Abstract
Background
The relative contribution of biologic subtype to locoregional recurrence (LRR) in patients who have been treated with neoadjuvant chemotherapy (NAC), mastectomy and postmastectomy radiotherapy (PMRT) is not clearly defined.
Methods
233 patients with Stage II-III breast cancer received NAC, mastectomy and PMRT between 2000-2009: 53% (n=123) had HR+ (ER or PR+/HER2−), 23% (n=53) had HER2+ (HER2+/HR+ or HR−), and 24% (n=57) had triple negative disease (TN: HR−/HER2−). The 5-year LRR rates were estimated by Kaplan-Meier methods. Cox regression analysis was performed to evaluate covariates associated with LRR.
Results
Median follow-up was 62 months. A pathologic complete response (pCR) was seen in 14% of patients. The 5-year LRR rate was 8% in the entire cohort. The LRR rate was 0% in patients with a pCR versus 9% in patients without a pCR (p=0.05). TN disease (HR=4.4, p=0.003) and pathologic node positivity (HR=9.8, p=0.03) were associated with LRR. Patients with TN disease had a higher LRR rate compared to patients with HER2+ and HR+ disease (20% versus 6% and 4%, p=0.005). In patients without a pCR, TN subtype was associated with increased LRR (26% versus 7% HER+ and 4% HR+, p<0.001).
Conclusions
Patients with TN breast cancer had the highest LRR rate after NAC, mastectomy and PMRT. While no LRR was observed in TN patients with pCR, TN patients with residual disease had significantly higher LRR risk. Patients with HR+ and HER2+ breast cancer had favorable LRR rates, regardless of NAC response, likely due to receipt of adjuvant systemic targeted therapies.
Introduction
Postmastectomy radiation therapy (PMRT) confers a survival benefit in breast cancer patients with positive lymph nodes, as supported by data from randomized trials and a large meta-analysis by the Early Breast Cancer Trialists’ Collaborative Group1-4. However, these trials did not routinely assess estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor-2 (HER2) status. In addition, all of the chemotherapy was delivered in the adjuvant setting. Despite their historical significance, these trials provide limited information for decision-making about PMRT based on breast cancer subtype, as well as patterns of locoregional recurrence (LRR) in patients who have received neoadjuvant chemotherapy (NAC).
Multiple studies have determined that the likelihood of achieving pathologic complete response (pCR) to NAC is associated with breast cancer subtype. Triple negative (TN) and trastuzumab-treated HER2 positive breast cancer patients demonstrate the highest rates of pCR5-7 Although pCR is a reliable surrogate for survival5,6,8, its relationship to LRR is less clearly defined. Other than the post-hoc analysis of the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-18/27 trials, the endpoint of LRR has not been specifically examined in randomized trials of breast cancer patients treated with NAC9. Furthermore, these trials did not include patients treated with PMRT, and only 40% of patients had hormone receptor status information, precluding analysis of the impact of biologic subtype and NAC response on LRR.
PMRT is a standard part of the multidisciplinary management of node positive breast cancer patients treated with NAC and mastectomy. However, there are limited data on identifying patients who remain at high risk for LRR despite standard local therapies. The purpose of our study was to evaluate the effect of breast cancer subtype and response to NAC on LRR risk.
Materials and Methods
Patients
After obtaining approval from the institutional review board, 437 patients with clinical Stage II-III breast cancer who underwent NAC, mastectomy and PMRT between 2000 and 2009 were identified. After excluding patients with inflammatory breast cancer (174 patients), receipt of axillary surgery prior to NAC (13 patients), missing RT information (10 patients), and history of prior non-skin malignancy (7 patients), 233 women comprised the study population.
The clinical stage at presentation was determined by physical examination and mammogram. Pathologic characteristics and treatment details were obtained from medical records. A pCR was defined as no residual invasive disease in the breast and axillary lymph nodes on pathologic examination. Patients were categorized into 3 groups: with hormone receptor positive disease (HR+: ER+ and/or PR+, HER2−), HER2 positive disease (HER2+: HER2+, ER or PR+/−), and triple negative disease (TN: ER−, PR− and HER2−).
