Abstract
Voltage-gated sodium channels (Nav) are critical regulators of neuronal excitability. Genes for the α-subunits of three sodium channel subtypes—SCN1A, SCN2A, and SCN3A—are all located on chromosome 2q24. A full-term boy with an unremarkable birth history presented at 1 month of age with unusual movements that had started on day of life 2. Exam was notable for lack of visual attention, hypotonia, and hyperreflexia. Electroencephalogram (EEG) showed an invariant burst suppression with multifocal spikes, ictal episodes with bicycling movements associated with buildups of rhythmic activity, and epileptic spasms. Work-up revealed a 1.77-Mb duplication at locus 2q24.3, encompassing the entirety of SCN2A and SCN3A, but not SCN1A. Phenobarbital led to rapid resolution of the clinical seizures and EEG background normalized other than rare sharp waves. Early-onset epileptic encephalopathy (EOEE), with neonatal seizures, burst suppression, and reversibility with phenobarbital, is part of the enlarging spectrum of Nav channelopathies. The delayed diagnosis provided an unusual opportunity to view the early natural history of this disorder and its remarkable responsiveness to barbiturate therapy. The clinical and EEG response to phenobarbital implicates seizures as the cause of the encephalopathy.
Introduction
Voltage gated sodium channels (VGSC) are critical regulators of neuronal excitability, and their role in pediatric epilepsy syndromes has been increasingly recognized (Oliva, Berkovic, & Petrou, 2012). Genes for the alpha-subunits of three of the main central nervous system sodium channel subtypes – SCN1A, SCN2A, and SCN3A – are all located on chromosome 2q24 (Goeggel Simonetti et al., 2012). Among the VGSCs, mutations in SCN1A have had the highest correlation with epilepsy, with over 650 variants associated with Dravet Syndrome and greater than twenty associated with Genetic Epilepsy with Febrile Seizures Plus (GEFS+); the majority of these are mutations that cause truncation of the protein (Oliva, et al., 2012). Increasingly, SCN2A variants have been found in patients with several epilepsy syndromes, including Benign Familial Neonatal Infantile Seizures (BFNIS), GEFS+, and Dravet-like disorders. Inherited missense mutations in SCN2A are associated with benign epilepsies while de novo nonsense or missense mutations tend to cause more severe disorders (Shi et al., 2012). Both sequence-based mutations and copy number variants involving SCN2A have been associated with an expanded phenotype, including Ohtahara syndrome, West syndrome, and unclassified early-onset epileptic encephalopathies (EOEEs) (Nakamura et al 2013).
We present a case of neonatal onset seizures not recognized until one month of life in a patient with a duplication of 2q24.3 affecting SCN2A and SCN3A but not SCN1A. This case provides an interesting example of the early clinical course of this epilepsy syndrome and was also remarkable for the impressive response of both seizures and the background EEG to phenobarbital monotherapy.
Case
At one month of age, a full-term boy with an unremarkable perinatal and birth history was brought to the emergency room by his mother due to concerns regarding unusual movements. The mother described two different types of events. The first type of movement had begun approximately two days after birth and was described as a “spasm” in which he had stiffening of the extremities and truncal flexion associated with a sharp cry and occasionally perioral cyanosis, followed by low amplitude abdominal clonic movements. These events lasted seconds and were followed by a period of marked hypotonia and several hours of sleep. They had increased in frequency from several times a day to twice per hour by the time of presentation. Mother had believed them to be exaggerated startle responses. The second type of movement had begun during the week before presentation. It was characterized by torsion of the upper body with twisting of the arms and eye deviation to the left lasting several seconds. These occurred both while awake and asleep and were usually followed by the spasms. Mother became concerned for intussusception and brought the child for emergency evaluation. Family history was notable for autism spectrum disorder in an older brother as well as suspicion of autism spectrum disorder in both parents.
Initial exam was notable for a child with normal growth parameters and several subtle dysmorphisms, including a white lock of hair on the occiput, a right ear pit and a left ear tag. He was irritable and having multiple of the events described above. Neurological examination otherwise demonstrated hypotonia, hyperreflexia, and lack of visual fixation.
