Abstract
Background
Peroxisome proliferator-activated receptor gamma coactivator-1 alpha (PGC-1α) coactivates multiple transcription factors and regulates several metabolic processes. In this study, we focused on the roles of PGC-1α in the apoptosis of endometrial cancer HEC-1A cells.
Materials and methods
PGC-1a expression in the HEC-1A cells was detected with real-time polymerase chain reaction and Western blot. Small interfering RNA directed against PGC-1α was designed and synthesized, and RNA interference technology was used to knock down PGC-1α mRNA and protein expression. Cell apoptosis, cell cycle, and mitochondrial membrane potential were then analyzed using flow cytometry. The expression of apoptotic proteins, Bcl-2 and Bax, was detected with Western blot.
Results
The specific downregulation of PGC-1α expression in the HEC-1A cells increased their apoptosis through the mitochondrial apoptotic pathway by reducing the expression of Bcl-2 and increasing the expression of Bax.
Conclusion
These results suggest that PGC-1α influences the apoptosis of HEC-1A cells and also provides a molecular basis for further investigation of the apoptotic mechanism in human endometrial cancer.
Keywords: endometrial cancer, PGC-1α, apoptosis, Bcl-2, Bax
Introduction
Endometrial cancer is one of the three major malignant tumors of the female reproductive system. In recent years, the incidence of endometrial cancer has increased, and it ranked sixth among new cases of female cancer throughout the world in 2012.1 Increasing research interest is being directed toward tumor cell apoptosis, and studies of endometrial cancer cell apoptosis can provide insight into the mechanism of endometrial cancer development and provide new directions for its clinical treatment.
Peroxisome proliferator-activated receptor gamma coactivator-1 alpha (PGC-1α) is a member of the peroxisome proliferator-activated receptor gamma coactivator-1 family and is the transcriptional coactivator of peroxisome proliferator-activated receptor gamma. PGC-1α displays tissue-specific expression and is more strongly expressed in tissues that are rich in mitochondria or have a high energy demand. It plays an important role in maintaining the balance between sugar, fat, and energy in the body; in the regulation of mitochondrial biosynthesis; and in other biological processes.2–4 In recent years, several studies have shown that the low PGC-1α expression is probably involved in malignant progression in a wide variety of tumor tissues, including breast cancer, colon cancer, and ovarian cancer.5–7 However, the study by Cormio et al and our previous study have shown that the expression of PGC-1α is elevated in endometrial carcinoma, and our preliminary study found that its expression is closely related to the clinical stage, depth of muscular infiltration, and lymph-node metastasis of the cancer.8,9
Apoptosis is a type of programmed cell death. There are two commonly described pathways of apoptosis: the intrinsic cell death pathway (the mitochondria-initiated pathway) and the extrinsic cell death pathway (the cell death receptor pathway).10 Mitochondria-dependent apoptosis is regulated by a group of proteins belonging to the Bcl-2 family, which can be divided into two major groups, the proapoptotic proteins (eg, Bax) and the antiapoptotic proteins (eg, Bcl-2). The balance between the pro- and antiapoptotic proteins determines whether apoptosis is initiated. Studies have shown that without apoptotic stress, Bcl-2 forms heterodimers with Bax to maintain the outer mitochondrial membrane integrity and block mitochondrial apoptosis. In the presence of apoptotic stimuli, the expression of proapoptotic protein Bax increased, following which they bind to prosurvival Bcl-2 proteins to release them from inhibition. Free Bax forms oligomers, leading to cytochrome c release from the intermembrane space of mitochondria to the cytoplasm. The released cytochrome c activates the caspase cascade to induce apoptosis.11
In this study, the role of PGC-1α in the apoptosis of endometrial carcinoma cells was investigated experimentally. We first observed that the specific downregulation of PGC-1α increased the apoptosis of HEC-1A cells. Our data showed that the downregulation of PGC-1α promoted cell apoptosis through the mitochondrial apoptotic pathway by reducing the expression of Bcl-2 and increasing the expression of Bax. Therefore, we suggest that PGC-1α plays an important role in cell apoptosis in endometrial cancer. Our results provided a molecular basis for further investigation of the mechanism of cell apoptosis in endometrial cancer.