Treatments
All patients received NAC consisting of an anthracycline (10%), taxane (6%), or both (84%). Of the HER2+ patients, 74% received trastuzumab, which was routinely offered for tumors >1cm and node-positive HER2+ breast cancer beginning in 2004. Prior to NAC, no patient had a sentinel lymph node biopsy (SLNB) or axillary lymph node dissection (ALND), and abnormal axillary lymph nodes were evaluated by sonogram and fine needle aspiration. Following NAC, all patients underwent mastectomy. Nearly all (99%) patients underwent ALND and 2 (1%) patients had only SLNB. The median number of axillary lymph nodes removed for all patients was 17 (range 2-50).
All patients received PMRT (median dose 5040cGy). Indications for PMRT were clinical Stage III disease irrespective of response to NAC, or clinical Stage II disease with residual disease after NAC. In addition to standard PMRT fields, which included the chest wall and supraclavicular fossa, 91 (39%) patients received an axillary boost, 47 (20%) received internal mammary lymph nodes irradiation, and 33 (14%) received a mastectomy scar boost. Among patients with ER+ breast cancer, 99% received anti-estrogen therapy after PMRT, and in HER2+ patients receiving trastuzumab treatment was continued postoperatively for a total duration of 12 months.
Statistical Analysis
LRR was defined as recurrence in the ipsilateral chest wall or internal mammary, supraclavicular, or axillary lymph nodes identified by biopsy or imaging. Patients who did not have LRR were censored at death or last follow-up, calculated from date of diagnosis. Disease-free survival (DFS) was defined as time to distant recurrence or LRR. The 5-year overall survival (OS), DFS, and LRR rates were estimated using Kaplan-Meier methods. Differences in clinicopathologic characteristics between breast cancer subtypes were evaluated by Chi-square test. Univariate Cox regression analysis for LRR was performed with the following factors: age, grade, biologic subtype, lymphovascular invasion and extracapsular extension, clinical and pathologic stage, number of pathologic positive lymph nodes, and pCR status. A propensity score-matched analysis was performed to assess the influence of biologic subtype on LRR (TN versus HR+ and HER2+).
Results
Patient Characteristics
The median patient age was 46 years (range 24-81 years). Table 1 details the patient and disease characteristics and treatment response categorized by biologic subtype. Of the 233 patients, 57 (24%) had TN tumors, 53 (23%) had HER2+ tumors, and 123 (53%) had HR+ tumors. Patients with HR+ disease were more likely to have invasive lobular histology (16%, p=0.003) compared to patients with HER2+ (2%) and TN (4%) disease. More patients with TN (77%) and HER2+ (79%) disease had high grade tumors compared to patients with HR+ (47%, p<0.0001) disease.
Table 1.
