Abstract
Ischiofemoral impingement (IFI) is an often unrecognized cause of hip pain caused by abnormal contact between the lesser trochanter and the ischium. To date, surgical treatment for those whose pain is not relieved by activity modification and steroid injections has not been defined. This study describes our imaging protocol and reports the results of arthroscopic, lesser trochanteric resections that were performed to treat this condition. Seven patients with symptomatic, MRI-documented IFI had ultrasound injections of ropivicaine and steroid into their ischiofemoral space. The injections provided complete but only transient relief of their groin and buttock pain and thus, all seven ultimately had an arthroscopic resection of their lesser trochanter. All hips were evaluated preoperatively and at 3, 6 and 12 months postoperatively with Byrd’s modified Harris hip scoring system. Average age of the seven patients was 46 years and there were five females and one male. Preoperative scores averaged 43 points. After surgery, all patients used crutches for 4–6 weeks, and had 6-week scores that averaged 58 points. The patients and their scores continued to improve and at 6 and 12 months, their scores averaged 86 and 91 points, and none had chronic hip flexor weakness or recurrence of their hip pain or snapping. Arthroscopic iliopsoas tenotomies in combination with a resection of the lesser trochanter will provide complete relief of the painful snapping, groin and buttock pain caused by ischiofemoral impingement.
INTRODUCTION
Ischiofemoral impingement (IFI) is an often unrecognized cause of pain and snapping in the hip, and is due to abnormal contact between the lesser trochanter and the lateral border of the ischium. To date, there are only a few reports regarding diagnosis and treatment of this problem [1–8] and surgical treatment for athletes and active individuals whose pain and snapping are not relieved by activity modification and ischiofemoral space injections has not been clearly defined [9–12]. This study describes our imaging protocol for evaluation of this problem, and reports the results of arthroscopic lesser trochanteric resections and iliopsoas tenotomies that were performed in seven patients to treat this condition.
METHODS
From a review of the senior author’s data base of 1285 consecutive hip arthroscopies, seven patients who had an arthroscopic iliopsoas tenotomy and a resection of the lesser trochanter performed between March 2013 and August 2014 to treat IFI and had a minimum follow-up of 1 year were identified.
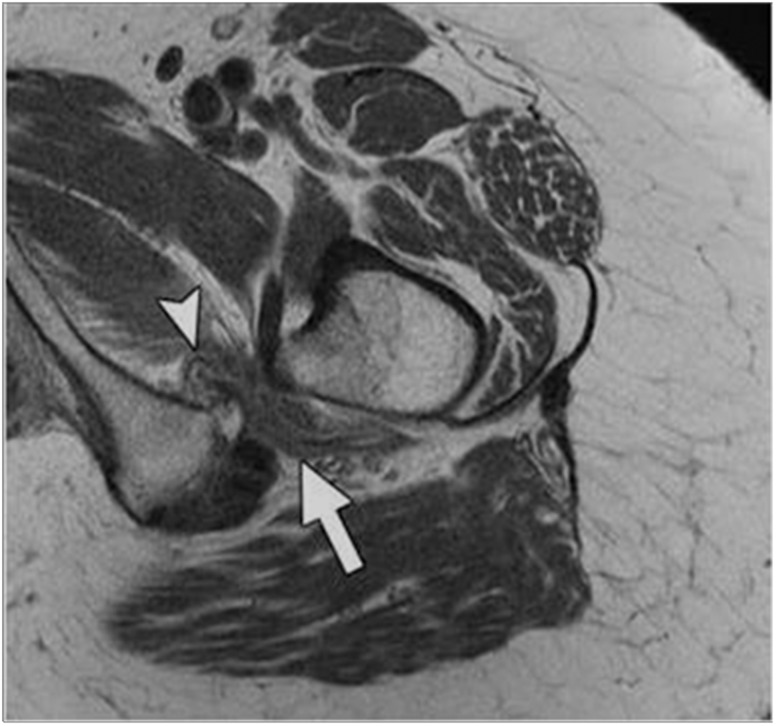
The seven pateints included in this study all had symptomatic, MRI-documented IFI (Fig. 1). The major symptoms in these patients were deep lower buttock pain, anterior groin pain which radiated posteriorly around the inner thigh to the lower buttock area (Fig. 2), tenderness to palpation of the ischiofemoral space, and either a positive IFI test [11], or a positive long-stride walking test [11].
