Abstract
Lymphatic malformations (LMs) are congenital lymphatic lesions that impose significant and costly morbidities on affected patients. Treatment options are limited due to incomplete understanding of LM pathobiology. Expression of an activated β2-adrenergic receptor has been described in LM tissue, suggesting that this pathway may contribute to the clinical manifestations of LM. We hypothesized that propranolol, a β-adrenergic receptor antagonist, might improve symptoms of patients with LMs and lymphatic anomalies. A retrospective chart review of patients treated with propranolol as an adjunct therapy was conducted; analyses included demographic characteristics, clinical features, and response to propranolol. Three patients with cystic and noncystic LMs displayed clinical improvement at a minimum dose of 0.7 mg/kg/d, whereas symptomatic relapses were observed when propranolol doses dropped below this threshold. Two patients with Klippel-Trenaunay syndrome demonstrated partial clinical responses with reduced edema. The fetus of a mother treated with propranolol from a gestational age of 35 weeks through delivery displayed arrested growth of a cervicofacial LM. Our retrospective review suggests that propranolol improved symptoms in a subset of patients with lymphatic anomalies. Propranolol treatment may also limit the growth of congenital LMs in utero.
Lymphatic malformations (LMs) arise due to defective lymphatic vessel development. LM subtypes include “common” or cystic (including macrocystic, microcystic, and mixed macrocystic/microcystic) LMs, generalized lymphatic anomalies, primary lymphedema, and Gorham-Stout disease (issva.org/classification).1–4 LMs can also form part of a mixed vascular malformation, as in Klippel-Trenaunay syndrome (KTS). LMs are associated with life-threatening sepsis, bleeding, and lymphorrhea. Despite significant morbidities, treatment options for LMs are inadequate because the etiology is poorly understood. Empiric approaches include sclerotherapy or surgical resection, but recurrence is common.5,6 Recently, sirolimus and sildenafil have demonstrated symptomatic improvement in patients with LMs.7–12 Literature on propranolol, a nonselective β-adrenergic receptor antagonist, is inconclusive; some reports have shown efficacy,13–17 whereas others found no effect in patients with lymphatic anomalies.18,19 We present a retrospective review of 4 patients with LM and 2 patients with KTS treated with propranolol as an adjunct therapy (with responses ranging from modest to clear symptomatic improvement) and, for the first time, treatment of a fetus with an LM.
We performed a retrospective study of 6 patients with lymphatic anomalies who received propranolol (Columbia University Institutional Review Board, AAAL7000 and AAAO8902). Five patients whose symptoms could not be managed with conventional therapy were offered propranolol as an adjunct. A fetus at risk for airway compromise was treated with propranolol via maternal administration. Before initiation of propranolol, patients underwent cardiac evaluations. If no contraindications were identified, propranolol was initiated (most commonly at 0.5 mg/kg/d). Gradual dose escalation (final dose range, 0.7–2 mg/kg/d) was performed as tolerated. The mother of the fetus with cervicofacial LM underwent cardiac evaluation and was treated with propranolol 10 mg 3 times daily.
Patient Presentations and Treatment Outcomes
Six LM/KTS patient records are summarized in Table 1. Propranolol (0.5–2 mg/kg/d) was administered from 1 to 46 months (Table 2). No major adverse events (grade 3 or 4) were recorded. Treatment outcomes are grouped according to presentation, as follows: (1) cystic and noncystic LMs (n = 3); (2) KTS (n = 2); and (3) prenatal LM (n = 1). In all 3 patients with LM, propranolol treatment was associated with clear symptomatic improvement. In the 2 patients with KTS, propranolol use was associated with decreased edema. The fetal LM did not increase in size during treatment, and the infant exhibited no airway compromise at delivery.
TABLE 1.
