Abstract
Objective
To evaluate the clinical effectiveness of bolus dose fentanyl and midazolam to treat episodic intracranial hypertension (ICH) in children with severe traumatic brain injury (TBI).
Design
Retrospective cohort.
Setting
Pediatric intensive care unit (PICU) in a university-affiliated children’s hospital Level I trauma center.
Patients
Thirty-one children aged 0–18 years with severe TBI (Glasgow Coma Scale Score ≤ 8) who received bolus doses of fentanyl and/or midazolam for treatment of episodic ICH.
Interventions
None.
Measurements and Main Results
The area under the curve (AUC) from high resolution intracranial pressure (ICP) pressure-time plots was calculated to represent cumulative ICH exposure: AUC for ICP above 20 mmHg (AUC-ICH) was calculated in 15 minute epochs before and after administration of fentanyl and/or midazolam for the treatment of episodic ICH. Our primary outcome measure, the difference between pre- and post-drug administration epochs (ΔAUC-ICH), was calculated for all occurrences. We examined potential covariates including age, injury severity, mechanism and time after injury; time after injury correlated with ΔAUC-ICH. In a mixed effects model, with patient as a random effect, drug/dose combination as a fixed effect, and time after injury as a covariate, ICH rose after administration of fentanyl and/or midazolam (overall aggregate mean ΔAUC-ICH= +17 mmHg*min, 95%CI 0–34 mmHg*min; p=0.04). The mean ΔAUC-ICH increased significantly after administration of high-dose fentanyl (p=0.02), low-dose midazolam (p=0.006) and high-dose fentanyl plus low-dose midazolam (0.007). Secondary analysis using age dependent thresholds showed no significant impact on cerebral perfusion pressure deficit (mean ΔAUC-CPP).
Conclusions
Bolus dosing of fentanyl and midazolam fails to reduce the ICH burden when administered for episodic ICH. Paradoxically, we observed an overall increase in ICH burden following drug administration, even after accounting for within subject effects and time after injury. Future work is needed to confirm these findings in a prospective study design.
Keywords: traumatic brain injury, intracranial hypertension, fentanyl, midazolam, pressure-time exposure
INTRODUCTION
Traumatic brain injury (TBI) is a leading cause of morbidity and mortality for children in the United States [1, 2]. The development of intracranial hypertension (ICH) may complicate TBI, compromising cerebral perfusion pressure (CPP) and leading to herniation syndromes, and death. CPP, determined by mean arterial blood pressure (MAP) and intracranial pressure (ICP), is defined by the relationship CPP = MAP – ICP. Based upon Level II-III evidence, the 2003 and 2012 clinical guidelines for TBI care recommend treatment of ICP above 20 mmHg [3, 4] and maintenance of CPP above estimated age appropriate thresholds [4, 5]. Notably, we have found that the timing and intensity of ICH-directed therapy favorably impacts functional outcome [6].
The most recent guidelines for management of severe TBI in pediatric patients do not make specific recommendations on the use of sedatives and analgesics, yet these medications are commonly used as first line therapy - 91% of practitioners report using sedatives as first tier ICH-directed therapy [4, 7, 8]. Specifically, fentanyl and midazolam are commonly used in the management of critically ill children, primarily for the sedative-analgesic effect that facilitates mechanical ventilation and lowers oxygen consumption in respiratory failure and shock states [9]. These drugs are also often used as first tier bolus therapy for the treatment of episodic ICH in pediatric TBI, despite evidence from both adult and pediatric studies that such drugs may actually increase ICP [10–13]. This paradoxical response is not fully understood, but may be due to: 1) vasoregulatory changes in cerebral blood flow from direct drug effect or in response to altered systemic hemodynamics [14]; 2) altered autonomic reflexes, or; 3) may vary with the root cause of ICH episodes (e.g. when pain or agitation is not the cause, then off-target drug effects dominate). In addition to concerns regarding a deleterious effect of opioids on ICH, evidence of altered gene expression and brain development in models of physiologic stress and association with postoperative delirium and cognitive decline in adult patients emphasize the need to limit opioid administration to situations with clear benefit [15–17]. Similar concerns apply to midazolam, which is associated with apoptotic neurodegeneration in the developing mouse brain [18], increased spreading depolarization clusters in adult patients with TBI [19], and increased opioid tolerance in mechanically ventilated children [20].
The aim of this study was to determine effectiveness of bolus fentanyl and midazolam in pediatric TBI patients with ICH by comparing the ICH pressure-time exposure before and after drug administration. High-resolution ICP monitoring enables quantification of both magnitude- and time-weighted exposure to ICH, the product of which is termed the pressure-time exposure (PTE). The PTE captures both the amplitude and duration of this secondary insult, and is therefore a more robust measure of ICH exposure than traditional measures such as absolute peak or mean ICP [21–23]. We likewise compared the changes to CPP in a time-weighted fashion. PTE was also determined during untreated occurrences of ICH and employed as within-subject control data in our analysis. With this approach, we were able to isolate the effects of the study drugs, accounting for subject effects and clinically relevant covariates.
MATERIALS AND METHODS
Setting and patient selection
The Institutional Review Board at Washington University approved all study procedures with a waiver of written informed consent. St. Louis Children’s Hospital is a 258-bed tertiary care facility with a 28-bed multidisciplinary medical-surgical-trauma intensive care unit, serving a large metropolitan area with a multistate catchment area.
We included patients admitted to the hospital between May 1, 2009 and December 31, 2011 who met the following inclusion criteria: age 0–18 years, admission to the Pediatric Intensive Care Unit (PICU) with severe traumatic brain injury (defined as Emergency Department post-resuscitation Glasgow Coma Scale Score ≤ 8), who underwent ICP monitoring with continuous high-resolution physiological data collection, including ICP, mean arterial blood pressure (MAP), and CPP. Patient demographic and injury severity data were abstracted from the medical record. An admission computed tomography (CT) Marshall CT score [24] was assigned by an experienced pediatric neurosurgeon. Time after injury was based on a best estimate of injury time from parent report or EMS run sheet.
Monitoring
All patients underwent ICP monitoring via either an intraparenchymal monitor (Camino ®, Integra™ LifeSciences, Plainsboro, NJ), or a ventriculostomy catheter (Codman ® BACTISEAL® EVD Catheter, Codman & Shurtleff, Inc., Raynham, MA), which were selected at the discretion of the attending neurosurgeon. Patients with ventriculostomy catheters and no concurrent intraparenchymal monitor were excluded from analysis because cerebrospinal fluid drainage precluded continuous ICP measurement. All intraparenchymal devices were zeroed according to manufacturer’s instructions..
