Abstract
Hip arthroscopy is a fast growing orthopedic field of expertise. As in any field of surgery adequate postoperative pain management regimes are of utmost importance. The purpose of this review is to provide an overview of current knowledge on anesthetic options for perioperative pain management for hip arthroscopy. We searched the Pubmed/Medline and Embase database for literature and included 10 studies for our analysis. Because of the variety of pain scales and different ways of measured pain no meta-analysis could be performed and a descriptive review is performed. There are several types of pain regimens that can mostly be divided in two groups: local anesthetics and nerve blocks. Included studies show a rather large variation in reported visual analogue scale scores, post anesthesia care unit admission time and opioid usage. There are several anesthetic options available for hip arthroscopy. Different studies use different dosages, anesthetic regimens and different protocols; this partly explains the differences between studies with similar techniques. Peripheral nerve blocks seems promising but regarding current literature no clear recommendation can be made about what the best perioperative pain management option is, an overview of all reported techniques is given.
INTRODUCTION
In recent decades hip arthroscopy gained popularity and the number of procedures performed increase every year by as much as 233% between 2007 and 2011 [1–3]. This is due to improved techniques [4] and the widening range of indications, such as femoroacetabular impingement, labral tears, chondral injuries, loose bodies, osteonecrosis and septic arthritis [5]. Hence, adequate postoperative pain management after these procedures gains relevance also. As with almost all relative new operation techniques it is not quite clear what the optimal perioperative pain protocol is.
There is a wide variation of reported pain scores after hip arthroscopy procedures. A study by Ward et al. [6] report of 90% of all patients experience moderate to severe pain after hip arthroscopy defined as a pain score of 7 or more on the visual analogue scale (VAS). Lee et al. [7] makes notice of even higher VAS scores (between 8 and 10) in the post anesthesia care unit (PACU). In a randomized trial of Zhang et al. [8] direct postoperative mean PACU pain scores are also above 7. These scores are considerably very high and consequently, higher VAS scores require more opioids that increase the incidence of postoperative nausea and vomiting. This can prolong hospital stay and cause unplanned re-admissions [9, 10]. Contradictory to these high pain scores is a study by Baker et al. [11], they report of a mean maximum VAS score of 2.4 (SD 2.9) in the PACU.
Pain after hip arthroscopy arises due to several factors. Perhaps the most logical division to make is to divide in two regions. First the intra-articular compartment where pain originates from the joint capsule (capsulotomy), a repaired labrum or bony resection. Outside the joint itself pain can be caused by traction, pain can originate from the portal tracts and possibly from extravasation of irrigation fluids through the capsulotomy that lead to soft tissue swelling.
In the extra-articular compartment pain prevention can exist of lowering the pump pressure since higher fluid infusion pressure is strongly correlated with postoperative pain after hip arthroscopy [12]. Furthermore, lowering swelling of the upper leg and minimizing traction time positively influences postoperative pain [12]. The anesthesiologist can facilitate this by providing good muscle relaxation and keeping the blood pressure as low as possible. The pain caused by the intra-articular structures is more difficult to control because this is the basic work that needs to be done to make the surgical procedure successful.
Different parts of the hip joint capsule have different sensory innervations; the femoral nerve innervates the anterior part of the hip joint, the obturator nerve gives branches to the anteromedial part and the sciatic nerve provides innervation to the posterior part of the joint [13]. This innervation of the hip joint provides multiple anesthetic options, however the best strategy remains unclear.
The aim of this systematic review is to provide an overview of the current scientific knowledge on perioperative pain management options in hip arthroscopy.
MATERIALS AND METHODS
Inclusion criteria
Types of studies: The search of the literature performed for this review was limited to published original reports concerning the perioperative pain regimens in hip arthroscopy. Studies from levels of evidence I to IV were included. Abstracts from scientific meetings, unpublished reports, case reports and review articles were not included.
Types of participants: Inclusion was limited to articles on male and female adult humans undergoing hip arthroscopy. No limitation on the reason for hip arthroscopy was made.
Types of intervention: All pain regimens, including peripheral nerve blockade, local anesthesia or pain medications used to treat perioperative pain around hip arthroscopy were included for this study.
Types of outcomes measures: Our primary outcome is pain measurement, reported as a numerical rating scale or VAS score. Registration of PACU time and measurement of opioid consumption are secondary outcomes. The minimum criterion for inclusion of the trial in the review was the adequate reporting of at least one of the outcome variables. Information regarding other outcome measures and adverse events was extracted and analysed when feasible.