Patient Baseline Characteristics
| Patient | Sex | Age at Diagnosis | Phenotype | Location | Symptoms/Signs | Previous Interventions |
|---|---|---|---|---|---|---|
| Pediatric LMs | ||||||
| 1 | M | Prenatal | Mixed macrocystic and microcystic LMs | Retroperitoneum | Edema | Debulking |
| Pelvis | Lymphatic drainage | |||||
| Perineum | Bleeding | |||||
| Left lower extremity | Sepsis | |||||
| Nonambulatory status | ||||||
| 2 | M | 5 y | Generalized lymphatic anomaly | Diffuse | Edema | Diuretics |
| Pleural/pericardial effusions Sepsis | Debulking | |||||
| 3 | M | 11 y | Lymphedema | Left foot | Lymphedema | None |
| Recurrent cellulitis | ||||||
| KTS | ||||||
| 4 | F | Prenatal | Capillary, venous, and LMs | Right lower extremity Peritoneum | Edema | Sclerotherapy |
| Lymphatic drainage | ||||||
| Bleeding | ||||||
| Sepsis | ||||||
| 5 | M | At birth | Capillary, venous, and LMs | Left lower extremity Perineum | Edema in toes | Debulking |
| Leakage from old incisions | Embolization of vein of Servelle | |||||
| Pain | ||||||
| Prenatal LMs | ||||||
| 6 | F | Prenatal | Mixed macrocystic and microcystic LMs | Cervicofacial | Prenatal radiographic finding | None |
| Concern for airway compromise | ||||||
F, female; M, male.
TABLE 2.
Propranolol: Clinical Efficacy
| Patient | Age at Therapy | Propranolol Dosage | Concurrent Intervention | Adverse Events | Clinical Outcome |
|---|---|---|---|---|---|
| Pediatric LMs | |||||
| 1 | 11 y | 0.58 → 1.25 mg/kg/d | Debulking | None | Decreased edema and drainage from old incisions |
| Decreased transfusions and unplanned hospitalizationsa | |||||
| Ambulatory | |||||
| 2 | 20 y | 0.43 → 0.7 mg/kg/d | None | Hair loss | Decreased edema (lost 5 kg)a |
| Increased function with minimal dyspnea climbing stairsa | |||||
| 3 | 11 y | 0.34 → 0.7 mg/kg/d | None | None | Lymphedema resolvedb |
| No episodes of recurrent cellulitisb | |||||
| KTS | |||||
| 4 | 1 mo | 1.5 → 2 mg/kg/d | Right trans-knee amputation | None | Decreased edema and drainage from skin lesions |
| 5 | 10 y | 0.98 → 1.31 mg/kg/d | Left above knee amputation | None | Decreased drainage from dermal LMs, decreased edema in toes |
| Prenatal LMs | |||||
| 6 | Prenatal | 10 mg TID to mother | None | None | Prenatal LM regressed |
| EXIT not needed | |||||
| No respiratory issues in NICU | |||||
EXIT, ex utero intrapartum treatment; TID, 3 times daily.
While patient was taking propranolol. Symptoms recurred after discontinuation.
Symptoms recurred when propranolol dose dropped to <0.55 mg/kg/d.