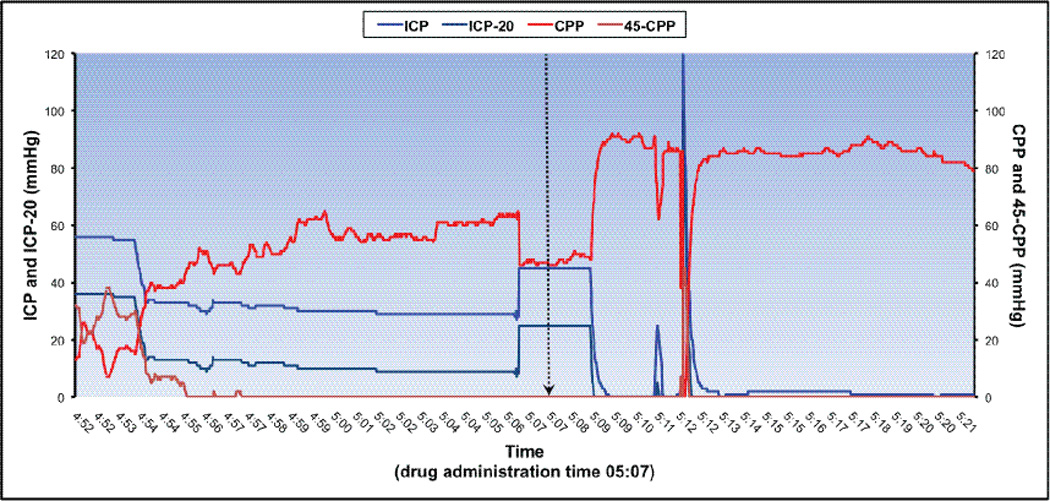
Arterial blood pressure was recorded continuously via either a radial or femoral arterial catheter, with the transducer zeroed at the phlebostatic axis; MAP and other hemodynamic parameters were measured using the IntelliVue MP70 bedside monitor (Philips Healthcare, Netherlands). CPP was derived from ICP and MAP waveforms, as MAP - ICP. Neurophysiologic parameters were recorded continuously at 0.56 Hz and digitally archived using the Mobius™ Multimodality Monitoring System (Integra™ LifeSciences, Plainsboro, NJ). Upon PICU discharge, data were downloaded to a secure institutional database. For analysis, the data were then imported into LABpilot software (Microdialysis AB, Sweden). Representative 30-minute tracings from two sample patients (one with favorable response to drug, and one lacking response) are shown in Figure 2. Data were collected until either monitor discontinuation or death.
Figure 2.
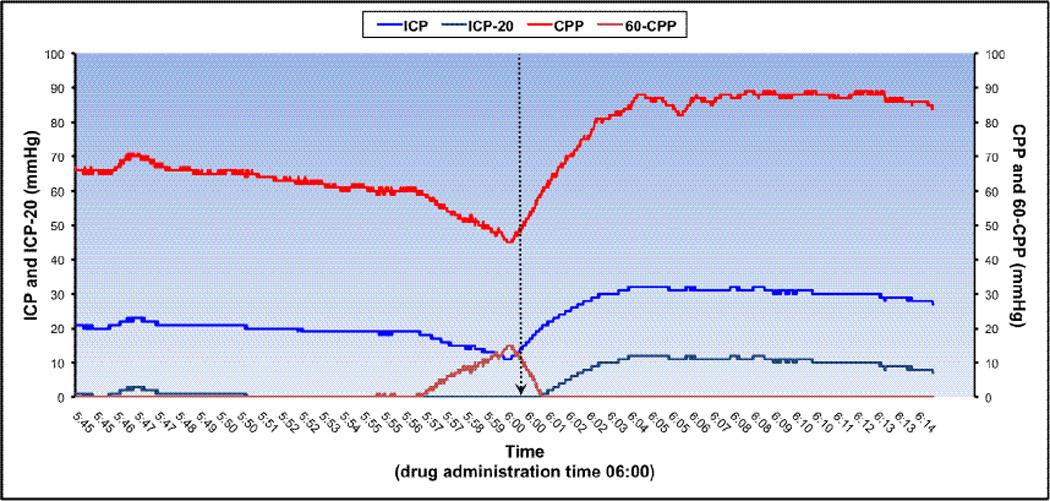
a. Example of a high-resolution tracing from a representative patient showing non-favorable response to treatment. Time axis represents a 30-minute recording interval, with equal 15-minute epochs before and after drug administration. Patient met criteria at 5:50. This patient was a 16-year-old male who suffered a gunshot wound, and presented with GCS 5. Fentanyl 2 mcg/kg and Midazolam 0.04 mg/kg (FH/ML) were given at time 06:00, denoted by the vertical arrow. Age-based CPP threshold was 60 mmHg.
b. Example of a high-resolution tracing from representative a patient showing favorable response to treatment. Time axis represents a 30-minute recording interval, with equal 15-minute epochs before and after drug administration. Patient met criteria at 4:57. This patient was a 3-month-old male who suffered a non-accidental trauma, and presented with GCS 6. Fentanyl 1 mcg/kg and Midazolam 0.1 mg/kg (FL/ML) were given at time 05:07, denoted by the vertical arrow. Age-based CPP threshold was 45 mmHg.
Patient Management
Patients were intubated, mechanically ventilated and sedated with fentanyl and midazolam infusions. Treatment of ICH was guided by an institutional protocol developed jointly by Pediatric Critical Care Medicine and Pediatric Neurosurgery (See Supplemental Digital Content) [6]. This protocol was consistent with the 2003 pediatric TBI guidelines and directed a tiered approach to ICH [25]. The drug choices and dose ranges reflect standard practice in our intensive care unit at the time, and are consistent with reported practices and published guidelines in both critically ill children and patients with severe TBI [26–28].
Data Collection
All data were de-identified prior to analysis. We determined if fentanyl and/or midazolam was administered in response to each ICH occurrence (defined as sustained ICP above 20 mmHg for greater than 5 minutes) by querying the electronic medication administration record (Allscripts, Chicago, IL). The pre- and post-dose administration study window (15 minute epochs before and after drug administration) was defined based on the pharmacodynamic properties of fentanyl and midazolam (study drugs) [29, 30]. Study drug administration occurrences were categorized using standard dose ranges commonly used in children with severe TBI at our institution (fentanyl high dose (FH): >1 mcg/kg, fentanyl low dose (FL): ≤ 1 mcg/kg, midazolam high dose (MH): >0.1 mg/kg, and midazolam low dose (ML): ≤ 0.1 mg/kg).
In order to isolate study drug effectiveness in treating episodic ICH, we excluded study drug administrations confounded by co-administration of other ICH-directed therapies. A priori, we defined these as (1) concurrent administration of other opioids or benzodiazepines within the 15 minute periods before or after fentanyl or midazolam administration, (2) bolus dosing of barbiturate, neuromuscular blockade, or osmotherapies (3% sodium chloride or mannitol) during the 15 minute period after study drug administration, or (3) pre-treatment osmotherapy 30 minutes prior to study drug administration. For remaining occurrences we calculated PTE for both ICP and CPP in 15 minute epochs before and after drug administration. We excluded sections of tracing with artifact, such as obvious baseline changes from zeroing, data dropout from electronic interface disruption, or extreme sustained, nonphysiologic elevations consistent with monitor malposition [21, 31]. The trapezoidal AUC method was used to calculate PTE from raw pressure versus time plots (PilotICU, M Dialysis AB, Johanneshov, Sweden) [21]. This method has been reported by other investigators, and has been used to interpolate AUC from intermittent ICP measurements. The AUC for ICP above 20 mmHg was defined as the ICH burden (AUC-ICH) based on current guidelines for treatment of ICH [4]. Only values of ICP over threshold of 20 mmHg are used to calculate AUC; no “negative” values from transient ICP reductions are included in the exposure calculation (as the AUC value for such episodes would be zero). The primary outcome measurement, the mean ΔAUC-ICH, was calculated as the difference between pre- and post-drug PTE.
Current TBI guidelines define a CPP target range, suggesting there may be age-specific thresholds with infants at the lower end and adolescents at the upper end [4]. We used age-specific normal estimates for MAP (50th percentile) and an ICP of 10mmHg to define the following CPP thresholds for AUC analysis: 45 mmHg for age < 2 years, 50 mmHg for age 2–6 years, 55 mmHg for ages 7–10 years, and 60 mmHg for age >10 years [32].
In order to include the ICP course of untreated ICH episodes in the model, we also performed AUC analyses on all untreated ICH episodes that met treatment thresholds (occurrences which represent clinical pathway violations). In many of these cases, however, it was evident from the medical record that the goals of care had been redirected due to futility or patients had met neurological criteria for death. In these situations, we closed the study period after the last recorded ICP-directed therapy to limit overrepresentation of prolonged periods of untreated ICH, in context judged to be futile. These untreated occurrences were coded as F0/M0, and included in the mixed model as an additional level of the fixed effect (drug). For these occurrences, we defined a time zero (t0) as the time at which treatment should have been given by criteria (sustained ICP over 20 mmHg for greater than 5 minutes), but was not. In these episodes, the pre and post epochs were the 15 minute intervals flanking t0.