Materials and methods
Materials
DharmaFECT transfection reagent was purchased from Thermo Fisher Scientific (Waltham, MA, USA). TRIzol Reagent was also from Thermo Fisher Scientific. The primary antibody against human PGC-1α was obtained from Santa Cruz Biotechnology Inc. (Dallas, TX, USA), and the primary antibodies against human Bcl-2 and Bax were obtained from Zhongshan Biotechnology Co. (Beijing, People’s Republic of China). Cell Cycle and Apoptosis Analysis Kits, Annexin V-FITC Apoptosis Detection Kit, and Mitochondrial Membrane Potential Assay Kit with JC-1 were all purchased from Beyotime Biotechnology Co. (Shanghai, People’s Republic of China). Fetal bovine serum was from HyClone (Logan, UT, USA).
Cell line and culture
The human endometrial cancer cell line HEC-1A was purchased from the Shanghai Institute of Cell Biology of the Chinese Academy of Sciences (Shanghai, People’s Republic of China). The cells were cultured as adherent monolayers in McCoy’s 5A medium (Thermo Fisher Scientific) containing 10% fetal bovine serum at 37°C under 5% CO2 in a saturated humidified incubator. This study protocol was approved by the Ethics Committee of China Medical University.
Transfection
Three small interfering RNAs (siRNAs) targeting different sites in the human PGC-1α mRNA (GenBank accession no NM_013261.3) were designed and synthesized by GenePharma Co., Ltd (Jiangsu, People’s Republic of China) to knock down PGC-1α expression, and a control siRNA that did not target PGC-1α mRNA was synthesized as the negative control (NC). Normal HEC-1A cells were used as the untreated control. All the siRNAs are listed in Table 1.
Table 1.
siRNA sequences used in this study
| Name | siRNA sequence |
|---|---|
| siRNA-1 | Sense: 5′-GUCCGAGUCACAACACUUATT-3′ |
| Antisense: 5′-UAAGUGUUGUGACUGCGACTT-3′ | |
| siRNA-2 | Sense: 5′-GGACAGUGAUUUCAGUAAUTT-3′ |
| Antisense: 5′-AUUACUGAAAUCACUGUCCTT-3′ | |
| siRNA-3 | Sense: 5′-CACCACUCCUCCUCAUAAATT-3′ |
| Antisense: 5′-UUUAUGAGGAGGAGUGGUGTT-3′ | |
| NC | Sense: 5′-UUCUCCGAACGUGUCACGUTT-3′ |
| Antisense: 5′-ACGUGACACGUUCGGAGAATT-3′ |
Abbreviations: siRNA, small interfering RNA; NC, negative control.
HEC-1A cells (8×105) were seeded in six-well plates and incubated overnight to allow their full extension and adherence before transfection. The cells were grown to 70%–90% confluence and then transfected with DharmaFECT transfection reagent according to the manufacturer’s instructions. siRNA (5 μM, 10 μL) was added to 190 μL of serum-free medium. At the same time, 5 μL of DharmaFECT transfection reagent was added to 195 μL of serum-free medium. The contents of each tube were then mixed and incubated for 5 minutes at room temperature. The siRNA was added to the DharmaFECT transfection reagent and incubated for 20 minutes at room temperature. Antibiotic-free complete medium (1,600 μL) was then added. Finally, the culture medium was removed from the wells of the six-well plates and 2 mL of the appropriate transfection medium was added to each well. The cells were collected for the further experiments after 72 hours of transfection.
Real-time polymerase chain reaction
Total RNA was isolated from the cells with TRIzol Reagent, according to the manufacturer's instructions, after 72 hours of transfection. First-strand complementary DNA was synthesized using the PrimeScript RT reagent Kit (Takara Holdings, Kyoto, Japan). PGC-1α gene expression was quantified relative to the endogenous expression level of GAPDH with real-time reverse transcription polymerase chain reaction (PCR) using the following primer sets: for PGC-1α, the forward primer was 5′-GACACAACACGGACAGAA-3′ and the reverse primer was 5′-CACAGGTATAACGGTAGGTAA-3′, and for GAPDH, the forward primer was 5′-GAAGGTGAAGGTCGGAGTC-3′ and the reverse primer was 5′-GAAGATGGTGATGGGATTTC-3′. An NC containing sterile water instead of the complementary DNA template was included with each set of PCRs. The PCR conditions were as follows: stage 1, 1 cycle of initial denaturation for 30 seconds at 95°C: and stage 2, 40 cycles of denaturation for 3 seconds at 95°C, followed by DNA synthesis for 30 seconds at 60°C. After amplification, real-time data acquisition and analysis were performed. The relative quantitative results were calculated using the method.