Case Reports
Cystic and Noncystic LMs: Clinical Improvement
Case 1 (Mixed Macrocystic/Microcystic LM)
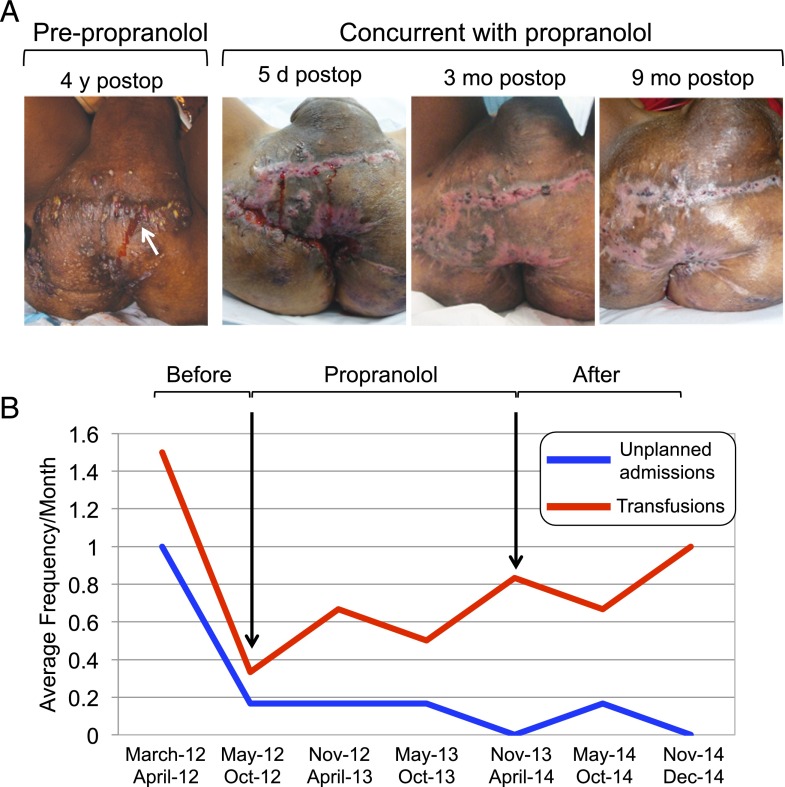
A 10-year-old boy with congenital LMs of the retroperitoneum, pelvis, perineum, and left lower extremity experienced multiple episodes of severe sepsis. He was unable to ambulate. Impaired healing of incision sites with persistent lymphorrhea persisted after a debulking procedure at 7 years of age (Fig 1A). Before his 11th birthday, the patient’s symptoms worsened, with increased edema, bleeding, and lymphorrhea, necessitating 3 hospitalizations and 4 transfusions (Fig 1B). Propranolol was offered and initiated (0.6 mg/kg/d). Decreased drainage and bleeding were noted within 24 hours. Unplanned emergency department visits and transfusion requirements decreased during treatment. After 2 weeks, LM edema decreased sufficiently to allow ambulation. Propranolol treatment continued (1.25 mg/kg/d).
FIGURE 1.
Propranolol improved morbidities of an 11-year-old patient with pleural, peritoneal, and perineal LMs (case 1). (A) Persistent drainage (arrow) from debulking before propranolol administration. Healing of incisions after debulking while taking propranolol at 5 days’, 3 months’, and 9 months’ postoperatively. (B) Frequency of blood transfusions (red) and unplanned hospital admissions (blue).
The patient experienced exacerbations during treatment with propranolol. One event was temporally associated with a viral syndrome and another with a decrease in effective dosage due to weight gain. Both events responded to an increase in the propranolol dosage. The patient underwent another debulking procedure during treatment, with healing and no lymphorrhea (Fig 1A). Propranolol was discontinued after 24 months due to noncompliance. His hospitalization and transfusion requirements increased (Fig 1B), and he lost the ability to ambulate. Sirolimus was initiated at 0.8 mg/m2 twice daily and up-titrated to a trough serum level of 7.1 ng/mL, with decreased lymphorrhea, bleeding, and 2 episodes of sepsis requiring hospitalization.
Case 2 (Generalized Lymphatic Anomaly)
A 19-year-old man presented with congenital bilateral lower extremity lymphedema, ascites, and dyspnea, controlled with furosemide and compression garments. He underwent surgical debulking at another institution, complicated by staphylococcal sepsis. His presenting symptoms included fever, cellulitis, worsening ascites, and pleuropericardial effusions, and he required pediatric ICU treatment. He was supported with biphasic positive airway pressure support, thoracostomy tubes, and pericardiocentesis. Propranolol treatment was initiated (0.43 mg/kg/d, increasing to 0.70 mg/kg/d). His condition improved, and he was discharged on propranolol monotherapy. Subsequent outpatient evaluation demonstrated improvement in lymphedema, a 5-kg weight loss, and the need for smaller compression garments and clothing due to decreased edema and ascites. The patient subsequently reported hair loss and chose to discontinue propranolol after 4 months. After discontinuation, he reported recurrent lymphedema and ascites, managed by paracentesis and intensified diuretic therapy. Despite these latter measures, his weight increased by 7 kg.