Fig. 1.
MRI study of the right hip in a patient with the clinical findings of IFI showing Quadratus Femoris muscle edema (arrow head) and narrowing of the ischiofemoral space (arrow) between the ischium and lesser trochanter.
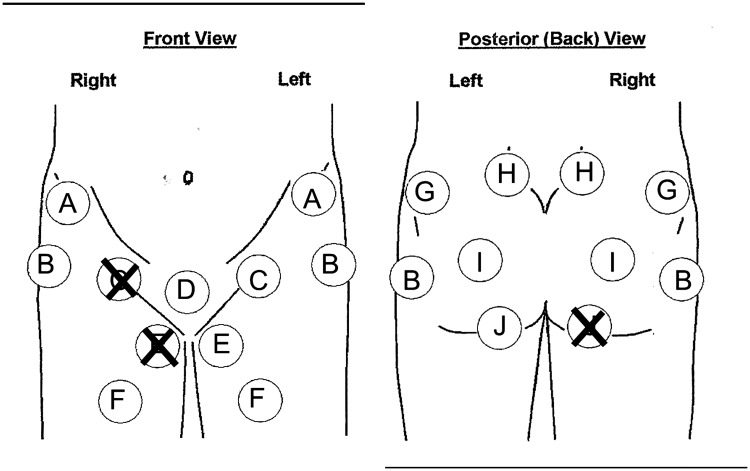
Fig. 2.
The pain ‘circle’ diagram had circles into which patients put an ‘X’ to indicate pain in the following areas: (A) anterior superior spine; (B) lateral greater trochanteric area; (C) central groin; (D) symphysis pubis; (E) proximal inner thigh; (F) anterior thigh; (G) posterior iliac crest; (H) sacroiliac joint; (I) sciatic notch and (J) ischial tuberosity. The circles were placed over anatomic locations commonly associated with hip pain. The patients would place X’s as shown above, to indicate how the anterior groin pain associated with their ischiofemoral impingement radiated posteriorly around the inner thigh to the lower buttock and ischial tuberosity area.
Ischiofemoral space tenderness was elicited by putting the patient in the prone or seated position and palpating the area just lateral to the ischial tuberosity. Patients with IFI will have marked tenderness with pressure applied lateral to the ischium over the ischiofemoral space, and the location of their pain lateral to the ischium is important in the diagnosis of IFI [11].
The IFI test was first described by Hatem et al. [11] who noted that the symptoms of IFI can be reproduced by a combination of extension, adduction and external rotation of the hip. In the current study, the test was performed with patients in the lateral decubitus position and the contralateral hip down on the table. The affected hip was then extended and adducted. The test was considered positive if the patients symptoms were reproduced when the hip was put in an extended and adducted position.
For the long-stride walking test, the patient was asked to walk taking long, exaggerated strides. The test was considered positive if their long strides reproduced their posterior buttock pain, and walking with short strides alleviated their pain [11].
Imaging
All seven patients were evaluated preoperatively with plain radiographs that included an anteroposterior view of the pelvis, and a cross-table lateral view of the involved hip.
Each patient also was evaluated with our MR hip arthrography protocol and ultrasound-guided injections into their ischiofemoral space. The details of both imaging protocols have been previously reported [13–15], and are summarized below.