Western blot
The cells were collected after 72 hours of transfection, washed twice with phosphate-buffered saline (PBS), incubated in radioimmunoprecipitation assay buffer (1× PBS, 1% NP40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, protease inhibitor cocktail) for 30 minutes on ice, and centrifuged at 12,000× g for 15 minutes at 4°C. The total proteins (30 μg) were resolved with sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes. Next, the membrane was blocked with 5% nonfat dry milk in Tris-buffered saline with Tween 20, pH 8.0, and then incubated with primary antibodies (PGC-1α, Bcl-2, Bax, and β-actin) overnight at 4°C. The appropriate horseradish peroxidase-conjugated secondary antibodies were used at 1:5,000 for all antibodies. The proteins were visualized with enhanced chemiluminescence reagent.
Annexin V-fluorescein isothiocyanate/propidium iodide double staining assay
The cells were collected after 72 hours of transfection, suspended in PBS, and counted. The cells (105) were centrifuged at 1,000× g for 5 minutes, and the supernatant was removed. The cells were suspended in 195 μL of annexin V-fluorescein isothiocyanate (FITC) binding buffer and 5 μL of annexin V-FITC and incubated in the dark at room temperature for 20 minutes. They were then centrifuged at 1,000× g for 5 minutes and resuspended in 190 μL of binding buffer and 10 μL of the propidium iodide (PI) working solution. Cell apoptosis was analyzed with a flow cytometer (BD FACSAria, San Jose, CA, USA).
Cell-cycle analysis
The cells were collected after 72 hours of transfection, centrifuged at 1,000× g for 5 minutes, and suspended in ice-cold PBS. The cells were again centrifuged at 1,000× g for 5 minutes, and the supernatant was removed. The cells were then fixed in 70% ice-cold ethanol at 4°C overnight. After centrifugation (1,000× g, 5 minutes), the cells were diluted with PBS and recentrifuged. The cells for each condition were then incubated with 500 μL of PI staining buffer at 37°C for 30 minutes in the dark. The cell cycles were analyzed with a flow cytometer at 488 nm.
Measurement of mitochondrial membrane potential
Changes in the mitochondrial membrane potential (Δψm) were assessed with a Mitochondrial Membrane Potential Assay Kit with JC-1. The cells were collected after 72 hours of transfection, washed with PBS, and incubated with a medium containing 20 μg/mL of JC-1 at 37°C for 20 minutes. The cells were washed twice with JC-1 binding buffer, suspended in 500 μL of JC-1 binding buffer, and then analyzed with flow cytometry.
Statistical analysis
Data are presented as mean ± SD values. Statistical comparisons were made with one-way analysis of variance and t-tests. Statistical significance was defined as P-values <0.05. All statistical analyses were performed with the SPSS Version 17.0 software (SPSS Inc., Chicago, IL, USA).
Results
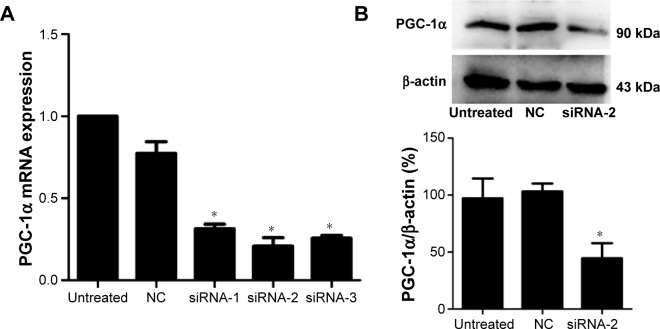
siRNA-2 downregulated PGC-1α expression in HEC-1A cells
After 72 hours of transfection, real-time PCR was used to detect the expression of PGC-1α mRNA in the HEC-1A cells. The three siRNAs downregulated the expression of PGC-1α mRNA in the HEC-1A cells, but siRNA-2 significantly reduced the expression of PGC-1α (Figure 1A). To confirm the interference of PGC-1α protein expression by siRNA-2, PGC-1α expression in the HEC-1A cells was examined with Western blot. PGC-1α protein expression was reduced after 72 hours of transfection with siRNA-2 (Figure 1B). The same result could be seen in human endometrial cancer RL95-2 cells in Figure S1A. All these results confirmed that siRNA-2 was an effective interferent that could be used for further experiments.
Figure 1.
PGC-1α expression in the HEC-1A cells after 72 hours of transfection with siRNAs.
