Abstract
Fam20c is essential for the normal mineralization of dentin and bone. The generation of odontoblast and osteoblast cell lines carrying floxed Fam20c allele can offer valuable tools for the study of the roles of Fam20c in the mineralization of dentin and bone. The limited capability of the primary odontoblasts and osteoblasts to proliferate necessitates the development of odontoblast and osteoblast cell lines serving as substitutes for the study of differentiation and mineralization of the odontoblasts and osteoblasts. In this study, we established and characterized immortalized mouse floxed Fam20c dental papilla mesenchymal and osteoblast cell lines. The isolated primary mouse floxed Fam20c dental papilla mesenchymal cells and osteoblasts were immortalized by the infection of lentivirus containing Simian Virus 40 T-antigen (SV40 T-Ag). The immortalization of floxed Fam20c dental papilla mesenchymal cells and osteoblasts was verified by the long-term passages and genomic integration of SV40 T-Ag. The immortalized floxed Fam20c dental papilla mesenchymal and osteoblast cell lines not only proliferated at a high rate and retained the morphology of their primary counterparts, but also preserved the dentin and bone specific gene expression as the primary dental papilla mesenchymal cells and osteoblasts did. Consistently, the capability of the primary floxed Fam20c dental papilla mesenchymal cells and osteoblasts to mineralize was also inherited by the immortalized dental papilla mesenchymal and osteoblast cell lines. Thus, we have successfully generated the immortalized mouse floxed Fam20c dental papilla mesenchymal and osteoblast cell lines.
Dentin and bone are two mineralized tissues that resemble each other in composition and mechanisms of formation. Odontoblasts and osteoblasts are the major cells necessary for the morphogenesis, maturation, and mineralization of dentin and bone, respectively. Odontoblasts and osteoblasts synthesize a series of extracellular matrix (ECM) proteins, that include type I collagen and SIBLINGs [Small Integrin-Binding Ligand N-linked Glycoproteins consisting of dentin sialophosphoprotein (DSPP), dental matrix protein 1 (DMP1), osteopontin (OPN), bone sialoprotein (BSP), and matrix extracellular phosphoglycoprotein (MEPE)] (Qin et al., 2004; Chen et al., 2008). These ECM molecules undergo post-translational modifications essential for the formation of dentin and bone. For example, disorders in the post-translational hydroxylation of type I pro-collagen resulting from the mutations of prolyl-3-hydroxylase-1 (P3H1, encoded by LEPRE1 gene) and/or cartilage-associated protein (CRTAP) cause autosomal recessive osteogenesis imperfecta (Vranka et al., 2004; Barnes et al., 2006; Morello et al., 2006). As one of the most important post-translational modifications, phosphorylation of SIBLINGs is essential for the normal mineralization of dentin and bone (Razzouk et al., 2002; Qin et al., 2004; Tartaix et al., 2004; Gericke et al., 2005).
FAM20C is a kinase that phosphorylates a series of secretory proteins including the SIBLINGs (Tagliabracci et al., 2012). FAM20C belongs to the “family with sequence similarity 20 (FAM20)” and is highly expressed in the differentiating and matured odontoblasts and osteoblasts (Wang et al., 2010). Deficiencies in human FAM20C cause Raine Syndrome manifesting as lethal osteosclerotic bone dysplasia or hypophosphatemic Rickets (Simpson et al., 2007; Simpson et al., 2009). Inactivation of Fam20c in mice results in mineralization disorders in dentin and bone. The defective mineralization in Fam20c-deficient mice is accompanied by altered phosphorus metabolism, reduced DMP1 expression and elevated serum FGF23 level (Wang et al., 2012a). A more recent study suggests that FAM20C phosphorylates Ser180 of FGF23, which prevents the O-glycosylation on Thr178 and allows the proteolysis of FGF23 (Tagliabracci et al., 2014). However, the understanding of the biochemical mechanisms by which FAM20C phosphorylates its substrates is still limited, partially due to the lack of valuable tools such as Fam20c-deficient cell lines. Mouse cell lines without the Fam20c allele derived from cells that form the presumptive mineralized tissues are the ideal tools to study the kinase roles of normal and mutant FAM20C.
In the present study, we developed and characterized the dental papilla mesenchymal (which can differentiate into odontoblasts) and osteoblast cell lines carrying floxed Fam20c allele. The primary dental papilla mesenchymal cells and osteoblasts isolated from Fam20cf/f mice were transformed into immortal cell lines by SV40T-Ag transfection (Wu et al., 2010;Wu et al., 2011). These cell lines displayed a stable capability for expansion as well as an identical gene expression profile to their primary cells. Using the benefit of the floxed Fam20c allele (Wang et al., 2012a), exon 6–9 can be excised by Cre recombinase to generate cell lines with the null Fam20c allele, which can be used for further experiments in the studies on FAM20C.
Materials and Methods
Genotyping of mice carrying floxed Fam20c allele (Fam20cf/f mouse)
The floxed Fam20c allele was generated by inserting Cre recombinase recognition sites (loxp) upstream to exon 6 and downstream to exon 9 into the mouse Fam20c gene. The Fam20cf/f mice were genotyped by polymerase chain reaction (PCR) assay as described previously. The floxed Fam20c allele produced a band of 400 bp, which was 100 bp shorter than that of the WT Fam20c allele (Wang et al., 2012a). The protocols for mouse utilization were approved by the Institutional Animal Care and Use Committee of Baylor College of Dentistry of Texas A&M University Health Sciences Center, TX, USA.