Statistical Analysis
All analyses were performed in SAS 9.3 (SAS Institute, Cary, NC). To quantify the ICH burden, the difference between pre- and post-drug AUC-ICH (the ΔAUC-ICH) was calculated for each drug administration occurrence. The ΔAUC-ICH was entered into a mixed random effects ANOVA model with drug-dose category as a fixed effect and with subject and subject*drug-dose interaction as random effects. Replicate episodes were nested within subject and drug-dose combination by using subject*drug-dose interaction as a random effect. Degrees of freedom were adjusted with the Kenward-Roger correction. Similar analyses were done for ΔAUC-CPP. This mixed effects model contains three variance components, between subjects, between drug-dose within subjects, and residuals within the subject*drug dose combination. This method accounts for repeated observations within the same subject and avoids overestimated degrees of freedom with biased low p-values. Occurrences of untreated ICH episodes were incorporated as an additional fixed effect level in the model, to serve as a within-subject control. Potential confounders including age, post-resuscitation GCS, time from injury to drug administration, mechanism of injury, administration of neuromuscular blockade and decompressive craniectomy were separately evaluated for association with the outcomes of interest (ΔAUC-ICH and ΔAUC-CPP). Variables were included in the mixed effects model if significant on univariate analysis. Continuous variables were compared with Pearson correlations. Categorical variables were compared with ANOVA or t-tests. Statistical significance was set at alpha=0.05. Since concurrent administration of neuromuscular blockade (NMB) could reduce ICH through avoidance of subclinical muscular rigidity due to fentanyl bolus administration, we used generalized linear mixed random effects logistic regression to account for this possible effect of NMB.
RESULTS
Patient demographics and injury characteristics are presented in Table 1. Overall intensity of ICP-directed therapy as measured by daily PILOT score is summarized in Table 3, and displayed in Figure 5. We analyzed data from 31 patients, representing 413 ICH occurrences (Figure 1). Of 92 patients screened, 33 patients were excluded because their GCS score improved shortly after admission and an ICP monitor was not placed. Thirty-eight patients met eligibility criteria. Of these, 7 patients were excluded from the analysis because only one type of drug-dose combination was administered, precluding evaluation in the mixed effects model. For the 31 patients included in the analysis, there were a total of 413 discrete occurrences of ICP above 20 mmHg lasting at least 5 minutes. Of these, 211 were treated with study drugs and 202 were not.
Table 1.
Demographic and injury characteristics (n=31)
| Variable | Value | |
|---|---|---|
| Male/female ratio | 65:35 | |
| Mean age in years (± SD) | 8 (± 6) | |
| Mean weight in kg (± SD) | 36 (± 26) | |
| Mechanism of injury (# and %) | MVC | 10 (32) |
| Fall | 1 (3) | |
| NAT | 8 (26) | |
| GSW | 3 (10) | |
| Other | 9 (29) | |
| Mean post-resuscitation GCS score (± SD) | 5 (± 3) | |
| Admission Marshall CT category (# and %) | I | 0 (0) |
| II | 17 (55) | |
| III | 6 (19) | |
| IV | 4 (13) | |
| V | 2(6) | |
| VI | 0 (0) | |
| In-hospital mortality n (%) | NR | 2 (6) 6 (19) |
Values represent numbers of patients, and values in parentheses represent percentages, unless otherwise noted. MVC, motor vehicle crash; NAT, non-accidental trauma; GSW, gunshot wound; GCS, Glasgow Coma Scale.
Table 3.
Summary of ICP directed therapies patients received during the study period.
| ICP Directed Therapies (PILOT Score Components) |
Patients receiving each ICP directed therapy (%) |
|||||
|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | ||
| Fever control | 26 | 15 | 17 | 0 | 0 | |
| Sedation | 100 | 100 | 100 | 100 | 100 | |
| Paralysis | 68 | 54 | 67 | 100 | 0 | |
| Hyperventilation | 68 | 54 | 56 | 25 | 0 | |
| Mannitol | 74 | 73 | 33 | 75 | 100 | |
| Hypertonic saline * | 74 | 81 | 89 | 100 | 100 | |
| CSF drainage | 10 | 19 | 11 | 25 | 0 | |
| Barbiturates * | 35 | 31 | 39 | 50 | 0 | |
| Hematoma evacuation | 23 | 4 | 0 | 0 | 0 | |
| Decompressive craniectomy |
13 | 12 | 0 | 0 | 0 | |
| Induced hypothermia | 26 | 27 | 24 | 50 | 100 | |
| Lumbar drainage | 0 | 4 | 0 | 0 | 0 | |
| Induced hypertension | 29 | 38 | 28 | 25 | 100 | |
|
Continuous infusions (% receiving) |
Hypertonic saline infusion ** | 52 | 65 | 67 | 75 | 100 |
| Pentobarbital infusion ** | 23 | 23 | 22 | 25 | 0 | |
| Vasoactive infusion | 42 | 54 | 39 | 25 | 100 | |
| Number patients | 31 | 26 | 18 | 4 | 1 | |
| Median PILOT score | 13 | 9 | 10 | 13.5 | 11 | |
Represents continuous infusion and bolus doses.
Percent of patients receiving continuous infusions of hypertonic saline or pentobarbital.
Figure 5.
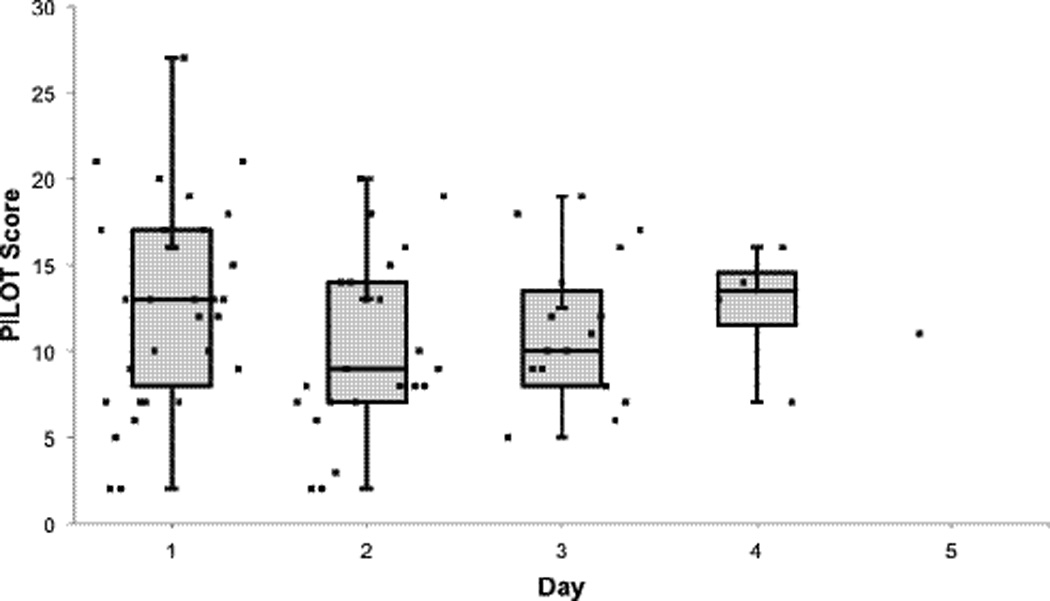
Median PILOT scores by day of therapy. Data represents median, interquartile range (IQR), and distribution of raw data points. Only one patient was receiving study drugs on day 5.