Case 3 (Primary Lymphedema)
A 9.5-year-old male patient presented with recurrent cellulitis of his left foot, requiring hospitalization and intravenous antibiotics. On examination, lymphedema of the affected lower left leg was noted, with a contralateral calf girth discrepancy of 1 cm. Propranolol was offered when the patient was 12 years old and initiated (0.5 mg/kg/d, increasing to 0.7 mg/kg/d). The patient noted decreased lower extremity lymphedema with no adverse effects.
The patient presented 1 year later with recurrent lymphedema and admitted noncompliance. Lymphedema resolved with directly observed propranolol treatment. Ten months later, the patient presented again with cellulitis, requiring admission for intravenous antibiotic treatment. During that time, he had undergone a growth spurt, which led to an effective dose decrease to 0.5 mg/kg/d. The cellulitis resolved with antibiotic treatment.
KTS: Limited Response
Case 4
A full-term female neonate was delivered with a massive lesion involving the right abdomen, flank, perineum, and right lower extremity, with cutaneous port wine stains, consistent with KTS. Magnetic resonance imaging/magnetic resonance angiography confirmed the presence of cystic LM components and venous anomalies. On day of life 11, she underwent doxycycline sclerotherapy of the macrocysts. At 2 weeks of age, propranolol was initiated, with a subsequent reduction in right lower extremity rubor 48 hours after treatment commenced. The patient was discharged but developed septic shock as an outpatient. She was resuscitated at an outside institution and transferred to our institution. After multidisciplinary consultation and family discussion, she underwent trans-knee disarticulation and diverting colostomy to decrease disease burden. Oral suppressive antibiotics were added to her regimen. The patient has remained infection-free for 46 months on propranolol (2 mg/kg/d). The right lower extremity stump incision is well healed, and improvement in edema was noted by her parents.
Case 5
A 10-year-old boy presented with left lower extremity KTS. Magnetic resonance imaging confirmed complex venous and lymphatic involvement. The patient’s functional status gradually deteriorated, with loss of the ability to ambulate. He underwent debulking procedures, but lymphedema recurred, with persistent lymphorrhea at incision sites. Propranolol treatment was initiated (1 mg/kg/d). A small decrease in incisional drainage was noted after 2 months of treatment. His lymphedema progressed after embolization of the vein of Servelle, with no improvement when propranolol was increased to 1.3 mg/kg/d.
Propranolol was tapered in preparation for an above-knee amputation. As propranolol was tapered (but before amputation), foot edema and drainage from the previous incisions worsened; both were quiescent during propranolol therapy. The patient underwent amputation, and incisional lymphorrhea responded to topical timolol gel. He is currently undergoing re-evaluation for resumption of propranolol treatment.
Prenatal LM: In Utero Treatment
Case 6 (Mixed Macrocystic/Microcystic LM)
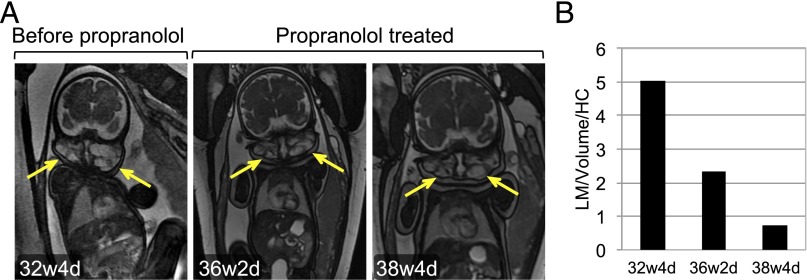
A 27-year-old woman was prenatally diagnosed with a fetus with a cervicofacial LM at 32 weeks’ gestation (Fig 2A), raising concern for airway obstruction. Propranolol was offered and treatment started (10 mg 3 times daily) at 35 weeks and continued until delivery (39 weeks). No adverse fetomaternal effects were noted.