Primary dental papilla mesenchymal cells and calvarial osteoblasts from Fam20cf/f mouse
The first molars of postnatal 4-day Fam20cf/f mice were collected for the isolation of dental papilla mesenchyme, which was digested in 3 mg/ml collagenase type I and 4 mg/ml of dispase for 30 min at 37°C. The digested dental mesenchyme was dispersed in a single-cell suspension by mechanical aspiration up and down for further ex vivo expansion. The calvarial bones of postnatal 1 day Fam20cf/f mice were isolated and cut into small 0.1 cm3 pieces. These pieces were attached to petri dishes for the emigration of osteoblasts from the calvarial bones. Both the primary dental papilla mesenchymal cells and calvarial osteoblasts were cultured with αMEM supplemented with 10% fetal bovine serum, 100 unit/ml pencillin and 100 µg/ml streptomycin at 37°C in humidified air containing 5% CO2. The medium was refreshed every 3 days until the cells reached confluence.
Lentivirus infection and selection of immortalized cells
The primary cells of passage 3 were infected by lentivirus containing SV40 T-Ag following the manufacturer’s protocol (Applied Biological Materials, In., Richmond, BC). After infection, the primary cells were re-plated at a low density to get separated clones from a single cell. Twelve clones from infected dental papilla mesenchymal cells and calvarial osteoblasts were selected for further examination and passages, respectively. After 30 passages over 6 months, all the selected clones were expanded into cell lines. One of the 12 clones was selected randomly from the immortalized dental papilla mesenchymal and osteoblast cell lines for the further analysis.
Cell morphology and proliferation
The primary cells of passage 3 and the immortal cell lines after passage 40 were selected for cell morphology and proliferation evaluation. The morphologies of the primary cells and immortalized cell lines were observed by a light inverted microscope. 5-bromo-2′-deoxyuridine (BrdU) was added to the medium in the 30 µM concentration to label the nuclei of the proliferating cells (Life technologies, Inc., Grand Island, NY). Four hours after the incorporation of BrdU, testing for BrdU positive nuclei in the primary cells and immortalized cell lines was performed with the BrdU assay Kit (Life technologies, Inc.).
RNA extraction and reverse transcription-PCR (RT-PCR)
Total RNA was extracted from passage 3 primary cells and passage 40 cell lines with RNA extraction Kit and total mRNA was reverse transcribed into cDNA with the RT-PCR Kit (Life Technologies, Inc.). The protocol for the amplification of Alp (Alkaline phosphatase), Atf4 (Activating transcription factor 4), Bsp (Bone sialoprotein), Col1a1 (Alpha 1 collagen type 1), Dlx3 (Distal-less homeobox 3), Dmp1(Dental matrix protein 1), Dspp (Dentin sialophosphoprotein), Gapdh (Glyceraldehyde-3-phosphate dehydrogenase), Mepe (Matrix extracellular phosphoglycoprotein), Ocn (Osteocalcin), Opn (Osteopontin), Osn (Osteonectin), Osx (Osterix), Runx2 (Runt-related transcription factor 2), and SV40 were reported previously (Wu et al., 2010; Wu et al., 2011). The cDNA of FAM20C transcripts was amplified with the primers (Forward: 5′-TGCGGAGATCGCTGCCTTCC-3′; Reverse: 5′-GCCACTGTCGTAGG GTGG CA-3′) at the annealing temperature of 55°C; the size of the PCR product was expected to be 450 bp.
Immunohistochemistry
For the detection of SV40 T-Ag and the dentin or bone-specific proteins, polyclonal (rabbit) antibodies against ATF4, DLX3, MEPE, OSN, OCN (Santa Cruz Biotechnology, Inc., Snata Cruz, CA), OPN and OSX (Abcam, Cambridge, MA), and the monoclonal (mouse) antibodies against SV40 T-Ag, RUNX2 and COL1α1 were applied (Abcam). The antibodies against BSP (a gift from Dr. Larry Fisher, National Institue of Dental and Craniofacial Research), DMP1 and DSP were applied as previously described (Baba et al., 2004; Huang et al., 2008; Liu et al., 2014). The secondary antibodies were goat anti-rabbit or goat anti-mouse antibodies conjugated with Biotin (Vector Laboratories, Inc., Burlingame, CA). In the negative control groups, the primary antibodies were not used, while the secondary antibodies were the same as in the experimental groups.
Alkaline phosphatase (ALP) assay and the mineralization assay
For the ALP activity assay, the primary cells and immortalized cell lines were cultured in the mineralization-inducing medium for a week (α-MEM supplemented with 10% fetal bovine serum, 100 unit/ml pencillin,100 µg/ml streptomycin, 50 µg/ml ascorbic acid and 10 mM sodium β-glycerophosphate). The in situ ALP staining was performed following the protocol described in the assay kit (Bio-Rad, Hercules, CA). For the mineralization assay, the primary cells and immortalized cell lines were cultured for 2–3 weeks in the mineralization-inducing medium. The mineralized cell culture was fixed with 4% PFA for 10 min, rinsed with phosphate buffered saline (PBS) and stained with Alizarin Red S (Sigma–Aldrich, St. Louis, MO).