Notes: (A) Real-time PCR analysis showed that all three siRNAs reduced the expression of PGC-1α after 72 hours of transfection compared with that in the NC siRNA-treated cells or in the untreated HEC-1A cells. siRNA-2 significantly reduced the expression of PGC-1α. (B) Western blot analysis showed that siRNA-2 reduced the expression of PGC-1α after 72 hours of transfection compared with that in the siRNA-transfected cells or in the untreated HEC-1A cells. Data are the mean ± SD values of three independent experiments. *P<0.05 (ANOVA).
Abbreviations: PGC-1α, peroxisome proliferator-activated receptor gamma coactivator-1 alpha; siRNA, small interfering RNA; PCR, polymerase chain reaction; NC, negative control; mRNA messenger RNA.
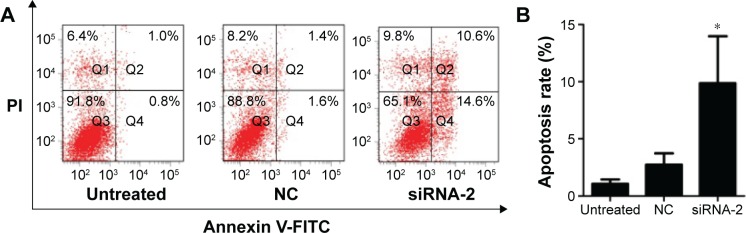
Knockdown of PGC-1α-induced apoptosis in HEC-1A cells
We evaluated the apoptotic effects of PGC-1α in the endometrial cancer HEC-1A cells using an annexin V-FITC/PI staining assay. The percentage of apoptotic cells among the HEC-1A cells transfected with siRNA-2 (9.87%±4.10%) was greater than those in the NC-treated (2.73%±1.00%) and untreated cells (1.07%±0.38%; Figure 2A and B). The same result could be seen in the RL95-2 cells in Figure S1B. These results suggest that PGC-1α influences apoptosis in the HEC-1A cells.
Figure 2.
Effects of PGC-1α knockdown on apoptosis of HEC-1A cells.
Notes: (A) Cell apoptosis was detected with annexin V-FITC/PI double staining and flow cytometry. (B) Values (intensity of fluorescent positive cells during early apoptotic events) are given as mean ± SD of triplicate experiments. *P<0.05 compared with the NC-treated and untreated control cells (ANOVA).
Abbreviations: PGC-1α, peroxisome proliferator-activated receptor gamma coactivator-1 alpha; FITC, fluorescein isothiocyanate; PI, propidium iodide; NC, negative control; siRNA, small interfering RNA.
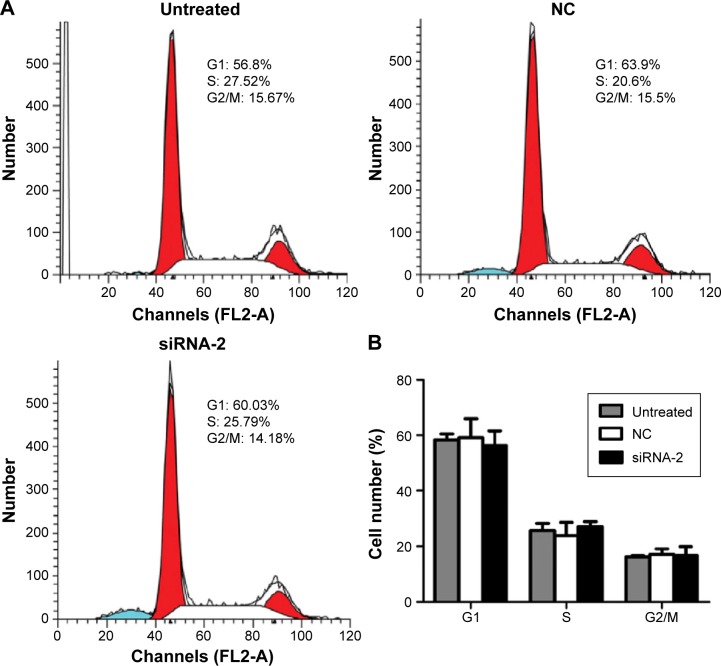
Knockdown of PGC-1α induced HEC-1A cell apoptosis by changing the mitochondrial membrane potential
To assess the effects of PGC-1α knockdown on the cell cycle, we analyzed the phases of the cell cycle after 72 hours of transfection of cells with siRNA-2. Comparing the NC-treated and untreated cells, there was no obvious change in the cell cycle after 72 hours of transfection of cells with siRNA-2 (Figure 3A and B). These results indicate that the downregulation of PGC-1α expression does not alter the cell cycle in the HEC-1A cells. The same result could be seen in the RL95-2 cells in Figure S1C. Therefore, PGC-1α may influence the apoptosis of HEC-1A cells without altering the cell cycle.