Figure 1.
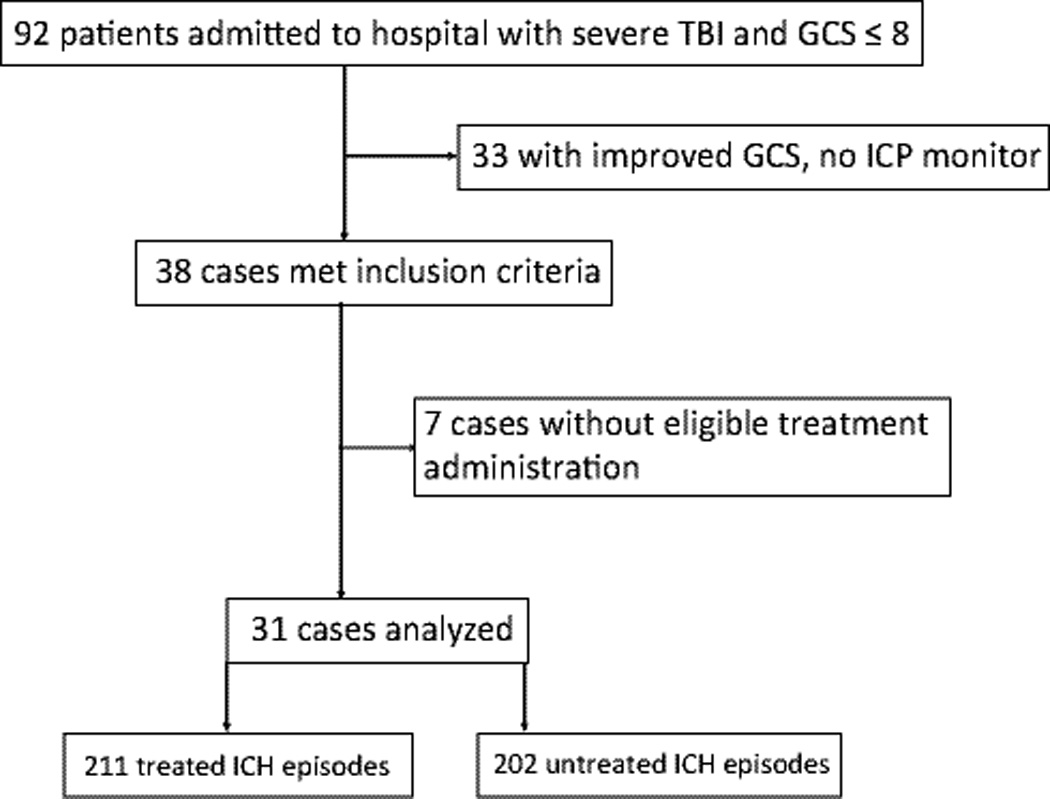
Patient flow diagram; TBI, traumatic brain injury; GCS, Glasgow Coma Scale; ICP, intracranial pressure; ICH, intracranial hypertension
Neither age nor post-resuscitation GCS correlated with ΔAUC-ICH (Table 2); time after injury significantly correlated with ΔAUC-ICH (r= −0.12; p=0.02) and was therefore included in the mixed effects model. Mechanism of injury, presence of neuromuscular blockade, and presence of decompressive craniectomy were analyzed as explanatory variables with ANOVA or Student’s t-test as appropriate. There were no significant differences in ΔAUC-ICH for these comparisons; these putative covariates were therefore not included in the final mixed effects model. There was no difference in use of NMB amongst fentanyl drug-dose combinations (p = 0.34).
Table 2.
Covariate analyses: continuous and categorical variables versus ΔAUC-ICH
| Variable | Analysis | p-value | |
|---|---|---|---|
| Age | Pearson correlation | 0.55 (r =0.02) | |
| Post-resuscitation GCS | 0.99 (r= 0.0006) | ||
| Time from injury to drug administration (hours) |
0.02 (r =0.12) | ||
| Mechanism of Injury | MVC | ANOVA | 0.98 |
| Fall | |||
| NAT | |||
| GSW | |||
| Other | |||
| Neuromuscular blockade (yes/no) | t-test | 0.17 | |
| Decompressive craniectomy (yes/no) | 0.28 | ||
Potential confounders were evaluated by univariate analyses. Statistical significance was set at alpha=0.05. MVC, motor vehicle crash; NAT, non-accidental trauma; GSW, gunshot wound; GCS, Glasgow Coma Scale; ANOVA, analysis of variance.
In our final ΔAUC-ICH model, 133 observations were excluded from the analysis because time of injury could not be accurately determined in the 8 corresponding subjects. Across all drug-dose combinations, in aggregate, mean AUC-ICH increased after administration of fentanyl and/or midazolam (mean ΔAUC-ICH= +17 mmHg*min, 95% CI 0–34 mmHg*min; p=0.04). Specifically, there was a significant increase in mean AUC-ICH after administration of the following drug-dose combinations: high-dose fentanyl (p=0.02), low-dose midazolam (p=0.006) and high-dose fentanyl plus low-dose midazolam (p=0.007). AUC-ICH increased for all other drug-dose combinations but the change did not reach statistical significance (Figure 3).
Figure 3.
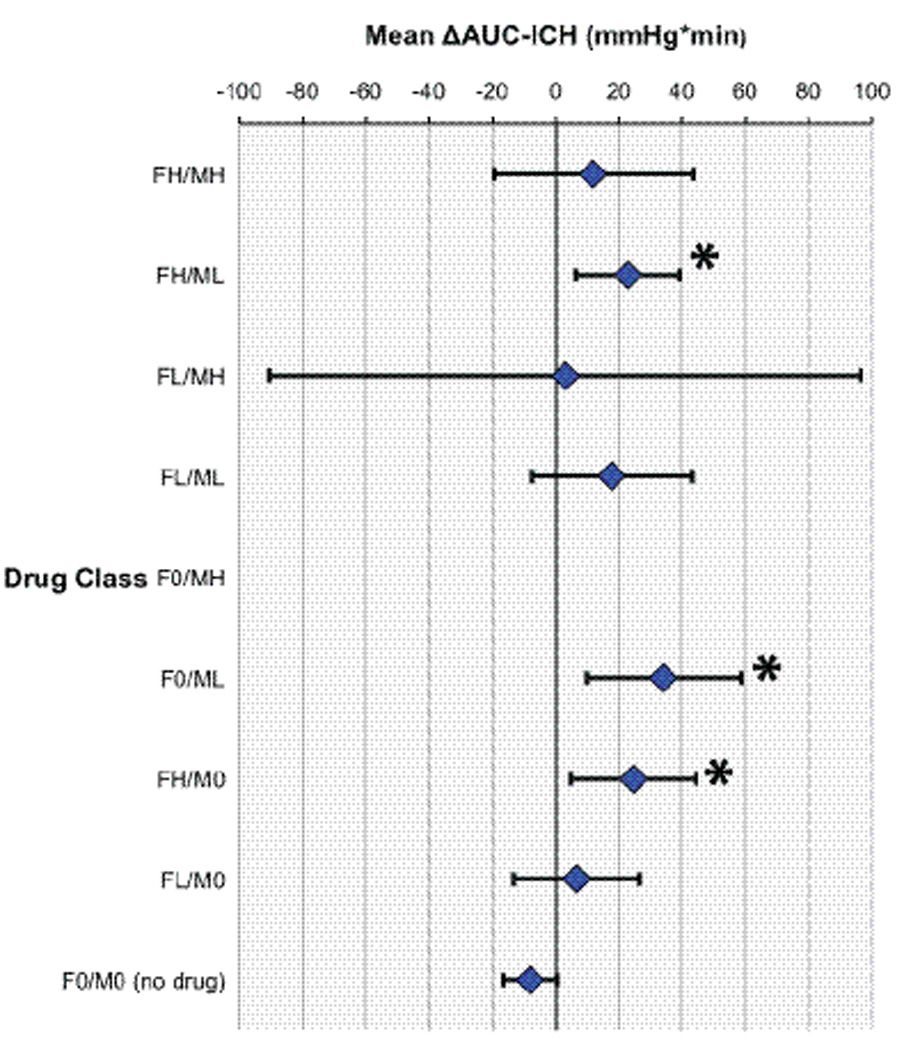
ΔAUC-ICH mixed model with subject as random effect and drug class as fixed effect. Drug classes represent each dose class contrasted with situation of no drug. Analyses are controlled for time after injury. Data represent least squares mean ΔAUC-ICH with upper and lower confidence bounds, * represents significance at p <0.05. For fentanyl doses; FH: >1 mcg/kg, FL: <1 mcg/kg, F0: none. For midazolam doses; MH: >0.1 mg/kg, ML: <0.1 mg/kg, M0: none. No data points for dose F0/MH (all instances of this drug class were excluded because time after injury data was not available for those patients).