Clinical and Pathologic Characteristics by Breast Cancer Subtype
| Characteristics | Patients | TN | HER2+ | HR+ | p= |
|---|---|---|---|---|---|
| Total (n) | 233 | 57 | 53 | 123 | |
| Age (median years, range) | 46 (24-81) | 45 (26-78) | 47 (24-69) | 47 (24-81) | 0.64 |
| Clinical stage before NAC (n [%]) | |||||
| T-stage | |||||
| 1-2 | 75 (32) | 19 (33) | 23 (43) | 33 (27) | 0.28 |
| 3-4 | 158 (68) | 38 (67) | 30 (57) | 90 (73) | |
| N-stage | |||||
| 0 | 28 (12) | 6 (11) | 4 (8) | 18 (15) | 0.35 |
| 1 | 127 (55) | 27 (47) | 35 (66) | 65 (53) | |
| 2 | 59 (25) | 17 (30) | 12 (22) | 30 (24) | |
| 3 | 19 (8) | 7 (12) | 2 (4) | 10 (8) | |
| Clinical stage | |||||
| II | 58 (25) | 10 (18) | 18 (34) | 30 (24) | 0.14 |
| III | 175 (75) | 47 (82) | 35 (66) | 93 (76) | |
| Histologic Type (n [%]) | |||||
| Ductal | 190 (82) | 51 (89) | 50 (94) | 123 (72) | 0.003 |
| Lobular | 23 (9) | 2 (4) | 1 (2) | 20 (16) | |
| Mammary | 20 (9) | 4 (7) | 2 (4) | 14 (11) | |
| Grade (n [%]) | |||||
| Well differentiated | 4 (2) | 0 (0) | 0 (0) | 4 (3) | <0.001 |
| Moderately differentiated | 38 (16) | 1 (2) | 6 (11) | 31 (25) | |
| Poorly differentiated | 144 (62) | 44 (77) | 42 (79) | 58 (47) | |
| Not reported | 46 (20) | 12 (21) | 5 (10) | 30 (24) | |
| Pathologic stage after NAC (n [%]) | |||||
| yT-stage | |||||
| 0 | 44 (19) | 19 (33) | 15 (28) | 10 (8) | <0.001 |
| 1 | 83 (36) | 18 (32) | 21 (40) | 44 (36) | |
| 2 | 65 (28) | 15 (26) | 11 (21) | 39 (32) | |
| 3 | 38 (16) | 3 (5) | 6 (11) | 29 (24) | |
| 4 | 3 (1) | 2 (4) | 0 (0) | 1 (1) | |
| yN-stage | |||||
| 0 | 69 (30) | 29 (51) | 21 (40) | 19 (15) | <0.001 |
| 1mic/1 | 71 (30) | 15 (26) | 16 (30) | 40 (33) | |
| 2 | 56 (24) | 7 (12) | 12 (22) | 37 (20) | |
| 3 | 37 (16) | 6 (11) | 4 (8) | 27 (22) | |
| Overall stage | |||||
| 0 | 32 (14) | 13 (23) | 11 (21) | 8 (7) | < 0.001 |
| I | 25 (11) | 10 (18) | 9 (17) | 6 (5) | |
| II | 74 (32) | 18 (32) | 15 (28) | 41 (33) | |
| III | 102 (44) | 16 (28) | 18 (32) | 68 (55) | |
| Number of LN sampled (median, range) | 17 (2-50) | 18 (4-50) | 16 (3-42) | 17 (2-43) | 0.27 |
| Number of positive LN (n [%]) | |||||
| 0 | 71 (30) | 29 (51) | 21 (40) | 21 (17) | <0.001 |
| 1-3 | 78 (34) | 16 (28) | 16 (30) | 38 (31) | |
| ≥ 4 | 84 (36) | 12 (21) | 16 (30) | 64 (52) | |
| LVI (n [%]) | |||||
| Yes | 126 (54) | 33 (58) | 23 (43) | 51 (41) | 0.11 |
| No | 107 (46) | 24 (42) | 30 (57) | 72 (59) | |
| ECE (n [%]) | |||||
| Yes | 86 (37) | 40 (70) | 36 (68) | 71 (58) | 0.19 |
| No | 147 (63) | 17 (30) | 17 (32) | 52 (42) | |
| pCR (n [%]) | |||||
| Yes | 32 (14) | 13 (23) | 11 (21) | 8 (7) | <0.001 |
| No | 201 (86) | 44 (77) | 42 (79) | 115 (93) |
Abbreviations: ECE= extracapsular extension, HER2+= human epidermal growth factor receptor-2 positive, HR+= hormonal receptor positive, LN= lymph nodes, LVI= lymphovascular invasion, N=node, NAC=neoadjuvant chemotherapy, pCR= pathologic complete response, T= tumor, TN= triple negative.
