Abstract
Background
MiRNA is widely recognized as the most important regulator in various diseases. However, there has been little research regarding miRNA expression and its involvement in ischemic stroke.
Material/Methods
In this study, we investigated the pattern of miRNA-34a-5p expression along with its clinical application in human ischemic stroke and in an in vivo rat model. We recruited 102 cerebral ischemia patients and 97 health controls for this study. Clinical data were gathered and recorded with the help of questionnaires. Blood samples were obtained from patients within 72 h after cerebral ischemia. National Institutes of Health Stroke Scale (NIHSS), Acute Stroke Treatment (TOAST), and infarct volume were used to analyze the correlation of miRNA-34a-5p expression and clinical information. In addition, blood samples and brain tissues were collected from an established middle cerebral artery occlusion (MCAO) model consisting of 20 adult male mice at 24 h after the MCAO. Expression level of miRNA-34a-5p was detected by real-time polymerase chain reactions.
Results
Results showed overexpression of miRNA-34a-5p in acute ischemic stroke patients blood samples compared to the controls (p<0.05). Also, large and small arterial strokes types demonstrated elevated miRNA-34a-5p expression levels. Further correlation analysis revealed a negative association between miRNA-34a-5p and NIHSS scores (r=−0.692 p<0.05) and infarct volume (r=−0.719, p<0.05). Moreover, in vivo experiment results showed significant up-regulated expression of miRNA-34a-5p in middle cerebral artery occlusion compared to controls, along with a positive correlation between miRNA-34a-5p in blood and brain (r=0.742, p<0.05).
Conclusions
Our results suggest there is a potential regulatory role of miRNA-34a-5p in acute ischemic stroke, which could serve as a therapeutic target or biomarker in stroke prognosis.
MeSH Keywords: Brain Ischemia, MicroRNAs, Stroke
Background
Ischemic stroke is a common disease that increasingly occurs with age, and it has the potential to become a heavy economic burden due to long-term disability [1]. Although the leading cause of ischeamic stroke remains unknown, previous studies have verified the association of multiple genes and proteins with the occurrence and development of stroke [2].
Previous studies have shown the importance of microRNAs (miRNAs), which are a group of short noncoding RNA molecules, and their potential involvement in the pathological process of stroke [3] due to their unique expression in regulation of ischemic preconditioning and ischemic postconditioning [4,5]. Moreover, 114 transformed miRNAs were discovered in middle cerebral artery occlusion (MCAO) rat brain samples [2]. Similar results were also reported by Dharap et al. [6]. A functional study verified that anti-miR-320a could lead to a dramatic decrease of infarct volume in cerebral ischemia [7]. Other population-based miRNA profiling analyses show the differential expression pattern in young stroke patients and controls [8]. miR-34a-5p was also reported to be the most enriched miRNA in mouse brains. In addition, overexpression of miR-34a-5p had the ability to modulate neuronal genes in neuroblastoma cell lines in vitro [9].
Although circulatory miR-34a-5p could be a reliable predictor for ischemic stroke, the clinical relationship between the altered miR-34a-5p and acute ischemic brain injury remains unknown. Therefore, the goal of this study was to explore the clinical role of miRNA in acute ischemic stroke.
Material and Methods
Study subjects
In this case-control study we enrolled 102 clinically and radiologically confirmed ischemia stroke patients from the Second Affiliated Hospital of Zhengzhou University during the period of July 2012 to January 2014, as well as 97 age- and sex-matched controls living in the same area as the patients. All ischemic strokes were diagnosed based on a detailed stroke history and a physical examination by professional clinical care providers. The diagnosis was confirmed with medical imaging diagnostic methods (CT scanning and MRI). All patients or family members gave informed consent. All study processes were approved by the Institutional Review Board of Zhengzhou University. We used the National Institutes of Health Stroke Scale (NIHSS) [10] system adapted to evaluate the severity of ischemic stroke. Trial of Org 10172 in Acute Stroke Treatment (TOAST) [11] was used to catalogue patient subtypes: large-artery atherosclerosis (LA), cardioembolism (CE), small artery stroke (SA), and undetermined etiology (UN). Infarct volumes were calculated by ABC/2 method [12].