Prior to MR imaging, the hips were injected with a standard solution of a long-acting anesthetic and contrast [13]. Prior to the injection, patients were asked to indicate on a hip pain-circle diagram [14] the activities that caused their hip pain, and the location(s) and severity [1–10] of their ‘hip’ pain (Fig. 2). Ten to 30 minutes after the injection, the patients again tried their painful activities and asked to indicate in which areas they still had pain and in which areas they had significant (80%) pain relief. As noted previously, all seven patients had deep gluteal and lower buttock pain (Fig. 2, Circle J), and six of the seven patients had anterior groin pain (Circle C) which often wrapped medially (Circle E) and posteriorly around the inner thigh to the ischial tuberosity (Circle J). After the injection, only two of the six patients with groin pain noted ‘some’ relief of their anterior pain, but none of the patients had any relief of their deep gluteal and lower buttock pain.
Following hip joint injections of anesthetic and contrast, all seven patients were imaged on the same 3.0T MRI scanner (Signa Excite HDx, GE Healthcare, Milwaukee, WI) using an eight-channel phased-array coil. All exams included fat-suppressed coronal T1 images (TR/TE, 700/8; echo train (ETL), 2; matrix, 288 × 224; FOV, 20 cm; and slice thickness, 4 mm with 0.4 mm interslice gap (4 mm/0.4 mm)), fat-suppressed coronal T2 images (TR/TE, 4600/80; ETL, 19; matrix, 320 × 224; FOV, 20 cm; and slice thickness, 4 mm/0.4 mm), fat-suppressed sagittal T1 images (TR/TE, 700/8; ETL, 2; matrix, 228 × 224; FOV, 18 cm; and slice thickness, 4 mm/0.4 mm), fat-suppressed oblique axial T1 images (TR/TE, 700/8; ETL, 2; matrix, 288 x 224; FOV, 18 cm; and slice thickness, 4 mm/0.4 mm) and axial T1 images (TR/TE, 633/8; ETL, 2; matrix, 228 × 224; FOV, 18 cm; and slice thickness, 4 mm and a gap of 0.4 mm). All seven patients had the MRI findings of IFI, including edema within the quadratus femoris muscle and a narrowed ischiofemoral or quadratus femoris space as described by Torriani et al. [4]. (Fig. 1). Four of the patients also had labral tears evident on their MRI’s.
The sonographic imaging and ischiofemoral space injections were performed at our institution by fellowship-trained musculoskeletal radiologists using linear transducers (7-4 MHz, 8-4 MHz, 10 L) and Philips 5000 or GE Logiq 9 ultrasound units.
The ultrasound-guided ischiofemoral space injection technique, which has been described previously, was as follows [15]. The patient was placed in the prone and the ultrasound probe was placed on the affected side and moved laterally to visualize the bony acoustic landmark of the lesser trochanter. The quadratus femoris (QF) muscle and sciatic nerve were then visualized and a 3.5 inch, 22-gauge spinal needle was inserted from lateral to medial in the plane of the transducer and passed in real-time lateral and deep to the sciatic nerve until the needle tip was placed in the QF muscle. At that point, a 4-ml solution containing 1 ml of kenalog, 1 ml of 1% preservative-free lidocaine and 3 ml of ropivicaine were injected. All seven patients had the aforementioned ultrasound-guided QF muscle injections, and in all cases, the injections relieved their groin and buttock pain but their relief was transient. Thus, all seven ultimately had an arthroscopic excision of their lesser trochanter.
Prior to surgery, all seven patients completed 6 or more months of nonoperative treatment which included: rest; avoidance of impingement positions; stretching and strengthening of the hip musculature and the use of non-steroidal anti-inflammatory medications and the aforementioned ultrasound-guided injections. When the injections and physical therapy failed to provide prolonged relief of their IFI symptoms, the patients were offered and elected to undergo hip arthroscopy and have an arthroscopic resection of their lesser trochanter.
Surgical technique
All seven patients had hip arthroscopy performed supine as described by Byrd; thus, all patients had arthroscopic evaluations of both the central and peripheral compartments of their hip joints. Four of the seven patients had labral tears. Two had non-repairable tears that were debrided, and two had tears that were repaired. Subsequently, osteoplasties were performed to treat pincer impingement in two, and combined CAM and pincer deformities in four patients, respectively. All patients then had an arthroscopic release of the iliopsoas tendon from their lesser trochanter, followed by an arthroscopic resection of their lesser trochanter.