Results
Immortalization of floxed Fam20c dental papilla mesenchymal cells and osteoblasts
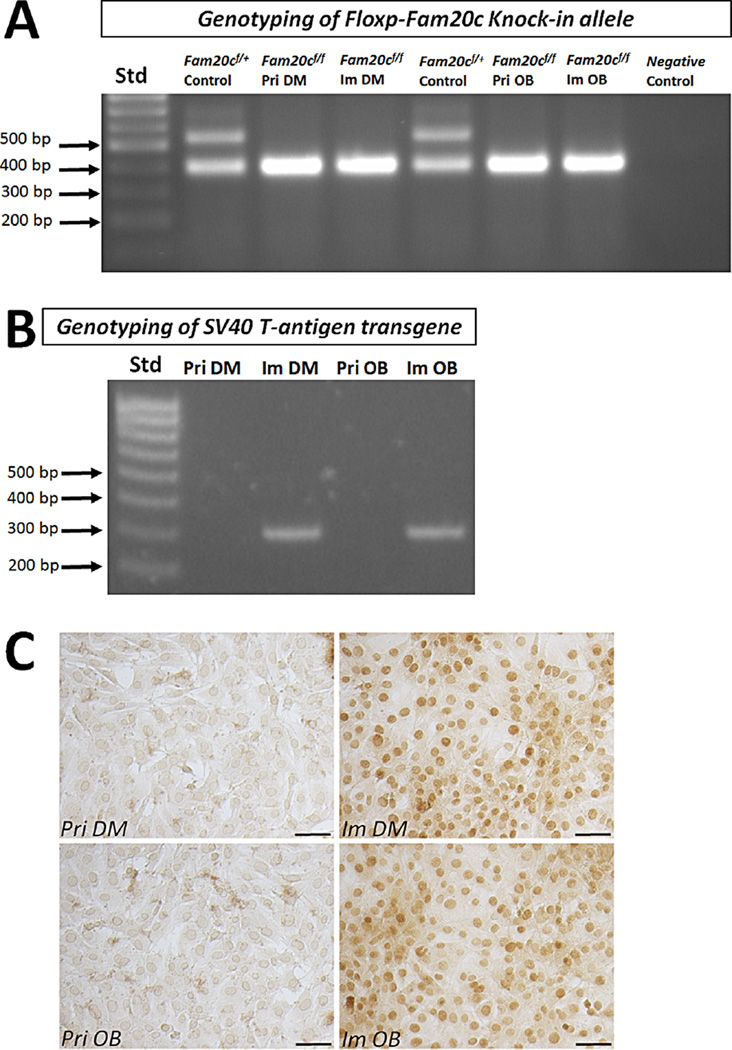
The homozygous Fam20cf/f mice used for cell isolation were confirmed by PCR as previously described (Wang et al., 2012a). The primary mesenchymal cells from the dental papilla and osteoblasts from the calvarium were isolated and re-confirmed by genotyping for their homozygous floxed Fam20c allele (Fig. 1A). To immortalize the dental papilla mesenchymal cells and osteoblasts, the lentivirus containing SV40 T-Ag gene was applied (Wu et al., 2010; Wu et al., 2011). After 10–14 days of the infection, colonies formed from the infected dental papilla mesenchymal cell and osteoblast culture. The colonies selected for the further 40 passages bypassed senescence without evidence of growth retardation in the following 5 months. One of the dental papilla mesenchymal cell lines and one of the osteoblast cell lines were selected for detailed characterization. Both the genotyping PCR with specific primers and immunohistochemistry with antibody against SV40 T-Ag demonstrated that SV40 T-Ag was detected only in the passaged dental papilla mesenchymal and osteoblast cell lines, but not in the primary cells (Fig. 1B and C).
Fig. 1.
Identification of floxed Fam20c alleles and SV40 transformation. A: Genomic DNA isolated from Fam20cf/+ control mouse (Lane 2 and 5), primary dental papilla (Lane 3, Pri DM) and calvarium (Lane 6, Pri OB) from Fam20cf/f mouse, and immortalized dental papilla mesenchymal cells (Lane 4, Im DM) and immortalized osteoblasts (Lane 7, Im OB) from the Fam20cf/f dental papilla mesenchyme and calvarium were amplified by PCR using primers specific for the floxed Fam20c alleles. (Lane 1 is DNA ladder labeled as Std; Lane 8 is negative control that contained only primers specific for the floxed Fam20c and no genomic DNA). B: Genomic DNA extracted from primary dental papilla (Lane 2) and calvarium (Lane 4) of Fam20cf/f mouse, and immortalized dental papilla mesenchymal cells (Lane 3) and osteoblasts (Lane 5) were amplified by primers specific for SV40 T-Ag. (Lane 1 is DNA ladder labeled as Std). C: Immunohistochemical staining with the antibody against SV40 T-Ag. The immunostaining of SV40 T-Ag was only present in the nuclei of immortalized dental papilla mesenchymal and osteoblast cell lines, but absent in the nuclei of primary dental papilla mesenchymal cells and calvarial osteoblasts. (Pri DM, primary dental papilla mesenchymal cells; Pri OB, primary calvarial osteoblasts; Im DM, immortalized dental papilla mesenchymal cell line; Im OB, immortalized osteoblast cell line; Scale bar, 100 µm).