Establishment of middle cerebral artery occlusion (MCAO) model
Twenty adult CD-1 mice (weight range 25–30 g) were raised to establish an in vivo MCAO model. Briefly, a silicone-coated 6-0 suture (Doccol Corporation, MA) was inserted from the external cerebral artery into the internal cerebral artery and placed at the opening of the middle cerebral artery in the anesthetized mice. A laser Doppler flow meter (Siemens, Germany) was used to confirm the occlusion status. Animal care and sacrifice methods were approved by the Animal Care and Use Committee of the Henan Medical Association.
Sample collection
We obtained 5-ml blood samples from acute ischemic stroke patients and stored the samples in a cooler. Total DNA, RNA, and miRNA were extracted within 12 h, following the manufacturer’s instructions (TRIzol, Invitrogen, USA). All genome samples were kept at −80°C. Cerebral tissues from the in vivo model were collected at the end of the experiment. We prepared and stained 2-mm coronal slices with 2, 3, 5-triphenyl tetrazolium chloride.
Real-time qRT-PCR
qRT-PCR was used to validate the differential expression of miRNAs between the 2 groups. In brief, 10 ng total RNA was reverse transcribed to cDNA using microRNA locked nucleic acid PCR primers specific for miR-34a-5p (Exiqon, Denmark) and the TaqMan reverse transcription kit (Applied Biosystems, USA). Diluted cDNA was subjected to qRT-PCR using the TaqMan MicroRNA Assay and TaqMan® Universal PCR Master Mix (ABI, Life Technologies) on a 7500 Real-Time PCR system (Applied Biosystems, USA). Relative quantification was performed using ΔΔCt method, and the data were normalized with U6 and RNU48 (Applied Biosystems) as endogenous controls. PCR was performed in triplicate for each sample. Relative miRNA expression levels were quantified using the 2−ΔΔCT method.
Statistical analysis
All numerical data are presented with mean ± standard deviation (SD) and performed with triplicate independent test. SPSS 13.0 software (SPSS Inc., Chicago, IL) was used to compare statistical differences in all test groups by using the t test. Pearson correlation analysis was performed to test the association of miRNA and clinicopathological features. P<0.05 was considered to be statistically significant.
Results
Demographic description of study population
Patients were diagnosed with ischemic stroke by a professional clinical provider and multiple radiology imaging results. Age, sex, life style, and disease history were recorded from a questionnaire or face-to-face interview. No significant differences were found between the ischemic stroke group and the normal control group (Table 1).
Table 1.
Clinical characteristics of patients
| Control | Acute ischemic stroke | p | ||||
|---|---|---|---|---|---|---|
| 1d | 2d | 3d | Total | |||
| Total | 97 | 26 | 31 | 45 | 102 | 1 |
| Age (years, range) | 6.52 (51~68) | 71.2 (47~81) | 66.5 (46~73) | 63.0 (54~72) | 65.1±10.0 | 0.053 |
| Male | 67 | 16 | 20 | 33 | 69 | 0.383 |
| Female | 30 | 10 | 11 | 12 | 33 | |
| Hypertension | 37 (38.14%) | 20 (76.9%) | 26 (76.4%) | 36 (80%) | 82 (80.4%) | <0.05 |
| Diabetes | 7 (7.2%) | 7 (26.9%) | 9 (26.4%) | 19 (42.2%) | 35 (34.3%) | <0.05 |
| Hyperlipidemia | 33 (34.0%) | 14 (53.8%) | 17 (50.0%) | 29 (64.4%) | 60 (58.8%) | <0.05 |
| Cardiopathy | 5 (5.1%) | 7 (26.9%) | 8 (23.5%) | 6 (13.3%) | 21 (20.6%) | <0.05 |
| NIHSS (Mean, range) | NA | 4.73 (1, 11) | 5.05 (0, 20) | 3.10 (0, 10) | 3.95 (0, 20) | NA |
| TS (%) | ||||||
| LA | NA | 10 (38.5%) | 16 (51.6%) | 19 (42.2%) | 45 (44.1%) | NA |
| CE | NA | 3 (11.5%) | 3 (9.7%) | 3 (6.744.4%) | 9 (8.8%) | NA |
| SA | NA | 3 (11.5%) | 3 (9.7%) | 5 (11.1%) | 11 (10.8%) | NA |
| UN | NA | 10 (38.5%) | 9 (29.0%) | 18 (40.0%) | 37 (36.3%) | NA |
NIHSS – National Institutes of Health Stroke Scale; NA – data not available; TS – TOAST subtype; LA – large-artery atherosclerosis; CE – cardioembolism; SA – small artery stroke; UN – undetermined etiology.