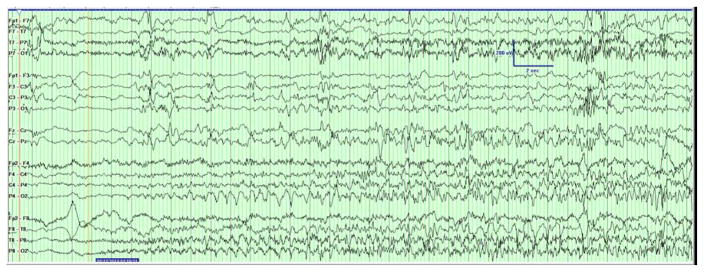
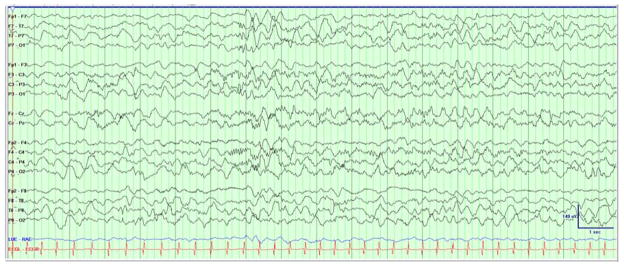
Two types of clinical events were captured on EEG. The first consisted of generalized tonic stiffening followed by bicycling movements lasting for seconds. Electrographically, the seizures started with a rhythmic alpha activity beginning paracentrally (either right or left) and spreading with irregular notched slowing to the midline then across to the opposite hemisphere in the parasagittal chain [Figure 1a]. The second type clinically consisted of epileptic spasms in clusters with corresponding diffuse high amplitude slow wave followed by attenuation and overriding beta activity [Figure 1b]. The background showed burst suppression, recurrent multifocal spikes, and absence of state changes or interstate variation in the burst-interburst ratio. [Figure 1c].
Figure 1. Initial EEG Demonstrating Seizures and Epileptic Encephalopathy.
(A) The patient had generalized tonic stiffening followed by bicycling movements lasting for seconds. The seizures started as semirhythmic 2- to 3-Hz slow waves beginning in the right occipital region (red arrow) that spread with irregular notched slowing to the midline, then across to the opposite hemisphere in the parasagittal chain (not shown). Filter settings: LLF 1 Hz, HFF 30 Hz, Notch 60 Hz; 20 seconds/page. (B) Epileptic spasms occurred in clusters corresponding to diffuse high-amplitude right-predominant slow waves (red arrows) followed by attenuation and overriding β activity. Filter settings: LLF 1 Hz, HFF 70 Hz, No Notch; 20 seconds/page. (C) EEG background showed burst suppression, with bursts of 2 to 4 seconds duration with mixed frequencies and sharp waves separated by interburst intervals of 4 to 6 seconds duration. Filter settings: LLF 1 Hz, HFF 70 Hz, Notch 60 Hz; 20 seconds/page.
MRI with spectroscopy and initial metabolic screens of plasma, urine, and CSF were normal [Table 1], other than a mildly low CSF pyridoxal-5-phosphate level of 19 associated with normal plasma pipecolic acid, urine alpha aminoadipic semialdehyde, and ultimately PNPO gene sequencing.
Table 1.
Metabolic Testing
| Fluid | Test |
|---|---|
| Serum | Lactate, pyruvate, CPK, NH3 (90), uric acid, amino acids, carnitine, acylcarnitines, VLCFA, plasma pipecolic acid, |
| CSF | Cell count, glucose, protein, lactic acid, amino acids, 5-MTHF, succinyladenosine, sialic acid, neurotransmitter metabolites, tetrahydrobiopterin & neopterin, 4-hydroxbutyric acid; Pyridoxal-5-Phospate was low (19, nl >30) |
| Urine | Organic acids, amino acids, acylglycine, s-sulfocysteine, urine AASA |
Phenobarbital therapy was introduced with immediate resolution of the longer tonic seizures upon a 20 mg/kg intravenous loading dose. Within three days of phenobarbital maintenance therapy, the spasms also resolved and the EEG background improved dramatically over the following month, with only rare scattered sharp waves within one week. Trials of pyridoxine, pyridoxal-5-phospate, and folinic acid had shown no benefit, and these agents were discontinued. Chromosomal microarray found a 1.77 Mb duplication at locus 2q24.3, encompassing the entirety of SCN2A and SCN3A, but not SCN1A. An epilepsy gene sequencing panel (GeneDx) showed the same finding without other mutations identified. Parents refused genetic testing.