Neoadjuvant Chemotherapy Response
Thirty-two (14%) patients had a pCR after NAC. Compared to patients with HR+ tumors, patients with TN and HER2+ tumors had higher rates of pCR: 13 of 57 (23%) patients with TN disease, 11 of 53 (21%) patients with HER2+ disease, and 8 of 123 (7%) patients with HR+ disease obtained a pCR (p<0.001). A pCR was more likely in patients with HER2+ tumors who received trastuzumab (10 of 40 patients, 25%) than those who did not receive trastuzumab (1 of 13 patients, 8%, p=0.18). Eight of the 28 (29%) patients with ER−/HER2+ disease had a pCR, compared to 3 of the 25 (12%, p=0.14) patients with ER+/HER2+ disease .
Treatment Outcome
The median follow-up was 62 months (range 7-161 months) during which time 22 patients had LRR, 83 had distant recurrences, and 85 died. The 5-year DFS and OS rates were 69% (95% confidence interval [CI]=60-78%) and 79% (95% CI= 74-84%), respectively. The 5-year LRR rate in the entire cohort was 8% (95% CI= 2-14%); 6 (27%) patients with LRR experienced an isolated first event and 16 (73%) patients presented with a simultaneous distant recurrence. Among the 22 patients with LRR, 7 had isolated chest wall recurrence, 10 had regional nodal recurrence, and 5 presented with both. Regional recurrence most frequently involved the internal mammary lymph nodes (7 patients), followed by supraclavicular (6 patients) and axillary (4 patients) lymph nodes. Table 2 details the radiation fields for each patient with a LRR. Despite being the most common nodal failure site, only 1 of the 7 patients with internal mammary lymph nodes failures had received dedicated internal mammary lymph nodes irradiation.
Table 2.
Irradiated Fields by Site of Locoregional Failure among 22 Patients with Locoregional Failure
| SCV RT/ SCV failures (n) | IMN RT/ IMN failures (n) | Axillary boost RT/ axillary failures (n) | CW scar boost/ CW failures (n) | |
|---|---|---|---|---|
| TN | 3 / 3 | 1 / 3 | 1 / 4 | 1/ 5 |
| HER2+ | 2 / 2 | 0 / 0 | 0 / 0 | 1 / 3 |
| HR+ | 1 / 1 | 0 / 4 | 0 / 0 | 1 / 4 |
| Total | 6 / 6 | 1 / 7 | 1 / 4 | 3/ 12 |
Abbreviations: CW= chest wall, IMN= internal mammary lymph nodes, RT= radiation therapy, SCV= supraclavicular fossa, other abbreviations same as Table 1.
On univariate analysis, patients with TN tumors (hazard ratio [HR]=4.4, p=0.003) and with pathologic node positive disease after NAC (ypN+; HR=9.8, p=0.03) had a higher risk of LRR. After using propensity score-matched analysis to adjust for differences in patient age, lymphovascular invasion, extracapsular extension, pCR status, clinical and pathologic stage, and number of pathologically positive lymph nodes, TN disease remained a significant risk factor for LRR (HR=3.7, p=0.008).