miR-34a-5p expression in study population and its relationship with TOAST subtypes, NIHSS scores, and infarct volume
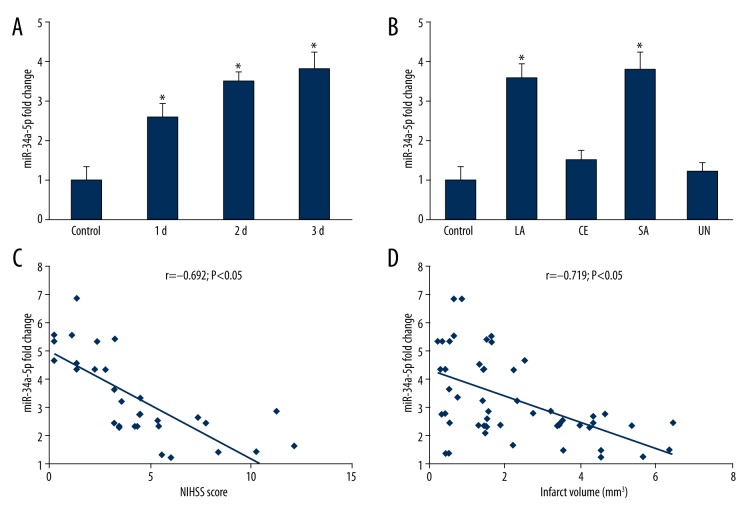
The expression of miR-34a-5p was higher during the 72 h in stroke patients compared with controls (Figure 1A). Additionally, the expression level was significantly enhanced in LA or SA stroke patients (Figure 1B). Moreover, miR-34a-5p expression levels were negatively associated with NIHSS scores (Figure 1C, r=−0.692, p<0.05) and infarct volume (Figure 1D, r=−0.719, p<0.05).
Figure 1.
miR-34a-5p expression patterns at multiple time points and the correlation with clinical data. (A) miR-34a-5p was highly expressed from day 1 to day 3 in stroke patients (26 in the day 1 group, 31 in the day 2 group, and 45 in the day 3 group). * p<0.05, compare with 97 controls. (B) Subtypes of strokes in our study included 45 LA patients, 9 CE patients, 11 SA patients, and 37 UN patients. Dramatic overexpression of miR-34a-5p was observed in LA and SA strokes patients *, p<0.05, compared with controls (n=97). LA – large-artery atherosclerosis; CE – cardioembolism; SA – small artery stroke; UN – undetermined etiology. (C) miR-34a-5p levels have a negative correlation with NIHSS scores in the scatter plot. r=−0.692, p<0.05. NIHSS – National Institutes of Health Stroke Scale. (D) miR-34a-5p levels were negatively associated with infarct volume of stroke patients. r=−0.719, p<0.05.