Search strategy for identification of studies
A research protocol was developed as described by Wright et al. [14], and used throughout the study process. This protocol was not registered. A literature search was performed throughout the Pubmed, Medline (Ovid) and Embase database on the 18th of August 2015. The search was independently performed by two authors (N.H.B. and D.H.). When using the following search terms: [(nerve block OR pain/diet therapy OR pain/drug therapy OR pain/prevention and control OR pain/therapy OR pain management OR analgesics) AND (arthroscopy) AND (hip OR hip joint)].
The references of retrieved publications were also manually checked to add studies potentially meeting the inclusion criteria and missed by the electronic search. Papers outside the English language were considered if translation was possible. We initially found 240 papers of which 17 were included for full text analysis. Of these papers 7 were excluded from final analysis for the following reasons: excluded 2 because of no reported outcomes, 2 because of no intervention described and 3 because of not specifically hip arthroscopy patients. The remaining 10 studies were included for analysis (Fig. 1).
Fig. 1.
Prisma flow chart.
RESULTS
Of the 10 studies included for analysis 5 are randomized controlled trials (RCTs) in which several techniques are evaluated (Table I).
Table I.
All included studies
| Study | Type | Group 1 | N | Group 2 | N | Outcomes |
|---|---|---|---|---|---|---|
| Morgenthaler et al. [15] | RCT | Intra-articular bupivacaine | 13 | Placebo | 13 | VAS and rescue medication |
| Baker et al. [16] | RCT | Portal infiltration | 33 | Intra-articular infiltration | 40 | VAS and rescue medication |
| Ward et al. [6] | RCT | Femoral nerve block | 20 | General anesthesia | 20 | PACU time, nausea, operation time |
| YaDeau et al. [17] | RCT | Lumbar plexus nerve block | 41 | General anesthesia | 41 | VAS, rescue medication, nausea/vomiting, hospital stay |
| Zhang et al. [8] | RCT | 200mg Celecoxib | 27 | Placebo | 26 | VAS and rescue medication |
| Krych et al. [18] | Prospective series | Fascia iliaca block | 30 | No control group | VAS, nausea, opioid use, patient satisfaction | |
| Potter et al. [19] | Prospective series | Fascia iliaca block | 53 | General anesthesia | 54 | VAS, PACU time, opioid use |
| Dold et al. [20] | Retrospective review | Femoral nerve block | 56 | General anesthesia | 40 | VAS, PACU time, opioid use |
| Baker et al. 2011 [11] | Retrospective chart review | Bupivacaine intra-articular | 85 | No control group | VAS, opiate requirement | |
| Schroeder et al. [21] | Retrospective review | Lumbar plexus block | 118 | General anesthesia | 118 | Peak PACU pain, PACU time, escape medication |
Because the various RCT’s use different scales and measured pain differently it was impossible to pool the data for a meta-analysis.
Local anesthetic infiltration
Intra-articular bupivacaine (injection of 20 ml 0.25% bupivacaine (50 mg) resulted in significant lower mean VAS scores and a decreased use of rescue medication in the first 20 h postoperatively when compared to placebo (injection of 20 ml 0.9% NaCl solution) in a small study of 26 patients [15]. Unfortunately, no power calculation was performed in this trial.
When comparing intra-articular bupivacaine versus portal infiltration with bupivacaine, postoperative VAS scores at 1 and 2 h were comparable [16]. The RCT of Baker et al. [16] was powered based on the lowest detectable change in VAS (1.3) and was calculated that 50 patients were required to observe a statistically significant difference (power 0.8, α = 0.05). Because of gender imbalance they included eventually a total of 73 patients. In this trial patients received either 10 ml 0.25% bupivacaine intra-articular after shutting of fluid irrigation through one of the arthroscopic cannula, or 10 ml 0.25% bupivacaine injected with an 18-gauge needle evenly spread between the portals. At 6 h postoperatively significant lower VAS scores were reported in the portal group (P = 0.0036). Although having lower VAS scores, the portal group required significant more intravenous morphine in the immediate postoperative period (2.33 mg of morphine versus 0.57 mg morphine, P = 0.036).
In a retrospective series by the same researchers 85 patients were assessed in whom 10 ml 0.5% bupivacaine was injected into the joint after surgery [11]. In this retrospective study the mean VAS at discharge from the recovery room was 1.4/10. In this paper no separate VAS scores the first hours after surgery were measured or recorded [11].
Peripheral nerve blocks
In a study by Ward et al. [6] 40 patients were to receive either morphine or a femoral nerve block postoperatively if their VAS score was 7 or higher. In total 36 of 40 patients reported a VAS of 7 or higher; a remarkable high number that indicates general anesthesia was insufficient for 90% of these patients. No power analysis was performed in this trial.