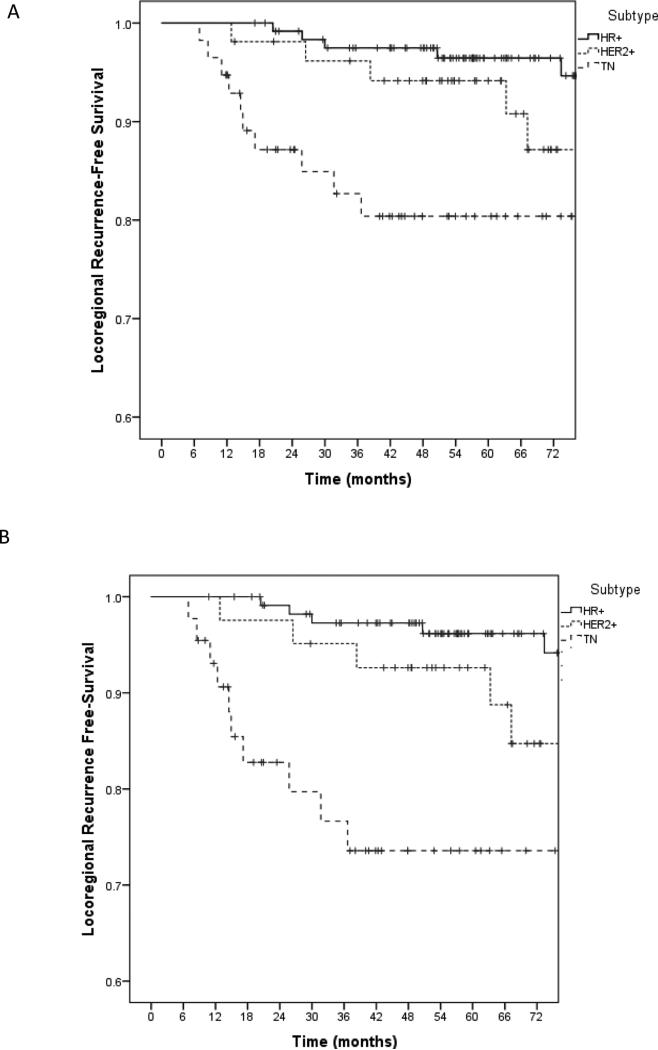
The interaction between biologic subtype, pCR and LRR was explored further. There was a significant difference in the 5-year LRR rates between patients based on biologic subtypes: 20% of the patients with TN disease, 6% of the patients with HER2+ disease, and 4% of the patients with HR+ disease experienced LRR (p=0.005, Figure 1a). No LRR was observed among the 32 patients who had a pCR (13 patients with TN, 11 with HER2+, and 8 with HR+ disease). In contrast, the 5-year LRR was 9% among the 201 patients who failed to achieve a pCR (p=0.05). Of the patients without a pCR, patients with TN tumors had significantly higher rate of LRR compared to patients with HER2+ and HR+ tumors: 10 of 44 patients with TN breast cancer experienced LRR at 5 years (5-year actuarial rate 26%), compared to 3 of 42 patients with HER2+ disease (7%), and 4 of 115 patients with HR+ disease (4%, p<0.001; Figure 1b).
Figure 1.
5-year locoregional recurrence-free survival by breast cancer subtype in overall study cohort (A), and in the patients who did not achieve a pCR (B).
Table 3 demonstrates LRR rates among breast cancer subtypes grouped by response to NAC and extent of pathologic nodal disease. Among the 69 patients with pathologically negative nodes after NAC, the rate of LRR was low among all subtypes, with one LRR among patients with HR+ tumors (19 patients) and no LRR in patients with TN (29 patients) and HER2+ (21 patients) tumors. Among the 164 ypN+ patients, LRR rates differed according to subtype, but not by the number of positive nodes. At 5 years, while none among the 40 patients with HR+ and 1-3 ypN+ disease developed LRR, 6 of the 16 patients with TN and 1-3 ypN+ disease experienced LRR (p<0.001). Similarly, while only 3 of the 64 patients with HR+ and ≥4 ypN+ disease developed LRR, 4 of the 12 patients with TN and ≥4 ypN+ disease had LRR at 5 years (p<0.001).
Table 3.
5-Year Actuarial LRR Rates According to Number of Positive Nodes and Response to Neoadjuvant Chemotherapy
| Patients | TN (LRR at 5 years/total patients [actuarial LRR]) | HER2+ (LRR at 5 years/total patients [actuarial LRR]) | HR+ (LRR at 5 years/total patients [actuarial LRR]) | p= |
|---|---|---|---|---|
| pCR | ||||
| yes | 0 / 13 (0%) | 0 / 11 (0%) | 0 / 8 (0%) | NA |
| no | 10 / 44 (26%) | 3 / 42 (7%) | 4 / 115 (4%) | <0.001 |
| Number of positive nodes | ||||
| 0 | 0 / 29 (0%) | 0 / 21 (0%) | 1 / 19 (5%) | 0.33 |
| 1-3 | 6 / 16 (46%) | 2 / 16 (13%) | 0 / 38 (0%) | <0.001 |
| 4+ | 4 / 12 (35%) | 1 / 16 (7%) | 3 / 64 (5%) | <0.001 |
Abbreviations: LRR= locoregional recurrence, NA= not applicable, other abbreviation same as Table 1.