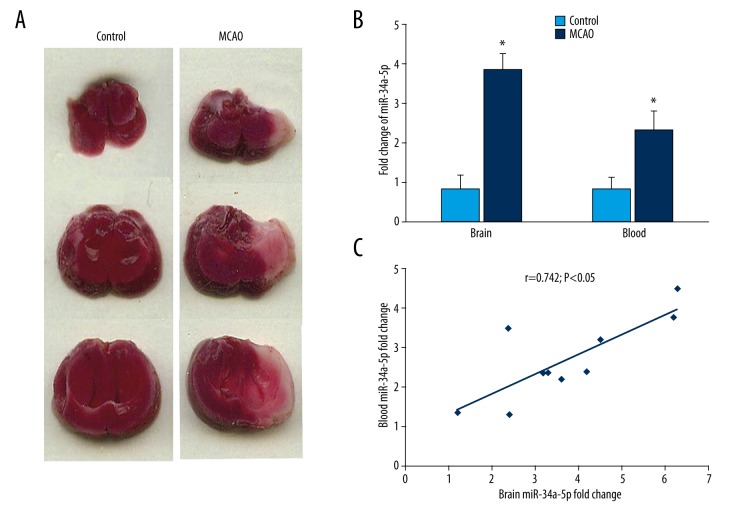
MCAO model brain sample images and relationship of brain and blood miR-34a-5p in ischemic in vivo model
Sample brain images from the MCAO rat model clearly exhibited cerebral damage compared with controls (Figure 2A). Overexpression of miR-34a-5p was observed in MCAO brain and blood samples compared to controls (Figure 2B, p<0.05). Spearman’s correlation analysis revealed a positive correlation between the plasma miR-34a-5p and the brain miR-34a-5p levels (Figure 2C, r=0.742, p<0.05).
Figure 2.
Correlation analysis of miR-34a-5p levels in blood and brain from the in vivo model. (A) Cerebral tissues from the in vivo model were collected at the end of the experiment. We prepared and stained 2-mm coronal slices with 2, 3, 5-triphenyl tetrazolium chloride. (B) The expression of miR-34a-5p in brain tissue and blood of MCAO mice at 24 h after ischemia. *, p<0.05, MCAO (n=10) vs. controls (n=10). Data represented as mean±SD in 3 independent experiments. (C) The positive correlation between blood and brain miR-34a-5p at 24 h after ischemia. r=0.742, p<0.05, n=10. MCAO – middle cerebral artery occlusion.
Discussion
Traditionally, the diagnosis of stroke mainly depended upon examination by a clinical care provider, and various neuron-imaging techniques [13–15]. However, these diagnostic methods are only able to confirm the disease status of patients and do no serve to predict disease. Therefore, there is great need for a reliable and easily detectable circulating biomarker for acute ischemic stroke risk and/or outcome prediction. Patient-based studies have already reported some alterations in circulatory miRNA expression in cerebral ischemia [16]. Additionally, the literature contains evidence of miRNA expression pattern in the circulatory system of ischemic patients [17]. For example, Long et al found decreased expression levels of circulating miR-30a, miR-126, and let-7b in ischemic stroke patients compared with the control group at 24 h, 1 week, 4 weeks, and 24 weeks, respectively [18]. However, in our study, circulatory miR-34a-5p expression was increased in patients at 72 h compared with controls. The changing expression levels in blood samples was also correlated with brain samples obtained from rat models, which demonstrates the ability of miR-34a-5p as a potential biomarker for acute ischemic stroke.
Generally, according to the TOAST classification, the subtypes of ischemic stroke include LA, CE, and SA. Based on this classification criterion, further analysis of miRNA expression patterns in these subtypes were conducted. Our results demonstrated that miR-34a-5p expression level was increased in LA and SA patients. Since the NIHSS scores was usually used to evaluate the severity level of ischemic stroke, the relationship of miRNA-34a expression level and NIHSS was assessed. Additionally, a negative correlation was discovered between miRNA-34a expression level and NIHSS in study patients. Our results suggest that miR-34a-5p is mainly sensitive for mild stroke. This negative association also implies the potential neuroprotective role of miR-34a-5p in stroke.