Using definitions described in the Data Collection section, we analyzed the effect of fentanyl and/or midazolam administration on the degree and time spent below age-dependent CPP thresholds (AUC-CPP deficit). In univariate analysis, post-resuscitation GCS correlated with ΔAUC-CPP deficit (r= −0.11; p=0.02) and was included in the final ΔAUC-CPP model. Analysis of AUC-CPP deficit showed no difference in exposure to CPP below age dependent thresholds after the administration of fentanyl and/or midazolam (Figure 4).
Figure 4.
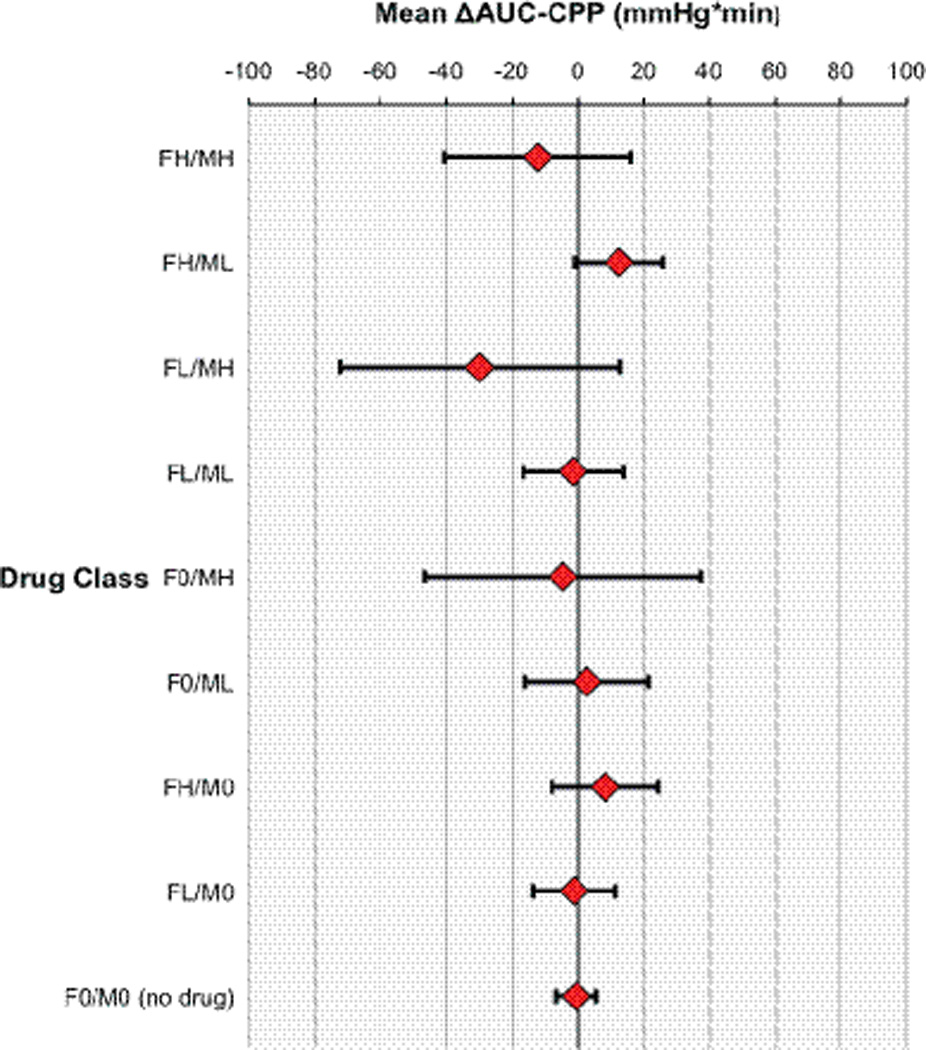
ΔAUC-CPP mixed model with subject as random effect and drug class as fixed effect. Drug classes represent each dose class contrasted with situation of no drug. Analyses are controlled for post-resuscitation GCS. Data represent least squares mean ΔAUC-CPP with upper and lower confidence bounds. No drug class was significant at p< 0.05. For fentanyl doses; FH: >1 mcg/kg, FL: <1 mcg/kg, F0: none.
For midazolam doses; MH: >0.1 mg/kg, ML: <0.1 mg/kg, M0: none.
DISCUSSION
Traumatic brain injury is often complicated by development of ICH, the magnitude and duration of which are correlated with worse outcomes [21–23, 33]. In order to test the hypothesis that bolus dosing of fentanyl and/or midazolam reduces ICP burden, we utilized high resolution monitoring data to compare time-weighted measures of ICH before and after drug administration.
We found that bolus administration of fentanyl and midazolam lacked effectiveness in the treatment of episodic ICH. In a mixed effects model, we found that the study drugs in fact increased ICH in the majority of drug/dose combinations. The aggregate increase in AUC-ICH [+17 mmHg*min] represented a +35% change in AUC-ICH from pre-treatment values. There was no significant change in CPP deficit following administration of these drugs. To our knowledge, this is the first investigation using high resolution data to show failure of these two commonly used agents to reduce the ICH burden in children with severe TBI.
Retrospective analysis of clinical decision making has significant limitations. Our main goal however was to evaluate the effectiveness of bolus fentanyl and midazolam administration in real world practice, where clinicians choose an intervention based on their clinical assessment. Retrospectively, it is not possible to determine whether the clinician’s decision to administer these drugs to treat ICH was physiologically justified, but we were able to evaluate the response to that decision. As highlighted in Figure 2, there is significant variance in clinical response to these drugs, both among and within patients. The observed response depends critically and temporally on the underlying cerebral hemodynamics at the time, as well as the clinical context. For example, if pain or agitation is the dominant stimulus at the time of drug administration, then response to drug may be favorable. Lacking the controlled conditions of a prospective trial, and physiologically driven drug administration criteria, our retrospective design cannot account for this variance.
Studies in adult patients demonstrate ICP increases following administration of fentanyl [10] and midazolam [12], as well as other commonly used opioids such as morphine [34], and sufentanil [11, 14]. These studies were limited by the use of low resolution ICP measurements, which may miss briefer episodes of ICH and lower the accuracy of AUC-ICH calculations. Additionally, previous studies evaluated the response to a single bolus dose of drug, and analyzed ICP for short duration windows. No before and after drug comparisons of ICH burden were made, rather they reported change relative to the absolute ICP immediately before drug administration. A cohort of severely injured adult patients was reported to show no ICP increase in response to fentanyl, morphine or sufentanil [35]. However this design evaluated the response to a titrated opioid infusion, not bolus therapy. Additionally, this cohort was also treated with induced hypocapnia, and had evidence of impaired cerebral vasoregulation, both of which likely influenced the ICP responses observed.