A single anesthesiologist using an ultrasound probe and a nerve stimulator performed all femoral nerve blocks. With a 21-gauge needle 25 ml of 0.25% bupivacaine with 1:200 000 epinephrine was administered.
Of the 20 patients who received femoral nerve blocks 18 were satisfied with their anesthesia compared to 14 out of 16 in the morphine group. The femoral nerve block patients stayed in the PACU for shorter times (177.85 min ± 17.34 versus 216.00 min ± 19.48, P < 0.0001) and suffered less postoperative nausea (10% versus 75%, P < 0.001) after surgery than those who received intravenous morphine. VAS scores after the intervention were not reported.
Dold et al. [20] published a retrospective review of 96 patients, of which 40 received general anesthesia and 56 patients a femoral nerve block preoperatively. A staff anesthesiologist using ultrasound guidance performed all block procedures. Between 15 and 25 ml of ropivacaine (0.33–0.75%) was injected to achieve circumferential spread around the femoral nerve. Mean pain scores were significantly lower 60 min postoperatively in the block group (2.48 versus 3.68, P = 0.02). Patients in the femoral nerve block group received a significantly lower total intraoperative and postoperative morphine-equivalent dose (2.04 mg versus 4.0 mg, P = 0.025). In the block group mean time to PACU discharge was 85,96 min (±29.79) versus 81,53 min (±26.07) for the no block group (P = 0.44).
Two independent series reviewed the effect of a fascia iliaca blockade (FIB). In a case series by Potter et al. [19], 53 patients received a fascia iliaca block and 54 patients received general anesthesia. Fascia iliaca nerve blocks were performed under ultrasound guidance by specialty-trained regional anesthesiologists. A 22-gauge needle was positioned 1 cm lateral of the femoral nerve and 30 ml of 0.25% bupivacaine with 5 mcg/ml of epinephrine. Expansion of the tissue plane and local anesthetic spread around the femoral nerve was visualized with ultrasound to confirm proper placement of the block.
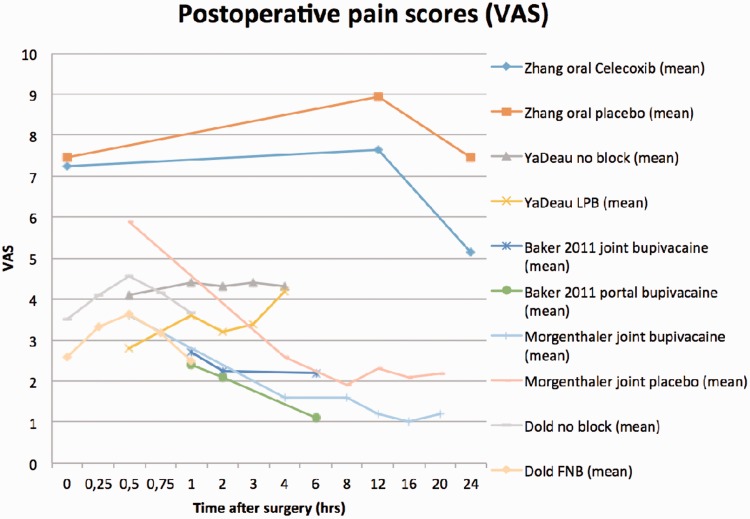
In the fascia iliaca block group initial PACU VAS scores were significantly higher than the no block group (7.2 ± 0.3 versus 5.5 ± 0.4, P = 0.001), however the fascia iliaca block group showed a significantly more dramatic decrease in VAS scores during PACU admission (-4.3 ± 0.2 versus -2.1 ± 0.3, P < 0.0001). At discharge of the PACU there was no significant difference in VAS between the two groups (2.8 ± 0.3 versus 3.4 ± 0.3, P = 0.15). Opioid use measured in morphine equivalent dose in the PACU was the same in both groups (4.4 mg ± 0.6 versus 4.4 mg ± 0.6, P = 0.98).
Krych et al. [18] included 30 patients who received a fascia iliaca block prior to surgery. There was no control group. Under ultrasound guidance a 22-gauge needle was advanced to enter the potential space between the iliacus muscle and the fascia iliaca. At this position 40 ml of 0.25% bupivacaine with 1:200 000 epinephrine was injected. Furthermore, all patients received additionally multimodal analgesics e.g. 10 mg i.v. ketamine and 15 mg i.v. ketorolac.