Discussion
In this series of 233 women with clinical Stage II-III breast cancer who underwent NAC, mastectomy and PMRT, we showed that rates of LRR vary significantly by biologic subtype (ER, PR, and HER2 status). While the 5-year LRR rate in the entire cohort was only 8%, patients with TN subtype had a LRR rate of 20%. Both TN subtype and ypN+ disease following NAC were negative predictors for LRR. Patients with TN breast cancer who failed to achieve a pCR had higher 5-year LRR rate compared to patients with HER2+ and HR+ breast cancer (26% versus 7% and 4%, p<0.001). These findings underscore the complex relationship between biologic subtype, response to NAC and clinical outcomes. Biologic subtype is proven to be an accurate predictor of survival in patients treated with NAC5-8. Less is known about the impact of biologic subtype and NAC response on LRR, resulting in controversy in the surgical and radiotherapeutic nodal management in patients receiving NAC9-13.
Our results add to a growing body of evidence that biologic subtype and response to NAC predict for LRR in patients with breast cancer. To date, the largest study examining these issues is the Collaborative Trials in Neoadjuvant Breast Cancer14 analysis which included 5,252 NAC patients (39% received mastectomy). At 42 months, the 5-year LRR rate was 6.8% in the entire cohort. On multivariate analysis, TN (HR=4.1, 95% CI=3.0-5.4) and ypN+ disease (HR=2.4, 95% CI=1.6-3.4) were independent predictors of LRR. Although only one-third of mastectomy patients received PMRT, the pooled analysis provides compelling evidence for biologic subtype and residual disease as significant risk factors for LRR.
Other studies examining predictors of LRR in breast cancer patients receiving mastectomy and NAC have been limited by lack of biologic subtype information and variable use of PMRT. In a study of 542 patients who underwent NAC and mastectomy15, ER negative breast cancer was associated with a higher risk of LRR (HR=2.1, p=0.04). However, HER2 status was not analyzed in study population, therefore limiting its applicability to current day practice. Meyers et al. demonstrated that a higher LRR rate was observed in patients with TN breast cancer (20%, p=0.06) after NAC and mastectomy compared to HER2+ (7%) and HR+ (5%) breast cancer10. Although 18% of patients did not receive PMRT, the authors’ findings corroborate the high LRR rate observed among patients with TN disease in our study. Wright et al. found that TN subtype was associated with a higher risk of LRR after NAC and mastectomy, compared to the non-TN subtype (HR=8.5, 95% CI=3.5-20.8)16. While these publications support the significance of biologic subtype in predicting LRR, the combined effects of biologic subtype and response to NAC were not elucidated.
One of the first studies to demonstrate a relationship between LRR and biologic subtype in patients receiving NAC was published among patients receiving breast conserving therapy. In 2012, Caudle et al. reported on LRR as a function of biologic subtype and pCR in 595 patients who received breast conserving therapy following NAC17. The authors found that TN (HR=5.7, 95% CI=2.6-112.3), HR−/HER2+ (HR=5.7, 95% CI=2.0-16.3), and the presence of ≥4 positive lymph nodes (HR=2.9, 95% CI=1.3-6.6) were associated with higher LRR. The 5-year LRR rate was significantly lower for patients achieving pCR: 1% for patients with TN tumors with a pCR versus 16% for patients with TN tumors without a pCR. Although this study predated the routine use of trastuzumab, it substantiates our finding that pCR status and biologic subtype may serve as a surrogate for LRR risk.