Welch et al. [19] conducted a series of studies on miR-34a-5p, and were the first to confirm its pro-apoptotic function. Other studies also showed similar results in which increased expression of miR-34a-5p led to elevated apoptosis in cells [20–22]. Together with our results, the overexpression of miR-34a-5p in the circulatory system of patients suggests the induction of brain cell apoptosis in acute ischemia stroke. Therefore, the effects of miR-34a-5p possibly involve the mechanism of Caspase-dependent apoptosis in acute ischemia stroke.
The expression of miR-34a-5p in blood and brain samples demonstrated a well-established positive correlation in ischemic mice in our study. These results provide evidence that plasma miR-34a-5p could be a potential biomarker in early ischemic diagnosis. Theoretically, miRNAs exist in various types of body fluids and it is not only limited to tissues [23,24]. Previous studies also confirmed that miRNA levels in blood are reproducible and are thus indicative of the disease state [25,26]. Moreover, some researchers found the common transcription of 20 miRNAs in blood and brain at 24-h reperfusion from reperfused MCAO rats [2]. These results suggest that plasma miRNA expression could be a stable marker for acute ischemia stroke.
Although we designed the study to have similar age, sex, and other demographic parameters in the patient group and control group to reduce potential selection bias, there are still some drawbacks in our study. Firstly, systematic miRNA microarray should be conducted to identify the highest expression alterations of miRNA(s) in patients, thereby increasing study strength mapping all changed miRNAs patterns. Secondly, the binding gene of miR-34a-5p needs to be investigated by in vitro luciferase process to find its therapeutic target.
Conclusions
Despite of few short-comings, the current study demonstrated the significant expressional changes of miR-34a-5p in acute ischemic stroke occurrence, subtypes, and infarct volume. Our findings reveal important alterations of miRNA in ischemic stroke and suggest that miR-34a-5p may be a viable molecular predictor in the process of acute ischemia stroke development. Further microarray studies, systematic pathway analysis, and luciferase-based functional studies with a larger sample sizes are needed to evaluate the clinical application of miRNA signatures.
Acknowledgement
We thank all clinical coordinators and the patients and their families for their participation in this study.
Footnotes
Conflict of Interest
All authors declared no conflict of interest.
Source of support: Departmental sources
References
- 1.Low SY, Ho YK, Too HP, et al. MicroRNA as potential modulators in chemoresistant high-grade gliomas. J Clin Neurosci. 2014;21:395–400. doi: 10.1016/j.jocn.2013.07.033. [DOI] [PubMed] [Google Scholar]
- 2.Jeyaseelan K, Lim KY, Armugam A. MicroRNA expression in the blood and brain of rats subjected to transient focal ischemia by middle cerebral artery occlusion. Stroke. 2008;39:959–66. doi: 10.1161/STROKEAHA.107.500736. [DOI] [PubMed] [Google Scholar]
- 3.Ouyang YB, Stary CM, Yang GY, et al. microRNAs: Innovative targets for cerebral ischemia and stroke. Curr Drug Targets. 2013;14:90–101. doi: 10.2174/138945013804806424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu XS, Michael C, Zhang RL, et al. MicroRNAs in cerebral ischemia-induced neurogenesis. J Neuropathol Exp Neurol. 2013;72:718–22. doi: 10.1097/NEN.0b013e31829e4963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rink C, Khanna S. MicroRNA in ischemic stroke etiology and pathology. Physiological Genomics. 2011;43:521–28. doi: 10.1152/physiolgenomics.00158.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dharap A, Bowen K, Place R, et al. Transient focal ischemia induces extensive temporal changes in rat cerebral MicroRNAome. J Cereb Blood Flow Metab. 2009;29:675–87. doi: 10.1038/jcbfm.2008.