FIGURE 2.
Propranolol therapy of a prenatal cervicofacial LM (case 6). (A) Coronal gradient-echo 2-dimensional magnetic resonance imaging scans of bilateral cervicofacial LM (arrows) at 32 weeks, 4 days (32w4d; pretreatment), and at 36 weeks 2 days (36w2d) and 38 weeks 2 days (38w2d) of gestation on propranolol therapy. (B) LM volume determined by ultrasonography normalized to head circumference (HC).
Repeat imaging demonstrated that while the fetus grew normally, LM dimensions stabilized. Ultrasonography suggested that LM volume may have decreased (Fig 2 A and B). Imaging confirmed fetal airway patency at 38 weeks, with uneventful delivery at 39 weeks’ gestational age.
Discussion
Based on anecdotal reports of propranolol efficacy in other lymphatic lesions,15–17 we performed a retrospective review of 5 patients with lymphatic anomalies who received propranolol as an adjunct therapy and 1 case of fetal cervicofacial LM whose mother received propranolol. Our initial experience suggests that propranolol improved symptoms in a subset of patients with cystic/noncystic LM/KTS; these included reduction of edema (n = 4; cases 1, 2, 3, and 4), bleeding (n = 2; cases 1 and 4), lymphorrhea (n = 3; cases 1, 4, and 5), and reduced hospitalizations in 1 patient. Both patients with KTS demonstrated partial relief of lymphatic-related symptoms, edema, and lymphorrhea during propranolol treatment (cases 4 and 5). Results from our series, in combination with previous reports,15,16 suggest that a prospective trial of propranolol in patients with LM is warranted.
Propranolol effects seemed to be dose dependent, with the lowest effective dose at 0.7 to 1 mg/kg/d. When propranolol was decreased or discontinued, qualitative experience and quantitative measures worsened. In case 1, cessation of therapy resulted in increased hospitalizations and transfusion requirements. In case 3, recurrent foot edema and cellulitis occurred with noncompliance. These findings are of particular importance in children, who are particularly susceptible to outgrowing medication doses.
Stabilization of a cervicofacial LM in a fetus who received propranolol via maternal administration was observed. Experience with propranolol treatment of fetal supraventricular tachycardias indicates that this option seems to be safe for mother and fetus.20,21 Cervicofacial LMs may obstruct the airway and require support perinatally, ranging from intubation to ex utero intrapartum treatment procedures,22 which entail potential fetomaternal risks.23,24 Maternal treatment with propranolol could be considered in such patients. However, neonatal LMs can also regress spontaneously,25 and maternal propranolol may be associated with intrauterine growth restriction.20 Further study of prenatal propranolol is necessary.
Recently, sirolimus, a mammalian target of rapamycin (mTOR) inhibitor, has been shown to be efficacious against life-threatening LMs.7,11,12 β2-adrenergic receptor signaling can activate mTOR downstream of cyclic adenosine monophosphate and cyclic adenosine monophosphate–dependent pathways.26 Propranolol may exert effects by attenuating mTOR activity, similar to sirolimus.
Conclusions
We concluded that a subset of patients in our series seemed to derive benefit from propranolol. These results support further studies to determine optimal means of assessing benefit, dosing, and identification of therapeutic alternatives for patients who do not improve.
Acknowledgments
The Lymphatics Work Group at CUMC includes Gerald Behr MD, Thomas Starc MD, Russ Miller MD, Maria Garzon MD, Julie Monteagudo MD, Connie Keung MD, and Peter Liou MD.
