Abstract
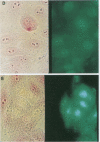
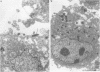
AIMS: Recent studies suggest that Helicobacter pylori is an invasive enteropathogen. However, the efficiency with which this pathogen invades mammalian cells remains unknown. Therefore, this study was designed to investigate the invasion frequencies of HEp-2 cells by clinical strains of H pylori. METHODS: An acridine orange assay and cultured HEp-2 cell monolayers were used to determine the HEp-2 cell penetration frequencies of 17 clinical isolates and one American Type Culture Collection (ATCC) strain of H pylori, and single clinical strains of Yersinia enterocolitica, Shigella flexneri, and a non-invasive ATCC Escherichia coli strain. RESULTS: The acridine orange assay demonstrated that invasion frequencies of HEp-2 cells by all H pylori isolates were significant and, in most instances, exceeded those for the S flexneri strain and equalled those for the Y enterocolitica strain. The assay also showed that internalised H pylori organisms remained viable for at least six hours, the maximum time that bacteria and HEp-2 cells were co-incubated. CONCLUSIONS: These results may have important implications for treatment and prevention strategies for this gastric pathogen. Furthermore, the acridine orange assay may be useful for assessing, in vitro, the ability of conventional and newer antibiotics, alone or in combination, to kill intracellular H pylori organisms.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bode G., Malfertheiner P., Ditschuneit H. Invasion of campylobacter-like organisms in the duodenal mucosa in patients with active duodenal ulcer. Klin Wochenschr. 1987 Feb 2;65(3):144–146. doi: 10.1007/BF01728609. [DOI] [PubMed] [Google Scholar]
- Bode G., Malfertheiner P., Ditschuneit H. Pathogenetic implications of ultrastructural findings in Campylobacter pylori related gastroduodenal disease. Scand J Gastroenterol Suppl. 1988;142:25–39. [PubMed] [Google Scholar]
- Bovallius A., Nilsson G. Ingestion and survival of Y. pseudotuberculosis in HeLa cells. Can J Microbiol. 1975 Dec;21(12):1997–2007. doi: 10.1139/m75-287. [DOI] [PubMed] [Google Scholar]
- Cover T. L., Blaser M. J. Purification and characterization of the vacuolating toxin from Helicobacter pylori. J Biol Chem. 1992 May 25;267(15):10570–10575. [PubMed] [Google Scholar]
- Crabtree J. E., Figura N., Taylor J. D., Bugnoli M., Armellini D., Tompkins D. S. Expression of 120 kilodalton protein and cytotoxicity in Helicobacter pylori. J Clin Pathol. 1992 Aug;45(8):733–734. doi: 10.1136/jcp.45.8.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler A. F., Havstad S., Ma C. K., Blaser M. J., Perez-Perez G. I., Schubert T. T. Accuracy of invasive and noninvasive tests to diagnose Helicobacter pylori infection. Gastroenterology. 1995 Jul;109(1):136–141. doi: 10.1016/0016-5085(95)90278-3. [DOI] [PubMed] [Google Scholar]
- Devenish J. A., Schiemann D. A. HeLa cell infection by Yersinia enterocolitica: evidence for lack of intracellular multiplication and development of a new procedure for quantitative expression of infectivity. Infect Immun. 1981 Apr;32(1):48–55. doi: 10.1128/iai.32.1.48-55.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckloff B. W., Podzorski R. P., Kline B. C., Cockerill F. R., 3rd A comparison of 16S ribosomal DNA sequences from five isolates of Helicobacter pylori. Int J Syst Bacteriol. 1994 Apr;44(2):320–323. doi: 10.1099/00207713-44-2-320. [DOI] [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr, Graham D. Y. Adherence and internalization of Helicobacter pylori by HEp-2 cells. Gastroenterology. 1992 May;102(5):1557–1567. doi: 10.1016/0016-5085(92)91714-f. [DOI] [PubMed] [Google Scholar]
- Evans D. G., Karjalainen T. K., Evans D. J., Jr, Graham D. Y., Lee C. H. Cloning, nucleotide sequence, and expression of a gene encoding an adhesin subunit protein of Helicobacter pylori. J Bacteriol. 1993 Feb;175(3):674–683. doi: 10.1128/jb.175.3.674-683.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leunk R. D., Johnson P. T., David B. C., Kraft W. G., Morgan D. R. Cytotoxic activity in broth-culture filtrates of Campylobacter pylori. J Med Microbiol. 1988 Jun;26(2):93–99. doi: 10.1099/00222615-26-2-93. [DOI] [PubMed] [Google Scholar]
- McDowell E. M., Trump B. F. Histologic fixatives suitable for diagnostic light and electron microscopy. Arch Pathol Lab Med. 1976 Aug;100(8):405–414. [PubMed] [Google Scholar]
- Miliotis M. D. Acridine orange stain for determining intracellular enteropathogens in HeLa cells. J Clin Microbiol. 1991 Apr;29(4):830–831. doi: 10.1128/jcm.29.4.830-831.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noach L. A., Rolf T. M., Tytgat G. N. Electron microscopic study of association between Helicobacter pylori and gastric and duodenal mucosa. J Clin Pathol. 1994 Aug;47(8):699–704. doi: 10.1136/jcp.47.8.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansonetti P. J., Ryter A., Clerc P., Maurelli A. T., Mounier J. Multiplication of Shigella flexneri within HeLa cells: lysis of the phagocytic vacuole and plasmid-mediated contact hemolysis. Infect Immun. 1986 Feb;51(2):461–469. doi: 10.1128/iai.51.2.461-469.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirigu F., Simonetta Capeccioni P., Dessi A., Usai P. Morphological study of the gastric antral mucosa colonized by campylobacter pylori. Ital J Gastroenterol. 1990 Feb;22(1):22–23. [PubMed] [Google Scholar]
- Small P. L., Isberg R. R., Falkow S. Comparison of the ability of enteroinvasive Escherichia coli, Salmonella typhimurium, Yersinia pseudotuberculosis, and Yersinia enterocolitica to enter and replicate within HEp-2 cells. Infect Immun. 1987 Jul;55(7):1674–1679. doi: 10.1128/iai.55.7.1674-1679.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Tricottet V., Bruneval P., Vire O., Camilleri J. P., Bloch F., Bonte N., Roge J. Campylobacter-like organisms and surface epithelium abnormalities in active, chronic gastritis in humans: an ultrastructural study. Ultrastruct Pathol. 1986;10(2):113–122. doi: 10.3109/01913128609014587. [DOI] [PubMed] [Google Scholar]
- Vaudaux P., Waldvogel F. A. Gentamicin antibacterial activity in the presence of human polymorphonuclear leukocytes. Antimicrob Agents Chemother. 1979 Dec;16(6):743–749. doi: 10.1128/aac.16.6.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyle F. A., Tarnawski A., Schulman D., Dabros W. Evidence for gastric mucosal cell invasion by C. pylori: an ultrastructural study. J Clin Gastroenterol. 1990;12 (Suppl 1):S92–S98. doi: 10.1097/00004836-199001001-00016. [DOI] [PubMed] [Google Scholar]