Morphology and proliferation of the primary and immortalized dental papilla mesenchymal and osteoblast cell lines
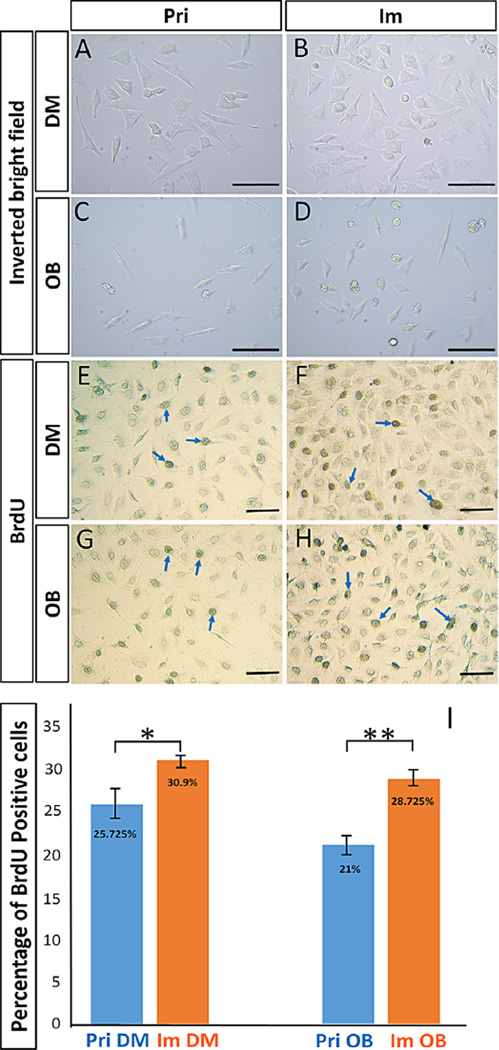
Under the light-inverted microscope, the immortalized dental papilla mesenchymal cell lines after passage 40 exhibited a fibroblast-like morphology similar to that of the primary Fam20cf/f dental papilla mesenchymal cells (Fig. 2A and B). Similarly, both the primary Fam20cf/f calvarial osteoblasts and the immortalized osteoblasts showed the typical fibroblast shape (Fig. 2C and D). However, both the immortalized dental papilla mesenchymal cells and osteoblasts proliferated at a significantly higher rate compared with their primary counterparts in the BrdU-labelling assays (Fig. 2E–I).
Fig. 2.
Comparison of cell morphologies and proliferation rates between Fam20cf/f primary and immortalized cell lines. A and B: The immortalized dental papilla mesenchymal cell line (B) showed a similar fibroblast-like morphology as to their primary dental papilla mesenchymal cells (A). C and D: The primary calvarial osteoblasts (C) and the immortalized osteoblast cell line (D) had the same fibroblast shape. E–H: Immunohistochemistry with the antibody against BrdU showed the BrdU positive nuclei (blue arrows) in the primary dental papilla mesenchymal cells (E), immortalized dental papilla mesenchymal cell line (F), primary calvarial osteoblasts (G) and immortalized osteoblast cell line (H). I: Statistical comparison of proliferation rates. The percentage of BrdU positive nuclei in the immortalized dental papilla mesenchymal cell line was 30.9% (SD = 2.228%), which was significantly higher than that of primary dental papilla mesenchymal cells (25.725%, SD = 3.3% P < 0.05); the difference in BrdU positive percentage between the immortalized osteoblast cell line (28.725%, SD = 0.95%) and primary calvarial osteoblasts (21.0%, SD = 3.915%) was also significant (P < 0.01). (Scale bar, 100 µm).
Tissue-specific gene expression in the primary and immortalized cells
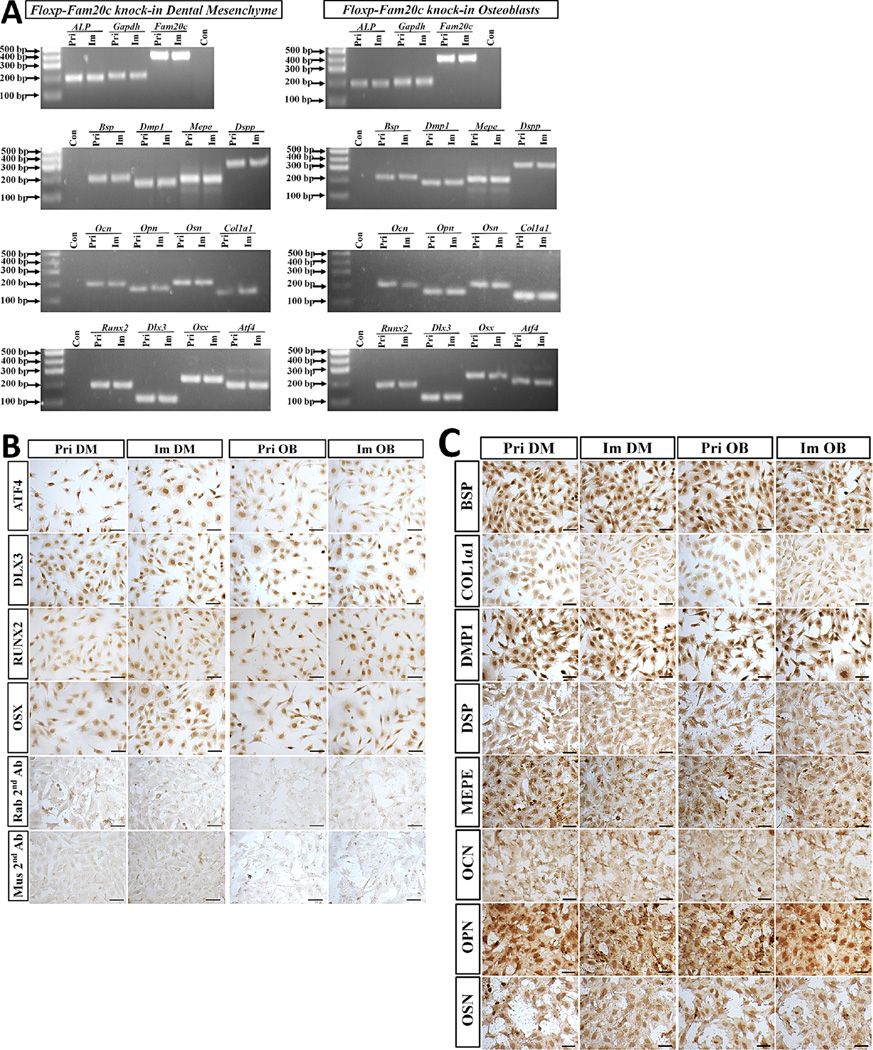
To examine if the immortalized cell lines have maintained their dentin-specific and bone-specific gene expression profile, RT-PCR assay was performed to detect the gene expression associated with the differentiation and mineralization of odontoblast and osteoblasts. The transcripts of Alp, Fam20c, Bsp, Dmp1, Mepe, Dspp, Ocn, Opn, Osn, Col1α1, Runx2, Dlx3, Osx, and Atf4 were detected in the primary dental papilla mesenchymal cells as well as in the immortalized dental papilla mesenchymal cell line (Fig. 3A). The proteins encoded by these genes were also detected by