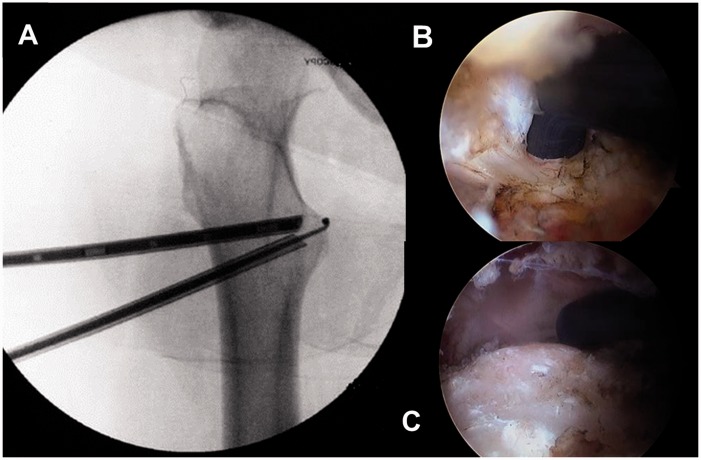
Details of the arthroscopic iliopsoas tendon release technique utilized in this study have been described previously [13]. In brief, after the hip arthroscopy was completed, traction was released, the knee was flexed to 20°, and the hip was maximally externally rotated to show the maximal width of the lesser trochanter (Fig. 3A). Under fluoroscopic guidance, an 18 gauge spinal needle was inserted from the lateral thigh and advanced until it was positioned on the anterior surface of the lesser trochanter. A nitanol wire replaced the needle and a 5.0-mm trochar and scope cannula were then advanced over the wire and onto the proximal aspect of the lesser trochanter. A second, distal portal was created in a similar manner and a second 5.0 mm cannula was inserted and placed in contact with the proximal cannula (Fig. 3A). A 30° arthroscope was placed in the proximal cannula, and a thermal probe was placed through the inferior cannula and advanced until it was visualized at the tip of the cannula (Fig. 3B). Once the scope and thermal probe were in place, it was kept in contact with the anterior surface of the lesser trochanter. This ensured safe access to the attachment of the iliopsoas tendon on the lesser trochanter, because with the probe on the bone, the only vessels that were encountered were the small descending branches of the lateral (femoral) circumflex artery. Throughout the approach to and the release of the tendon, sequential fluoroscopic images were obtained to ensure proper positioning of the cannulas and thermal probe.
Fig. 3.
(A–C) A fluoroscopic view showing the best position for the proximal cannula which contains the 30º arthroscope (A), and the position of the inferior cannula through which the thermal probe is inserted and advanced until it is visualized at the tip of the cannula (B). The tendon and muscle attachments are removed from the lesser trochanter (C) prior to its resection.
Once the thermal probe was visualized, the probe on cutting current was used to clear the soft tissues on the anterior surface of the lesser trochanter until the iliopsoas tendon was visualized (Fig. 3B). After the tendon had been clearly identified, it was released from its attachment to the anterior, superior, and medial surfaces of the lesser trochanter (Fig. 3C). In one case, the tendon was split into two separate bundles at the site of insertion. This was not unexpected since a prior anatomical study by Tatu et al. found that the tendon was partially or completely split at its site of insertion in 4 (17%) of their 24 specimens [16].