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sepramaniam S, Armugam A, Lim KY. MicroRNA 320a functions as a novel endogenous modulator of aquaporins 1 and 4 as well as a potential therapeutic target in cerebral ischemia. J Bio Chem. 2010;285:29223–30. doi: 10.1074/jbc.M110.144576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan KS, Armugam A, Sepramaniam S. Expression profile of microRNAs in young stroke patients. PLoS One. 2009;4:e7689. doi: 10.1371/journal.pone.0007689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agostini M, Tucci P, Steinert JR, et al. microRNA-34a regulates neurite outgrowth, spinal morphology, and function. Proc Natl Acad Sci USA. 2011;108:21099–104. doi: 10.1073/pnas.1112063108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Special report from the National Institute of Neurological Disorders and Stroke: Classification of cerebrovascular diseases III. Stroke. 1990;21:637–76. doi: 10.1161/01.str.21.4.637. [DOI] [PubMed] [Google Scholar]
- 11.Adams HP, Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke Definitions for use in a multicenter clinical trial TOAST Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 12.Sims JR, Gharai LR, Schaefer PW, et al. ABC/2 for rapid clinical estimate of infarct, perfusion, and mismatch volumes. Neurology. 2009;72:2104–10. doi: 10.1212/WNL.0b013e3181aa5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whiteley W, Tian Y, Jickling GC. Blood biomarkers in stroke: Research and clinical practice. Int J Stroke. 2012;7:435–39. doi: 10.1111/j.1747-4949.2012.00784.x. [DOI] [PubMed] [Google Scholar]
- 14.Jickling GC, Sharp FR. Blood biomarkers of ischemic stroke. Neurotherapeutics. 2011;8:349–60. doi: 10.1007/s13311-011-0050-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao D, Bi G, Feng J, et al. Association of serum chemerin levels with acute ischemic stroke and carotid artery atherosclerosis in a Chinese population. Med Sci Monit. 2015;21:3121–28. doi: 10.12659/MSM.895866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gan CS, Wang CW, Tan KS. Circulatory microRNA-145 expression is increased in cerebral ischemia. Genet Mol Res. 2012;11:147–52. doi: 10.4238/2012.January.27.1. [DOI] [PubMed] [Google Scholar]
- 17.Wang F, Long G, Zhao C, et al. Plasma microRNA-133a is a new marker for both acute myocardial infarction and underlying coronary artery stenosis. J Trans Med. 2013;11:222. doi: 10.1186/1479-5876-11-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Long G, Wang F, Li H, et al. Circulating miR-30a, miR-126 and let-7b as biomarker for ischemic stroke in humans. BMC Neurol. 2013;13:178. doi: 10.1186/1471-2377-13-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Welch C, Chen Y, Stallings RL. MicroRNA-34a functions as a potential tumor suppressor by inducing apoptosis in neuroblastoma cells. Oncogene. 2007;26:5017–22. doi: 10.1038/sj.onc.1210293. [DOI] [PubMed] [Google Scholar]
- 20.Raver-Shapira N, Marciano E, Meiri E, et al. Transcriptional activation of miR-34a contributes to p53-mediated apoptosis. Mol Cell. 2007;26:731–43. doi: 10.1016/j.molcel.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 21.Chang TC, Wentzel EA, Kent OA, et al. Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol Cell. 2007;26:745–52. doi: 10.1016/j.molcel.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sano T, Reynolds JP, Jimenez-Mateos EM, et al. MicroRNA-34a up regulation during seizure-induced neuronal death. Cell Death Dis. 2012;3:e287. doi: 10.1038/cddis.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ha M, Kim VN. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol. 2014;15:509–24. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- 24.Zhu J, Yue H, Qiao C, et al. Association between single-nucleotide polymorphism (SNP) in miR-146a, miR-196a2, and miR-499 and risk of ischemic stroke: A meta-analysis. Med Sci Monit. 2015;21:3658–63. doi: 10.12659/MSM.895233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Creemers EE, Tijsen AJ, Pinto YM. Circulation microRNAs: Novel biomakers and extracellular communicators in cardivascular disease? Circ Res. 2012;110:483–95. doi: 10.1161/CIRCRESAHA.111.247452. [DOI] [PubMed] [Google Scholar]
- 26.Chen X, Ba Y, Ma L. Characterization of microRNAs in serum: A novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18:997–1006. doi: 10.1038/cr.2008.282. [DOI] [PubMed] [Google Scholar]