immunohistochemistry in the primary and immortal dental papilla mesenchymal cells (Fig. 3B and C). Both RT-PCR and immunohistochemistry results consistently confirmed the expression of Alp, Fam20c, Bsp, Dmp1, Mepe, Dspp, Ocn, Opn, Osn, Col1α1, Runx2, Dlx3, Osx, and Atf4 in the primary calvarial osteoblasts and immortalized osteoblasts (Fig. 3A–C). These data confirmed that the immortalized Fam20cf/f dental papilla mesenchymal and osteoblast cell lines have sustained their dentinogenic and osteogenic characteristics, respectively, even after a long term passage and culture.
Fig. 3.
Dentin-specific and bone-specific gene expression in the primary and immortalized cells. A: Total RNA extracted from primary dental papilla mesenchymal cells, primary calvarial osteoblasts, immortalized dental papilla mesenchymal and osteoblast cell lines were reversely transcribed into cDNA, and then, amplified by PCR using the primers specific for Alp, Fam20c, Bsp, Dmp1, Mepe, Dspp, Ocn, Opn, Osn, Col1, Runx2, Dlx3, Osx, and Atf4. B and C: Immunohistochemistry with antibodies against ATF4, DLX3, RUNX2, OSX, BSP, COL1A1, DMP1, DSP, MEPE, OCN, OPN, and OSN in the primary dental papilla mesenchymal cells, primary calvarial osteoblasts, immortalized dental papilla mesenchymal, and osteoblast cell lines. In the negative controls, only the secondary antibodies against Rabbit (Rab 2nd Ab) and mouse (Mus 2nd Ab) IgG, while no primary antibodies were applied. (Pri DM, primary dental papilla mesenchymal cells; Pri OB, primary calvarial osteoblasts; Im DM, immortalized dental papilla mesenchymal cell line; Im OB, immortalized osteoblast cell line; Scale bar = 100 µm).
Induced differentiation and mineralization of the immortalized Fam20cf/f dental papilla mesenchymal and osteoblast cell lines
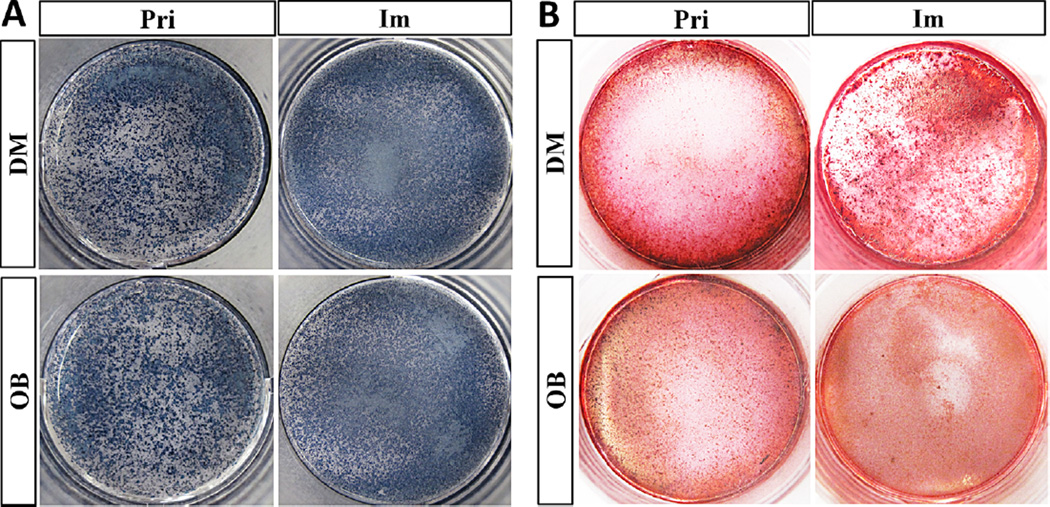
To further confirm the dentinogenic and osteogenic characteristics in the transformed Fam20cf/f dental papilla mesenchymal cells and osteoblasts, the activity of ALP, the marker for both the odontoblast and osteoblast differentiation, was examined in the immortalized cell lines. After one week’s culture in the mineralization-inducing medium, positive staining of ALP activity was found in the primary dental papilla mesenchymal cells, primary calvarial osteoblasts, the induced immortalized dental papilla mesenchymal and osteoblast cell lines (Fig. 4A). When the mineralization-inducing culture extended to 2 weeks, the primary dental papilla mesenchymal cells, primary calvarial osteoblasts, the induced immortalized dental papilla mesenchymal and osteoblast cell lines formed mineralized nodules (Fig. 4B).