Patients reported a mean VAS score of 3.5 ± 2.3 in the post-operative recovery room. Mean VAS scores were measured the first 5 days postoperatively, scores ranged between 4.7 ± 2.5 (Day 0) and 3.4 ± 1.9 (Day 5). Pain scores were measured at 6.00 p.m. on Day 0, it is not mentioned what the time was when patients underwent surgery. Regarding their postoperative pain control 20 patients (67%) were very satisfied and 10 patients (33%) were satisfied. Time to discharge from the PACU was not measured in this study; no complications were reported in this study.
In a RCT of 82 patients, YaDeau et al. [17] administered a LBP in 41 patients versus combined spinal epidural anesthesia in 41 patients. In this trial, an adequate sample size/power analysis calculation was performed and indicated 40 patients per group (two-sided α = 0.05, β = 0.20). Control patients were prepared for a LPB but no needle was inserted. LPB was performed for LPB patients after sedation and a 21-gauge needle was inserted. After obtaining quadriceps stimulation at <0.5 mA, 30 ml 0.25% bupivacaine with 1:200 000 epinephrine was administered.
Mean PACU pain scores in rest were significantly lower in the block group than the combined spinal epidural group (3.3 ± 2.2 versus 4.2 ± 1.8). They recorded no difference in PACU analgesic use, PACU pain during movement or patient satisfaction. Furthermore, they recorded no differences in pain scores, patient satisfaction or total analgesic use post discharge.
Schroeder et al. [21] determined the effect of an LBP versus general anesthesia in a retrospective series of 236 patients. Lumbar plexus blocks were performed in a room dedicated to regional anesthesia techniques. Following skin analgesia with 1% lidocaine a 21-gauge needle was inserted and a current was applied via a nerve stimulator. The needle was manipulated until a current of 1.0 mA or less was able to elicit a contraction of the ipsilateral quadriceps muscle. 20–30 ml of 0.5% ropivacaine with 3 mcg/ml epinephrine was administered.
118 patients were included in the LPB group and matched to 118 patients in a no block group. In the block group peak PACU pain scores were significantly lower than the non-block group (5.0 (2.0–6.0) versus 5.3 ± 0.2, P = 0.023, scores given as median). PACU admission time was also significant lower in the block group (217.5 min (175.5–264.5) versus 240 min (183.5–299.5), P = 0.044, scores given as median). Perioperative opioid administration was significant lower in the block group than the non-block group [5.0 mg (2.5–7.0) versus 7.5 mg (5.0–10.0) P < 0.0001, scores given as median].
Zhang et al. [8] performed a RCT in which 27 patients received 200 mg Celecoxib 1 h preoperatively and 26 patients received 200 mg of a placebo. No power calculation was done in this study. VAS scores at 12 h (7.65 versus 8.93) and 24 h (5.13 versus 7.45) postoperatively were significantly lower in the Celecoxib group (P < 0.05). Medication usage was also significantly lower in the Celecoxib group (2.56 pills versus 4.35 pills, P < 0.05).
No difference was found in average time to discharge of the recovery room: 147 min for the Celecoxib group versus 152 min for the placebo group (P > 0.05).
DISCUSSION
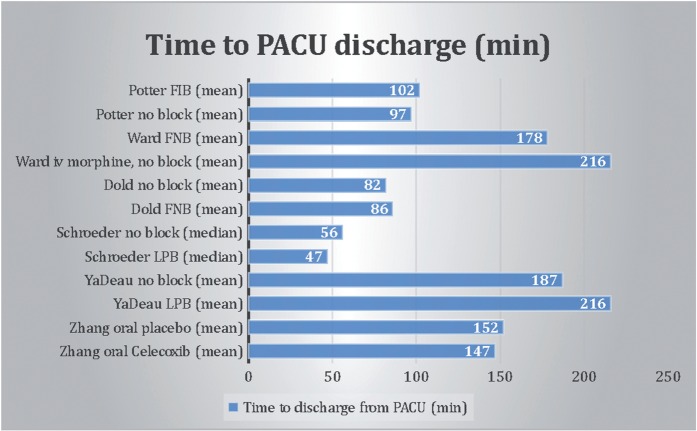
This review shows there are several perioperative anesthetic treatment options with similar results regarding VAS scores and mean time to discharge from the PACU (Figs 2 and 3). Unfortunately, pooling of the data is not possible because there is too much variation in pain scale recordings and analgesic use between the different studies. Figure 2 shows great variation in PACU discharge time, even between studies with the same technique.
Fig. 2.
Time to PACU discharge (minutes).
Fig. 3.
Mean postoperative VAS scores.