Figure 3.
Effects of PGC-1α knockdown on the cell cycle in the HEC-1A cells.
Notes: (A) cell-cycle distribution in the HEC-1A cells after 72 hours of transfection with siRNA-2 or NC, or in the untreated control cells. (B) Percentages of cells in the G1, S, and G2/M phases of the cell cycle (n=3). Values are given as the mean ± SD of triplicate experiments. P>0.05 (ANOVA).
Abbreviations: PGC-1α, peroxisome proliferator-activated receptor gamma coactivator-1 alpha; siRNA, small interfering RNA; NC, negative control.
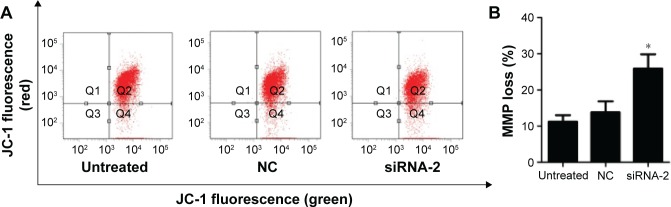
The loss of mitochondrial membrane potential is recognized as an early event in cells undergoing mitochondria-mediated apoptosis. We investigated the mitochondrial membrane potential with the fluorescent probe JC-1 and a flow cytometer. Our results show that the percentage of cells displaying green fluorescence increased significantly after 72 hours of transfection with siRNA-2 (25.9%±3.96%) compared with the percentages among the NC-treated (11.25%±1.77%) and untreated cells (13.85%±3.04%; Figure 4A and B). These results suggest that the knockdown of PGC-1α reduces the mitochondrial membrane potential. Therefore, PGC-1α may affect the apoptosis of HEC-1A cells via the mitochondria-mediated pathway. The same result could be seen in the RL95-2 cells in Figure S1D.
Figure 4.
Effects of PGC-1α knockdown on the mitochondrial membrane potential in the HEC-1A cells.
Notes: (A) Cells were stained with JC-1 and analyzed with flow cytometry after 72 hours of transfection with siRNA-2 or NC siRNA. siRNA-2 treatment increased the number of cells with low Δψm. (B) Quantification of the results showed that siRNA-2 treatment increased the number of cells with low Δψm. Results are shown as the mean ± SD values of three independent experiments. *P<0.05 compared with the NC-treated and untreated control cells (ANOVA).
Abbreviations: PGC-1α, peroxisome proliferator-activated receptor gamma coactivator-1 alpha; siRNA, small interfering RNA; NC, negative control; MMP, mitochondrial membrane potential.
Knockdown of PGC-1α changed the expression of Bcl-2 and Bax
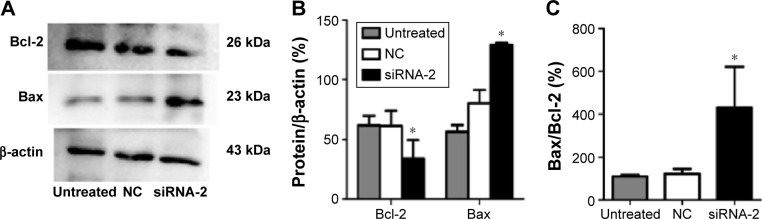
The protein regulators Bcl-2 and Bax belong to the Bcl-2 family, which is known to contain apoptosis-regulatory factors. Bcl-2 inhibits cell apoptosis, whereas the overexpression of Bax promotes apoptosis. The interactions between the members of the Bcl-2 family play an important role in the mitochondria-mediated apoptotic pathway. To determine the effects of PGC-1α on the apoptosis of HEC-1A cells, the expression of the apoptosis-related proteins Bax and Bcl-2 was analyzed with Western blot. The expression of Bcl-2 decreased, and the expression of Bax increased after 72 hours of transfection with siRNA-2 (Figure 5A–C) compared with their expression in the NC-treated and untreated control cells. These results suggest that the knockdown of PGC-1α affects cell apoptosis through the mitochondria-mediated pathway by reducing the expression of Bcl-2 and increasing the expression of Bax. The same result could be seen in the RL95-2 cells (Figure S2A–C).
Figure 5.
Knockdown of PGC-1α increased Bax expression and reduced Bcl-2 expression in the HEC-1A cells.