Fig. 4.
Alkaline phosphatase (ALP) activity and the formation of mineralized nodules in the Fam20cf/f primary and immortalized cell lines. A: After 1 week’s culture in the mineralization-inducing medium, in situ cytochemsitry of ALP activity showed positive staining in the primary dental papilla mesenchymal cells, primary calvarial osteoblasts, and the induced immortalized dental papilla mesenchymal and osteoblast cell lines. B: After 2 weeks of mineralization-inducing culture, condensed mineralized nodules were also detected by Alizarin Red S staining in the primary dental papilla mesenchymal cells, primary calvarial osteoblasts, the induced immortalized dental papilla mesenchymal and osteoblast cell lines.
Discussion
Odontoblasts and osteoblasts play important roles in the formation, mineralization and repair of dentin and bone, respectively. However, due to the Hayflick limit (Hayflick and Moorhead, 1961), the cultured primary cells undergo senescence rapidly and are often unable to provide an adequate number of cells for analyses. Quite a few odontoblast and osteoblast cell lines have been developed from human and mouse, which are used to elucidate the mechanism of the formation and mineralization of dentin and bone under physiological and pathological condition (McKay et al., 1996; Hulley et al., 1998; MacDougall et al., 1998; Costa et al., 1999; Shea et al., 2000; He et al., 2004). Using the benefits of the SV40 T-antigen and the lentivirus vectors, the primary cells were reported to be transformed into immortal cell lines that still retained the original genotypic and phenotypic characteristics (Wu et al., 2010; Wu et al., 2011). The immortalized dental papilla mesenchymal and osteoblast cell lines transformed by SV40 T-antigen have been applied in the physiological and pathological research in the cell differentiation, mineralization, regeneration, gene expression regulation, and crosstalk of signaling pathways in dentin and bone (Galler et al., 2006; Iwata et al., 2007; Hoffman et al., 2010).
The Fam20c-deficient mice suffered from hypophosphataemic rickets which affected dentin and bone (Wang et al., 2012a, b). Several studies indicated that FAM20C regulates mineralization by phosphorylating the SIBLING proteins and FGF23 (Ishikawa et al., 2012; Tagliabracci et al., 2012; Tagliabracci et al., 2014). However, the exact mechanisms of how FAM20C acts in regulating dentinogenesis and osteogenesis are unclear, and there is a discrepancy between the osteosclerotic phenotype associated with some human FAM20C mutations and the rickets phenotype of Fam20c-deficient mice. By introducing the Cre recombinase, the Fam20cf/f dental papilla mesenchymal and osteoblast cell lines can be transformed into Fam20c-deficient dental papilla mesenchymal and osteoblast cell lines which can thus serve as a valuable tool to study the mechanisms of how FAM20C works in the phosphorylation of secretory proteins, cell differentiation and the potential crosstalk among different signaling pathways as well as in the elucidation of pathogenesis underlying the development of Raine syndrome. For example, by transfecting the constructs expressing FAM20C with different mutations identified in Raine syndrome patients into the Fam20c-null dental papilla mesenchymal and osteoblast cell lines, investigators can determine the effects of various mutations on the kinase activity of the different mutant FAM20C enzymes. To ensure that the information obtained from the immortalized cell lines is applicable to the primary cells, the genotypes and phenotypes of the primary cells must be maintained in the transformed (immortalized) cell lines. In our study, the primary and the immortalized dental papilla mesenchymal and osteoblasts exhibited similar morphologies. The strong ALP activity and capability of forming mineralized nodules were also retained in the SV40 T-antigen-positive dental papilla mesenchymal and osteoblast cell lines. The gene expression assay showed that both the immortalized dental papilla mesenchymal cells and the osteoblasts expressed osteonectin, osteocalcin, collagen Type I, and SIBLINGs, which are the main components of the ECM molecules involved in the differentiation and mineralization of odontoblasts and osteoblasts. The accelerated proliferation rate in the transformed cell lines, which was also observed in other SV40 T-antigen transformed cell lines, is attributed to the promotion of SV40 T-antigen on the cell cycle as described previously (Porcu et al., 1992). Moreover, the transcription factors key to dentinogenesis and osteogenesis, including Runx2, Osx, Atf4, and Dlx3, were expressed in the immortalized dental papilla mesenchymal and osteoblasts as well as in their primary counterparts. Taken together, we concluded that the immortalized Fam20cf/f dental papilla mesenchymal and osteoblast cell lines have maintained the characteristics of the primary dental papilla mesenchymal cells and osteoblasts. We envision that these Fam20cf/f cell lines will serve as an excellent tool for the study of FAM20C function in dentinogenesis and osteogenesis.
Acknowledgments
We are grateful to Jeanne Santa Cruz for her assistance with editing this article. This work was supported by NIH Grant R01DE022549 (to CQ).