The use of opioids in traumatic brain injury is controversial in adult neurointensive care and neuroanesthesia. It is well established that several opioids can increase ICP and/or reduce CPP in adult neurosurgical patients with TBI, subarachnoid hemorrhage [36], and brain tumors [37]. The mechanism for this response is not fully understood, although opioid-mediated vasodilation has been proposed (leading to increased brain blood volume in the presence of abnormal brain compliance) [34]. In recognition of these data, and the lack of evidence supporting use in children, the 2003 consensus guidelines recommended that use of fentanyl in pediatric TBI be left to physician discretion [7]. The most recent guidelines do not specifically address the use of fentanyl due to the continued lack of clinical studies in children [4].
Data on the effects of midazolam in patients with ICH is limited. In a study of 12 adult patients with severe TBI, non significant changes in ICP following bolus midazolam were reported; notably however, ICP increased significantly when baseline pressure was less than 18 mmHg [12]. This study only compared baseline measurements to intermittent post-bolus values and was limited by small sample size and the lack of a control group. Current guidelines do not make specific recommendations on the use of midazolam or other benzodiazepines in children with severe TBI [4].
We selected high resolution ΔAUC-ICH as our primary outcome variable. By taking into account both the level and duration of the insult, this approach allows more robust determination of the ICH exposure [21]. Automated collection of high resolution ICP recordings is reliable and accurate, and combined assessment of ICH amplitude and duration predicts outcome better than peak or mean ICP values alone [22]. While this methodology has been used to develop bedside early warning systems [23], this is the first application of high resolution ΔAUC-ICH analysis to evaluate response to therapy in children.
In this cohort of patients, bolus administration of fentanyl and/or midazolam had no significant effect on ΔAUC-CPP. This finding is difficult to explain with the available data. As opposed the single ICH threshold used in our ΔAUC-ICH analysis, the ΔAUC-CPP analysis used four age-dependent thresholds. Time after injury correlated with ΔAUC-ICH, but not with ΔAUC-CPP, which correlated with post-resuscitation GCS. These differences preclude direct comparisons between the effect of drug administration on ΔAUC-ICH and ΔAUC-CPP. Interestingly, our data suggests that delta AUC-ICH decreases as time after injury increases. Given that the slope of this correlation approaches zero, further interpretation of this relationship is beyond the scope of our study. Future controlled prospective studies will be needed to adequately address the effect of time after injury on the response to these medications.
As noted in Figure 3, there was variance in effect seen amongst different drug/dose combinations, suggesting possible synergistic effects of combined therapy. However, these specific drug combinations were given across multiple patients, so unknown patient factors may be confounding these relationships. Additionally, the confidence bounds for the 3 significant combinations overlap, suggesting equivalency of effect.
Our analysis depends upon the accuracy of drug dose administration times as entered into the electronic medication administration record (eMAR). Our institutional policy specifies that eMAR times represent time of drug administration, not medication order time, order sign-off time or time of documentation entry into the medical record. Our inability to validate the accuracy of eMAR charting could introduce error. However, it seems unlikely that it would introduce a systematic bias, skewing AUC changes in a unidirectional manner that would alter the interpretation of our results. Several aspects of our study should be considered when assessing the external validity of our findings. As noted in Table 1, mortality in this cohort was 19%, which is higher that reported in recent clinical reports [6, 38]. Of the six patients who died, three had inflicted TBI, one had a gunshot wound to the head, two experienced cardiac arrest prior to PICU admission and one patient had GCS=3 with fixed and dilated pupils on admission. The mortality in our cohort is comparable to previous pediatric TBI studies that included these high mortality risk patients [39, 40]. We chose to include them because our goal was to evaluate sedation administration in routine clinical practice. Future prospective clinical trials with adequate inclusion and exclusion criteria are needed.
We also excluded from analysis 7 patients who only received one type of study drug combination. The statistical rationale for this was that these patients would have had confounding between the fixed and random effects, such that their data could not contribute to the estimation of drug effect in the mixed model. This could be a potential limitation if these patients differed fundamentally in their disease severity or responsiveness to therapy. It should be noted however, that receiving only a single type of drug combination does not imply less intense therapy, just less variance in therapy.
Additionally, during the study period, a proportion of patients received continuous vasoactive infusions (Table 3). Regardless of the indication, the administration of vasoactive support may influence the cerebral hemodynamic response to fentanyl and midazolam. This effect can only be addressed in a prospective clinical trial where hemodynamic effects of vasoactive medications are measured and accounted for.
We limited our analyses to patients who were monitored with intraparenchymal devices, and excluded those with only an intraventricular catheter. Patients with intraventricular catheters often undergo intermittent or even continuous CSF drainage, which requires interruption of pressure transduction. Since this precludes collection of complete high-resolution data, these patients were excluded. If there were substantial differences in the responsiveness of these patients to opioids or benzodiazepines, then excluding them from analysis could introduce systematic bias, albeit of unclear direction.
In order to isolate the effect of midazolam and fentanyl on ICH, we excluded any study drug administration event that was confounded by administration of other ICH-directed therapies, including treatment with osmotic agents in the preceding 30 minute window. In so doing, we cannot evaluate possible interactions between these medications and other ICH-directed therapies (i.e. decompressive craniectomy). We feel however that this approach added rigor to our study design, allowing us to analyze isolated effects of the drugs under study. Our study is limited by the fact that clinicians may have used interventions such as acute hyperventilation to treat episodic ICH, confounding the drug effect under investigation. While our medical record review did not show such interventions, they may have occurred and not been captured in the medical record.
We included untreated episodes of ICH in our final mixed model. As mentioned, these episodes represented either protocol deviation, or in many cases cessation of further therapy due to medical futility. This approach was used to add statistical rigor to the analysis. Methodologically, assessing both treated and untreated episodes within each subject allows greater power by increasing design connectivity between subjects. To avoid bias due to overrepresentation of futile cases, untreated ICH episodes were selected from time prior to the last treated episode. Excluding such terminal data from analysis is consistent with the approach used by other investigators [21].
Finally, our study could not identify all possible subject, drug, or disease process effects that might explain the paradoxical increase in ICP pressure-time burden we observed following study drug administration. Our retrospective design limits the ability to do this in a rigorous fashion. For example, certain drug/dose combinations may have altered cerebral hemodynamics through reduction in systemic blood pressure and cerebral perfusion pressure. Autoregulatory processes might then be expected to lower cerebral vascular resistance, and augment cerebral blood flow and volume, which in an injured and poorly compliant brain, would lead to increased ICH. Future prospective work could investigate this possibility by including concurrent measurements of cerebral blood flow and volume. Our analysis did show no difference in the use of continuous NMB infusion amongst different drug/dose combinations. This has explanatory relevance, in that NMB could conceivably have ablated subclinical muscle rigidity, and the consequent increase in ICH, during fentanyl bolus administration. Given our retrospective study design, it is not possible to determine whether continuous infusions of NMB were achieving adequate paralysis and ablation of subclinical motor activity.
CONCLUSIONS
Anecdotal clinical experience shows that individual patients may occasionally respond favorably to bolus dosing of fentanyl and midazolam. However, in this cohort of patients, bolus dosing of fentanyl and midazolam failed to significantly reduce ICH exposure when given in response to episodic ICH. In fact, we observed an increase in ICH following fentanyl and midazolam administration; this increase was significant for several drug-dose combinations, even after accounting for random subject effects as well as the time between injury and drug administration. Our results are consistent with previous reports on the use of bolus fentanyl and midazolam in adults with severe TBI and ICH. Our findings highlight the need for rigorous prospective evaluation of the administration of bolus fentanyl and midazolam for treatment of ICH in children with severe TBI.