Notes: (A–C) Western blot ratio analysis of Bax and β-actin, Bcl-2 and β-actin, and Bax and Bcl-2 is shown. In the siRNA-2 group, Bcl-2 expression was lower than in the NC-treated and untreated cells, whereas Bax expression was higher. Results are shown as the mean ± SD values of three independent experiments. *P<0.05 compared with the NC-treated and untreated control cells (ANOVA).
Abbreviations: PGC-1α, peroxisome proliferator-activated receptor gamma coactivator-1 alpha; siRNA, small interfering RNA; NC, negative control; Bcl-2, B-cell lymphoma 2; Bax, Bcl-2 Associated X Protein.
Discussion
PGC-1α is a member of the peroxisome proliferator-activated receptor gamma coactivator-1 family, which has three members: PGC-1α, peroxisome proliferator-activated receptor gamma coactivator-1 beta, and PGC-1-related coactivator.12 PGC-1α was first identified in the mouse brown adipose tissue with the yeast two-hybrid technology by Puigserver in 1998.13 It plays an important role in the processes of mitochondrial biosynthesis, glucose transport, and cell apoptosis. In recent years, studies have shown that PGC-1α is involved in the development of some malignant tumors. Its expression is lower in some cancers than in normal tissues, including colon, breast, and ovarian cancer.5–7 However, its expression in other cancers is higher than in normal tissues. The study by Cormio et al and our previous study suggested that PGC-1α expression is higher in endometrial cancer tissues than in normal endometrial tissues.8,9
The annexin V-FITC/PI staining assay was always used to evaluate the apoptotic effects. Several studies have suggested that the lower left quadrant represents the surviving cells, the lower right quadrant represents the early apoptotic cells, the upper right quadrant represents the necrotic or postapoptotic cells, and the upper left quadrant represents the allowed detection error by using the annexin V-FITC/PI staining assay.14,15 In this study, we found that the downregulation of PGC-1α expression induced early apoptosis of HEC-1A cells. It is well known that anaerobic glycolysis is the main way through which adenosine triphosphate is provided to cancer cells, even under oxygen-sufficient conditions. This phenomenon is known as the Warburg effect, and it is probably attributable to the damaged mitochondrial function within the cancer cells. Several studies have speculated that PGC-1α expression is lower in some cancer tissues, reducing the generation of mitochondria and increasing glycolysis, which are consistent with the Warburg effect.5–7 However, in recent years, studies have indicated that mitochondrial function is required for transformation and tumor growth.16–18 A study by Bhalla et al has shown that loss of PGC-1α protects against carcinogenesis and that PGC1α coordinately regulates mitochondrial and fatty acid metabolism to promote tumor growth.19 Therefore, we speculate that the downregulation of PGC-1α expression induces HEC-1A cell apoptosis by regulating some mitochondrial functions.
It is well known that blocking the cell cycle induces cell apoptosis. In this study, we found that the downregulation of PGC-1α expression did not induce cell apoptosis by affecting the cell cycle. Two separate pathways leading to cell apoptosis have been clearly documented: the extrinsic and intrinsic pathways. The intrinsic pathway is mediated by the mitochondria.20 A decline in the mitochondrial membrane potential is regarded as an important landmark in early cell apoptosis. In this study, the mitochondrial membrane potential was lower after the cells were transfected with siRNA-2 than in the NC-treated or untreated cells, which suggests that the downregulation of PGC-1α expression induces cell apoptosis via the mitochondria-mediated pathway in the HEC-1A cells.
In recent years, several studies have suggested that the permeabilization of the mitochondrial outer membrane plays an important role in mitochondria-mediated cell apoptosis. The interaction between members of the Bcl-2 family regulates mitochondrial outer membrane permeabilization and thus induces cell apoptosis.11,21 In this study, we found that the expression of the prosurvival protein Bcl-2 was lower and that of the proapoptotic protein Bax was higher after the downregulation of PGC-1α expression in the HEC-1A cells. This indicates that the downregulation of PGC-1α induces cell apoptosis by affecting the expression of Bcl-2 and Bax and changing the permeability of the mitochondrial membrane.
Conclusion
The downregulation of PGC-1α expression may induce HEC-1A cell apoptosis through the mitochondria-mediated pathway by regulating the expression of Bcl-2 and Bax. We infer that PGC-1α plays a dual role in the development of cancers. PGC-1α stands at the crossroads of many pathways as the control of mitochondria biogenesis, tumor cell energy, and protection of tumor cells against apoptosis via a mitochondrial pathway, representing a good target for therapeutic intervention. However, the specific regulatory mechanism is still unclear and will be investigated in our future studies.