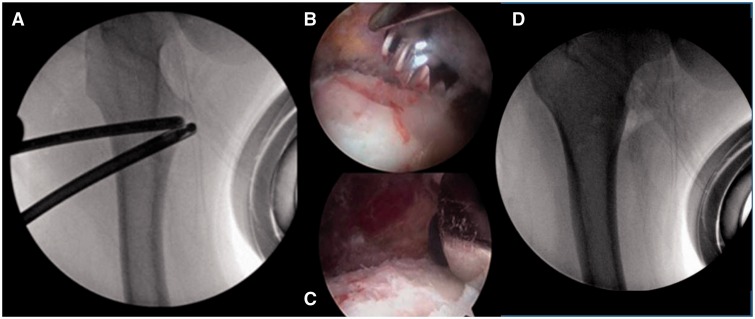
After the lesser trochanter was cleared of iliopsoas muscle and tendon, a 5.5 mm burr was inserted over the hip arthroscopy sled and placed on the anterior surface of the lesser trochanter (Fig. 4A). The lesser trochanter was then resected with the 5.5 mm burr (Fig. 4B and C). Visualization of the entire lesser trochanter was facilitated by flexing, abducting, and externally rotating the hip. The entire lesser trochanter was removed to prevent persistence of lesser trochanteric impingent due to inadequate resection of bone; this may occur with a partial resection since a thorough dynamic post-resection assessments for impingement cannot be completed with the patient in the supine position on the operating room table.
Fig. 4.
(A–D) Fluoroscopic view of the right hip showing the 5.5 mm burr placed on the anterior surface of the lesser trochanter; arthroscopic views (B–C) showing the progressive resection of the lesser trochanter; and a fluoroscopic view (D) demonstrating the completed resection of the lesser trochanter.
Prior to wound closure, anteroposterior and oblique fluoroscopic images were obtained to ensure that the entire lesser trochanter had been removed, Fig. 4D).
Rehabilitation protocol
Postoperatively, the patients’ weight bearing status was based on their control and coordination of their hip muscles. Thus, patients were instructed to use their crutches or other assistive devices until they had regained active flexion of their hip, and achieved a normal gait pattern (no limp) and coordinated muscle activity. Active hip flexion exercises were initiated when the patient could flex their hip when in a seated position. Active-assisted range of motion and abductor muscle strengthening exercises were initiated during the third postoperative week, and by 12 weeks after surgery, athletes and highly motivated individuals started walking and jogging and gradually progressed to running as tolerated. Over the next 3 months, the athletes worked to achieve good balance and control of their extremities and no pain with sport specific movements, including impact activities and to improve hip flexor strength to where it was at least 90% of the contralateral side.
Outcome assessment
All seven hips included in this study were examined by the senior author and assessed with Byrd's 100-point modified Harris hip scoring system (mHHS) prior to arthroscopy, and at 3, 6 and 12 months after surgery. Operative notes, operative hip sheets (drawings), intraoperative photos, and pre- and postoperative clinical notes were available for review for all seven patients.
Prior to initiating this study, appropriate IRB approval was obtained, and this study was performed in compliance with HIPPA regulations.
RESULTS
The average age of the seven patients was 46 years (range 15–66 years), and there were six females and one male. Four of the seven patients were involved in regular sports activities which included soccer, softball, and dance. All seven patients complained of pain with sitting, and posterior buttock pain. Other aggravating activities included walking, running, kicking a ball and climbing stairs. Six of the seven patients also had anterior groin pain which radiated posteriorly around the inner thigh to the lower buttock area (Fig. 2), and four of the seven patients had a ‘deep’ snapping sensation in their hip. In these patients, their deep snapping sensation could be reproduced by repeatedly internally and externally rotating the hip in the extended, adducted position utilized for performing the IFI test [11]. Thus, we theorized that it is the psoas tendon trapping and then abruptly rolling over the lateral edge of the swollen quadratus femoris muscle that produces the snap. However, we were unable to perform dynamic ultrasound evaluations during the performance of the IFI test to confirm our theory. All seven patients also had tenderness to palpation of the ischiofemoral space, and five patients (71%) had either a positive IFI test, or a positive long-stride walking test. In four patients, both tests were positive.