In our study, 46% of patients with TN breast cancer with residual disease in 1-3 lymph nodes developed LRR at 5 years, whereas patients with 1-3 positive lymph nodes with HER2+ and HR+ breast cancer had favorable 5-year LRR rates of 13% and 0%, respectively (p<0.001, Table 3). A similar trend was observed in patients with ≥4 positive lymph nodes. The favorable outcomes seen in patients with HR+ and HER2+ breast cancer are most likely due to the receipt of ongoing adjuvant anti-estrogen and anti-HER2 therapies. Furthermore, the discrepant outcomes observed between incomplete responders with TN versus other subtypes of breast cancer raises the question of how to tailor axillary treatment in such patients. The ongoing ALLIANCE A011202 trial18 is designed to elucidate whether radiation alone can provide sufficient regional control in patients with ypN+ disease following NAC. Our findings suggest that outcomes with radiation alone may differ by subtype since high LRR rate was observed among TN patients with ypN+ disease treated with both ALND and radiotherapy in our study, who are the subject of the ALLIANCE trial.
We observed isolated internal mammary lymph nodes recurrence as the most common site of regional failure. Among the 7 patients with internal mammary lymph node recurrence, only 1 had received irradiation to this site. These findings suggest that the internal mammary nodes should be routinely targeted among patients receiving nodal irradiation following NAC, particularly in TN breast cancer patients who have high rates of LRR. The low number of LRR events and small patient subsets preclude us from concluding whether or not routine omission of internal mammary lymph nodes irradiation is acceptable in HR+ and HER2+ breast cancer patients, who had low rates of LRR even in the setting of incomplete response to NAC.
In conclusion, our study provides strong evidence that biology plays a critical role in determining locoregional outcomes of patients receiving NAC. Response to NAC and biologic subtype are predictors of LRR in patients who have received mastectomy and PMRT. The poor-responders to NAC with TN breast cancer represent a population at high risk for LRR and maximal locoregional therapy should be considered in these patients. Furthermore, clinical trials evaluating novel treatment approaches to improve locoregional control, such as concurrent PARP inhibitors or chemotherapy with PMRT, are warranted in these patients. Conversely, the favorable LRR rates in patients with HR+ breast cancer suggests that the extent of locoregional therapy may be limited in such patients, even when pCR is not obtained. Larger studies evaluating clinicopathologic variables on LRR in these favorable-risk subsets will help us determine the appropriate treatments in these patients.
Synopsis.
Patients with triple negative breast cancer with residual disease after neoadjuvan chemotherapy had higher rate of locoregional recurrence (26%, p<0.001) compared to patients with HER2+ (7%) and hormone receptor positive (4%) breast cancer.
Footnotes
Conflict of Interest Statement: There is no conflict of interest of any of the authors.