Supplementary materials
PGC-1α expression in RL95-2 cells after their transfection with siRNA-2, and effects of PGC-1α knockdown on apoptosis, cell cycle and the mitochondrial membrane potential in RL95-2 cells. (A) Western blotting analysis showed that siRNA-2 reduced the expression of PGC-1α 72 h after transfection compared with that in cells transfected with the negative control siRNA or in untreated RL95-2 cells of three independent experiments. (B) Cell apoptosis was detected with annexin V-FITC/PI double staining and flow cytometry in RL95-2 cells. Values (intensity of fluorescent positive cells during early apoptotic events). (C) Cell-cycle distribution in RL95-2 cells 72 h after transfection with siRNA-2 or NC, or in untreated control cells. Percentages of cells in G1, S, and G2/M phases of the cell cycle. (D) Cells were stained with JC-1 and analyzed with flow cytometry 72 h after transfection with siRNA-2 or the negative control siRNA. siRNA-2 treatment increased the number of cells with low Δψm. Quantification of the results showed that siRNA-2 treatment increased the number of cells with low Δψm. We analyzed three independent experiments which compared with the NC-treated and untreated control cells.
Notes: Data are the means ± SD of three independent experiments comparing treatment and untreated control cells. *P<0.05 (ANOVA).
Abbreviations: PGC-1α, peroxisome proliferator-activated receptor gamma coactivator-1 alpha; NC, negative control; siRNA, small interfering RNA; PI, propidium iodide; AV-FITC, Annexin V-fluorescein isothiocyanate; MMP, mitochondrial membrane potential.
Knockdown of PGC-1α increased Bax expression and reduced Bcl-2 expression in RL95-2 cells. (A, B and C) Western blot ratio analysis of Bax and β-actin, Bcl-2 and β-actin, and Bax and Bcl-2. In the siRNA-2 group, Bcl-2 expression was lower than in the NC-treated and untreated cells, whereas Bax expression was higher.
Notes: Results are shown as the means ± SD of three independent experiments. *P<0.05 compared with the NC-treated and the untreated control cells (ANOVA).
Acknowledgments
This study was supported by the National Natural Science Foundation of China (grant nos 82172874 and 81472438), the Department of Science and Technology of Liaoning Province (grant no 2013225079), and the Shenyang City Science and Technology Bureau (grant no F12-227-1-62).
Footnotes
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Lin J, Handschin C, Spiegelman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005;1(6):361–370. doi: 10.1016/j.cmet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Villena JA. New insights into PGC-1 coactivators: redefining their role in the regulation of mitochondrial function and beyond. FEBS J. 2015;282(4):647–672. doi: 10.1111/febs.13175. [DOI] [PubMed] [Google Scholar]
- 4.Jones AW, Yao Z, Vicencio JM, Karkucinska-Wieckowska A, Szabadkai G. PGC-1 family coactivators and cell fate: roles in cancer, neurodegeneration, cardiovascular disease and retrograde mitochondria-nucleus signalling. Mitochondrion. 2012;12(1):86–99. doi: 10.1016/j.mito.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 5.Watkins G, Douglas-Jones A, Mansel RE, Jiang WG. The localisation and reduction of nuclear staining of PPARgamma and PGC-1 in human breast cancer. Oncol Rep. 2004;12(2):483–488. [PubMed] [Google Scholar]
- 6.Feilchenfeldt J, Brundler MA, Soravia C, Totsch M, Meier CA. Peroxisome proliferator-activated receptors (PPARs) and associated transcription factors in colon cancer: reduced expression of PPARgamma-coactivator 1 (PGC-1) Cancer Lett. 2004;203(1):25–33. doi: 10.1016/j.canlet.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Ba Y, Liu C, et al. PGC-1alpha induces apoptosis in human epithelial ovarian cancer cells through a PPARgamma-dependent pathway. Cell Res. 2007;17(4):363–373. doi: 10.1038/cr.2007.11. [DOI] [PubMed] [Google Scholar]
- 8.Cormio A, Guerra F, Cormio G, et al. The PGC-1alpha-dependent pathway of mitochondrial biogenesis is upregulated in type I endometrial cancer. Biochem Biophys Res Commun. 2009;390(4):1182–1185. doi: 10.1016/j.bbrc.2009.10.114. [DOI] [PubMed] [Google Scholar]