Outcomes
The preoperative scores of the seven patients averaged 43 points (Range 20–76 points). After surgery, the patients had hip flexor weakness, used crutches for 2–4 weeks and had 6-week scores that averaged 58 points. Their scores continued to improve and at 6 months averaged 86 points, and at 12 months averaged 91 points (Range 76–100 points). The patient that scored 76 points had relief of her buttock pain, but had persistent ‘hip’ pain due to chronic greater trochanteric bursitis. After an average follow-up of 20 months (range 12–28 months), none of the patients had tenderness to palpation of the ischiofemoral space, and none had a positive IFI test or a positive long-stride walking test. In addition, none of the patients had a recurrence of their snapping, or groin or buttock pain, and all of the athletes returned to full participation in their sport which included high school soccer and softball (she played catcher), at an average of 5.6 months (range 4–7 months) after surgery.
There were no complications in our series of patients. At their 1-year follow-up visits, none had hip flexor weakness and there were no cases of post-tenotomy heterotopic ossification or re-formation of the lesser trochanter (Fig. 5).
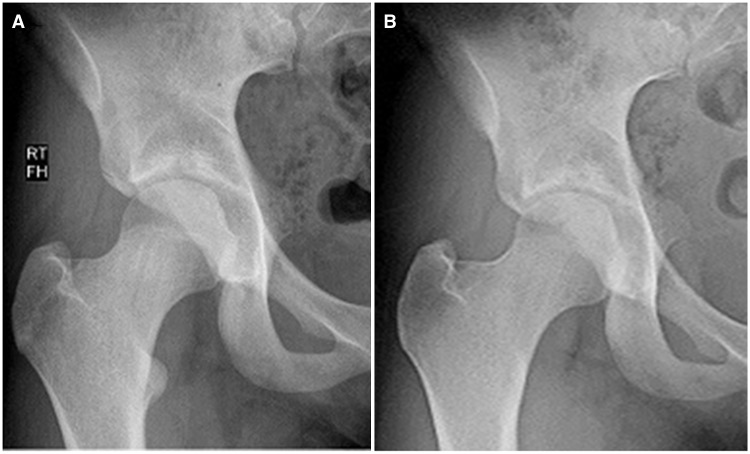
Fig. 5.
(A–B) Preop (A) and 6-month postop (B) radiographs showing the complete resection of the lesser trochanter in a 15-year-old high school track athlete. There was no regrowth of bone or heterotrophic ossification at the lesser trochanter at 6 months or on similar films obtained 1 year after surgery. The athlete returned to competition and ran in her state championship, symptom-free 6 months after her hip arthroscopy.
DISCUSSION
Diagnosis of IFI is often delayed because the patient’s history (anterior and posterior hip pain, deep snapping sensation) and limited clinical findings are not definitive. In patients with groin and buttock pain and MRI findings of IFI, ultrasound-guided anesthetic injections of the quadratus femoris (QF) muscle and ischiofemoral space will confirm that IFI is the cause of the patient’s hip pain. Ultrasound-guided anesthetic-steroid injections into the QF muscle and ischiofemoral space will be diagnostic and may provide months of pain relief [15]. Backer et al. compared the results of 20 patients that had both the MRI findings and clinical confirmation of pain relating to IFI. US-guided injections were performed in seven patients, (the injection group) and 13 patients did not have injections (the control group). During the 2-week period after injection, pain reduction was significantly better in the injection group with a mean reduction of 1.7 points (range 1–2 points) compared to a reduction of 0.8 points (range 0–2 points) for the control group (P < 0.01) [14].
The results of the current study indicate that if the aforementioned non-operative measures fail, an arthroscopic resection of the lesser trochanter will provide long-term relief of the hip pain caused by this type of extra-articular bony impingement. To date however, there are only four published reports regarding the arthroscopic treatment of IFI [9–12]. In 2014, Safran and Ryu described a case of IFI in a 19-year-old female athlete who had an endoscopic release of her iliopsoas tendon and a resection of the lesser trochanter through an anterior approach. Two years after her endoscopy, she was running two miles every day without pain, had 5-/5 hip flexor strength, and had an International Hip Outcome Tool (iHOT) score of 85 points, which represented an improvement of 53 points [9].