References
- 1.Overgaard M, Hansen PS, Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. New Engl J Med. 1997 Oct 2;337(14):949–955. doi: 10.1056/NEJM199710023371401. [DOI] [PubMed] [Google Scholar]
- 2.Overgaard M, Jensen MB, Overgaard J, et al. Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet. 1999 May 15;353(9165):1641–1648. doi: 10.1016/S0140-6736(98)09201-0. [DOI] [PubMed] [Google Scholar]
- 3.Ragaz J, Olivotto IA, Spinelli JJ, et al. Locoregional radiation therapy in patients with high-risk breast cancer receiving adjuvant chemotherapy: 20-year results of the British Columbia randomized trial. Jnci-J Natl Cancer I. 2005 Jan 19;97(2):116–126. doi: 10.1093/jnci/djh297. [DOI] [PubMed] [Google Scholar]
- 4.Ebctcg, McGale P, Taylor C, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014 Jun 21;383(9935):2127–2135. doi: 10.1016/S0140-6736(14)60488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008 Mar 10;26(8):1275–1281. doi: 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- 6.von Minckwitz G, Untch M, Blohmer JU, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012 May 20;30(15):1796–1804. doi: 10.1200/JCO.2011.38.8595. [DOI] [PubMed] [Google Scholar]
- 7.Bonnefoi H, Litiere S, Piccart M, et al. Pathological complete response after neoadjuvant chemotherapy is an independent predictive factor irrespective of simplified breast cancer intrinsic subtypes: a landmark and two-step approach analyses from the EORTC 10994/BIG 1-00 phase III trial. Ann Oncol. 2014 Jun;25(6):1128–1136. doi: 10.1093/annonc/mdu118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Symmans WF, Peintinger F, Hatzis C, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007 Oct 1;25(28):4414–4422. doi: 10.1200/JCO.2007.10.6823. [DOI] [PubMed] [Google Scholar]
- 9.Mamounas EP, Anderson SJ, Dignam JJ, et al. Predictors of Locoregional Recurrence After Neoadjuvant Chemotherapy: Results From Combined Analysis of National Surgical Adjuvant Breast and Bowel Project B-18 and B-27. J Clin Oncol. 2012 Nov 10;30(32):3960–3966. doi: 10.1200/JCO.2011.40.8369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meyers MO, Klauber-DeMore N, Ollila DW, et al. Impact of Breast Cancer Molecular Subtypes on Locoregional Recurrence in Patients Treated with Neoadjuvant Chemotherapy for Locally Advanced Breast Cancer. Ann Surg Oncol. 2011 Oct;18(10):2851–2857. doi: 10.1245/s10434-011-1665-8. [DOI] [PubMed] [Google Scholar]
- 11.Nagar H, Mittendorf EA, Strom EA, et al. Local-Regional Recurrence with and without Radiation Therapy after Neoadjuvant Chemotherapy and Mastectomy for Clinically Staged T3n0 Breast Cancer. Int J Radiat Oncol. 2011 Nov 1;81(3):782–787. doi: 10.1016/j.ijrobp.2010.06.027. [DOI] [PubMed] [Google Scholar]
- 12.Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proceedings of the National Academy of Sciences of the United States of America. 2001 Sep 11;98(19):10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parker JS, Mullins M, Cheang MC, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. 2009 Mar 10;27(8):1160–1167. doi: 10.1200/JCO.2008.18.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mamounas EP, Cortazar P, Zhang L, Von Minckwitz G, Mehta K. Locoregional recurrence (LRR) after neoadjuvant chemotherapy (NAC): Pooled-analysis results from the collaborative trials in neoadjuvant breast cancer (CTNeoBC). J Clin Oncol. 2014;32(suppl 26) abstr 61. [Google Scholar]
- 15.Huang EH, Tucker SL, Strom EA, et al. Predictors of locoregional recurrence in patients with locally advanced breast cancer treated with neoadjuvant chemotherapy, mastectomy, and radiotherapy. International journal of radiation oncology, biology, physics. 2005 Jun 1;62(2):351–357. doi: 10.1016/j.ijrobp.2004.09.056. [DOI] [PubMed] [Google Scholar]
- 16.Wright JL, Takita C, Reis IM, et al. Predictors of locoregional outcome in patients receiving neoadjuvant therapy and postmastectomy radiation. Cancer. 2013 Jan 1;119(1):16–25. doi: 10.1002/cncr.27717. [DOI] [PubMed] [Google Scholar]
- 17.Caudle AS, Yu TK, Tucker SL, et al. Local-regional control according to surrogate markers of breast cancer subtypes and response to neoadjuvant chemotherapy in breast cancer patients undergoing breast conserving therapy. Breast Cancer Res. 2012;14(3) doi: 10.1186/bcr3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Comparison of Axillary Lymph Node Dissection With Axillary Radiation for Patients With Node-Positive Breast Cancer Treated With Chemotherapy. NCT01901094. 2014 ( https://clinicaltrials.gov/ct2/show/record/NCT01901094)