- 9.Ren Z, Yang H, Wang C, Ma X. The effects of PGC-1alpha on the proliferation and energy metabolism of malignant endometrial cancer cells. Onco Targets Ther. 2015;8:769–774. doi: 10.2147/OTT.S79960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong RS. Apoptosis in cancer: from pathogenesis to treatment. J Exp Clin Cancer Res. 2011;30:87. doi: 10.1186/1756-9966-30-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hardwick JM, Soane L. Multiple functions of BCL-2 family proteins. Cold Spring Harb Perspect Biol. 2013;5(2):a008722. doi: 10.1101/cshperspect.a008722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scarpulla RC. Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim Biophys Acta. 2011;1813(7):1269–1278. doi: 10.1016/j.bbamcr.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92(6):829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 14.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods. 1995;184(1):39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 15.Chen S, Cheng AC, Wang MS, Peng X. Detection of apoptosis induced by new type gosling viral enteritis virus in vitro through fluorescein annexin V-FITC/PI double labeling. World J Gastroenterol. 2008;14(14):2174–2178. doi: 10.3748/wjg.14.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frezza C, Gottlieb E. Mitochondria in cancer: not just innocent bystanders. Semin Cancer Biol. 2009;19(1):4–11. doi: 10.1016/j.semcancer.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 17.Fogal V, Richardson AD, Karmali PP, Scheffler IE, Smith JW, Ruoslahti E. Mitochondrial p32 protein is a critical regulator of tumor metabolism via maintenance of oxidative phosphorylation. Mol Cell Biol. 2010;30(6):1303–1318. doi: 10.1128/MCB.01101-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Funes JM, Quintero M, Henderson S, et al. Transformation of human mesenchymal stem cells increases their dependency on oxidative phosphorylation for energy production. Proc Natl Acad Sci U S A. 2007;104(15):6223–6228. doi: 10.1073/pnas.0700690104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhalla K, Hwang BJ, Dewi RE, et al. PGC1alpha promotes tumor growth by inducing gene expression programs supporting lipogenesis. Cancer Res. 2011;71(21):6888–6898. doi: 10.1158/0008-5472.CAN-11-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu L, Zhang Z, Xing D. Cell death via mitochondrial apoptotic pathway due to activation of Bax by lysosomal photodamage. Free Radic Biol Med. 2011;51(1):53–68. doi: 10.1016/j.freeradbiomed.2011.03.042. [DOI] [PubMed] [Google Scholar]
- 21.Xiong S, Mu T, Wang G, Jiang X. Mitochondria-mediated apoptosis in mammals. Protein Cell. 2014;5(10):737–749. doi: 10.1007/s13238-014-0089-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PGC-1α expression in RL95-2 cells after their transfection with siRNA-2, and effects of PGC-1α knockdown on apoptosis, cell cycle and the mitochondrial membrane potential in RL95-2 cells. (A) Western blotting analysis showed that siRNA-2 reduced the expression of PGC-1α 72 h after transfection compared with that in cells transfected with the negative control siRNA or in untreated RL95-2 cells of three independent experiments. (B) Cell apoptosis was detected with annexin V-FITC/PI double staining and flow cytometry in RL95-2 cells. Values (intensity of fluorescent positive cells during early apoptotic events). (C) Cell-cycle distribution in RL95-2 cells 72 h after transfection with siRNA-2 or NC, or in untreated control cells. Percentages of cells in G1, S, and G2/M phases of the cell cycle. (D) Cells were stained with JC-1 and analyzed with flow cytometry 72 h after transfection with siRNA-2 or the negative control siRNA. siRNA-2 treatment increased the number of cells with low Δψm. Quantification of the results showed that siRNA-2 treatment increased the number of cells with low Δψm. We analyzed three independent experiments which compared with the NC-treated and untreated control cells.
Notes: Data are the means ± SD of three independent experiments comparing treatment and untreated control cells. *P<0.05 (ANOVA).
Abbreviations: PGC-1α, peroxisome proliferator-activated receptor gamma coactivator-1 alpha; NC, negative control; siRNA, small interfering RNA; PI, propidium iodide; AV-FITC, Annexin V-fluorescein isothiocyanate; MMP, mitochondrial membrane potential.
Knockdown of PGC-1α increased Bax expression and reduced Bcl-2 expression in RL95-2 cells. (A, B and C) Western blot ratio analysis of Bax and β-actin, Bcl-2 and β-actin, and Bax and Bcl-2. In the siRNA-2 group, Bcl-2 expression was lower than in the NC-treated and untreated cells, whereas Bax expression was higher.
Notes: Results are shown as the means ± SD of three independent experiments. *P<0.05 compared with the NC-treated and the untreated control cells (ANOVA).