Glossary
- KTS
Klippel-Trenaunay syndrome
- LM
lymphatic malformation
- mTOR
mammalian target of rapamycin
Footnotes
Dr Wu designed the clinical retrospective study, reviewed patient charts, and drafted the initial manuscript; Dr Hooper reviewed patient charts; Dr Laifer-Narin reviewed in utero imaging studies and critically reviewed the manuscript; Dr Simpson collected, reviewed, and analyzed the in utero patient data and critically reviewed the manuscript; Dr Kandel conceptualized and designed the clinical retrospective study, supervised the collection of data, and critically reviewed and edited the manuscript; Dr Shawber conceptualized and supervised the clinical retrospective and immunohistochemical studies, reviewed and interpreted the data, and critically reviewed the manuscript; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by the National Institutes of Health (K08HL102068-01 [J.K.W.] and R21EB016515-01 ([J.J.K., C.J.S.]). Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: Dr Hooper is now employed by Baxalta; the other authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Wassef M, Blei F, Adams D, et al. ; ISSVA Board and Scientific Committee . Vascular Anomalies Classification: recommendations from the International Society for the Study of Vascular Anomalies. Pediatrics. 2015;136(1). Available at: www.pediatrics.org/cgi/content/full/136/1/e203 [DOI] [PubMed] [Google Scholar]
- 2.Lala S, Mulliken JB, Alomari AI, Fishman SJ, Kozakewich HP, Chaudry G. Gorham-Stout disease and generalized lymphatic anomaly—clinical, radiologic, and histologic differentiation. Skeletal Radiol. 2013;42(7):917–924 [DOI] [PubMed] [Google Scholar]
- 3.Trenor CC III, Chaudry G. Complex lymphatic anomalies. Semin Pediatr Surg. 2014;23(4):186–190 [DOI] [PubMed] [Google Scholar]
- 4.Maclellan RA, Greene AK. Lymphedema. Semin Pediatr Surg. 2014;23(4):191–197 [DOI] [PubMed] [Google Scholar]
- 5.Raveh E, de Jong AL, Taylor GP, Forte V. Prognostic factors in the treatment of lymphatic malformations. Arch Otolaryngol Head Neck Surg. 1997;123(10):1061–1065 [DOI] [PubMed] [Google Scholar]
- 6.Bai Y, Jia J, Huang XX, Alsharif MJ, Zhao JH, Zhao YF. Sclerotherapy of microcystic lymphatic malformations in oral and facial regions. J Oral Maxillofac Surg. 2009;67(2):251–256 [DOI] [PubMed] [Google Scholar]
- 7.Hammill AM, Wentzel M, Gupta A, et al. Sirolimus for the treatment of complicated vascular anomalies in children. Pediatr Blood Cancer. 2011;57(6):1018–1024 [DOI] [PubMed] [Google Scholar]
- 8.Swetman GL, Berk DR, Vasanawala SS, Feinstein JA, Lane AT, Bruckner AL. Sildenafil for severe lymphatic malformations. N Engl J Med. 2012;366(4):384–386 [DOI] [PubMed] [Google Scholar]
- 9.Singh P, Singh P, Mundy D. Giant neonatal thoraco-abdominal lymphatic malformations treated with sildenafil: a case report and review of the literature. J Neonatal Perinatal Med. 2013;6(1):89–92 [DOI] [PubMed] [Google Scholar]