Literature Cited
- Baba O, Qin C, Brunn JC, Jones JE, Wygant JN, McIntyre BW, Butler WT. Detection of dentin sialoprotein in rat periodontium. Eur J Oral Sci. 2004;112:163–170. doi: 10.1111/j.0909-8836.2004.00110.x. [DOI] [PubMed] [Google Scholar]
- Barnes AM, Chang W, Morello R, Cabral WA, Weis M, Eyre DR, Leikin S, Makareeva E, Kuznetsova N, Uveges TE, Ashok A, Flor AW, Mulvihill JJ, Wilson PL, Sundaram UT, Lee B, Marini JC. Deficiency of cartilage-associated protein in recessive lethal osteogenesis imperfecta. N Engl J Med. 2006;355:2757–2764. doi: 10.1056/NEJMoa063804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Chen L, Jahangiri A, Chen B, Wu Y, Chuang HH, Qin C, MacDougall M. Expression and processing of small integrin-binding ligand N-linked glycoproteins in mouse odontoblastic cells. Arch Oral Biol. 2008;53:879–889. doi: 10.1016/j.archoralbio.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa CA, Vaerten MA, Edwards CA, Hanks CT. Cytotoxic effects of current dental adhesive systems on immortalized odontoblast cell line MDPC-23. Dent Mater. 1999;15:434–441. doi: 10.1016/s0109-5641(99)00071-8. [DOI] [PubMed] [Google Scholar]
- Galler KM, Schweikl H, Thonemann B, D’Souza RN, Schmalz G. Human pulp-derived cells immortalized with Simian Virus 40 T-antigen. Eur J Oral Sci. 2006;114:138–146. doi: 10.1111/j.1600-0722.2006.00327.x. [DOI] [PubMed] [Google Scholar]
- Gericke A, Qin C, Spevak L, Fujimoto Y, Butler WT, Sùrensen ES, Boskey AL. Importance of phosphorylation for osteopontin regulation of biomineralization. Calcif Tissue Int. 2005;77:45–54. doi: 10.1007/s00223-004-1288-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He WX, Niu ZY, Zhao SL, Jin WL, Gao J, Smith AJ. TGF-beta activated Smad signalling leads to a Smad3-mediated down-regulation of DSPP in anodontoblast cell line. Arch Oral Biol. 2004;49:911–918. doi: 10.1016/j.archoralbio.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- Hoffman BE, Newman-Tarr TM, Gibbard A, Wang S, Hanning C, Pratta MA, Boyle RJ, Kumar S, Majumdar MK. Development and characterization of a human articular cartilage-derived chondrocyte cell line that retains chondrocyte phenotype. J Cell Physiol. 2010;222:695–702. doi: 10.1002/jcp.21994. [DOI] [PubMed] [Google Scholar]
- Huang B, Sun Y, Maciejewska I, Qin D, Peng T, McIntyre B, Wygant J, Butler WT, Qin C. Distribution of SIBLING proteins in the organic and inorganic phases of rat dentin and bone. Eur J Oral Sci. 2008;116:104–112. doi: 10.1111/j.1600-0722.2008.00522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulley PA, Gordon F, Hough FS. Inhibition of mitogen-activated protein kinase activity and proliferation of an early osteoblast cellline (MBA 15.4) by dexamethasone: Role of protein phosphatases. Endocrinology. 1998;139:2423–2431. doi: 10.1210/endo.139.5.6020. [DOI] [PubMed] [Google Scholar]
- Ishikawa HO, Xu A, Ogura E, Manning G, Irvine KD. The Raine syndrome protein FAM20C is a Golgi kinase that phosphorylates bio-mineralization proteins. PLoS ONE. 2012;7:e42988. doi: 10.1371/journal.pone.0042988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata T, Yamakoshi Y, Simmer JP, Ishikawa I, Hu JC. Establishment of porcine pulp-derived cell lines and expression of recombinant dentin sialoprotein and recombinant dentin matrix protein-1. Eur J Oral Sci. 2007;115:48–56. doi: 10.1111/j.1600-0722.2007.00426.x. [DOI] [PubMed] [Google Scholar]
- Liu PH, Zhang H, Liu C, Wang XF, Chen L, Qin CL. Inactivation of Fam20C in cell expressing Type I collagen causes periodontal disease in Mice. PLoS ONE. 2014;9:e114396. doi: 10.1371/journal.pone.0114396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDougall M1, Selden JK, Nydegger JR, Carnes DL. Immortalized mouse odontoblast cell line MO6-G3 application for in vitro biocompatibility testing. Am J Dent. 1998;11:S11–S16. [PubMed] [Google Scholar]
- McKay GC, Macnair R, MacDonald C, Grant MH. Interactions of orthopaedic metals with an immortalized rat osteoblast cell line. Biomaterials. 1996;17:1339–1344. [PubMed] [Google Scholar]
- Morello R, Bertin TK, Chen Y, Hicks J, Tonachini L, Monticone M, Castagnola P, Rauch F, Glorieux FH, Vranka J, Bächinger HP, Pace JM, Schwarze U, Byers PH, Weis M, Fernandes RJ, Eyre DR, Yao Z, Boyce BF, Lee B. CRTAP is required for prolyl 3- hydroxylation and mutations cause recessive osteogenesis imperfecta. Cell. 2006;127:291–304. doi: 10.1016/j.cell.2006.08.039. [DOI] [PubMed] [Google Scholar]