Infiltration techniques
A knee arthroscopy study shows that portal infiltration might be a good alternative for intra-articular infiltration regarding postoperative pain [22]. It is imaginable that pain after hip arthroscopy arises more from soft tissue swelling compared with knee arthroscopy; during hip arthroscopy traction is used and portals have to penetrate through a thicker soft tissue mass. In the only portal infiltration study included in this analysis, VAS scores were significantly lower 6 h after surgery compared with the intra-articular group, there was no difference in scores at 1 and 2 h after surgery.
Several studies reported the outcomes of intra-articular infiltration directly after hip arthroscopy [11, 15, 16]. Patients receiving intra-articular bupivacaine had significantly lower VAS scores compared to a placebo group. Unfortunately, separate VAS scores were not recorded over a longer period of time.
Previous knee arthroscopy studies investigated the effect of local joint injection with morphine or clonidine and showed improved results regarding pain scores after surgery [23, 24]. In a knee arthroscopy study measuring the effect of different types of intra-articular anesthetics, both groups with intra-articular levobupivacaine and tramadol or fentanyl showed significant better results than the levobupivacaine alone or control group [25].
Thus, the effect of intra-articular infiltration seems evident but the local anesthetic may also damage chondrocytes. Studies by Chu et al. [26–28] show that bupivacaine can have toxic effects on chondrocytes. A recent in vivo canine study by Sherman et al. [29] reported that the viability of chondrocytes is significantly decreased after intra-articular injection of local anesthetic-corticosteroids combinations.
Alternative to intra-articular infiltration of corticosteroids is the injection of magnesium. In a group of patients undergoing knee arthroscopy significant lower pain scores were reported with the injection of magnesium compared to a placebo [30]. As to our knowledge no hip studies with magnesium are performed so far. Furthermore, an in vitro study performed by Baker et al. [31] compared chondrocyte cell viability after injection of local anesthetic (bupivacaine, levobupivacaine, lidocaine or ropivacaine) with and without adding magnesium sulfate. The addition of magnesium resulted in higher levels of cell survival compared with the local anesthetic alone. The risk of chondrotoxic damage induced by intra-articular infiltration seems plausible and one must weigh this to the benefit of relative small changes in VAS scores. If local anesthetic is given it might be wise to add magnesium sulfate to reduce local toxic effects on chondrocyte cell viability [31].
The group of Zhang et al. [8] is the only group that investigated the effect of a COX-2 inhibitor. They recorded significant lower VAS scores in the Celecoxib group than the placebo group. Therapeutic effects are likely because of the selective inhibition of cyclooxygenase 2, while the toxic effects are induced by inhibition of cyclooxygenase 1. A selective cyclooxygenase 2 inhibitor could definitely play a role in a postoperative pain regimen since it show better results in pain scores than placebo.
Peripheral nerve blockade
LPB for total hip replacement seems a promising technique to reduce postoperative pain adequately with a low rate of complications [33, 34]. In the study of YaDeau et al. [17], LPB significantly reduced pain scores in the PACU, it is questionable if this difference is clinically relevant since pain scores were similar in both groups 4 h after surgery. Schroeder et al. [21] reported in their retrospective review significantly lower immediate and peak PACU pain scores in the LPB group. Unfortunately, no separate VAS scores were measured over a longer period of time. In a meta-analysis of hip surgeries a LPB resulted in lowered pain scores 4–8 h after surgery compared to opioids alone. In that analysis no convincing evidence was reported to support LPB over spinal, epidural or general anesthesia.
It is remarkable that there is a great difference in both LPB studies for measured time to PACU discharge while both performed more or less the same anesthetic procedure. In Yadeau’s group mean time to discharge was 216 min (±86) for the LPB patients, in Schroeder’s study it was 47 min (39,5–62,0). Yadeau’s study reports that patients were discharged following predetermined criteria, it is not mentioned what these criteria were. Schroeder’s group does not mention PACU discharge criteria.
Paravertebral blocks might be a theoretical alternative offering some advantages over the LPB; it is a more simple technique, LPB is associated with some serious complications (epidural or spinal spread resulting in hypotension, retroperitoneal hematoma) and in contrast to the LPB the quadriceps muscle strength is maintained with a paravertebral block. A case-report suggests that L1-L2 paravertebral blocks might have a similar effect on postoperative VAS pain scores for hip arthroscopy patients [7, 35]. Overall, a paravertebral block might provide the patient with better options for early mobilization and possibly early same day hospital discharge although so far there are no clinical trials performed which support this.