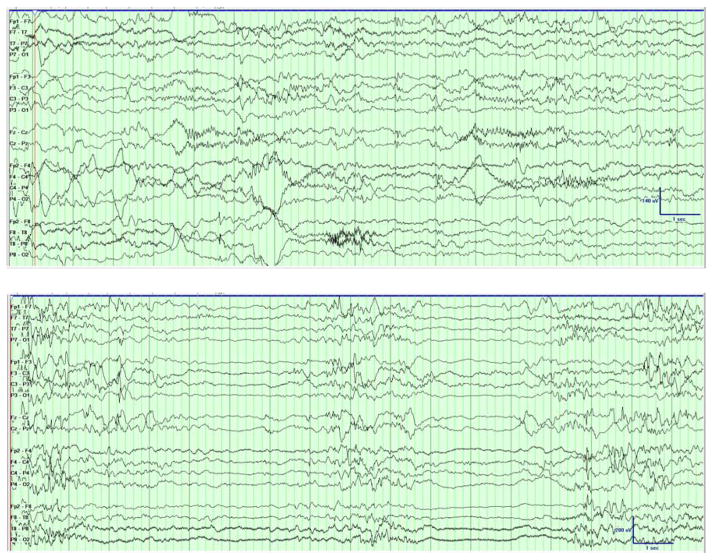
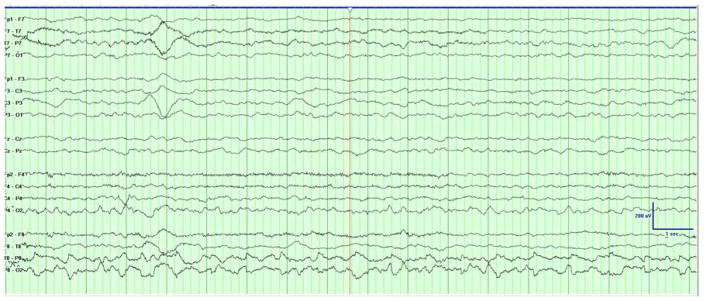
On follow-up, at two months of age, the child was seizure free with exam notable only for a mild spastic catch to the extremities with possible decrease in visual fixation. The EEG showed normal background features in wakefulness [Figure 2a] and sleep [Figure 2b], with rare scattered sharp waves. Upon family’s insistence, the phenobarbital was weaned off at four months of age. At follow-up at six months of age, one month after the final dose of medication, the child was seizure with mild global delay, functioning at about a 4-month level, and was continuing to make developmental progress.
Figure 2.
Follow-up EEG Demonstrating Normal Background with Rare Sharp Waves
Discussion
We present a case of early onset epileptic encephalopathy (EOEE) which appeared to meet criteria for Ohtahara syndrome, associated with tonic seizures, epileptic spasms, and burst-suppression pattern on EEG which showed a dramatic response to phenobarbital monotherapy. This provides an unusual glimpse into the early course of EOEE with an unusually long pretreatment course followed by treatment responsiveness.
The proband in this report had a 1.77 Mb duplication of 2q24.3 affecting SCN2A and SCN3A. Simonetti et al (Goeggel Simonetti, et al., 2012) reviewed nine cases of early onset epilepsy associated with larger duplications (1.5–12Mb) affecting the 2q24 gene cluster. Eight of the cases had duplication affecting all or part of SCN1A as well as the entirety of SCN2A and SCN3A; these children all presented with neonatal seizures and seven of the eight had moderate to profound delay or intellectual disability on follow-up (Heron et al., 2010; Okumura et al., 2011; Raymond et al., 2011). The single case (Vecchi et al., 2011) with a 12Mb duplication affecting SCN2A and SCN3A but not SCN1A had later onset of initial seizures at 3 months of age with eventual re-emergence of seizures later in childhood as well as intellectual disability at follow-up. Of interest, while our patient had a more severe epilepsy at presentation, his duplication was considerably smaller (1.71Mb vs. 12Mb). This may be secondary to the fact that there was significant mosaicism in the prior case, with only 40–51% of blood cells expressing the duplication. Overall, our case provides further evidence that duplications affecting SCN2A and SCN3A but not SCN1A can be associated with neonatal epilepsy and that they can present with a phenotype of a significant epileptic encephalopathy.