Howse et al. described a posterolateral approach for decompression of the ischiofemoral space. They used a cutting block technique to do a partial resection of the lesser trochanter for decompression of the ischiofemoral space [12]. They noted that their technique is minimally invasive and preserves the attachment of the lesser trochanter. In 2015, Jo and O’Donnell described their technique for the endoscopic resection of the lesser trochanter for the treatment of IFI in a 17-year-old college student. They performed a partial resection of the lesser trochanter through two anterolateral portals that were established at the level of the lesser trochanter. They reported that the patient’s resting pain and pain with adduction and external rotation disappeared within 1 week, and that pain relief was maintained at 4 months follow-up [10].
The largest cases series reported to date was that of Hatem et al. who reported the 2-year results of five patients with IFI who underwent endoscopic partial resections of their lesser trochanter through a posterior approach [11]. They accessed the lesser trochanter by resecting and creating a small window in the quadratus femoris muscle at the level of the lesser trochanter. They then resected the posterior one-third of the lesser trochanter. Two-years postoperatively, their patients’ mean modified Harris Hip Score had improved from 51 to 94 points, and the average time to return to sports for their five patients was 4.4 months. They concluded that endoscopic partial resection of the lesser trochanter was effective for the treatment of IFI in patients with ‘consistent clinical and imaging diagnostic findings.’ However, diagnostic injections were not performed in any their patients [11].
Our study evaluated the clinical results of seven patients with IFI that had arthroscopic iliopsoas tenotomies performed in conjunction with resection of the lesser trochanter through an anterior approach. The 1-year modified Harris hip scores (mHHS) for these patients averaged 91 points and 1 year after surgery, none of the patients had recurrent snapping of their tendon, hip flexor weakness or heterotopic bone formation.
The current study differed from that of Hatem et al. in two aspects. First, all seven patients had a diagnostic and potentially therapeutic injection of a long-acting anesthetic (ropivicaine) and steroid (kenalog) into their QF muscle and ischiofemoral space. In all cases, the injections relieved their groin and buttock pain and confirmed the diagnosis of IFI, but their relief was transient, and all seven ultimately had an arthroscopic resection of their lesser trochanter. As noted previously none of the patients in Hatem et al.’s study had diagnostic injections [11]. Second, all seven patients in the current study had an anterior approach to their lesser trochanter followed by a lesser trochanteric iliopsoas tenotomy and a complete resection of their lesser trochanter. The five patients in Hatem et al.’s study had a posterior approach to the lesser trochanter, a partial resection of the quadratus femoris muscle, a partial release of the iliopsoas tendon, and only a partial resection (posterior one-third) of their lesser trochanter [11].
There were no complications in our series of patients. At their 1-year follow-up visits, all of the patients had returned to their preoperative work and sports activities, and none had hip flexor weakness and there were no cases of post-tenotomy heterotopic ossification (Fig. 4).
Limitations
The strengths of this study are that it is a consecutive series of patients that had: (i) diagnostic ultrasound-guided injections to confirm the diagnosis of IFI; (ii) arthroscopic resection of their lesser trochanter to treat their IFI; (iii) their hip arthroscopy performed by one surgeon and (iv) their outcomes prospectively evaluated with Byrd’s modified Harris hip scoring system. The limitations of the study are that: (i) it is retrospective review of the data on this case series of patients; (ii) a limited number of patients had the index procedure performed and (iii) six of the seven patients had treatment of intra-articular abnormalities which could have been responsible for part of the improvement in their mHHS.