Supplementary Material
Acknowledgments
The authors thank Christopher Brenner, RN for assistance with eMAR queries, Barbara Miller, RN for high resolution physiological data collection and extraction, and Lori Barganier, RN and Tina Day, CRA for clinical data extraction and database management.
Dr. Pineda received support for article research from the National Institutes of Health (NIH). His institution received grant support from The Robert Wood Johnson Foundation, Saint Louis Children’s Hospital Foundation, Sean Glanvill Foundation, and the NIH (Washington University IDDRC; NIH/NICHD P30 HD062171). Dr. Wallendorf is employed by the Washington University School of Medicine. His institution received grant support from the Division of Biostatistics (IDDRC). Dr. Doctor consulted for Terumo BCT and Novartis and lectured for Terumo BCT. His institution received grant support from the NIH, AHA, Children’s Discovery Institute, Entegrion, and Terumo BCT.
Financial Support: This study and Dr. Pineda were supported by the St. Louis Children’s Hospital Foundation, the Sean Glanvill Foundation, the Harold Amos Medical Faculty Development Program (Robert Wood Johnson Foundation), and the Intellectual and Developmental Disabilities Research Center at Washington University (NIH/NICHD P30 HD062171).
Footnotes
COPYRIGHT FORM DISCLOSURES:
The remaining authors have disclosed that they do not have any potential conflicts of interest.
Supplemental Digital Content. Table 1 and Table 2 outline our institutional approach to management of severe pediatric TBI.
REFERENCES
- 1.Langlois JA, Rutland-Brown W, Thomas KE. The incidence of traumatic brain injury among children in the United States: differences by race. J Head Trauma Rehabil. 2005;20(3):229–238. doi: 10.1097/00001199-200505000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Schneier AJ, Shields BJ, Hostetler SG, Xiang H, Smith GA. Incidence of pediatric traumatic brain injury and associated hospital resource utilization in the United States. Pediatrics. 2006;118(2):483–492. doi: 10.1542/peds.2005-2588. [DOI] [PubMed] [Google Scholar]
- 3.Adelson PD, Bratton SL, Carney NA, Chesnut RM, du Coudray HE, Goldstein B, Kochanek PM, Miller HC, Partington MD, Selden NR, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 6. Threshold for treatment of intracranial hypertension. Pediatr Crit Care Med. 2003;4(3 Suppl):S25–S27. [PubMed] [Google Scholar]
- 4.Kochanek PM, Carney N, Adelson PD, Ashwal S, Bell MJ, Bratton S, Carson S, Chesnut RM, Ghajar J, Goldstein B, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents--second edition. Pediatr Crit Care Med. 2012;13(Suppl 1):S1–S82. doi: 10.1097/PCC.0b013e31823f435c. [DOI] [PubMed] [Google Scholar]
- 5.Adelson PD, Bratton SL, Carney NA, Chesnut RM, du Coudray HE, Goldstein B, Kochanek PM, Miller HC, Partington MD, Selden NR, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 8. Cerebral perfusion pressure. Pediatr Crit Care Med. 2003;4(3 Suppl):S31–S33. [PubMed] [Google Scholar]
- 6.Pineda JA, Leonard JR, Mazotas IG, Noetzel M, Limbrick DD, Keller MS, Gill J, Doctor A. Effect of implementation of a paediatric neurocritical care programme on outcomes after severe traumatic brain injury: a retrospective cohort study. Lancet neurology. 2013;12(1):45–52. doi: 10.1016/S1474-4422(12)70269-7. [DOI] [PubMed] [Google Scholar]
- 7.Adelson PD, Bratton SL, Carney NA, Chesnut RM, du Coudray HE, Goldstein B, Kochanek PM, Miller HC, Partington MD, Selden NR, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 9. Use of sedation and neuromuscular blockade in the treatment of severe pediatric traumatic brain injury. Pediatr Crit Care Med. 2003;4(3 Suppl):S34–S37. [PubMed] [Google Scholar]
- 8.Dean NP, Boslaugh S, Adelson PD, Pineda JA, Leonard JR. Physician agreement with evidence-based recommendations for the treatment of severe traumatic brain injury in children. J Neurosurg. 2007;107(5 Suppl):387–391. doi: 10.3171/PED-07/11/387. [DOI] [PubMed] [Google Scholar]
- 9.Kress JP, O’Connor MF, Pohlman AS, Olson D, Lavoie A, Toledano A, Hall JB. Sedation of critically ill patients during mechanical ventilation. A comparison of propofol and midazolam. Am J Respir Crit Care Med. 1996;153(3):1012–1018. doi: 10.1164/ajrccm.153.3.8630539. [DOI] [PubMed] [Google Scholar]
- 10.de Nadal M, Ausina A, Sahuquillo J, Pedraza S, Garnacho A, Gancedo VA. Effects on intracranial pressure of fentanyl in severe head injured patients. Acta Neurochir Suppl. 1998;71:10–12. doi: 10.1007/978-3-7091-6475-4_3. [DOI] [PubMed] [Google Scholar]
- 11.Sperry RJ, Bailey PL, Reichman MV, Peterson JC, Petersen PB, Pace NL. Fentanyl and sufentanil increase intracranial pressure in head trauma patients. Anesthesiology. 1992;77(3):416–420. doi: 10.1097/00000542-199209000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Papazian L, Albanese J, Thirion X, Perrin G, Durbec O, Martin C. Effect of bolus doses of midazolam on intracranial pressure and cerebral perfusion pressure in patients with severe head injury. Br J Anaesth. 1993;71(2):267–271. doi: 10.1093/bja/71.2.267. [DOI] [PubMed] [Google Scholar]
- 13.Tobias JD. Increased intracranial pressure after fentanyl administration in a child with closed head trauma. Pediatr Emerg Care. 1994;10(2):89–90. doi: 10.1097/00006565-199404000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Werner C, Kochs E, Bause H, Hoffman WE, Schulte am Esch J. Effects of sufentanil on cerebral hemodynamics and intracranial pressure in patients with brain injury. Anesthesiology. 1995;83(4):721–726. doi: 10.1097/00000542-199510000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Juul SE, Beyer RP, Bammler TK, Farin FM, Gleason CA. Effects of neonatal stress and morphine on murine hippocampal gene expression. Pediatr Res. 2011;69(4):285–292. doi: 10.1203/PDR.0b013e31820bd165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Traudt CM, Tkac I, Ennis KM, Sutton LM, Mammel DM, Rao R. Postnatal morphine administration alters hippocampal development in rats. J Neurosci Res. 2012;90(1):307–314. doi: 10.1002/jnr.22750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fong HK, Sands LP, Leung JM. The role of postoperative analgesia in delirium and cognitive decline in elderly patients: a systematic review. Anesth Analg. 2006;102(4):1255–1266. doi: 10.1213/01.ane.0000198602.29716.53. [DOI] [PubMed] [Google Scholar]
- 18.Young C, Jevtovic-Todorovic V, Qin YQ, Tenkova T, Wang H, Labruyere J, Olney JW. Potential of ketamine and midazolam, individually or in combination, to induce apoptotic neurodegeneration in the infant mouse brain. British journal of pharmacology. 2005;146(2):189–197. doi: 10.1038/sj.bjp.0706301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hertle DN, Dreier JP, Woitzik J, Hartings JA, Bullock R, Okonkwo DO, Shutter LA, Vidgeon S, Strong AJ, Kowoll C, et al. Effect of analgesics and sedatives on the occurrence of spreading depolarizations accompanying acute brain injury. Brain : a journal of neurology. 2012;135(Pt 8):2390–2398. doi: 10.1093/brain/aws152. [DOI] [PubMed] [Google Scholar]