Our patient had a burst suppression pattern that resolved within one month of initiating phenobarbital. Prior cases with 2q24 duplications have shown mixed responses to phenobarbital, with at least one case (Raymond, et al., 2011) showing good control of seizures while other cases had a poor response but improved with other medications, in particular carbamazepine (Vecchi, et al., 2011) and valproate (Goeggel Simonetti, et al., 2012; Okumura, et al., 2011; Vecchi, et al., 2011). Given the rapid clinical and electrographic improvement with treatment, we suspect that the patient’s encephalopathic background is better explained as secondary to one month of uncontrolled seizures rather than as a primary effect of the duplication, though of course an interaction between the two cannot be excluded
One of the limitations includes the lack of parental testing to confirm the pathogenicity of this mutation. However, given that this is an established pathogenic area and the lack of other causes of this epileptic encephalopathy after thorough work up, we believe this mutation to be the cause of this phenotype.
This case is a yet another example of the phenotypic variation seen in VGSC mutations and more specifically SCN2A and SCN3A. Phenobarbital was found to be effective in treatment of the seizures and the epileptic encephalopathy EEG pattern in a short period of time, which is a new and clinically useful finding.
Acknowledgments
P. L. P. is supported by NINDS R01 NS82286.
References
- Goeggel Simonetti B, Rieubland C, Courage C, Strozzi S, Tschumi S, Gallati S, et al. Duplication of the sodium channel gene cluster on 2q24 in children with early onset epilepsy. Epilepsia. 2012;53(12):2128–2134. doi: 10.1111/j.1528-1167.2012.03676.x. [DOI] [PubMed] [Google Scholar]
- Heron SE, Scheffer IE, Grinton BE, Eyre H, Oliver KL, Bain S, et al. Familial neonatal seizures with intellectual disability caused by a microduplication of chromosome 2q24.3. Epilepsia. 2010;51(9):1865–1869. doi: 10.1111/j.1528-1167.2010.02558.x. [DOI] [PubMed] [Google Scholar]
- Holland KD, Kearney JA, Glauser TA, Buck G, Keddache M, Blankston JR, et al. Mutation of sodium channel SCN3A in a patient with cryptogenic pediatric partial epilepsy. Neurosci Lett. 2008;433(1):65–70. doi: 10.1016/j.neulet.2007.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini C, Scheffer IE, Nabbout R, Mei D, Cox K, Dibbens LM, et al. SCN1A duplications and deletions detected in Dravet syndrome: implications for molecular diagnosis. Epilepsia. 2009;50(7):1670–1678. doi: 10.1111/j.1528-1167.2009.02013.x. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Kato M, Osaka H, et al. Clinical spectrum of SCN2A mutations expanding to Ohtahara syndrome. Neurology. 2013;81(11):992–8. doi: 10.1212/WNL.0b013e3182a43e57. [DOI] [PubMed] [Google Scholar]
- Okumura A, Yamamoto T, Shimojima K, Honda Y, Abe S, Ikeno M, et al. Refractory neonatal epilepsy with a de novo duplication of chromosome 2q24.2q24.3. Epilepsia. 2011;52(7):e66–69. doi: 10.1111/j.1528-1167.2011.03139.x. [DOI] [PubMed] [Google Scholar]
- Oliva M, Berkovic SF, Petrou S. Sodium channels and the neurobiology of epilepsy. Epilepsia. 2012;53(11):1849–1859. doi: 10.1111/j.1528-1167.2012.03631.x. [DOI] [PubMed] [Google Scholar]
- Raymond G, Wohler E, Dinsmore C, Cox J, Johnston M, Batista D, et al. An interstitial duplication at 2q24.3 involving the SCN1A, SCN2A, SCN3A genes associated with infantile epilepsy. Am J Med Genet A. 2011;155A(4):920–923. doi: 10.1002/ajmg.a.33929. [DOI] [PubMed] [Google Scholar]
- Shi X, Yasumoto S, Kurahashi H, Nakagawa E, Fukasawa T, Uchiya S, et al. Clinical spectrum of SCN2A mutations. Brain Dev. 2012;34(7):541–545. doi: 10.1016/j.braindev.2011.09.016. [DOI] [PubMed] [Google Scholar]
- Vanoye CG, Gurnett CA, Holland KD, George AL, Kearney JA. Novel SCN3A variants associated with focal epilepsy in children. Neurobiol Dis. 2014;62:313–322. doi: 10.1016/j.nbd.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecchi M, Cassina M, Casarin A, Rigon C, Drigo P, De Palma L, et al. Infantile epilepsy associated with mosaic 2q24 duplication including SCN2A and SCN3A. Seizure. 2011;20(10):813–816. doi: 10.1016/j.seizure.2011.07.008. [DOI] [PubMed] [Google Scholar]