CONCLUSIONS
Arthroscopic iliopsoas tenotomies in combination with a resection of the lesser trochanter are safe, outpatient procedures that will provide long-term relief of the painful snapping, and groin and posterior buttock pain caused by this type of extra-articular bony impingement.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1.Johnson KA. Impingement of the lesser trochanter on the ischial ramus after total hip arthroplasty. Report of three cases. J Bone Joint Surg Am 1977; 59: 268–9. [PubMed] [Google Scholar]
- 2.Kassarjian A. Signal abnormalities in the quadratus femoris muscle: Tear or impingement? AJR Am J Roentgenol 2008; 190: W379. [DOI] [PubMed] [Google Scholar]
- 3.Patti JW, Ouellette H, Bredella MA. et al. Impingement of lesser trochanter on ischium as a potential cause for hip pain. Skeletal Radiol 2008; 37: 939–41. [DOI] [PubMed] [Google Scholar]
- 4.Torriani M, Souto SCL, Thomas BJ. et al. Ischiofemoral impingement syndrome: An entity with hip pain and abnormalities of the quadratus femoris muscle. AJR Am J Roentgenol 2009; 193: 186–90. [DOI] [PubMed] [Google Scholar]
- 5.Tosun O, Algin O, Yalcin N. et al. Ischiofemoral impingement: Evaluation with new MRI parameters and assessment of their reliability. Skelet Radiol 2012; 41: 575–87. [DOI] [PubMed] [Google Scholar]
- 6.Ali AM, Whitwell D, Ostlere SJ. Case report: Imaging and surgical treatment of a snapping hip due to ischiofemoral impingement. Skelet Radiol 2011; 40: 653–6. [DOI] [PubMed] [Google Scholar]
- 7.Truong WH, Murnaghan L, Hopyan S. et al. Ischioplasty for femoroischial impingement. J Bone Joint Surg Am 2012; 2: 2–6. [DOI] [PubMed] [Google Scholar]
- 8.Ganz R, Slongo T, Turchetto L. et al. The lesser trochanter as a cause of hip impingement: Pathophysiology and treatment options. Hip Int 2013; 23: 35–41. [DOI] [PubMed] [Google Scholar]
- 9.Safran M, Ryu J. Ischiofemoral impingement of the hip: A novel approach to treatment. Knee Surg Sport Traumatol Arthrosc 2014; 22: 781–5. [DOI] [PubMed] [Google Scholar]
- 10.Jo S, O’Donnell JM. Endoscopic lesser trochanteric resection for treatment of ischiofemoral impingement. Jhps 2015; 2: 184–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hatem MA, Palmer IJ, Martin HD. Diagnosis and 2-Year Outcomes of Endoscopic Treatment for Ischiofemoral Impingement. Arthroscopy 2015; 31: 239–46. [DOI] [PubMed] [Google Scholar]
- 12.Howse EA, Sandeep M, Tamam C. et al. Ischiofemoral Space Decompression Through Posterolateral Approach: Cutting Block Technique. Arthrosc Tech 2014; 3: e661–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flanum ME, Keene JS, Blankenbaker DG. et al. Arthroscopic treatment of the painful “internal” snapping hip: Results of a new endoscopic technique and imaging protocol. Am J Sports Med 2007; 35: 770–9. [DOI] [PubMed] [Google Scholar]
- 14.Arnold DR, Keene JS, Blankenbaker DG. et al. Hip Pain Referral Patterns in Patients with Labral Tears: Analysis Based on Intra-Articular Anesthetic Injections, Hip Arthroscopy and a New Pain “Circle” Diagram. Phy Sports Med 2011; 39: 1–7. [DOI] [PubMed] [Google Scholar]
- 15.Backer MW, Lee KS, Blankenbaker DG. et al. Correlation of ultrasound-guided corticosteroid injection of the quadratus femoris with MRI findings of ischiofemoral impingement. AJR Am J Roentgenol 2014; 203: 589–93. [DOI] [PubMed] [Google Scholar]
- 16.Tatu L, Parratte B, Vuillier M. et al. Descriptive anatomy of the femoral portion of The iliopsoas muscle: Anatomical basis of anterior snapping of the hip. Surg Radiol Anat 2001; 23: 371–4. [DOI] [PubMed] [Google Scholar]