- Porcu P, Ferber A, Pietrzkowski Z, Roberts CT, Adamo M, LeRoith D, Baserga R. The growth-stimulatory effect of simian virus 40 T antigen requires the interaction of insulinlike growth factor 1 with its receptor. Mol Cell Biol. 1992;12:5069–5077. doi: 10.1128/mcb.12.11.5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzouk S, Brunn JC, Qin C, Tye CE, Goldberg HA, Butler WT. Osteopontin posttranslational modifications, possibly phosphorylation, are required for in vitro bone resorption but not osteoclast adhesion. Bone. 2002;30:40–47. doi: 10.1016/s8756-3282(01)00637-8. [DOI] [PubMed] [Google Scholar]
- Shea LD, Wang D, Franceschi RT, Mooney DJ. Engineered bone development from a pre-osteoblast cell line on three-dimensional scaffolds. Tissue Eng. 2000;6:605–617. doi: 10.1089/10763270050199550. [DOI] [PubMed] [Google Scholar]
- Simpson MA, Hsu R, Keir LS, Hao J, Sivapalan G, Ernst LM, Zackai EH, Al-Gazali LI, Hulskamp G, Kingston HM, Prescott TE, Ion A, Patton MA, Murday V, George A, Crosby AH. Mutations in FAM20C are associated with lethal osteosclerotic bone dysplasia (Raine syndrome), highlighting a crucial molecule in bone development. Am J Hum Genet. 2007;81:906–912. doi: 10.1086/522240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson MA, Scheuerle A, Hurst J, Patton MA, Stewart H, Crosby AH. Mutations in FAM20C also identified in non-lethal osteosclerotic bone dysplasia. Clin Genet. 2009;75:271–276. doi: 10.1111/j.1399-0004.2008.01118.x. [DOI] [PubMed] [Google Scholar]
- Tagliabracci VS, Engel JL, Wen J, Wiley SE, Worby CA, Kinch LN, Xiao J, Grishin NV, Dixon JE. Secreted kinase phosphorylates extracellular proteins that regulate biomineralization. Science. 2012;336:1150–1153. doi: 10.1126/science.1217817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliabracci VS, Engel JL, Wiley SE, Xiao J, Gonzalez DJ, Nidumanda Appaiah H, Koller A, Nizet V, White KE, Dixon JE. Dynamic regulation of FGF23 by Fam20C phosphorylation, GalNAc-T3 glycosylation, and furin proteolysis. Proc Natl Acad Sci USA. 2014;111:5520–5525. doi: 10.1073/pnas.1402218111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaix PH, Doulaverakis M, George A, Fisher LW, Butler WT, Qin C, Salih E, Tan M, Fujimoto Y, Spevak L, Boskey AL. In vitro effects of dentin matrix protein-1 on hydroxyapatite formation provide insights into in vivo functions. J Biol Chem. 2004;279:18115–18120. doi: 10.1074/jbc.M314114200. [DOI] [PubMed] [Google Scholar]
- Qin C, Baba O, Butler WT. Post-translational modifications of sibling proteins and their roles in osteogenesis and dentinogenesis. Crit Rev Oral Biol Med. 2004;15:126–136. doi: 10.1177/154411130401500302. [DOI] [PubMed] [Google Scholar]
- Qin C, Baba O, Butler WT. Post-translational modifications of sibling proteins and their roles in osteogenesis and dentinogenesis. Crit Rev Oral Biol Med. 2004;15:126–136. doi: 10.1177/154411130401500302. [DOI] [PubMed] [Google Scholar]
- Vranka JA, Sakai LY, Bächinger HP. Prolyl 3-hydroxylase 1, enzyme characterization and identification of a novel family of enzymes. J Biol Chem. 2004;279:23615–23621. doi: 10.1074/jbc.M312807200. [DOI] [PubMed] [Google Scholar]
- Wang X, Hao J, Xie Y, Sun Y, Hernandez B, Yamoah AK, Prasad M, Zhu Q, Feng JQ, Qin C. Expression of FAM20C in the osteogenesis and odontogenesis of mouse. J Histochem Cytochem. 2010;58:957–967. doi: 10.1369/jhc.2010.956565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wang S, Li C, Gao T, Liu Y, Rangiani A, Sun Y, Hao J, George A, Lu Y, Groppe J, Yuan B, Feng JQ, Qin C. Inactivation of a novel FGF23 regulator, FAM20C, leads to hypophosphatemic rickets in mice. PLoS Genet. 2012;8:e1002708. doi: 10.1371/journal.pgen.1002708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wang S, Lu Y, Gibson MP, Liu Y, Yuan B, Feng JQ, Qin C. FAM20C plays an essential role in the formation of murine teeth. J Biol Chem. 2012;287:35934–35942. doi: 10.1074/jbc.M112.386862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LA, Feng J, Wang L, Mu YD, Baker A, Donly KJ, Gluhak-Heinrich J, Harris SE, MacDougall M, Chen S. Immortalized mouse floxed Bmp2 dental papilla mesenchymal cell lines preserve odontoblastic phenotype and respond to BMP2. J Cell Physiol. 2010;225:132–139. doi: 10.1002/jcp.22204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LA, Feng J, Wang L, Mu YD, Baker A, Donly KJ, Harris SE, MacDougall M, Chen S. Development and characterization of a mouse floxed Bmp2 osteoblast cell line that retains osteoblast genotype and phenotype. Cell Tissue Res. 2011;343:545–558. doi: 10.1007/s00441-010-1120-3. [DOI] [PMC free article] [PubMed] [Google Scholar]