The femoral, sciatic and obturator nerves all innervate the hip joint. However, the contribution of each nerve to postoperative pain after hip arthroscopy is unclear. The femoral nerve seems responsible for a major part of the innervation of the hip capsule [13]. A femoral nerve blockade (FNB) has already been identified and used as an adequate anesthetic technique for knee surgery [36, 37].
The use of a FNB for hip surgery improved pain control the first hours after surgery [38]. Dold et al. [20] reported lower pain scores in the FNB group 60 min after surgery. Total morphine dose in the PACU and maximal pain rating in the surgical day care unit were also significantly lower in the FNB group. This study lacked standard protocols, patients received different dosages and combinations of analgesics, antiemetic prophylaxis, and varying concentrations and volumes of local anesthetics were used.
Ward et al. [6] reported significantly less nausea, shorter PACU times and higher rates of satisfaction in their FNB group compared to intravenous morphine. Unfortunately, no VAS scores were reported after intervention and only a dichotomous yes or no was scored for patient satisfaction. In the study of Ward et al. [6] a FNB was given if PACU pain scores were 7 or higher, in Dold’s study patients received a pre-operative block. This might explain great postoperative differences in PACU pain scores between both studies. It might also be the reason for the rather large difference in mean time to PACU discharge, 85.96 min (±29.79) in Dold’s study versus 177.85 min (±34) in Ward’s group. Although promising, due to the methodological shortcomings it is hard to draw a definite conclusion on FNB for hip arthroscopy.
In femur fracture or total hip arthroplasty studies a FIB resulted in decreased pain scores and lower opioids consumption postoperatively after surgery [39–42]. In our analysis two studies evaluated the effect on pain scores of a FIB after hip arthroscopy. In the group of Krych et al. [18], 30 consecutive patients received a FIB and all patients left the hospital the same day of surgery, with mean Day 0 reported VAS scores 4.7. This study lacked a control group and multimodal analgesia was used, therefore it cannot be said what the sole effect was of the FIB.
In the study of Potter et al. [19], patients received a FIB postoperatively in the PACU if they reported inadequate pain control. Therefore it is logical that initial VAS scores in the PACU are higher in the FIB group, though the dramatic change in VAS scores seems promising. No pain scores the first hours after surgery were recorded and the lack of randomization is limitations of this study. We believe it is hard to recommend this technique based on the available data.
LIMITATIONS
Limitations of these studies: often case series, no control groups, randomization not clarified, methods poorly explained, small groups, different pain scores used, not always well specified nor time of measurement. VAS scores for shorter periods of time measured. Patient satisfaction measured as dichotomous outcomes. The included RCT’s should be analysed with care since 3 of the 5 comparative studies did not report any power analysis [6, 15, 8]. Only Baker et al. [16] and Yadeau et al. [17] performed an adequate calculation, where Baker et al. added a total of 23 patients for gender imbalance. We think the analysis of Yadeau is the most accurate and the other studies might be underpowered.
There is large heterogeneity in performed nerve blocks, different dosages and techniques, different patient groups and reported pain scores. Furthermore, different studies that investigate the same anesthetic technique, report large differences in PACU discharge time. It is imaginable that different institutions use different protocols and guidelines when a patient is ready for PACU discharge. This might explain the rather large difference in PACU admission time.
CONCLUSION