- 20.Anand KJ, Clark AE, Willson DF, Berger J, Meert KL, Zimmerman JJ, Harrison R, Carcillo JA, Newth CJ, Bisping S, et al. Opioid analgesia in mechanically ventilated children: results from the multicenter Measuring Opioid Tolerance Induced by Fentanyl study. Pediatr Crit Care Med. 2013;14(1):27–36. doi: 10.1097/PCC.0b013e318253c80e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vik A, Nag T, Fredriksli OA, Skandsen T, Moen KG, Schirmer-Mikalsen K, Manley GT. Relationship of “dose” of intracranial hypertension to outcome in severe traumatic brain injury. J Neurosurg. 2008;109(4):678–684. doi: 10.3171/JNS/2008/109/10/0678. [DOI] [PubMed] [Google Scholar]
- 22.Kahraman S, Dutton RP, Hu P, Xiao Y, Aarabi B, Stein DM, Scalea TM. Automated measurement of “pressure times time dose” of intracranial hypertension best predicts outcome after severe traumatic brain injury. J Trauma. 2010;69(1):110–118. doi: 10.1097/TA.0b013e3181c99853. [DOI] [PubMed] [Google Scholar]
- 23.Kahraman S, Hu P, Stein DM, Stansbury LG, Dutton RP, Xiao Y, Hess JR, Scalea TM. Dynamic three-dimensional scoring of cerebral perfusion pressure and intracranial pressure provides a brain trauma index that predicts outcome in patients with severe traumatic brain injury. J Trauma. 2011;70(3):547–553. doi: 10.1097/TA.0b013e31820c768a. [DOI] [PubMed] [Google Scholar]
- 24.Marshall LF, Marshall SB, Klauber MR, Van Berkum Clark M, Eisenberg H, Jane JA, Luerssen TG, Marmarou A, Foulkes MA. The diagnosis of head injury requires a classification based on computed axial tomography. J Neurotrauma. 1992;9(Suppl 1):S287–S292. [PubMed] [Google Scholar]
- 25.Adelson PD, Bratton SL, Carney NA, Chesnut RM, du Coudray HE, Goldstein B, Kochanek PM, Miller HC, Partington MD, Selden NR, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 17. Critical pathway for the treatment of established intracranial hypertension in pediatric traumatic brain injury. Pediatr Crit Care Med. 2003;4(3 Suppl):S65–S67. [PubMed] [Google Scholar]
- 26.Gish EC, Harrison D, Gormley AK, Johnson PN. Dosing evaluation of continuous intravenous fentanyl infusions in overweight children: a pilot study. J Pediatr Pharmacol Ther. 2011;16(1):39–46. [PMC free article] [PubMed] [Google Scholar]
- 27.Rosen DA, Rosen KR. Midazolam for sedation in the paediatric intensive care unit. Intensive Care Med. 1991;17(Suppl 1):S15–S19. doi: 10.1007/BF01731149. [DOI] [PubMed] [Google Scholar]
- 28.Brain Trauma F, Bratton SL, Chestnut RM, Ghajar J, McConnell Hammond FF, Harris OA, et al. American Association of Neurological S, Congress of Neurological S, Joint Section on N, Critical Care AC. Guidelines for the management of severe traumatic brain injury. XI. Anesthetics, analgesics, and sedatives. J Neurotrauma. 2007;24(Suppl 1):S71–S76. doi: 10.1089/neu.2007.9985. [DOI] [PubMed] [Google Scholar]
- 29.Buhrer M, Maitre PO, Crevoisier C, Stanski DR. Electroencephalographic effects of benzodiazepines. II. Pharmacodynamic modeling of the electroencephalographic effects of midazolam and diazepam. Clinical pharmacology and therapeutics. 1990;48(5):555–567. doi: 10.1038/clpt.1990.192. [DOI] [PubMed] [Google Scholar]
- 30.Scott JC, Stanski DR. Decreased fentanyl and alfentanil dose requirements with age. A simultaneous pharmacokinetic and pharmacodynamic evaluation. The Journal of pharmacology and experimental therapeutics. 1987;240(1):159–166. [PubMed] [Google Scholar]
- 31.Hemphill JC, 3rd, Barton CW, Morabito D, Manley GT. Influence of data resolution and interpolation method on assessment of secondary brain insults in neurocritical care. Physiol Meas. 2005;26(4):373–386. doi: 10.1088/0967-3334/26/4/004. [DOI] [PubMed] [Google Scholar]
- 32.Haque IU, Zaritsky AL. Analysis of the evidence for the lower limit of systolic and mean arterial pressure in children. Pediatr Crit Care Med. 2007;8(2):138–144. doi: 10.1097/01.PCC.0000257039.32593.DC. [DOI] [PubMed] [Google Scholar]
- 33.Jagannathan J, Okonkwo DO, Yeoh HK, Dumont AS, Saulle D, Haizlip J, Barth JT, Jane JA, Jane JA., Jr Long-term outcomes and prognostic factors in pediatric patients with severe traumatic brain injury and elevated intracranial pressure. J Neurosurg Pediatr. 2008;2(4):240–249. doi: 10.3171/PED.2008.2.10.240. [DOI] [PubMed] [Google Scholar]
- 34.de Nadal M, Munar F, Poca MA, Sahuquillo J, Garnacho A, Rossello J. Cerebral hemodynamic effects of morphine and fentanyl in patients with severe head injury: absence of correlation to cerebral autoregulation. Anesthesiology. 2000;92(1):11–19. doi: 10.1097/00000542-200001000-00008. [DOI] [PubMed] [Google Scholar]
- 35.Lauer KK, Connolly LA, Schmeling WT. Opioid sedation does not alter intracranial pressure in head injured patients. Can J Anaesth. 1997;44(9):929–933. doi: 10.1007/BF03011963. [DOI] [PubMed] [Google Scholar]
- 36.Schmittner MD, Vajkoczy SL, Horn P, Bertsch T, Quintel M, Vajkoczy P, Muench E. Effects of fentanyl and S(+)-ketamine on cerebral hemodynamics, gastrointestinal motility, and need of vasopressors in patients with intracranial pathologies: a pilot study. J Neurosurg Anesthesiol. 2007;19(4):257–262. doi: 10.1097/ANA.0b013e31811f3feb. [DOI] [PubMed] [Google Scholar]
- 37.Marx W, Shah N, Long C, Arbit E, Galicich J, Mascott C, Mallya K, Bedford R. Sufentanil, alfentanil, and fentanyl: impact on cerebrospinal fluid pressure in patients with brain tumors. J Neurosurg Anesthesiol. 1989;1(1):3–7. [PubMed] [Google Scholar]
- 38.Adelson PD, Wisniewski SR, Beca J, Brown SD, Bell M, Muizelaar JP, Okada P, Beers SR, Balasubramani GK, Hirtz D, et al. Comparison of hypothermia and normothermia after severe traumatic brain injury in children (Cool Kids): a phase 3, randomised controlled trial. Lancet neurology. 2013;12(6):546–553. doi: 10.1016/S1474-4422(13)70077-2. [DOI] [PubMed] [Google Scholar]
- 39.Ducrocq SC, Meyer PG, Orliaguet GA, Blanot S, Laurent-Vannier A, Renier D, Carli PA. Epidemiology and early predictive factors of mortality and outcome in children with traumatic severe brain injury: experience of a French pediatric trauma center. Pediatr Crit Care Med. 2006;7(5):461–467. doi: 10.1097/01.PCC.0000235245.49129.27. [DOI] [PubMed] [Google Scholar]
- 40.Vavilala MS, Bowen A, Lam AM, Uffman JC, Powell J, Winn HR, Rivara FP. Blood pressure and outcome after severe pediatric traumatic brain injury. J Trauma. 2003;55(6):1039–1044. doi: 10.1097/01.TA.0000101759.23607.57. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


