar]
- 5.Tsang CM, Lau EPW, Di K, et al. Berberine inhibits Rho GTPases and cell migration at low doses but induces G2 arrest and apoptosis at high doses in human cancer cells. Int J Mol Med. 2009;24:131–38. doi: 10.3892/ijmm_00000216. [DOI] [PubMed] [Google Scholar]
- 6.Sinha S, Yang W. Cellular signaling for activation of Rho GTPase Cdc42. C Signal. 2008;20:1927–34. doi: 10.1016/j.cellsig.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Ismail IA, Kang HS, Lee H-J, et al. DJ-1 upregulates breast cancer cell invasion by repressing KLF17 expression. Br J Cancer. 2014;110:1298–306. doi: 10.1038/bjc.2014.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hang L, Min W, Min L, et al. Expression and role of DJ-1 in leukemia. Biochem Biophys Research Commun. 2008;375:477–83. doi: 10.1016/j.bbrc.2008.08.046. [DOI] [PubMed] [Google Scholar]
- 9.Mei T, Cui YZ, Song GH, et al. Proteomic analysis identifies MMP-9, DJ-1 and A1BG as overexpressed proteins in pancreatic juice from pancreatic ductal adenocarcinoma patients. BMC Cancer. 2008;8:1–11. doi: 10.1186/1471-2407-8-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arnouk H, Merkley MA, Podolsky RH, et al. Characterization of Molecular Markers Indicative of Cervical Cancer Progression. Proteomics Clin Appl. 2009;3:516–27. doi: 10.1002/prca.200800068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuen HF, Chan YP, Law S, et al. DJ-1 could predict worse prognosis in esophageal squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2008;17:3593–602. doi: 10.1158/1055-9965.EPI-08-0214. [DOI] [PubMed] [Google Scholar]
- 12.Zhu XL, Wang ZF, Lei WB, et al. DJ-1: A novel independent prognostic marker for survival in glottic squamous cell carcinoma. Cancer Sci. 2010;101:1320–25. doi: 10.1111/j.1349-7006.2010.01531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou L, Snow B, Binari RC, Manoukian AS. DJ-1, a novel regulator of the tumor suppressor PTEN: Cancer Cell. Cancer Cell. 2005;7:263–73. doi: 10.1016/j.ccr.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 14.Yilmaz OH, Valdez R, Theisen BK, et al. PTEN-dependence distinguishes haematopoietic stem cells from leukemia-initating cells. Nature. 2006;441:475–82. doi: 10.1038/nature04703. [DOI] [PubMed] [Google Scholar]
- 15.Du J, Wang LN, Li CX, et al. MicroRNA-221 targets PTEN to reduce the sensitivity of cervical cancer cells to gefitinib through the PI3K/Akt signaling pathway. Tumor Biology. 2015 doi: 10.1007/s13277-015-4247-8. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Elbashir SM, Harborth J, Weber K, Tuschl T. Analysis of gene function in somatic mammalian cells using small interfering RNAs. Methods. 2002;26:199–213. doi: 10.1016/S1046-2023(02)00023-3. [DOI] [PubMed] [Google Scholar]
- 17.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 18.Zhen S, Hua L, Takahashi Y, et al. In vitro and in vivo growth suppression of human papillomavirus 16-positive cervical cancer cells by CRISPR/Cas9. Biochem Biophys Res Commun. 2014;450:1422–26. doi: 10.1016/j.bbrc.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 19.Bronsert P, Enderle-Ammour K, Bader M, et al. Cancer cell invasion and EMT marker expression: A three-dimensional study of the human cancer-host interface. J Pathol. 2014;234:410–22. doi: 10.1002/path.4416. [DOI] [PubMed] [Google Scholar]
- 20.Xiangyi H, Zhong Z, Jianfang L, et al. DJ-1 promotes invasion and metastasis of pancreatic cancer cells by activating SRC/ERK/uPA. Carcinogenesis. 2012;33:555–62. doi: 10.1093/carcin/bgs002. [DOI] [PubMed] [Google Scholar]
- 21.Laderach DJ, Gentilini LD, Laura G, et al. A unique galectin signature in human prostate cancer progression suggests galectin-1 as a key target for treatment of advanced disease. Cancer Res. 2013;73:86–96. doi: 10.1158/0008-5472.CAN-12-1260. [DOI] [PubMed] [Google Scholar]
- 22.Douglas H, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 23.Davidson B, Hadar R, Schlossberg A, et al. Expression and clinical role of DJ-1, a negative regulator of PTEN, in ovarian carcinoma. Hum Pathol. 2008;39:87–95. doi: 10.1016/j.humpath.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 24.Lee H, Choi SK, Ro JY. Overexpression of DJ-1 and HSP90α, and loss of PTEN associated with invasive urothelial carcinoma of urinary bladder: Possible prognostic markers. Oncol Lett. 2012;3:507–12. doi: 10.3892/ol.2011.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y, Cui J, Zhang C-h, et al. High-expression of DJ-1 and loss of PTEN associated with tumor metastasis and correlated with poor prognosis of gastric carcinoma. Int J Med Sci. 2013;10:1689–97. doi: 10.7150/ijms.7292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawate T, Iwaya K, Koshikawa K, et al. High levels of DJ-1 protein and isoelectric point 6.3 isoform in sera of breast cancer patients. Cancer Sci. 2015;106(7):938–43. doi: 10.1111/cas.12673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mirantes C, Eritja N, Dosil MA, et al. An inducible knockout mouse to model the cell-autonomous role of PTEN in initiating endometrial, prostate and thyroid neoplasias. Dis Model Mech. 2013;6:710–20. doi: 10.1242/dmm.011445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fong P, Meng L-R. Effect of mTOR inhibitors in nude mice with endometrial carcinoma and variable PTEN expression status. Med Sci Monit Basic Res. 2014;20:146–52. doi: 10.12659/MSMBR.892514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petros K, Papi RM, Xeni P, et al. Tumor suppressor PTEN in breast cancer: heterozygosity, mutations and protein expression. Anticancer Res. 2014;34:1387–400. [PubMed] [Google Scholar]
- 30.Maraiz H. Effects of stromal cell derived factor on PTEN. 2014. https://www.lap-publishing.com/
- 31.Song MS, Salmena L, Pandolfi PP. The functions and regulation of the PTEN tumour suppressor. Nat Rev Mol Cell Biol. 2012;13:283–96. doi: 10.1038/nrm3330. [DOI] [PubMed] [Google Scholar]
- 32.Amit S, Swain WA, Donna R, et al. Phospho-Akt expression is associated with a favorable outcome in non-small cell lung cancer. Clin Cancer Res. 2005;11:2930–36. doi: 10.1158/1078-0432.CCR-04-1385. [DOI] [PubMed] [Google Scholar]
- 33.Kulik G, Klippel A, Weber MJ. Antiapoptotic signalling by the insulin-like growth factor I receptor, phosphatidylinositol 3-kinase, and Akt. Mol Cell Biol. 1997;17:1595–606. doi: 10.1128/mcb.17.3.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brunet A, Bonni A, Zigmond MJ, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–68. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 35.Hossein A, Rousseaux MWC, Marcogliese PC, et al. DJ-1 protects the nigrostriatal axis from the neurotoxin MPTP by modulation of the AKT pathway. Proc Natl Acad Sci USA. 2010;107:3186–91. doi: 10.1073/pnas.0914876107. [DOI] [PMC free article] [PubMed] [Google Scholar]