There is a wide variation in anesthetic techniques available for pain management after hip arthroscopy; some techniques can even be combined. Based on the available literature no clear recommendations on which technique to use can be made, because comparison of studies is impossible. Future randomized trials are needed to evaluate the best pain management for hip arthroscopy. We recommend that future studies use validated VAS pain score registration at regular intervals, use standardized anesthetic block techniques and follow standard perioperative analgesic protocols.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1.Colvin AC, Harrast J, Harner C. Trends in hip arthroscopy. J Bone Joint Surg Am 2012; 94: e23. [DOI] [PubMed] [Google Scholar]
- 2.Montgomery SR, Ngo SS, Hobson T. et al. Trends and demographics in hip arthroscopy in the United States. Arthroscopy 2013; 29:661–5. [DOI] [PubMed] [Google Scholar]
- 3.Sing DC, Feeley BT, Tay B. et al. Age-related trends in hip arthroscopy: a large cross sectional analysis. Arthroscopy 2015; 31:2307–13.e2. [DOI] [PubMed] [Google Scholar]
- 4.Safran MR. Advances in hip arthroscopy. Sports Med Arthrosc 2010; 18:55.. [DOI] [PubMed] [Google Scholar]
- 5.Khanduja V, Villar RN. Arthroscopic surgery of the hip: current concepts and recent advances. J Bone Joint Surg Br 2006; 88:1557–66. [DOI] [PubMed] [Google Scholar]
- 6.Ward JP, Albert DB, Altman R. et al. Are femoral nerve blocks effective for early postoperative pain management after hip arthroscopy? Arthroscopy 2012; 28:1064–9. [DOI] [PubMed] [Google Scholar]
- 7.Lee EM, Murphy KP, Ben-David B. Postoperative analgesia for hip arthroscopy: combined L1 and L2 paravertebral blocks. J Clin Anesth 2008; 20:462–5. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Z, Zhu W, Zhu L. et al. Efficacy of celecoxib for pain management after arthroscopic surgery of hip: a prospective randomized placebo-controlled study. Eur J Orthop Surg Traumatol 2014; 24:919–23. [DOI] [PubMed] [Google Scholar]
- 9.Pavlin DJ, Chen C, Penaloza DA. et al. Pain as a factor complicating recovery and discharge after ambulatory surgery. Anesth Analg 2002; 95:627–34. [DOI] [PubMed] [Google Scholar]
- 10.Segerdahl M, Warren-Stomberg M, Rawal N. et al. Clinical practice and routines for day surgery in Sweden: results from a nation-wide survey. Acta Anaesthesiol Scand 2008; 52:117–24. [DOI] [PubMed] [Google Scholar]
- 11.Baker JF, Byrne DP, Hunter K. et al. Post-operative opiate requirements after hip arthroscopy. Knee Surg Sports Traumatol Arthrosc 2011; 19:1399–402. [DOI] [PubMed] [Google Scholar]
- 12.Tan CO, Chong YM, Tran P. et al. Surgical predictors of acute postoperative pain after hip arthroscopy. BMC Anesthesiol 2015; 15:96.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Birnbaum K, Prescher A, Hessler S. et al. The sensory innervation of the hip joint–an anatomical study. Surg Radiol Anat 1997; 19:371–5. [DOI] [PubMed] [Google Scholar]
- 14.Wright RW, Brand RA, Dunn W. et al. How to write a systematic review. Clin Orthop Relat Res 2007; 455:23–9. [DOI] [PubMed] [Google Scholar]
- 15.Morgenthaler K, Bauer C, Ziegeler S. et al. Intra-articular bupivacaine following hip joint arthroscopy. Effect on postoperative pain. Anaesthesist 2007; 56:1128–32. [DOI] [PubMed] [Google Scholar]
- 16.Baker JF, McGuire CM, Byrne DP. et al. Analgesic control after hip arthroscopy: a randomized, double-blinded trial comparing portal with intra-articular infiltration of bupivacaine. Hip Int 2011; 21:373–7. [DOI] [PubMed] [Google Scholar]
- 17.YaDeau JT, Tedore T, Goytizolo EA. et al. Lumbar plexus blockade reduces pain after hip arthroscopy: a prospective randomized controlled trial. Anesth Analg 2012; 115:968–72. [DOI] [PubMed] [Google Scholar]
- 18.Krych AJ, Baran S, Kuzma SA. et al. Utility of multimodal analgesia with fascia iliaca blockade for acute pain management following hip arthroscopy. Knee Surg Sports Traumatol Arthrosc 2014; 22:843–7. [DOI] [PubMed] [Google Scholar]
- 19.Potter MQ, Sun GS, Fraser JA. et al. Psychological distress in hip arthroscopy patients affects postoperative pain control. Arthroscopy 2014; 30:195–201. [DOI] [PubMed] [Google Scholar]
- 20.Dold AP, Murnaghan L, Xing J. et al. Preoperative femoral nerve block in hip arthroscopic surgery: a retrospective review of 108 consecutive cases. Am J Sports Med 2014; 42:144–9. [DOI] [PubMed] [Google Scholar]
- 21.Schroeder KM, Donnelly MJ, Anderson BM. et al. The analgesic impact of preoperative lumbar plexus blocks for hip arthroscopy. A retrospective review. Hip Int 2013; 23:93–8. [DOI] [PubMed] [Google Scholar]