- 10.Danial C, Tichy AL, Tariq U, et al. An open-label study to evaluate sildenafil for the treatment of lymphatic malformations. J Am Acad Dermatol. 2014;70(6):1050–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adams DM, Trenor CC III, Hammill AM, et al. Efficacy and Safety of Sirolimus in the Treatment of Complicated Vascular Anomalies. Pediatrics. 2016;137(2):e20153257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vlahovic AM, Vlahovic NS, Haxhija EQ. Sirolimus for the treatment of a massive capillary-lymphatico-venous malformation: a case report. Pediatrics. 2015;136(2). Available at: www.pediatrics.org/cgi/content/full/136/2/e513 [DOI] [PubMed] [Google Scholar]
- 13.Leboulanger N, Garel C, Borde IT, Garabedian EN, Denoyelle F. Propranolol therapy for hemorrhagic lymphangioma of the tongue. Arch Otolaryngol Head Neck Surg. 2011;137(8):813–815 [DOI] [PubMed] [Google Scholar]
- 14.Poralla C, Specht S, Born M, Müller A, Bartmann P, Müller A. Treatment of congenital generalized lymphangiectasia with propranolol in a preterm infant. Pediatrics. 2014;133(2). Available at: www.pediatrics.org/cgi/content/full/133/2/e439 [DOI] [PubMed] [Google Scholar]
- 15.Ozeki M, Fukao T, Kondo N. Propranolol for intractable diffuse lymphangiomatosis. N Engl J Med. 2011;364(14):1380–1382 [DOI] [PubMed] [Google Scholar]
- 16.Ozeki M, Kanda K, Kawamoto N, et al. Propranolol as an alternative treatment option for pediatric lymphatic malformation. Tohoku J Exp Med. 2013;229(1):61–66 [DOI] [PubMed] [Google Scholar]
- 17.Nir V, Guralnik L, Livnat G, et al. Propranolol as a treatment option in Gorham-Stout syndrome: a case report. Pediatr Pulmonol. 2014;49(4):417–419 [DOI] [PubMed] [Google Scholar]
- 18.Maruani A, Brown S, Lorette G, Pondaven-Letourmy S, Herbreteau D, Eisenbaum A. Lack of effect of propranolol in the treatment of lymphangioma in two children. Pediatr Dermatol. 2013;30(3):383–385 [DOI] [PubMed] [Google Scholar]
- 19.Akyüz C, Ataş E, Varan A. Treatment of a tongue lymphangioma with sirolimus after failure of surgical resection and propranolol. Pediatr Blood Cancer. 2014;61(5):931–932 [DOI] [PubMed] [Google Scholar]
- 20.Pruyn SC, Phelan JP, Buchanan GC. Long-term propranolol therapy in pregnancy: maternal and fetal outcome. Am J Obstet Gynecol. 1979;135(4):485–489 [DOI] [PubMed] [Google Scholar]
- 21.Rubin PC. Current concepts: beta-blockers in pregnancy. N Engl J Med. 1981;305(22):1323–1326 [DOI] [PubMed] [Google Scholar]
- 22.Laje P, Peranteau WH, Hedrick HL, et al. Ex utero intrapartum treatment (EXIT) in the management of cervical lymphatic malformation. J Pediatr Surg. 2015;50(2):311–314 [DOI] [PubMed] [Google Scholar]
- 23.Butwick A, Aleshi P, Yamout I Obstetric hemorrhage during an EXIT procedure for severe fetal airway obstruction. Can J Anaesth 2009;56(6):437–442 [DOI] [PubMed]
- 24.Lazar DA, Olutoye OO, Moise KJ Jr, et al. Ex-utero intrapartum treatment procedure for giant neck masses—fetal and maternal outcomes. J Pediatr Surg. 2011;46(5):817–822 [DOI] [PubMed] [Google Scholar]
- 25.Perkins JA, Maniglia C, Magit A, Sidhu M, Manning SC, Chen EY. Clinical and radiographic findings in children with spontaneous lymphatic malformation regression. Otolaryngol Head Neck Surg. 2008;138(6):772–777 [DOI] [PubMed] [Google Scholar]
- 26.Sato M, Dehvari N, Oberg AI, et al. Improving type 2 diabetes through a distinct adrenergic signaling pathway involving mTORC2 that mediates glucose uptake in skeletal muscle. Diabetes. 2014;63(12):4115–4129 [DOI] [PubMed] [Google Scholar]