- 22.Townshend D, Emmerson K, Jones S. et al. Intra-articular injection versus portal infiltration of 0.5% bupivacaine following arthroscopy of the knee: a prospective, randomized double-blinded trial. J Bone Joint Surg Br 2009; 91:601–3. [DOI] [PubMed] [Google Scholar]
- 23.Rosseland LA, Stubhaug A, Skoglund A. et al. Intra-articular morphine for pain relief after knee arthroscopy. Acta Anaesthesiol Scand 1999; 43:252–7. [DOI] [PubMed] [Google Scholar]
- 24.Reuben SS, Connelly NR. Postoperative analgesia for outpatient arthroscopic knee surgery with intraarticular clonidine. Anesth Analg 1999; 88:729–33. [DOI] [PubMed] [Google Scholar]
- 25.Sayin P, Dobrucali H, Turk HS. et al. Effects of intra-articular levobupivacaine, fentanyl-levobupivacaine and tramadol-levobupivacaine for postoperative pain in arthroscopic knee surgery. Acta Orthop Traumatol Turc 2015;49:267–73. [DOI] [PubMed] [Google Scholar]
- 26.Chu CR, Izzo NJ, Papas NE. et al. In vitro exposure to 0.5% bupivacaine is cytotoxic to bovine articular chondrocytes. Arthroscopy 2006; 22:693–9. [DOI] [PubMed] [Google Scholar]
- 27.Chu CR, Izzo NJ, Coyle CH. et al. The in vitro effects of bupivacaine on articular chondrocytes. J Bone Joint Surg Br 2008; 90:814–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chu CR, Coyle CH, Chu CT. et al. In vivo effects of single intra-articular injection of 0.5% bupivacaine on articular cartilage. J Bone Joint Surg Am 2010; 92:599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sherman SL, James C, Stoker AM. et al. In vivo toxicity of local anesthetics and corticosteroids on chondrocyte and synoviocyte viability and metabolism. Cartilage 2015; 6:106–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bondok RS, Abd El-Hady AM. Intra-articular magnesium is effective for postoperative analgesia in arthroscopic knee surgery. Br J Anaesth 2006; 97:389–92. [DOI] [PubMed] [Google Scholar]
- 31.Baker JF, Byrne DP, Walsh PM. et al. Human chondrocyte viability after treatment with local anesthetic and/or magnesium: results from an in vitro study. Arthroscopy 2011; 27:213–7. [DOI] [PubMed] [Google Scholar]
- 32.Hawkey CJ. COX-2 inhibitors. Lancet 1999; 353:307–14. [DOI] [PubMed] [Google Scholar]
- 33.Stevens RD, Van Gessel E, Flory N. et al. Lumbar plexus block reduces pain and blood loss associated with total hip arthroplasty. Anesthesiology 2000; 93:115–21. [DOI] [PubMed] [Google Scholar]
- 34.Touray ST, de Leeuw MA, Zuurmond WW. et al. Psoas compartment block for lower extremity surgery: a meta-analysis. Br J Anaesth 2008; 101:750–60. [DOI] [PubMed] [Google Scholar]
- 35.Ilkhchoui Y, Arndt CD, Koshkin E. et al. Preoperative L1 and L2 paravertebral block is an effective postoperative analgesia for hip arthroscopy in a multimodal analgesic regimen. BMJ Case Rep 2013; 2013: pii: bcr2013010496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paul JE, Arya A, Hurlburt L. et al. Femoral nerve block improves analgesia outcomes after total knee arthroplasty: a meta-analysis of randomized controlled trials. Anesthesiology 2010; 113:1144–62. [DOI] [PubMed] [Google Scholar]
- 37.Mall NA, Wright RW. Femoral nerve block use in anterior cruciate ligament reconstruction surgery. Arthroscopy 2010; 26:404–16. [DOI] [PubMed] [Google Scholar]
- 38.Fournier R, Van Gessel E, Gaggero G. et al. Postoperative analgesia with “3-in-1” femoral nerve block after prosthetic hip surgery. Can J Anaesth 1998; 45:34–8. [DOI] [PubMed] [Google Scholar]
- 39.Candal-Couto JJ, McVie JL, Haslam N. et al. Pre-operative analgesia for patients with femoral neck fractures using a modified fascia iliaca block technique. Injury 2005; 36:505–10. [DOI] [PubMed] [Google Scholar]
- 40.Foss NB, Kristensen BB, Bundgaard M. et al. Fascia iliaca compartment blockade for acute pain control in hip fracture patients: a randomized, placebo-controlled trial. Anesthesiology 2007; 106:773–8. [DOI] [PubMed] [Google Scholar]
- 41.Godoy-Monzon D, Iserson KV, Vazquez JA. Single fascia iliaca compartment block for post-hip fracture pain relief. J Emerg Med 2007; 32:257–62. [DOI] [PubMed] [Google Scholar]
- 42.Stevens M, Harrison G, McGrail M. A modified fascia iliaca compartment block has significant morphine-sparing effect after total hip arthroplasty. Anaesth Intensive Care 2007; 35:949–52. [DOI] [PubMed] [Google Scholar]